Optimized Extraction of Passiflora ligularis Pectins: Characterization and Application in Moisturizing Cosmetic Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Exploratory Extraction
2.3. Experimental Design
2.4. Pectin Characterization
2.5. Swelling Capacity and Water Retention Capacity
2.6. ATR-FTIR and NMR Spectroscopy
2.7. Preparation of Cosmetic Formulations
2.8. Preliminary Stability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Exploratory Extraction
3.2. Experimental Design
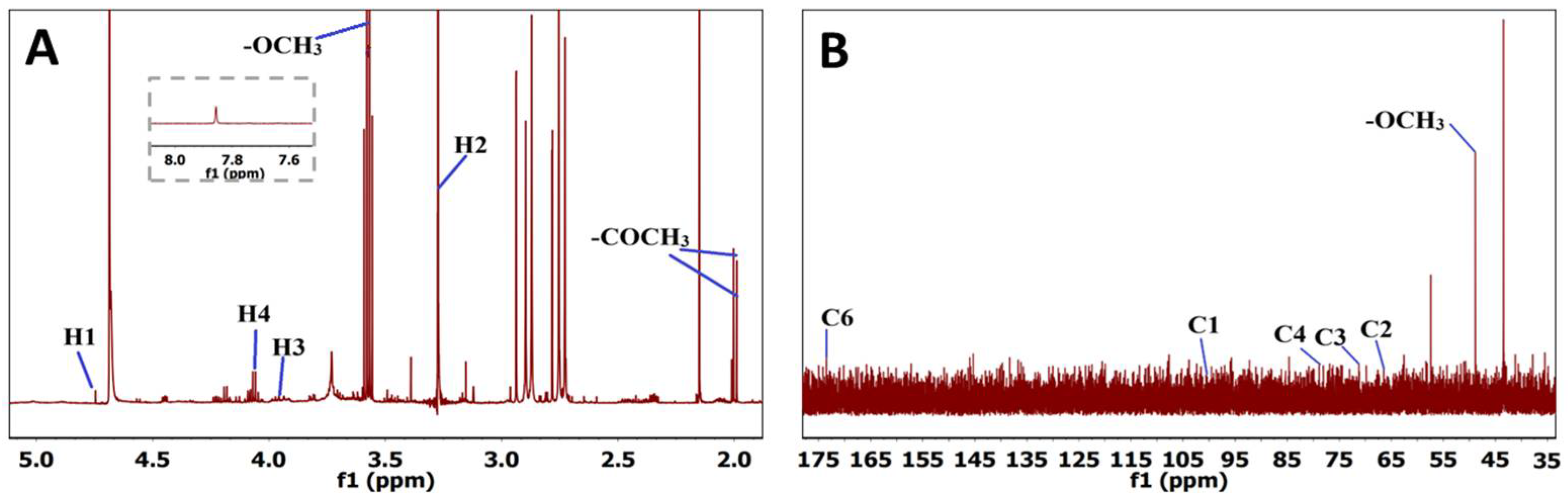
3.3. Pectin Characterization
3.4. Cosmetic Gel Formulations and Preliminary Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.C.; Ma, S.H.; Chang, Y.T.; Chen, C.C. Clinical Evaluation of Skin Aging: A Systematic Review. Arch. Gerontol. Geriatr. 2025, 139, 105995. [Google Scholar] [CrossRef] [PubMed]
- Klotz, T.; Moran, H.; Vu, P.; Maddern, G.; Wagstaff, M. Commonly Recommended Moisturising Products: Effect on Transepidermal Water Loss and Hydration in a Scar Model. Burns 2025, 51, 107629. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Xiong, R.; Wang, J.; Zhang, X.; Liu, J.; Shen, J. A Dehydratable and Rapidly Rehydratable Hydrogel for Stable Storage and On-Demand Moisturizing Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 138102. [Google Scholar] [CrossRef]
- Luna-Perez, Y.; Puertas-Mejia, M.A.; Mejia-Giraldo, J.C. Marine Macroalgae: A Source of Chemical Compounds with Photoprotective and Antiaging Capacity—An Updated Review. J. Appl. Pharm. Sci. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Lin, L.; Zheng, G. Extraction, Moisturizing Activity and Potential Application in Skin Cream of Akebia Trifoliata (Thunb.) Koidz Polysaccharide. Ind. Crops Prod. 2023, 197, 116613. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Nagel, A.; Winkler, C.; Carle, R.; Endress, H.; Rentschler, C.; Neidhart, S. Processes Involving Selective Precipitation for the Recovery of Purified Pectins from Mango Peel. Carbohydr. Polym. 2017, 174, 1144–1155. [Google Scholar] [CrossRef]
- Koubala, B.B.; Kansci, G.; Mbome, L.I.; Crépeau, M.J.; Thibault, J.F.; Ralet, M.C. Effect of Extraction Conditions on Some Physicochemical Characteristics of Pectins from “Améliorée” and “Mango” Mango Peels. Food Hydrocoll. 2008, 22, 1345–1351. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; González-Correa, C.H. Folin-Ciocalteu Reaction Alternatives for Higher Polyphenol Quantitation in Colombian Passion Fruits. Int. J. Food Sci. 2021, 2021, 8871301. [Google Scholar] [CrossRef]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Dominguéz, M.C. Extraction of Valuable Compounds from Granadilla (Passiflora ligularis Juss) Peel Using Pressurized Fluids Technologies. Sustain. Chem. Pharm. 2023, 34, 101135. [Google Scholar] [CrossRef]
- Rey, D.; Fernandes, T.A.; Sulis, P.M.; Gonçalves, R.; Sepúlveda, R.M.; Silva Frederico, M.J.; Aragon, M.; Ospina, L.F.; Costa, G.M.; Silva, F.R.M.B. Cellular Target of Isoquercetin from Passiflora Ligularis Juss for Glucose Uptake in Rat Soleus Muscle. Chem. Biol. Interact. 2020, 330, 109198. [Google Scholar] [CrossRef] [PubMed]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Domínguez, M.C. Valorization of Granadilla Waste (Passiflora ligularis, Juss.) by Sequential Green Extraction Processes Based on Pressurized Fluids to Obtain Bioactive Compounds. J. Supercrit. Fluids 2023, 194, 105833. [Google Scholar] [CrossRef]
- Barreto, G.E.; Púa, A.L.; De Alba, D.D.; Pión, M.M. Extracción y Caracterización de Pectina de Mango de Azúcar (Mangifera indica L.) Extraction and Characterization of Pectin from Sugar Mango (Mangifera indica L.). Temas Agrar. 2017, 2, 77–84. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An Emerging New Bioactive Food Polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Freitas de Oliveira, C.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Ferreira Marczak, L.D. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT-Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Silva, N.C.; Benites, E.A.; Carlos, J.; Gomero, M. Extracción y Caracterización de Pectinas Obtenidas a Partir de Frutos de La Biodiversidad Peruana Extraction and Characterization of Pectins in Several Types of Fruits of the Peruvian Biodiversity. Ing. Ind. 2008, 26, 175–199. [Google Scholar] [CrossRef]
- Kley Valladares-Diestra, K.; Porto de Souza Vandenberghe, L.; Ricardo Soccol, C. A Biorefinery Approach for Pectin Extraction and Second-Generation Bioethanol Production from Cocoa Pod Husk. Bioresour. Technol. 2022, 346, 126635. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical Properties of Pectin from Malus Domestica ‘Fălticeni’ Apple Pomace as Affected by Non-Conventional Extraction Techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave Heating Extraction of Pectin from Lime Peel: Characterization and Properties Compared with the Conventional Heating Method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Dixit, S.S.; Muruganandam, L.; Ganesh Moorthy, I. Pectin from Fruit Peel: A Comprehensive Review on Various Extraction Approaches and Their Potential Applications in Pharmaceutical and Food Industries. Carbohydr. Polym. Technol. Appl. 2025, 9, 100708. [Google Scholar] [CrossRef]
- Rentería-Ortega, M.; de Colín-Alvarez, M.L.; Gaona-Sánchez, V.A.; Chalapud, M.C.; García-Hernández, A.B.; León-Espinosa, E.B.; Valdespino-León, M.; Serrano-Villa, F.S.; Calderón-Domínguez, G. Characterization and Applications of the Pectin Extracted from the Peel of Passiflora Tripartita Var. Mollissima. Membranes 2023, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.J.; Sarkar, S.; Sharmin, T.; Mondal, S.C. Extraction of Pectin from Powdered Citrus Peels Using Various Acids: An Analysis Contrasting Orange with Lime. Appl. Food Res. 2024, 4, 100614. [Google Scholar] [CrossRef]
- Shourove, J.H.; Jon, P.H.; Samadder, M.; Chy, M.W.R.; Miah, M.S.; Fahim, R.H.; Islam, G.M.R. Extraction of Pectin from Watermelon Rinds Using Sequential Ultrasound-Microwave Technique: Optimization Using RSM and ANN Modeling and Characterization. Int. J. Biol. Macromol. 2025, 307, 141905. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Qi, J.; Jiang, W.; Yang, X. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- European Commission CosIng—Cosmetics Ingredients. Available online: https://ec.europa.eu/growth/tools-databases/cosing/advanced (accessed on 5 February 2024).
- Marque, C.; Pensé-Lhéritier, A.-M.; Bacle, I. Chapter 9—Sensory Methods for Cosmetics Evaluation. In Nonfood Sensory Practices; Pensé-lhéritier, A.-M., Bacle, I., Delarue, J., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 169–196. [Google Scholar] [CrossRef]
- Mérat, E.; Roso, A.; Dumaine, M.; Sigurani, S. Sensory Evaluation of Cosmetic Functional Ingredients. Nonfood Sesory Pract. 2022, 197–216. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Winkler, R.; Puertas-Mejía, M. Novel UV Filters from Pentacalia Pulchella Extracts with Photoprotective Properties and Antioxidant Activity. Photochem. Photobiol. Sci. 2021, 20, 1585–1597. [Google Scholar] [CrossRef]
- Agencia Nacional de Vigilancia Sanitaria. Guía de Estabilidad de Productos Cosméticos; Agencia Nacional de Vigilancia Sanitaria: Brasilia, Brasil, 2005; pp. 1–52.
- ISO/TR 18811:2018; Cosmetics—Guidelines on the Stability Testing of Cosmetic Products. ISO—International Standards Organization: Geneva, Switzerland, 2018; pp. 1–16.
- Pablo Díaz-Castillo, J.; Jhoana Mier Giraldo, H.; Fernando Sánchez, M.; Nuñez Hernandez, G.; Lucia Camargo Gómez, C.; Janneth Moyano Bonilla, L. Estudios de Estabilidad de Productos Cosméticos Recomendaciones Para El Desarrollo de Supervisión y Coordinación: Programa de Calidad Para El Sector Cosméticos-Safe+; Consultor Nacional Calidad Cosméticos, Programa Safe+ de ONUDI Investigación y Escritura; Organización de las Naciones Unidas para el Desarrollo Industrial: Viena, Austria, 2018; pp. 1–90. [Google Scholar]
- Nadar, C.G.; Arora, A.; Shastri, Y. Sustainability Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. Au 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of New Cellulose Nanomaterial from Red Algae Marine Biomass Gelidium Elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef]
- Yapo, B.M. Biochemical Characteristics and Gelling Capacity of Pectin from Yellow Passion Fruit Rind as Affected by Acid Extractant Nature. J. Agric. Food Chem. 2009, 57, 1572–1578. [Google Scholar] [CrossRef]
- Rolin, G.B.C.; Seymour, J.P.K. Pectins and Their Manipulation; Blackwell: Oxford, UK, 2002. [Google Scholar]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and Characterization of Pectin from Passion Fruit Peels. Agric. Agric. Sci. Procedia 2014, 2, 231–236. [Google Scholar] [CrossRef]
- Mao, Y.; Robinson, J.P.; Binner, E.R. Current Status of Microwave-Assisted Extraction of Pectin. Chem. Eng. J. 2023, 473, 145261. [Google Scholar] [CrossRef]
- Sarah, M.; Hasibuan, I.M.; Misran, E.; Maulina, S. Optimization of Microwave-Assisted Pectin Extraction from Cocoa Pod Husk. Molecules 2022, 27, 6544. [Google Scholar] [CrossRef] [PubMed]
- Indriani, R.; Legowo, A.M.; Susanti, S. Characteristics of Pectin Isolated from Mango (Mangifera indica) and Watermelon (Citrullus vulgaris) Peel. J. Appl. Food Technol. 2012, 4, 31–34. [Google Scholar] [CrossRef]
- Susanti, S.; Legowo, A.M.; Nurwantoro, N.; Silviana, S.; Arifan, F. Comparing the Chemical Characteristics of Pectin Isolated from Various Indonesian Fruit Peels. Indones. J. Chem. 2021, 21, 1057–1062. [Google Scholar] [CrossRef]
- Reichembach, L.H.; de Oliveira Petkowicz, C.L. Extraction and Characterization of a Pectin from Coffee (Coffea arabica L.) Pulp with Gelling Properties. Carbohydr. Polym. 2020, 245, 116473. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, W.; Ai, B.; Zheng, L.; Zheng, X.; Xiao, D.; Sheng, Z.; Yang, J.; Wang, S. Passion Fruit Peel-Derived Low-Methoxyl Pectin: De-Esterification Methods and Application as a Fat Substitute in Set Yogurt. Carbohydr. Polym. 2025, 347, 122664. [Google Scholar] [CrossRef]
- Valdivia-Rivera, S.; Herrera-Pool, I.E.; Ayora-Talavera, T.; Lizardi-Jiménez, M.A.; García-Cruz, U.; Cuevas-Bernardino, J.C.; Cervantes-Uc, J.M.; Pacheco, N. Kinetic, Thermodynamic, Physicochemical, and Economical Characterization of Pectin from Mangifera indica L. Cv. Haden Residues. Foods 2021, 10, 2093. [Google Scholar] [CrossRef]
- Lin, Y.; An, F.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and Rheological Characterization of Pectin from Passion Fruit (Passiflora edulis f. flavicarpa) Peel Extracted by High-Speed Shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Contreras-Esquivel, J.C.; Aguilar, C.N.; Montanez, J.C.; Brandelli, A.; Espinoza-Perez, J.D.; Renard, C.M.G.C. Pectin from Passion Fruit Fiber and Its Modification by Pectinmethylesterase. J. Food Sci. Nutr. 2010, 15, 57–66. [Google Scholar] [CrossRef]
- Kozioł, A.; Środa-Pomianek, K.; Górniak, A.; Wikiera, A.; Cyprych, K.; Malik, M. Structural Determination of Pectins by Spectroscopy Methods. Coatings 2022, 12, 546. [Google Scholar] [CrossRef]
- Yang, X.; Wang, K.; Zhong, Y.; Cui, W.; Jia, X.; Yin, L. Synthesis, Characterization and Application of Sugar Beet Pectin-Ferulic Acid Conjugates in the Study of Lipid, DNA and Protein Oxidation. Int. J. Biol. Macromol. 2025, 307, 141358. [Google Scholar] [CrossRef]
- Lin, Y.; He, H.; Huang, Q.; An, F.; Song, H. Flash Extraction Optimization of Low-Temperature Soluble Pectin from Passion Fruit Peel (Passiflora edulis f. flavicarpa) and Its Soft Gelation Properties. Food Bioprod. Process. 2020, 123, 409–418. [Google Scholar] [CrossRef]
- Kar, M.; Chourasiya, Y.; Maheshwari, R.; Tekade, R.K. Chapter 2—Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Oxford, UK, 2019; pp. 29–83. [Google Scholar] [CrossRef]

| Ingredient (INCI Name) | Concentration (% w/w) | Main Function |
|---|---|---|
| Aqua | 93.1 | Solvent |
| Pectin (P. ligularis) | 5.0 | Binder/Emulsion stabilizer |
| Phenoxyethanol, Ethylhexylglycerin | 1.0 | Antimicrobial/Preservative |
| Carbomer | 0.5 | Rheology modifier |
| Ascorbic acid | 0.4 | Antioxidant |
| Sodium Hydroxide | q.s. | pH adjuster |
| INCI Name | Concentration (% w/w) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Main Function | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | ||
| Aqua | Solvent | q.s. to 100% | ||||||||
| Pectin (P. ligularis) | 5.0 | Moisturizing/Gelling agent | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Phenoxyethanol and Ethylhexyglycerin | 1.0 | Preservative | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Ascorbic Acid | 0.4 | Antioxidant | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Sodium Hydroxide (1 M) | q.s. | pH adjuster | q.s. * | q.s. * | q.s. * | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Carbomer | 0.5/1.0 | Rheology modifier | 0.0 | 0.5 | 1.0 | 1.0 | 0.0 | 0.0 | 1.0 | 1.0 |
| Triethanolamine (TEA) | 2.8 | pH adjuster | 0.0 | 0.0 | 0.0 | 2.4 (pH6.5) | 2.4 (pH5.5) | 2.4 (pH5.5) | 2.2 (pH6.0) | 2.0 (pH5.5) |
| Guar Hydroxypropyltrimonium Chloride | 0.4/1.0 | Rheology modifier | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 1.0 | 0.0 | 0.0 |
| Condition | Parameter |
|---|---|
| Centrifugation | 3000 rpm, 30 min |
| High temperature | 40 °C, 24 h |
| Room temperature | 25 °C, 24 h |
| Refrigeration | 2–8 °C, 24 h |
| Freezing | −5 °C, 24 h |
| Sunlight (UVA, UVB, Visible) | 2 h |
| Experiment | Method | Solvent | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| E1 | Reflux | Water | 60 | 15 | 6.387 ± 0.400 |
| E2 | Reflux | Water | 60 | 25 | 6.149 ± 1.400 |
| E3 | Reflux | Water | 50 | 15 | 6.067 ± 1.100 |
| E4 | Reflux | Water | 50 | 25 | 5.847 ± 1.800 |
| E5 | Reflux | Citric acid | 60 | 15 | 0.499 ± 0.300 |
| E6 | Reflux | Citric acid | 60 | 25 | 0.513 ± 0.080 |
| E7 | Reflux | Citric acid | 50 | 15 | 1.886 ± 0.200 |
| E8 | Reflux | Citric acid | 50 | 25 | 0.965 ± 0.300 |
| E9 * | Microwave | Water | 60 | 15 | 19.300 ± 0.100 |
| E10 * | Microwave | Water | 60 | 25 | 22.435 ± 3.500 |
| E11 | Microwave | Water | 50 | 15 | 13.544 ± 6.800 |
| E12 * | Microwave | Water | 50 | 25 | 14.530 ± 0.300 |
| E13 | Microwave | Citric acid | 60 | 15 | 45.970 ± 5.500 |
| E14 * | Microwave | Citric acid | 60 | 25 | 32.855 ± 0.700 |
| E15 | Microwave | Citric acid | 50 | 15 | 47.373 ± 3.500 |
| E16 | Microwave | Citric acid | 50 | 25 | 25.496 ± 2.200 |
| Parameter | Result ± SD |
|---|---|
| Moisture (%) | 0.13 ± 0.01 |
| Acidity (%) | 0.42 ± 0.01 |
| Methoxyl content (%) | 9.05 ± 0.01 |
| Degree of esterification (%) | 57.6 ± 0.03 |
| Swelling capacity (mL/g) | 12.46 ± 0.01 |
| Water retention (%) | 12.26 ± 0.01 |
| Formulation | Gelling System | pH Range | Centrifugation (Day 1) | Heat Stability (40 °C) | Cold Stability (2–8 °C) | Viscosity Change | Sensory Changes (Color/Odor/Texture) |
|---|---|---|---|---|---|---|---|
| F1 | Pectin only | 4.5–5.0 | Stable | Significant loss | Moderate precipitation | ↓↓ | Color/odor altered |
| F2 | Carbomer (pH 6.0) | 6.0–6.2 | Stable | Moderate stability | Strong precipitation | ↓↓ | Odor decreased, texture affected |
| F3 | Carbomer (pH 6.2) | 6.0–6.3 | Stable | Moderate stability | Strong precipitation | ↓↓ | Odor decreased, pH fluctuation |
| F4 | Carbomer (pH 6.5) | 6.3–6.5 | Stable | Moderate stability | Strong precipitation | ↓↓ | Texture/graininess |
| F5 | Guar gum | 5.0–5.5 | Stable | Major instability | Precipitation | ↓↓↓ | Residues on skin, odor altered |
| F6 | Guar gum | 5.5–6.0 | Stable | Major instability | Precipitation | ↓↓↓ | Fluid consistency, odor loss |
| F7 | Carbomer (neutralized, pH ≥ 6.5) | 6.5–6.7 | Stable | High stability | Mild precipitation | ↓ | Good color/odor retention |
| F8 | Carbomer (neutralized, pH ≥ 6.5) | 6.6–6.8 | Stable | High stability | Mild precipitation | ↓ | Best preserved texture & odor |
| Formulation | Thermal Stability | Cold Stability | pH Stability | Sensory Attributes (Odor, Texture, Spreadability) | Overall Performance |
|---|---|---|---|---|---|
| F1 | Low | Moderate | Stable | Altered color/odor, rigid texture | Poor |
| F2–F4 | Moderate | Low | Unstable | Reduced odor, precipitation, texture changes | Limited |
| F5–F6 | Very low | Low | Stable | Residues on skin, loss of viscosity, odor loss | Poor |
| F7 | High | Moderate | Moderate | Good texture/odor retention | Good |
| F8 | High | Moderate | Moderate | Excellent texture, odor, and spreadability | Best (selected) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restrepo-Zapata, M.C.; Chacón-Pabón, P.A.; Montoya-Henao, E.; Muñoz-Castiblanco, D.T.; Mejía-Giraldo, J.C. Optimized Extraction of Passiflora ligularis Pectins: Characterization and Application in Moisturizing Cosmetic Products. Cosmetics 2025, 12, 261. https://doi.org/10.3390/cosmetics12060261
Restrepo-Zapata MC, Chacón-Pabón PA, Montoya-Henao E, Muñoz-Castiblanco DT, Mejía-Giraldo JC. Optimized Extraction of Passiflora ligularis Pectins: Characterization and Application in Moisturizing Cosmetic Products. Cosmetics. 2025; 12(6):261. https://doi.org/10.3390/cosmetics12060261
Chicago/Turabian StyleRestrepo-Zapata, Maria Camila, Paola Andrea Chacón-Pabón, Estefanía Montoya-Henao, Deysi Tatiana Muñoz-Castiblanco, and Juan Camilo Mejía-Giraldo. 2025. "Optimized Extraction of Passiflora ligularis Pectins: Characterization and Application in Moisturizing Cosmetic Products" Cosmetics 12, no. 6: 261. https://doi.org/10.3390/cosmetics12060261
APA StyleRestrepo-Zapata, M. C., Chacón-Pabón, P. A., Montoya-Henao, E., Muñoz-Castiblanco, D. T., & Mejía-Giraldo, J. C. (2025). Optimized Extraction of Passiflora ligularis Pectins: Characterization and Application in Moisturizing Cosmetic Products. Cosmetics, 12(6), 261. https://doi.org/10.3390/cosmetics12060261






