Abstract
Benefit/risk management of skin exposure to sunlight, especially ultraviolet (UV) rays, is mainly driven by photoaging, cancer incidence, and the requirement for vitamin D3 synthesis. Antioxidant phytocompounds are considered to be a valuable source of molecules to protect skin from UV-induced damage, but their impact on other UV-related metabolic pathways is rarely described. In this study, an indigoid-rich Persicaria tinctoria extract (PTE) was evaluated on three consequences of UV exposure: DNA damage and inflammation, vitamin D3 content, and melanogenesis. A moderate UV exposure was applied on skin models, corresponding to approximately 1 h exposure in the spring in western Europe. UV-induced DNA damage and inflammation were measured through the quantification of cyclobutane pyrimidine dimers (CPDs) and cytokines. Response to heat stress was quantified through the release of prostaglandin. Then, the impact of PTE on vitamin D3 and melanin synthesis was observed. PTE decreased by −56% in the number of cells presenting CPDs. PTE decreased the production of pro-inflammatory cytokine IL-6 (−59%) and stimulated the release of the protective cytokine IL-1Ra (+49%). It decreased PGE2 release by −27%. In skin explants, PTE boosted the vitamin D3 concentration (+345%). Several genes involved in melanogenesis were up-regulated by PTE (MC1R × 2.46, MITF × 1.69, TYR × 2.06, MLPH × 1.53). It promoted melanin content by +126% and by +86% when associated with SPF 30. The extract decreased the amount of protective eumelanin, leading to visible skin tanning of reconstructed human epidermis (L*-15%, ITA −125%). As a new finding, PTE minimized DNA damage and inflammation caused by a daily dose of UV, and surprisingly, promoted vitamin D3 and eumelanin synthesis, suggesting that it represents an opportunity to reconcile skin protection and the physiological need for sunlight.
1. Introduction
Natural light reaching the earth is composed of ultraviolet rays with ultraviolet A (UVA, 400–320 nm), accounting for 95% of the UV spectrum, and highly energetic ultraviolet B (UVB, 290–200 nm) for 5% [1]. Both types of radiation trigger harmful effects on skin structure. UVB mainly penetrate the epidermis and are responsible for sunburn-related inflammatory responses [2]. Directly absorbed by DNA, UVB also display mutagenic and carcinogenic properties, notably through the production of cyclobutane pyrimidine dimers (CPDs) [3]. UVA penetrate skin deeper, up to the dermis, and are now considered as a major cause of photoaging, notably by producing oxidative stress and damaging lipids, proteins or DNA [4,5,6].
Both acute sunlight exposure and non-extreme daily UV rays are the leading causes of photodamage and participate in the long-term process of photoaging [7,8,9]. Chronic sub-erythemal UV radiation increases similar markers observed with acute doses as sunburn cell formation and accumulation of the p53 protein related to DNA damage. Within the epidermal compartment, oxidative stress favors lipids peroxidation and alters skin barrier function [10]. In the dermis, such low repeated UV exposure decreases structural components such as fibrillin, pro-collagen 1, and glycosaminoglycan deposition, contributing to wrinkle appearance [8]. Chronic UV rays could also be more prone to affecting the immune function [11]. Therefore, the use of broad-spectrum sunscreen and antioxidants in the everyday life is recommended to limit photodamages [12,13,14].
On the other hand, sunlight contributes to the emergence of life on earth, providing energy for carbohydrate production through photosynthesis [15]. Natural light provides health benefits for the human skin as well. UVB contributes to vitamin D3 synthesis, an essential molecule, of which its deficiency is associated with unfavorable outcomes and severe diseases (growth retardation; infectious, autoimmune or cardiovascular diseases; cancer) [16,17]. In addition, insufficient sun exposure is considered to be a public health problem [17,18]. Determining an adequate UV dose to provide sufficient vitamin D3 and avoid skin photodamage is not trivial and depends on the skin phototype [1,19]. It is demonstrated that short sunlight exposure in summer (about 20 min, 7–80 mJ/cm2, depending on studies) provides sufficient vitamin D3, but few data are available for the other seasons where UV irradiance decreases and is associated with vitamin D3 deficiency [20,21]. Skin pigmentation has evolved in the human lineage by meeting two essential requirements: providing relative photoprotection with melanin pigment in latitude with high UV, and sustaining UVB-related synthesis of vitamin D3 in low-UV environments [22]. Melanin is synthesized in melanocytes, located in the basal layer of the epidermis [23,24]. Pigment is produced in specific organelles, the melanosomes, which are transferred to epidermal keratinocytes, ensuring melanin migration from the deepest layer of epidermis towards the surface [25]. Regulation of melanogenesis involves complex pathways, which can be initiated by UV radiation and UV-induced DNA damage [1]. One of these pathways is triggered by the neurohormone αMSH (melanocyte-stimulating hormone) interacting with the MC1R receptor (melanocortin 1 receptor). A pivotal element of these signals’ integration is MITF (microphtalmia-associated transcription factor), which directly regulates expression of tyrosinase genes encoding enzymes in melanin metabolic pathway [26]. Melanin contributes to skin protection by absorbing 50 to 75% of UV rays: it exists as an inverse correlation between pigmentation and skin cancer incidence, as well as CPDs, but photoprotection remains essential for all phototypes to efficiently reduce damage [1,27]. Two molecules constitute melanin: dark brown eumelanin and red/yellow pheomelanin [28]. Eumelanin is more stable, persisting in keratinocytes of dark skin and is highly efficient as a ROS scavenger. It is therefore considered as the main photoprotective form of melanin.
Botanicals represent a valuable source of skin-protective molecules against UV-induced photoaging [29]. Persicaria tinctoria (Aiton), also named Polygonum tinctorium, is an indigo plant from which a blue powder called indigo naturalis is extracted [30]. It is part of Chinese and Korean traditional medicine, with interesting properties against inflammation, psoriasis, and atopic dermatitis [30,31,32,33]. The main components of indigo naturalis are indole alkaloids, such as indirubin. This molecule is responsible for the anti-psoriatic activity of the plant extract by decreasing hyper-proliferation of epidermal keratinocytes, and displays antioxidant and anti-inflammatory effects [34,35,36,37].
In this work, a dose of UVA and UVB was determined to be representative of 1 h of daylight exposure during spring in Europe. Applied on skin explants, this dose did not affect melanin synthesis but increased the formation of pro-carcinogenic CPDs. Indigoid-rich P. tinctoria extract (PTE) was evaluated in different skin models regarding two main deleterious effects of UV: DNA damage and inflammation. The impact of PTE on vitamin D3 content in skin and melanin synthesis was also explored.
2. Materials and Methods
2.1. Persicaria Tinctoria Extract (PTE)
Persicaria tinctoria was cultivated in France and fresh aerial parts were harvested between mid-July to mid-September. Indican, a hydrophilic and colorless indoxyl-β-D-glucoside located in vacuoles, was extracted with hot water from fresh leaves. The extraction procedure is available in the patent application WO2024223872. Simultaneous extraction of β-glucosidases from chloroplasts caused the hydrolysis of the glucose moiety of indican. The aglycone is reactive and spontaneously dimerized into indigoids molecules. After solid–liquid filtration to remove plant residuals, calcium hydroxide was added to reach pH 9–11 and, in association with aeration, allowed to accelerate the dimerization process in favor of the formation of indirubin and indigotin that precipitated. The solution was finally filtered, dried, and ground before sieving to obtain indigo blue powder. Indirubin was selectively extracted from the indigo powder. The enriched fraction, formulated in moringa oil, was standardized within a range of indirubin content between 250 and 450 ppm. The ingredient was stabilized with an antioxidant rosemary extract titrated in carnosic acid. Following a toxicological study, it was determined that the maximum usage dose for topical body application is 0.8% of PTE and this concentration was selected to explore the multifaceted consequences of the UV exposure on human skin.
2.2. Quantification of Cyclobutane Pyrimidine Dimers (CPDs) Under Moderate Daily UV Exposure
2.2.1. Skin Explants Culture and Preparation
Human skin explants were obtained with informed consent from abdominal surgery (44 years old female donor, phototype IV). The explants were kept alive by culturing on biocompatible plastic grids into standard 24-well plates in an air–liquid interface with 600 µL of skin culture medium (Genoskin®, Toulouse, France) at 37 °C in 5% CO2 humidified air. The culture medium (600 µL) was renewed every 24 h. Skin explants were topically treated with placebo emulsion and emulsion (20 µL) containing PTE at 0.8% following this composition: aqua/water (83.3%), Emulium® Delta (5%), isodecyl neopentanoate (4.5%), phenoxyethanol (1%), ± Polygonum tinctorium leaf/stem extract (0.8%), dimethicone (0.3%) and fragrance (0.1%). The control condition was left untreated. After 3 days of incubation, the skin explants were rinsed with 600 µL of PBS (phosphate-buffered saline), repeated twice, and PBS were then replaced by 600 µL HBSS for performing the UV irradiation as follows: irradiated at 4.5 J/cm2 of UVA (λ = 365 nm) and 0.15 J/cm2 of UVB (λ = 312 nm) (Bio-Sun Vilber, Wittlich, Germany). Then, the treatment (20 µL) was topically applied again for 24 h, before a second irradiation at the same doses. Twenty-four hours after the second irradiation, the skin explants surfaces were rinsed with PBS, then fixed in formaldehyde solution 20 mL with 3 triplicates for 48 h at 4 °C.
2.2.2. Immunostaining of CPDs
The fixed samples were then dehydrated in successive ethanol baths of increasing concentrations from 0 to 99%, then a last batch of Xylene, before being embedded in paraffin by using an automated PEARL system (LEICA). The transversal sections were performed using a microtome (5 μm thickness, 2 sections per slide, 1 slide per skin explant) and kept at room temperature until analysis. The sections were deparaffinized by successive batches staring by Xylene, decreasing concentration of ethanol from 99% to 0%, and then water by using atomized system Autostainer (Leica Biosystems, Argenteuil, France). The sections were incubated at 95 °C for 20 min in an unmasking citrate solution 1× to optimize antigen–antibody interaction. The slides were cooled down at room temperature in the same solution. After saturation with TBS-Tween-2% BSA (200 µL), the sections were incubated overnight at 5 °C with the primary antibody (Cosmobio, Tokyo, Japan; 1/500 in TBSB) solution directed against the marker of interest (CPDs). After washing twice with PBS (300 µL), the binding sites recognized by the primary antibody were revealed by a secondary fluorescent antibody (GAR-568) diluted at 1/500 in TBSB. The sections were mounted with Prolong-containing DAPI to color the nuclei. Then, the sections were observed using a ZEISS 710 confocal microscope (ZEISS, Jena, Germany). Images were captured and processed with ZEN software (Blue Edition 2.6, objective lens ×20). The number of CPDs’ positive cells was counted using ImageJ software. The formation of CPDs was quantified by CPDs’ positive cell number normalized to the area of nuclear layers of the epidermis.
2.3. Quantification of Cytokines and Prostaglandin Under UV and Heat Stress
2.3.1. UV-Induced Inflammation in NHEKs- IL6 and IL-1Ra Quantification
NHEKs isolated from human skin in our laboratory were seeded in a type I collagen pre-coated 12-well plate at 65 000 cells per well in triplicate. The cells were incubated for 48 h in complete medium (DermaLife® supplemented with factors, CellSystems®, Germany) 500 µL and 1% of antibiotics at 37 °C with 5% CO2. At the end of the incubation, the cells were rinsed twice with PBS (500 µL) and pre-incubated with PTE at 0.01% (v/v) for 24 h in DermaLife® complete medium without hydrocortisone and 1% of antibiotics at 37 °C with 5% CO2. PTE was pre-diluted at 50% in dimethyl sulfoxide (DMSO), then diluted in basal medium to obtain the final concentration 0.01%. Dexamethasone at 1 µM was used as a reference inhibitor of inflammation. After 24 h of pre-incubation, the cells were rinsed with PBS (500 µL), and HBSS (Hank’s balanced salt solution) medium (500 µL) was added to each well. Cells were then stressed with 75 mJ/cm2 UVB irradiation (λ = 312 nm) and incubated for 24 h at 37 °C with 5% CO2 in complete medium without hydrocortisone. Untreated skin cells cultivated with the medium were used as control. At the end of the culture, the cultured media were collected and centrifuged at 2000 g for 10 min at 4 °C to eliminate dead cells. The media were stored at −20 °C. An MTT assay was performed to evaluate the treatments’ cytotoxicity and for the normalization of cytokines quantification. IL-6 and IL-1Ra were quantified using Luminex assay (Bio-techne®, Minneapolis, MN, USA).
2.3.2. Heat Stress Condition in NHEKs—PGE2 Quantification
NHEKs were seeded at 65 000 cells in a type I collagen pre-coated 12-well plate. For performing the experiments, keratinocytes were cultivated in monolayers until they reached confluency. Cells were pre-incubated during 24 h in absence (control) or in the presence of capsazepine at 10 nM used as reference inhibitor of PGE2 production, or with PTE at 0.01% (v/v). PTE was pre-diluted at 50% DMSO, then diluted in basal medium to obtain the final concentration 0.01%. After this pre-incubation step, cells were incubated for 5 min at 60 °C. Cells were then incubated again for 24 h at 37 °C in absence (control) or in the presence of treatments. At the end of this incubation period, cell culture media were collected and stored at −20 °C until their measurement for PGE2 concentration using ELISA kit (Bio-techne®, Minneapolis, MN, USA). Cells lysates were extracted and proteins contained in the cell lysates were quantified using a spectro-colorimetric method (Bradford method).
2.4. Quantification of Vitamin D3 in Skin Explants Treated with PTE and Under Moderate Daily UV Exposure
2.4.1. Skin Explants Culture and Preparation
Human skin explants were obtained with the informed consent from breast and abdominoplasty surgeries (54 and 50 years old Caucasian female donors). The explants were cultured on biocompatible plastic grids into standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) 600 µL at 37 °C with 5% CO2. The culture medium (600 µL) was renewed every 24 h. Skin explants were topically treated with 20 µL PTE diluted at 0.8% in Miglyol 812® (IOI Oleochemical, Marl, Germany) or with Miglyol 812® only, as vehicle control. After 3 days of incubation, the skin explants were rinsed in PBS twice with 600 µL and the medium was replaced by HBSS for UV irradiation as follows: irradiated at 4.5 J/cm2 of UVA (λ = 365 nm) and 0.15 J/cm2 of UVB (λ = 312 nm), and without. Then, the treatment was applied again for 24 h after a second irradiation at the same dosages. Twenty-four hours after the second irradiation, the skin explants’ surfaces were rinsed with PBS, then frozen at −80 °C to be stored before further investigations.
2.4.2. HPLC Quantification of Vitamin D3
Into a 2 mL Eppendorf tube, approximately 100 mg of exactly weighed skin cut into the smallest possible pieces were introduced. Methanol (500 µL) was added, and the mixture was homogenized under ice with Ultra-Turrax® disperser (IKA T25, Digital, Staufen, Germany) at 2440 g at 4 °C for 10 min. Again, 500 µL methanol was added and the resulting mixture was vortexed for 10 min at 30 Hz and protected from light. It was then centrifuged for 5 min at 20,000× g at 4 °C. Supernatant (100 µL) was collected and evaporated until dryness under nitrogen dioxide. The residue was suspended in 100 µL of internal standard (IS) solution for HPLC analysis. HPLC experiment was conducted using a column Cosmosil 5-MS-II C18 (5 µm, 150 × 4.6 mm), with column oven at 20 °C, and an injection volume of 20 µL. Elution was in isocratic mode using methanol at 1 mL/min for 20 min. The mass spectrometry detection mode was APCI positive, and acquisition mode found SIM vitamin D3 (385.3 amu), and IS vitamin D3 (390.4 amu).
2.5. Gene Expression Analysis on Skin Explants Treated with PTE
Human skin explants were obtained with informed consent from breast surgeries (54 years old Caucasian female donor). The explants were kept alive by culturing on biocompatible plastic grids into standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan), at 37 °C with 5% CO2. The culture medium (600 µL) was renewed every 24 h. Skin explants were topically treated with 20 µL of PTE diluted at 0.8% in Miglyol 812® or Miglyol 812® only, as vehicle control. After 2 days of incubation, the skin explants were frozen at −80 °C in RNAlater solution (Thermofisher, Waltham, MA, USA). Skin explants were then cut and inserted in microtubes containing beads (lysing matrix) and 1 mL of Extract-all with 5.1 M of guanidine thiocyanate. Microtubes were then placed in a FastPrep-24™ 5G bead-beating grinder and lysis system, and 3 cycles of 30 s were used to grind the skin pieces. Total RNA was then extracted following Extract-all method. RNA quality was controlled using a 4150 TapeStation (Agilent, Santa Clara, CA, USA) and a reverse transcription was performed to obtain cDNA. RT-qPCR was realized on 10 ng of cDNA per well on specific plates designed to study transcriptomic expression of different genes involved in skin pigmentation or skin response to UV exposure. The results were normalized according to IPO8 (Importin 8) and EIF2B1 (Eukaryotic Translation Initiation Factor 2B Subunit Alpha) housekeeping genes. Data were expressed relative to vehicle (Miglyol 812®) control.
2.6. Quantification of Melanin in Skin Explants Treated with PTE Under Moderate Daily UV Exposure, Using Fontana Masson Stain
2.6.1. Skin Explants Culture and Preparation
Human skin explants were obtained with informed consent from abdominal surgery (45 years old Caucasian female donor). The explants were kept alive by culturing on biocompatible plastic grids into standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) at 37 °C with 5% CO2. The culture medium (600 µL) was renewed every 24 h. Skin explants were topically treated with 20 µL of placebo emulsion and emulsion containing PTE at 0.8%. The control condition was left untreated. After 3 days of incubation, the skin explants were rinsed with PBS and irradiated at 4.5 J/cm2 of UVA (λ = 365 nm) and 0.15 J/cm2 of UVB (λ = 312 nm). Then, the treatment was applied again for 24 h after a second irradiation at the same dosages. Twenty-four hours after the second irradiation, the skin explants’ surfaces were rinsed with PBS, then fixed in formalin for histological analyses. The experiment was conducted in triplicate. The emulsion follows the composition described in Section 2.2.1.
2.6.2. Fontana–Masson Staining and Quantification of Melanin Content by Imagery Analysis
The skin explants fixed in formalin were dehydrated and embedded in paraffin. Slices of 4 µm were cut, then dewaxed to be stained by Fontana–Masson silver method for melanin visualization. Four images per explant were collected with automated bright field and digital confocal imaging system (ImageXpress® PICO, Molecular Devices, San Jose, CA, USA). Photomicrographs (jpeg format) of stained tissue sections were opened in the GIMP-GNU open-source software program. The brown-to-black color signals corresponding to melanin pigment amount were selected, copied, and pasted into a new image on a white background. Images were saved in jpeg format. These images were subsequently opened in the ImageJ open-source software program. The image was then inverted, and the mean intensity was measured.
2.7. Quantification of Eumelanin and Pheomelanin in Skin Explants Treated with PTE Under Moderate Daily UV Exposure, Using LC-MS
2.7.1. Skin Explants Culture and Preparation
The skin explants were obtained with the informed consent from breast surgeries (54 years old Caucasian female donor). The explants were kept alive by culturing on biocompatible plastic grids into standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) at 37 °C with 5% CO2. The culture medium (600 µL) was renewed every 24 h. Skin explants were topically treated with 20 µL of PTE diluted at 0.8% in Miglyol 812® or Miglyol 812® only, as vehicle control. After 3 days of incubation, the skin explants were rinsed with PBS and irradiated at 4.5 J/cm2 of UVA (λ = 365 nm) and 0.15 J/cm2 of UVB (λ = 312 nm). Then, the treatment was applied again for 24 h after a second irradiation, at the same dosages. Twenty-four hours after the second irradiation, the skin explants’ surfaces were rinsed with PBS, then frozen at −80 °C for eumelanin quantification. The experiment was conducted in triplicate.
2.7.2. Quantitative Analysis of Eumelanin and Pheomelanin Using HPLC
Eumelanin and pheomelanin are well-known and common pigments found in nature. However, their complex polymer structure and high thermostability complicate their direct chemical identification. A widely used analytical method is their indirect determination using HPLC with UV detection of both types of melanin by their most abundant oxidation products: pyrrole-2,3-dicarboxylic acid (PDCA), pyrrole-2,3,5-tricarboxylic acid (PTCA), thiazole-4,5-dicarboxylic acid (TDCA), and thiazole-2,4,5-tricarboxylic acid (TTCA). In this study, the exploration of eumelanin oxidation was focused on pyrrole-2,3-dicarboxylic acid (PDCA) and pyrrole-2,3,5-tricarboxylic acid (PTCA) production and thiazole-4,5-dicarboxylic acid (TDCA) for pheomelanin [38]. For the measurement of eumelanin and pheomelanin degradation products by LC-MS, the analytes of interest were isolated from the matrix by an aqueous extraction. The extracts were then analyzed using a liquid chromatography system coupled to a mass spectrometry ISQ detector (Ultimate 3000 HPLC system, Thermo Fisher Scientific, Waltham, MA, USA) using Chromeleon 7.2 software.
2.8. Evaluation of Skin Pigmentation on Pigmented-Reconstructed Human Epidermis Treated with PTE Under Moderate Daily UV Exposure
2.8.1. Culture and Preparation
Reconstructed human epidermis (RHE) containing melanocytes from a donor of Asian descent (MelanoDerm™, NHM-A/MEL-300-A batch 37212 kit A, MatTek In Vitro Life Science Laboratories, Slovak Republic) were kept alive in an air–liquid interface with culture medium (EPI-100-NMM-133, MatTek) at 37 °C with 5% CO2. The culture medium was renewed every day. MelanoDerm™ were topically treated with PTE diluted at 0.8% in Miglyol 812® or Miglyol 812® only, as vehicle control. After 7 days of incubation, MelanoDerm™ were rinsed with PBS and irradiated at 1.5 J/cm2 of UVA (λ = 365 nm) and 0.05 J/cm2 of UVB (λ = 312 nm). Then, the treatment was applied again for 3 days of culture. A second irradiation at the same doses was performed and treatment was applied again. Twenty-four hours after the second irradiation, culture medium was collected, stored at −20 °C, and the MelanoDerm™ were rinsed with PBS, then frozen at −80 °C for further analyses.
2.8.2. L* and ITA* Parameters Measurement
Pictures of MelanoDerm™ were taken at the beginning of the culture and at the end of the culture. Adobe Photoshop® software was used to determine the L* (clarity, 0 black to 100% white), a* (green-red spectrum), and b* (blue-yellow spectrum) from a smoothed and blurred picture, and individual typology angle (ITA) was calculated using the following formula: ITA = [Arc tan ((L* − 50)/b*)] × 180/π.
2.9. Evaluation of Pigmentation in Skin Explants Treated with PTE in SPF 30 Sunscreen Formula Under Moderate Daily UV Exposure
Human skin explants were obtained with informed consent from breast surgeries (45 years old Caucasian female donor). The explants were kept alive by culturing on biocompatible plastic grids into standard 24-well plates in an air–liquid interface with skin culture medium (Givaudan) at 37 °C with 5% CO2. The culture medium (600 µL) was renewed every 24 h. Skin explants were topically treated with 20 µL of oily sunscreen formula (placebo control) or formula containing PTE at 0.8%. The composition of the formula followed this composition: Dub Helioptima® (19%), Alcohol, Octinoxate, Bis-Ethylhexylophenol Methoxyphenyl Triazine, Ethylhexyl Salicylate, Butyl Methoxybenzoylmethane, ± Polygonum tinctorium Leaf/Stem Extract 0.8%, Tocopheryl Acetate. After 3 days of incubation, the skin explants were irradiated at 4.5 J/cm2 of UVA (λ = 365 nm) and 0.15 J/cm2 of UVB (λ = 312 nm). Then, the treatment was applied again for 24 h before a second irradiation at the same dosages. Twenty-four hours after the second irradiation, the skin explants surfaces were rinsed with PBS and then fixed in formalin for Fontana–Masson’s staining (described above in Section 2.6.2.). The experiment was conducted in triplicate.
2.10. Statistical Analysis
For all studies, a Shapiro–Wilk test was used to verify whether the raw data followed the Gaussian Law. In case of normally distributed data, the mean values were compared using either an unpaired or paired Student’s t-test. In case of non-normally distributed data, a Wilcoxon (paired) or Kruskal–Wallis test. followed by a Mann–Whitney U (unpaired) test were used for paired data or unpaired data, respectively. Regardless of the test, it was considered as a significant result: p < 0.1 with #, p < 0.05 with *, p < 0.01 with ** and p < 0.001 with ***.
3. Results
3.1. UVA/UVB Dose Determination
The determined UV dose corresponds to a moderate daily UV exposure in skin explants: 4.5 J/cm2 of UVA and 0.15 J/cm2 of UVB in two applications per 24 h, using two representative wavelengths of the UVA (365 nm) and UVB (312 nm) spectra [39]. These values took into account the predominance of UVA (95%) in sunlight [40]. UVA/UVB ratio varies throughout the day and depends on latitude, season, or geo-orbital factors such as ozone layer characteristics and meteorological conditions. In this study, UVA/UVB ratio was set at 30, in the range 23–32 defined by previous studies [7,39]. This treatment corresponds approximately to 1 h daily UV exposure during spring, as observed in Europe in April [41]. It is expected to be lower than the minimal erythema dose determined by previous authors: for Christiaens and collaborators, it corresponded to 1h20 exposure in mean UV daylight conditions for phototype I; Seité and collaborators determined it was 3.4 ± 0.55 J/cm2 to 12 ± 2.5 J/cm2 for Caucasian skin depending on the UVA/UVB ratio [7,8,39]. This UV dose was confirmed to not induce melanogenesis, as a biomarker of skin physiological response (Figure 1a), but was sufficient to trigger CPDs formation (Figure 1b).
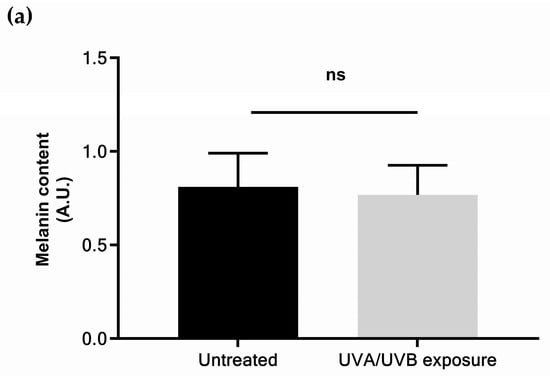
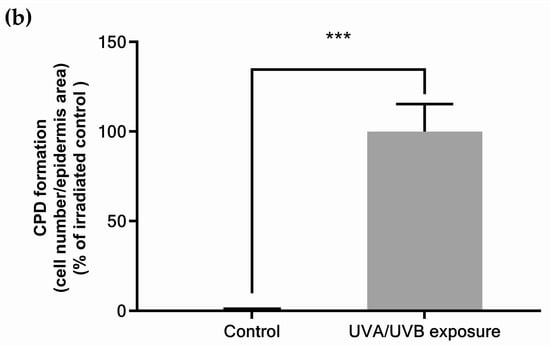
Figure 1.
Physiological responses of skin explants to moderate UV exposure: (a) melanin content; (b) quantification of cyclobutane pyrimidine dimers detected by immunostaining and quantified by fluorescence emission measurement. Values are of mean ± standard error (SEM). Mann–Whitney statistical test, *** p < 0.001, ns p > 0.1.
3.2. PTE Mitigates the Deleterious Effects of Sun Exposure
3.2.1. PTE Decreases DNA Damage
Cyclobutane pyrimidine dimers (CPDs) are a major type of DNA lesion resulting from UV damage [42]. Skin explants were exposed to a moderate dose of UVA/UVB and CPDs were determined by immunostaining (Figure 2). Pre-treatment with PTE-containing emulsion significantly decreased CPDs by −56% compared to a stressed placebo. These data indicate that PTE limits DNA damage caused by UV exposure.
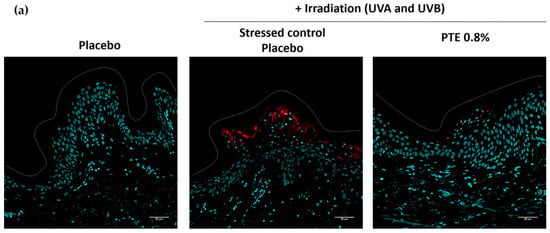
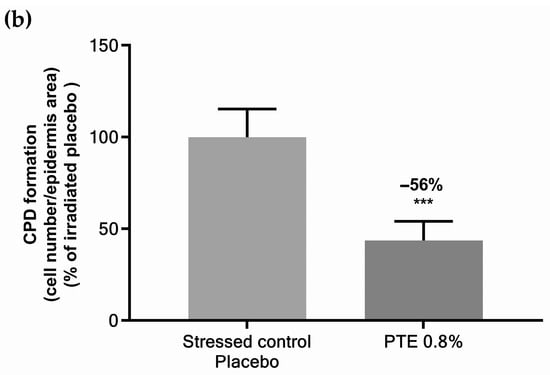
Figure 2.
(a) Immunostaining of cyclobutane pyrimidine dimers (CPD) and (b) related quantification in skin explants stressed by UVA/UVB irradiation and pre-treated with Persicaria tinctoria extract 0.8% in emulsion or with placebo emulsion. Values are mean ± standard error (SEM). Mann–Whitney statistical test, *** p < 0.001.
3.2.2. PTE Decreases UV-Induced Inflammation and Heat Stress-Induced PGE2
Normal human epidermal keratinocytes (NHEKs) underwent UVB irradiation to produce an inflammatory response [43]. The release of pro-inflammatory cytokine IL-6 and anti-inflammatory IL-1Ra was quantified in the culture medium of UV-irradiated NHEKs (Figure 3a,b). PTE reduced the release of IL-6 by −59% and induced IL-1Ra by +72% in the culture medium of UV-irradiated NHEKs compared to vehicle control.
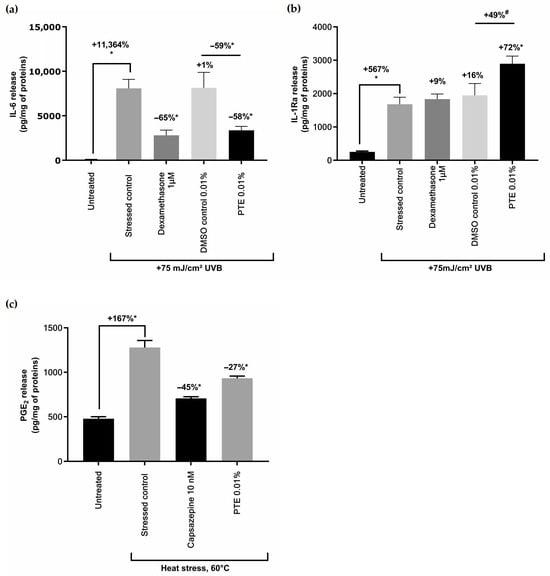
Figure 3.
Effect of Persicaria tinctoria extract (PTE) on cytokines and PGE2: (a) quantification of interleukin 6 (IL-6) and (b) interleukin 1 receptor antagonist (IL-1Ra) released in the culture medium of keratinocytes stressed with UVB 75 mJ/cm2, and not pre-treated (stressed control) or pre-treated with Dexamethasone (anti-inflammatory reference compound), vehicle (DMSO), or PTE 0.8% in vehicle; quantification of (c) PGE2 protaglandin in heat-stressed keratinocytes or untreated keratinocytes, or exposed to heat stress only or in combination with a positive reference decreasing PGE2 (Capsazepine) or with PTE. Values are of mean ± standard error (SEM). Mann–Whitney statistical test, * p < 0.05, # p < 0.1. Percentages on top of bars are calculated in comparison to untreated conditions while the percentages indicated on top of black lines are calculated between values indicated by the black line.
NHEKs were also stressed using heat in order to mimic the exposure to another component of sunlight, the infrared rays. PTE significantly decreased the release of prostaglandin PGE2 induced by heat stress compared to the stressed control (Figure 3c).
3.3. PTE Increases Vitamin D3 Content
UV rays from sunlight promote the synthesis of vitamin D3 in human skin [16]. Vitamin D3 was quantified in skin explants treated with PTE 0.8% and irradiated with UV (Figure 4). In the placebo treatment, vitamin D3 content increased by +148.5% compared to untreated explants, suggesting the UVA/UVB dose was sufficient to increase the molecule concentration in skin. When treated with PTE, it rose by +345% compared to irradiated placebo control, meaning PTE outperformed UV exposure alone in the simulation of this metabolic pathway.
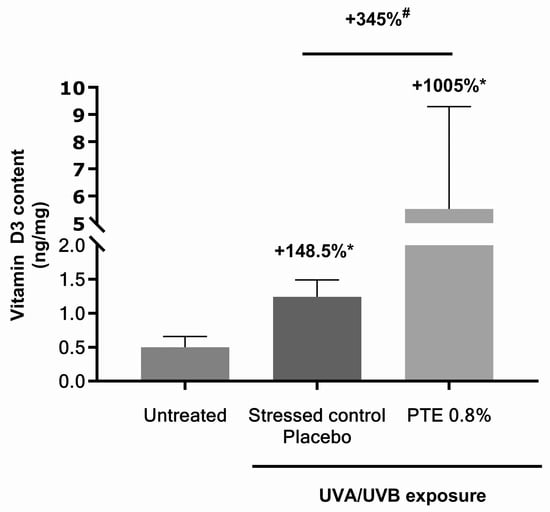
Figure 4.
Vitamin D3 quantification in skin explants untreated or treated with vehicle alone or with Persicaria tinctoria extract 0.8% in vehicle (Miglyol 812®), under moderate UVA/UVB exposure. Values are of mean ± standard error (SEM). Mann–Whitney statistical test, # p < 0.1, * p < 0.05.
3.4. PTE Promotes Melanogenesis
3.4.1. PTE Increases Expression of Genes Involved in Melanogenesis and Its Regulation
Skin explants were treated with PTE 0.8% without UV exposure, and total RNA were extracted to perform gene expression analysis on transcript encoding for proteins involved in melanogenesis. Results are presented in Figure 5. Four transcripts were significantly up-regulated in skin explants treated with PTE: MC1R (melanocortin 1 receptor) expression increased by 2.46-fold, MITF by 1.69-fold (microphtalmia-associated transcription factor), TYR (tyrosinase) by 2.06-fold, and MLPH (melanophilin) by 1.53-fold. MC1R is a membrane receptor of melanocytes binding αMSH (alpha-melanocyte stimulating hormone) and a central regulator of eumelanin synthesis [23,28]. MITF is the pivotal transcription factor of melanogenesis, integrating the multiple signaling pathways that regulate melanogenesis. TYR is a melanogenic enzyme catalyzing L-tyrosine conversion into L-DOPA, and of the latter into L-DOPAquinone, the precursor molecule of eumelanogenesis and pheomelanogenesis. MLPH controls the transfer rate of melanosomes from melanocytes to keratinocytes [26]. Therefore, PTE increased the expression of genes that promote melanogenesis.
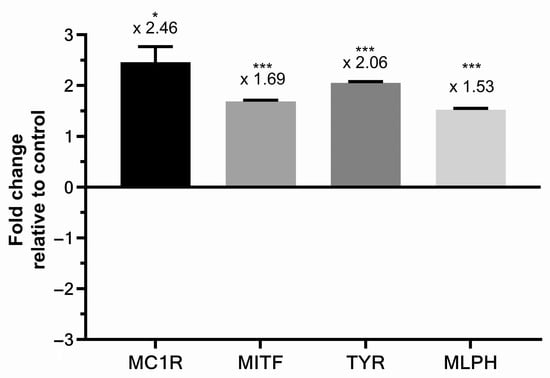
Figure 5.
Gene expression analysis of melanocortin 1 receptor (MC1R), microphtalmia-associated transcription factor (MITF), tyrosinase (TYR), and melanophilin (MLPH) transcripts in skin explants treated with Persicaria tinctoria extract 0.8% in Miglyol 812®, expressed in fold change relative to control (vehicle, Miglyol 812®). Values are of mean ± standard error (SEM). Mann–Whitney statistical test, * p < 0.5, *** p < 0.001.
3.4.2. PTE Enhances Melanin Content and Skin Color Under Moderate Daily UV Dose Exposure
Melanin was quantified in skin explants treated with PTE 0.8% formulated in cream or with placebo and exposed to daily dose of UV radiation. Results are presented in Figure 6a. Melanin content in explant was boosted by +222% vs. untreated condition and +126% vs. placebo, boosting the effect of UV irradiation. Human skin melanin is in fact composed of different molecules, mainly eumelanin (brownish black) and pheomelanin (reddish yellow). Eumelanin, considered to be photoprotective, predominates in all skin phototypes, but in light skin, its content is considerably decreased [6]. Eumelanin and pheomelanin were quantified in skin explants treated with PTE and UV irradiation (Figure 6b,c). PTE induced a significant increase in eumelanin (+60% vs. control), while pheomelanin increased, not significantly, by 23%. Pigmented-RHE were similarly treated with PTE 0.8% and daily dose of UV radiation. Skin color was evaluated by measuring L* (brightness, from 0 = black to 100 = white) and ITA (the lower the value is, the darker the skin) parameters that are related to melanin content in skin (Figure 6d,e). PTE induced a decrease in the L* (Figure 6d) and ITA (Figure 6e) parameters indicating PTE enhanced skin tanning in this model, in accordance with the boost in melanin content observed in skin explants.
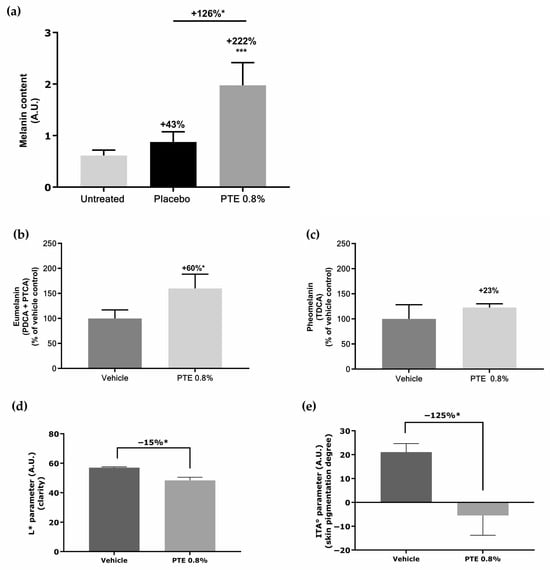
Figure 6.
Impact of Persicaria tinctoria extract (PTE) on melanin content and skin color: (a) quantification of total melanin in skin explants treated with PTE 0.8% formulated in cream or with placebo (cream without PTE) under UVA/UVB exposure; (b) quantification of eumelanin and (c) phaeomelanin in skin explants after treatment with PTE 0.8% in vehicle (Miglyol 812®) or with vehicle alone, under UVA/UVB exposure; (d) L* and (e) ITA* parameters obtained from the macroscopic pictures of pigmented-RHE (reconstructed human epidermis) treated with Persicaria tinctoria extract at 0.8% in vehicle (Miglyol 812®), or with vehicle alone and irradiated with a daily UVA/UVB. Values are of mean ± standard error (SEM). Mann–Whitney statistical test, * p < 0.05, *** p < 0.001.
3.4.3. PTE Promotes Melanin Synthesis Under SPF Protection
Experts’ recommendations to manage daily sun exposure include the use of sunscreen [27,44]. From the perspective of using PTE in sun products, its effect on melanin synthesis when formulated with UV filter SPF 30 was evaluated (Figure 7). The presence of sunscreen did not impact the action of PTE which increased melanin content by +86% when compared to placebo.
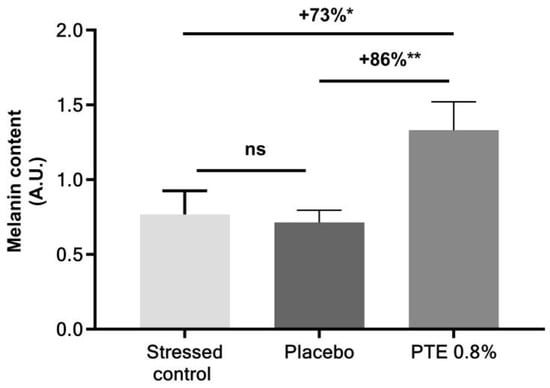
Figure 7.
Melanin content in skin explants under a moderate daily UVA/UVB exposure, receiving no other treatment (stressed control) or treated with Persicaria tinctoria extract (PTE) 0.8% in formula or with placebo (formula without PTE), including SPF 30. Values are of mean ± standard error (SEM). Mann–Whitney statistical test, * p < 0.05, ** p < 0.01, ns p > 0.1.
4. Discussion
Daylight is composed of several wavelengths which can affect human skin physiology. UV rays, mainly comprising UVA accompanied by UVB, reach the earth’s surface and penetrate tissue. Skin tanning and sunburn are well-known visible outcomes, which are often associated with extreme solar radiation. It is now established that both acute and chronic UV exposure during occupational and recreational activities contributes to skin photodamage and aging [2,7,8]. Deleterious consequences of UV exposure include oxidative stress altering DNA, proteins, or lipids, inflammation or direct DNA damage through CPDs formation. These events contribute to the appearance of clinical signs of aging (epidermis thickening, hyperpigmentation, dermal sagging, wrinkles, …) and can ultimately lead to carcinoma or melanoma. Sun avoidance and photoprotection with clothes or sunscreen are recommended for all skin phototypes [19,27]. Even moderate sun exposure leads to biological damage such as DNA lesions [9,42]. Paradoxically, sunlight exposure promotes beneficial aspects, including the synthesis of vitamin D3 [45].
Except for people working outside all day, sun exposure is lower than the total UV dose received by the earth’s surface in one day. Indoor workers, who are more susceptible to vitamin D3 deficiency, receive about 2.2-fold less UV radiation than outdoor workers [46,47]. For example, in Paris in April, the UV daylight dose is 68.31 J/cm2 per day [7]. Taking into account the duration of sunshine irradiance at this time of the year, we define a dose of 4.5 J/cm2 UVA and 0.15 J/cm2 UVB as representative of a daily exposure of 1 h for a western Europe inhabitant in April. This dose was also defined according to biological response of the skin: it did not modify the melanin content in skin but was able to trigger CPDs. Due to their high energy, UVB contribute more to DNA damage than UVA [48]. In the solar spectrum, UVB below 300 nm is strongly diminished, so wavelengths between 300 and 320 are more likely to induce CPDs. UVA from 340 to 400 nm represents 75% of solar UVA radiation and their negative impact on skin are well characterized [49]. According to these observations, the wavelengths of 312 nm and 365 nm were selected in this study, and it constitutes a limitation as they did not represent the full UVA and UVB from solar radiation reaching the ground surface.
Natural compounds are a valuable source of protective compounds against UV-induced oxidative stress, inflammation, and accumulation of DNA damage [29]. As part of traditional Chinese and Korean medicine, P. tinctoria was previously prescribed for the treatment of atopic dermatitis and psoriasis. More recently, the antioxidant indirubin, a main component of P. tinctoria extract, attracted attention for the anti-proliferative and pro-apoptotic properties of its glycosides against carcinoma and melanoma cells [50]. In this study, a P. tinctoria extract (PTE), standardized in indirubin, was evaluated on the modulation of two skin biological responses to UV: DNA lesions in the form of CPDs, and inflammation, which both contribute to skin cancer risk [48,51]. PTE decreased the proportion of cells containing CPDs in skin explants (−56%) and the production of pro-inflammatory cytokine IL-6 (−59%) in monolayer keratinocytes together with an increase in anti-inflammatory IL-1Ra [52]. The anti-inflammatory effects of indirubin were recently reviewed, showing that IL-6 decrease could be achieved through different pathways involving nuclear factor κ-B, aryl hydrocarbon receptor or Janus kinase/signal transducer, and activator of transcription [37]. This natural compound was recently demonstrated to be an agonist of the aryl hydrocarbon receptor (AHR), a regulator of skin barrier function that modulates inflammatory response [53,54]. Oxidative stress contribute to the inflammatory response and its regulation is notably by AHR, which can be modulated by phytochemicals [55]. This could be an avenue for future studies exploring the pathways triggered by PTE. The extract down-regulated the release of prostaglandin triggered by heat stress, mimicking the influence of other solar radiations, such as infrared [56]. Taken together, these data suggest that PTE has promising effects in reducing sunlight-associated damage in skin; these are probably related to the presence of indirubin.
Besides these protective effects of PTE, we also demonstrated that it strongly increased vitamin D3 content in skin (+345%). To our knowledge, no plant extract was previously described to exert such effect. The human body’s vitamin D requirement is mainly covered by the skin metabolism under UVB exposure [45,57,58]. Deficiency in vitamin D3 is at the root of several pathologies and is considered as a potential public health problem related to low sunlight exposure [17,45,47]. Thanks to its role in immunomodulation, anti-inflammatory or antioxidant pathways, and in keratinocytes regulation, vitamin D3 contributes to skin photoprotection and prevents aging [58]. With this ability to stimulate vitamin D3 biosynthesis under low UV light, PTE could find applications, especially in winter, and during spring or autumn, when the UV irradiance is low and vitamin deficiency is high [59,60].
Melanin produced by melanocytes is responsible for skin color and is composed of different molecules called eumelanin and phaeomelanin [24]. Skin pigmentation has a protective role and is associated with reduced DNA damage [42,61]. Eumelanin especially contributes to skin protection against the mutagenic properties of UV rays by absorbing radiation and decreasing oxidative stress [3,28]. Plant extracts are mainly studied for their anti-melanogenic effects, but the search for natural solutions, for example, to treat hypopigmentation, revealed several species and their isolated phytocompounds down-regulate genes involved in melanogenesis or increase melanin content in cellular models [62,63,64]. As a new finding, PTE promoted the expression of several of these genes in the absence of UV treatment: MC1R, MITF, TYR, and MLPH, with the highest increase observed for the first one. As described in the introduction, MITF is a pivotal transcription factor controlling enzymes synthesizing melanin as TYR, suggesting PTE directly promotes melanogenesis. The increase in MLPH indicates PTE may not only play a role in melanin synthesis but also in its transport to the upper layer of epidermis [65]. MC1R is a melanocyte receptor, responding to UV-induced α-MSH, which enhances the expression of enzymes responsible for eumelanin synthesis [3,28]. Thus, the rise in melanin, and more particularly the protective eumelanin, caused by PTE in the skin may be related to the increased expression of MC1R. It would be of interest to evaluate PTE on red-haired skin, for which mutation in MC1R inactivates it. One of the strengths of this work is to provide demonstration of melanin and skin tanning increase in skin explants and reconstructed epidermal models, while pro-melanogenic effects of plant extracts are generally demonstrated on monolayer cellular models. The pro-melanogenic activity of PTE was even efficient under SPF 30 and conducted to a visible modification of skin color. Our team previously presented data on the positive impact of PTE on mood, and its ability to stimulate β-endorphin synthesis in skin [66]. This peptide is promoted by UV and explains UV-seeking behavior, which is also driven by skin tanning addiction [67]. In this sense, PTE could also contribute to satisfying consumer desires for the positive effects of sunlight, without increasing the duration of exposure.
5. Conclusions
The interest in plant extracts in modulating skin response to UV is generally addressed by dealing with their antioxidant and anti-inflammatory properties or their action on melanogenesis, but do not address the vitamin D3 metabolism, although it is an important health-promoting aspect of sunlight. This work aimed at describing the effect of a Persicaria tinctoria extract on the multifaceted aspects of the biological response of skin to UV. Our findings revealed that PTE decreased biomarkers of DNA damage and inflammation, suggesting it could prevent photoaging. Also, it promoted skin pigmentation by stimulating melanin and skin tanning, especially enhancing the protective eumelanin. On top of these protecting effects, the extract was originally observed to stimulate a positive aspect of sunlight by increasing vitamin D3, which is involved in healthy skin metabolism. These results encourage the evaluation of plant metabolites in all aspects of UV-induced skin metabolism. This indigoid-rich extract would be complementary to sunscreens in daily solar protection formulas and represents an opportunity to reconcile the management of sunlight risks for skin and vitamin D3 metabolism.
Author Contributions
Conceptualization: M.d.T. and A.S.; methodology, validation, and formal analysis: B.S.-P. and M.d.T.; investigation: A.D. and J.M.; supply of surgical material: J.T.; writing—original draft preparation: M.d.T.; writing—review and editing: M.d.T., C.Z. and A.S.; funding acquisition and resources: R.R.; project administration and supervision: A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The explants used in this study are considered as “biological waste” resulting from surgical interventions, complying with French regulations regarding the import, export, storage, and preparation of human biological materials for research purposes, and hold all necessary authorizations Approval Code: DC-2023-5658; Approval Date: 15-12-2023. The manuscript does not include clinical studies. Therefore, the ethical approval is not applicable.
Informed Consent Statement
For each surgical skin explant, informed consent was obtained from patients.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed at the corresponding author.
Acknowledgments
We would like to thank Qima Life Sciences (Gençay, France) and Ephyscience (Nantes, France) for their support in conducting some experiments.
Conflicts of Interest
Morgane de Tollenaere, Catherine Zanchetta, Anaïs Durduret, Jessy Martinez, Bénédicte Sennelier-Portet, Amandine Scandolera, and Romain Reynaud the authors are employees of the company Givaudan France. The author Jean Tiguemounine is employed by Plastic surgery, Polyclinique Courlancy. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AHR | Aryl Hydrocarbon Receptor |
| αMSH | Melanocyte-Stimulating Hormone |
| CPD | Cyclobutane Pyrimidine Dimers |
| MC1R | Melanocortin 1 Receptor |
| MITF | Microphtalmia-Associated Transcription Factor |
| MLPH | Melanophilin |
| PTE | Persicaria tinctoria Extract |
| SPF | Sun Protection Factor |
| TYR | Tyrosinase |
| UV | Ultraviolet |
References
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; Del Bino, S.; Marionnet, C.; Verschoore, M. New Insights in Photoaging, UVA Induced Damage and Skin Types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Jiang, G. Research Progress on Skin Photoaging and Oxidative Stress. Postępy Dermatol. I Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to Non-Extreme Solar UV Daylight: Spectral Characterization, Effects on Skin and Photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef]
- Seité, S.; Medaisko, C.; Christiaens, F.; Bredoux, C.; Compan, D.; Zucchi, H.; Lombard, D.; Fourtanier, A. Biological Effects of Simulated Ultraviolet Daylight: A New Approach to Investigate Daily Photoprotection. Photodermatol. Photoimmunol. Photomed. 2006, 22, 67–77. [Google Scholar] [CrossRef]
- Seité, S.; Fourtanier, A.; Moyal, D.; Young, A.R. Photodamage to Human Skin by Suberythemal Exposure to Solar Ultraviolet Radiation Can Be Attenuated by Sunscreens: A Review. Br. J. Dermatol. 2010, 163, 903–914. [Google Scholar] [CrossRef]
- Gabe, Y.; Takeda, K.; Tobiishi, M.; Kikuchi, S.; Tsuda, K.; Haryuu, Y.; Nakajima, Y.; Inomata, Y.; Nakamura, S.; Murase, D.; et al. Evaluation of Subclinical Chronic Sun Damage in the Skin via the Detection of Long-Lasting Ultraweak Photon Emission. Ski. Res. Technol. 2021, 27, 1064–1071. [Google Scholar] [CrossRef]
- Weill, F.S.; Cela, E.M.; Ferrari, A.; Paz, M.L.; Leoni, J.; Gonzalez Maglio, D.H. Skin Exposure to Chronic but Not Acute UV Radiation Affects Peripheral T-Cell Function. J. Toxicol. Environ. Health A 2011, 74, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily Photoprotection to Prevent Photoaging. Photodermatol. Photoimmunol. Photomed. 2021, 37, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Flament, F.; Mercurio, D.G.; Catalan, E.; Bouhadanna, E.; Delaunay, C.; Miranda, D.F.; Passeron, T. Impact on Facial Skin Aging Signs of a 1-Year Standardized Photoprotection over a Classical Skin Care Routine in Skin Phototypes II–VI Individuals: A Prospective Randomized Trial. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Seité, S.; Christiaens, F.; Bredoux, C.; Compan, D.; Zucchi, H.; Lombard, D.; Fourtanier, A.; Young, A. A Broad-Spectrum Sunscreen Prevents Cumulative Damage from Repeated Exposure to Sub-Erythemal Solar Ultraviolet Radiation Representative of Temperate Latitudes. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 219–222. [Google Scholar] [CrossRef]
- Green, N.J.; Xu, J.; Sutherland, J.D. Illuminating Life’s Origins: UV Photochemistry in Abiotic Synthesis of Biomolecules. J. Am. Chem. Soc. 2021, 143, 7219–7236. [Google Scholar] [CrossRef]
- Rossberg, W.; Saternus, R.; Wagenpfeil, S.; Kleber, M.; März, W.; Reichrath, S.; Vogt, T.; Reichrath, J. Human Pigmentation, Cutaneous Vitamin D Synthesis and Evolution: Variants of Genes (SNPs) Involved in Skin Pigmentation Are Associated with 25(OH)D Serum Concentration. Anticancer Res. 2016, 36, 1429–1437. [Google Scholar]
- Alfredsson, L.; Armstrong, B.K.; Butterfield, D.A.; Chowdhury, R.; de Gruijl, F.R.; Feelisch, M.; Garland, C.F.; Hart, P.H.; Hoel, D.G.; Jacobsen, R.; et al. Insufficient Sun Exposure Has Become a Real Public Health Problem. Int. J. Environ. Res. Public Health 2020, 17, 5014. [Google Scholar] [CrossRef]
- Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? In Sunlight, Vitamin D and Skin Cancer; Reichrath, J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–36. ISBN 978-3-030-46227-7. [Google Scholar]
- Lindqvist, P.G.; Epstein, E.; Landin-Olsson, M. Sun Exposure—Hazards and Benefits. Anticancer Res. 2022, 42, 1671–1677. [Google Scholar] [CrossRef]
- Webb, A.R.; Kift, R.; Berry, J.L.; Rhodes, L.E. The Vitamin D Debate: Translating Controlled Experiments into Reality for Human Sun Exposure Times. Photochem. Photobiol. 2011, 87, 741–745. [Google Scholar] [CrossRef]
- Felton, S.J.; Cooke, M.S.; Kift, R.; Berry, J.L.; Webb, A.R.; Lam, P.M.W.; de Gruijl, F.R.; Vail, A.; Rhodes, L.E. Concurrent Beneficial (Vitamin D Production) and Hazardous (Cutaneous DNA Damage) Impact of Repeated Low-Level Summer Sunlight Exposures. Br. J. Dermatol. 2016, 175, 1320–1328. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. Human Skin Pigmentation as an Adaptation to UV Radiation. Proc. Natl. Acad. Sci. USA 2010, 107, 8962–8968. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.-M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Del Bino, S.; Ito, S.; Sok, J.; Nakanishi, Y.; Bastien, P.; Wakamatsu, K.; Bernerd, F. Chemical Analysis of Constitutive Pigmentation of Human Epidermis Reveals Constant Eumelanin to Pheomelanin Ratio. Pigment Cell Melanoma Res. 2015, 28, 707–717. [Google Scholar] [CrossRef]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of Skin Tanning in Different Racial/Ethnic Groups in Response to Ultraviolet Radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The Biochemistry of Melanogenesis: An Insight into the Function and Mechanism of Melanogenesis-Related Proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Lim, H.W.; Goh, C.-L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection According to Skin Phototype and Dermatoses: Practical Recommendations from an Expert Panel. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Swope, V.B.; Abdel-Malek, Z.A. MC1R: Front and Center in the Bright Side of Dark Eumelanin and DNA Repair. Int. J. Mol. Sci. 2018, 19, 2667. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant Extracts and Natural Compounds Used against UVB-Induced Photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Sun, Q.; Leng, J.; Tang, L.; Wang, L.; Fu, C. A Comprehensive Review of the Chemistry, Pharmacokinetics, Pharmacology, Clinical Applications, Adverse Events, and Quality Control of Indigo Naturalis. Front. Pharmacol. 2021, 12, 664022. [Google Scholar] [CrossRef]
- Min, G.-Y.; Kim, J.-H.; Kim, T.-I.; Cho, W.-K.; Yang, J.-H.; Ma, J.-Y. Indigo Pulverata Levis (Chung-Dae, Persicaria Tinctoria) Alleviates Atopic Dermatitis-like Inflammatory Responses In Vivo and In Vitro. Int. J. Mol. Sci. 2022, 23, 553. [Google Scholar] [CrossRef]
- Han, N.-R.; Park, J.-Y.; Jang, J.-B.; Jeong, H.-J.; Kim, H.-M. A Natural Dye, Niram Improves Atopic Dermatitis through down-Regulation of TSLP. Environ. Toxicol. Pharmacol. 2014, 38, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Han, N.-R.; Kang, S.W.; Moon, P.-D.; Jang, J.-B.; Kim, H.-M.; Jeong, H.-J. Genuine Traditional Korean Medicine, Naju Jjok (Chung-Dae, Polygonum Tinctorium) Improves 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis-like Lesional Skin. Phytomedicine 2014, 21, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-L.; Lin, Y.-K.; Tsai, C.-N.; Wang, T.-M.; Chen, T.-Y.; Pang, J.-H.S. Indirubin, An Acting Component of Indigo Naturalis, Inhibits EGFR Activation and EGF-Induced CDC25B Gene Expression in Epidermal Keratinocytes. J. Dermatol. Sci. 2012, 67, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-J.; Di, T.-T.; Wang, Y.; Wang, M.-X.; Meng, Y.-J.; Lin, Y.; Xu, X.-L.; Li, P.; Zhao, J.-X. Indirubin Ameliorates Imiquimod-Induced Psoriasis-like Skin Lesions in Mice by Inhibiting Inflammatory Responses Mediated by IL-17A-Producing Γδ T Cells. Mol. Immunol. 2018, 101, 386–395. [Google Scholar] [CrossRef]
- Qi, T.; Li, H.; Li, S. Indirubin Improves Antioxidant and Anti-Inflammatory Functions in Lipopolysaccharide-Challenged Mice. Oncotarget 2017, 8, 36658–36663. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Huang, W.; Rao, X.; Lai, Y. Pharmacological Properties of Indirubin and Its Derivatives. Biomed. Pharmacother. 2022, 151, 113112. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Advanced Chemical Methods in Melanin Determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef]
- Christiaens, F.J.; Chardon, A.; Fourtanier, A.; Frederick, J.E. Standard Ultraviolet Daylight for Nonextreme Exposure Conditions. Photochem. Photobiol. 2005, 81, 874–878. [Google Scholar] [CrossRef]
- Tran, T.T.-N.; Schulman, J.; Fisher, D.E. UV and Pigmentation: Molecular Mechanisms and Social Controversies. Pigment Cell Melanoma Res. 2008, 21, 509–516. [Google Scholar] [CrossRef]
- Home/Root—EUROSUN Project. Available online: https://i-pri.org/ (accessed on 21 April 2025).
- Tadokoro, T.; Kobayashi, N.; Zmudzka, B.Z.; Ito, S.; Wakamatsu, K.; Yamaguchi, Y.; Korossy, K.S.; Miller, S.A.; Beer, J.Z.; Hearing, V.J. UV-Induced DNA Damage and Melanin Content in Human Skin Differing in Racial/Ethnic Origin. FASEB J. 2003, 17, 1177–1179. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosuppression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Rigel, D.S.; Taylor, S.C.; Lim, H.W.; Alexis, A.F.; Armstrong, A.W.; Chiesa Fuxench, Z.C.; Draelos, Z.D.; Hamzavi, I.H. Photoprotection for Skin of All Color: Consensus and Clinical Guidance from an Expert Panel. J. Am. Acad. Dermatol. 2022, 86, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A Global Perspective for Health. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Schmalwieser, A.W.; Casale, G.R.; Colosimo, A.; Schmalwieser, S.S.; Siani, A.M. Review on Occupational Personal Solar UV Exposure Measurements. Atmosphere 2021, 12, 142. [Google Scholar] [CrossRef]
- Coppeta, L.; Papa, F.; Magrini, A. Are Shiftwork and Indoor Work Related to D3 Vitamin Deficiency? A Systematic Review of Current Evidences. J. Environ. Public Health 2018, 2018, 8468742. [Google Scholar] [CrossRef]
- Pfeifer, G.P. Mechanisms of UV-Induced Mutations and Skin Cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Rebl, H.; Sawade, M.; Hein, M.; Bergemann, C.; Wende, M.; Lalk, M.; Langer, P.; Emmert, S.; Nebe, B. Synergistic Effect of Plasma-Activated Medium and Novel Indirubin Derivatives on Human Skin Cancer Cells by Activation of the AhR Pathway. Sci. Rep. 2022, 12, 2528. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Ultraviolet Radiation and Chronic Inflammation—Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life 2021, 11, 326. [Google Scholar] [CrossRef]
- Jensen, L.E. Targeting the IL-1 Family Members in Skin Inflammation. Curr. Opin. Investig. Drugs 2010, 11, 1211–1220. [Google Scholar]
- Faber, S.C.; Soshilov, A.A.; Giani Tagliabue, S.; Bonati, L.; Denison, M.S. Comparative In Vitro and In Silico Analysis of the Selectivity of Indirubin as a Human Ah Receptor Agonist. Int. J. Mol. Sci. 2018, 19, 2692. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gallego, N.; Sánchez-Madrid, F.; Cibrian, D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells 2021, 10, 3176. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Chiba, T.; Ito, T.; Nakahara, T.; Tsuji, G. Antioxidants for Healthy Skin: The Emerging Role of Aryl Hydrocarbon Receptors and Nuclear Factor-Erythroid 2-Related Factor-2. Nutrients 2017, 9, 223. [Google Scholar] [CrossRef]
- Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.-E.; Lee, Y.M.; Park, C.-H.; Chung, J.H. Effects of Infrared Radiation and Heat on Human Skin Aging In Vivo. J. Investig. Dermatol. Symp. Proc. 2009, 14, 15–19. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: An Ancient Hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Levis, S.; Gomez, A.; Jimenez, C.; Veras, L.; Ma, F.; Lai, S.; Hollis, B.; Roos, B.A. Vitamin D Deficiency and Seasonal Variation in an Adult South Florida Population. J. Clin. Endocrinol. Metab. 2005, 90, 1557–1562. [Google Scholar] [CrossRef]
- Eloi, M.; Horvath, D.V.; Szejnfeld, V.L.; Ortega, J.C.; Rocha, D.A.C.; Szejnfeld, J.; Castro, C.H.M. Vitamin D Deficiency and Seasonal Variation over the Years in São Paulo, Brazil. Osteoporos. Int. 2016, 27, 3449–3456. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takahashi, K.; Zmudzka, B.Z.; Kornhauser, A.; Miller, S.A.; Tadokoro, T.; Berens, W.; Beer, J.Z.; Hearing, V.J. Human Skin Responses to UV Radiation: Pigment in the Upper Epidermis Protects against DNA Damage in the Lower Epidermis and Facilitates Apoptosis. FASEB J. 2006, 20, 1486–1488. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Kucharska, E.; Zajdel, R. The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds. Molecules 2022, 27, 4360. [Google Scholar] [CrossRef]
- Goenka, S. Oleuropein Is a Stimulator of Melanocyte Dendricity: Potential for Treatment of Hypopigmentation. Biologics 2025, 5, 8. [Google Scholar] [CrossRef]
- Pratiwi, D.; Mariya, S.; Rayendra, R.; Setiyono, A. Phytochemical Analysis and Pro-Melanogenic Activity of Nigella Sativa Extract in B16F10 Cells: A Natural Candidate for Vitiligo Treatment. Pharmacogn. J. 2025, 17, 307–313. [Google Scholar] [CrossRef]
- Myung, C.H.; Lee, J.E.; Jo, C.S.; Park, J.I.; Hwang, J.S. Regulation of Melanophilin (Mlph) Gene Expression by the Glucocorticoid Receptor (GR). Sci. Rep. 2021, 11, 16813. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Durduret, A.; Chapuis, E.; Martinez, J.; Sennelier-Portet, B.; Scandolera, A.; Reynaud, R. Sunshine in a Bottle: Harnessing the Power of Persicaria Tinctoria Extract for Radiant Skin and Enhanced Well-Being—SOFW—Verlag Für Chemische Industrie. Available online: https://www.sofw.com/en/hikashop-menu-for-categories-listing/product/1944-sunshine-in-a-bottle-harnessing-the-power-of-persicaria-tinctoria-extract-for-radiant-skin-and-enhanced-well-being (accessed on 10 June 2025).
- Fell, G.L.; Robinson, K.C.; Mao, J.; Woolf, C.J.; Fisher, D.E. Skin β-Endorphin Mediates Addiction to Ultraviolet Light. Cell 2014, 157, 1527–1534. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).