Safety Profile and Efficacy of Biosea® Revive Serum for Hair Growth Through In Vitro Assessment and Clinical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BRS and Particle Size Analysis
2.2. In Vitro Assays
2.2.1. Hair Follicle Cell Safety and Proliferation Test
2.2.2. Inhibition of Reactive Oxygen Species (ROS) Generation Assay
2.2.3. 5α-Reductase Protein Expression Induced by Dihydrotestosterone (DHT) in Human Hair Follicle Dermal Papilla Cells
2.3. Clinical Trial
2.3.1. Study Design
2.3.2. Safety Evaluation
2.3.3. Hair Analysis Measurement
3. Results
3.1. In Vitro Study
3.1.1. Determination of Particle Size by DLS
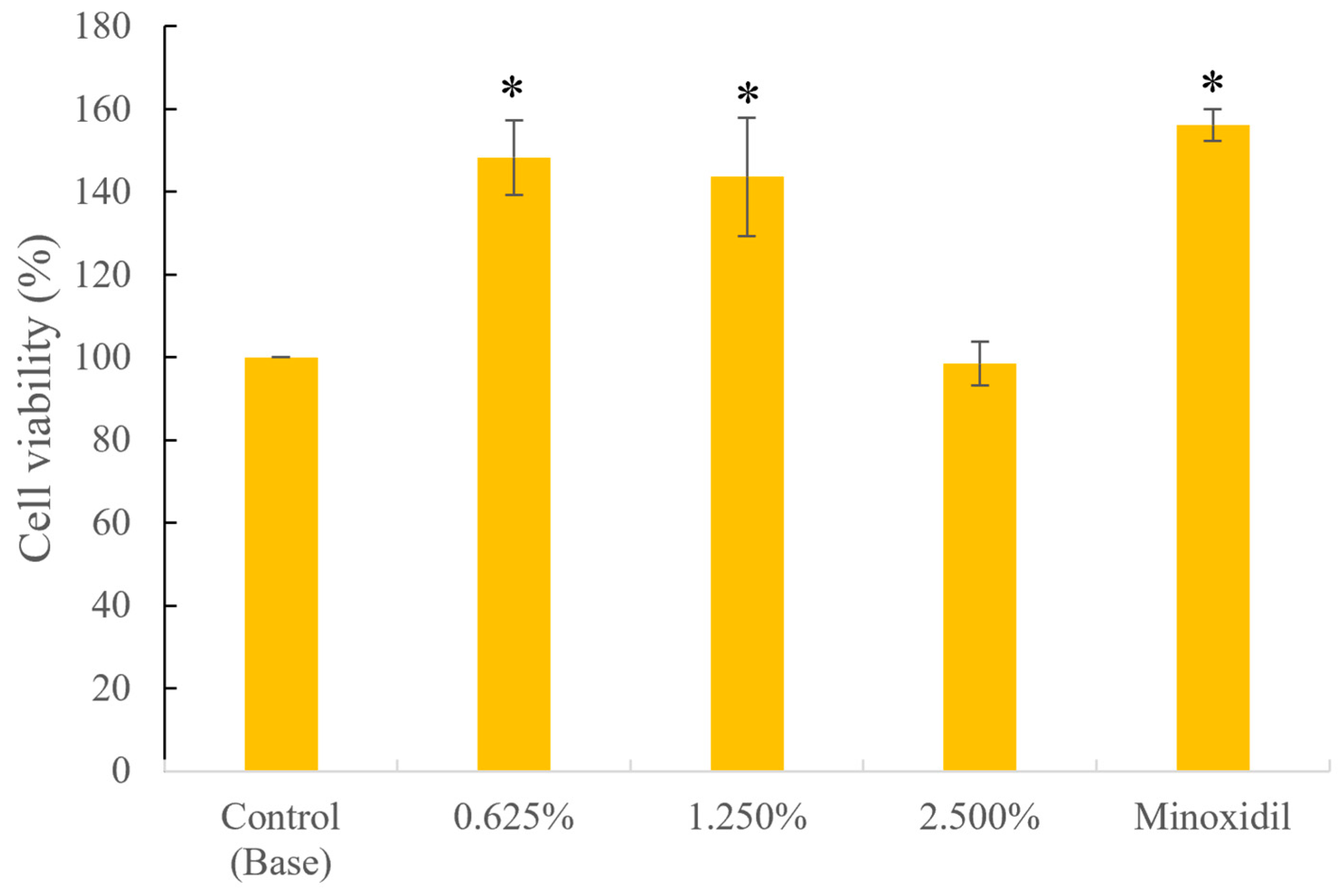
3.1.2. Hair Follicle Cell Safety and Proliferation Activity
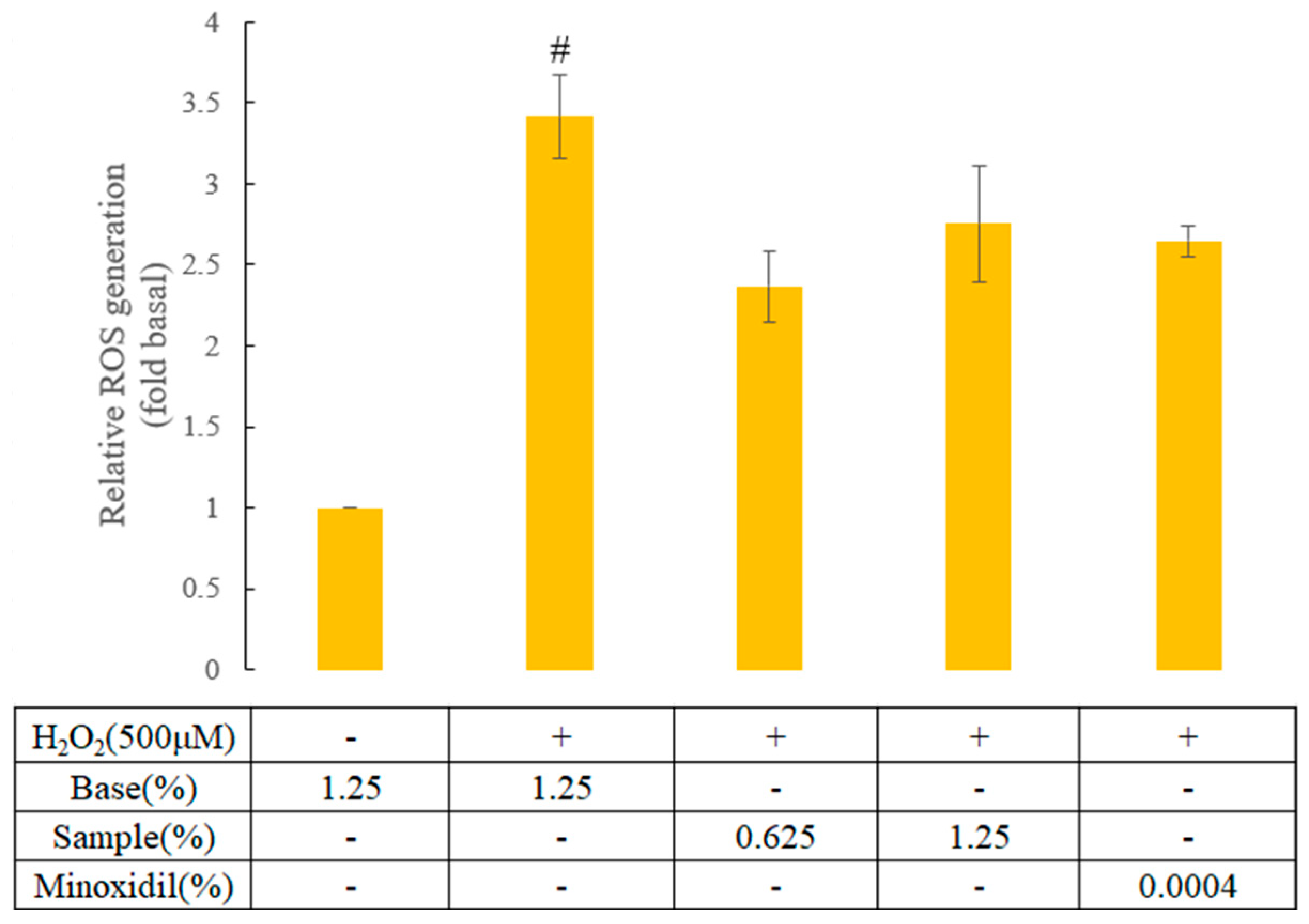
3.1.3. Antioxidant Activity
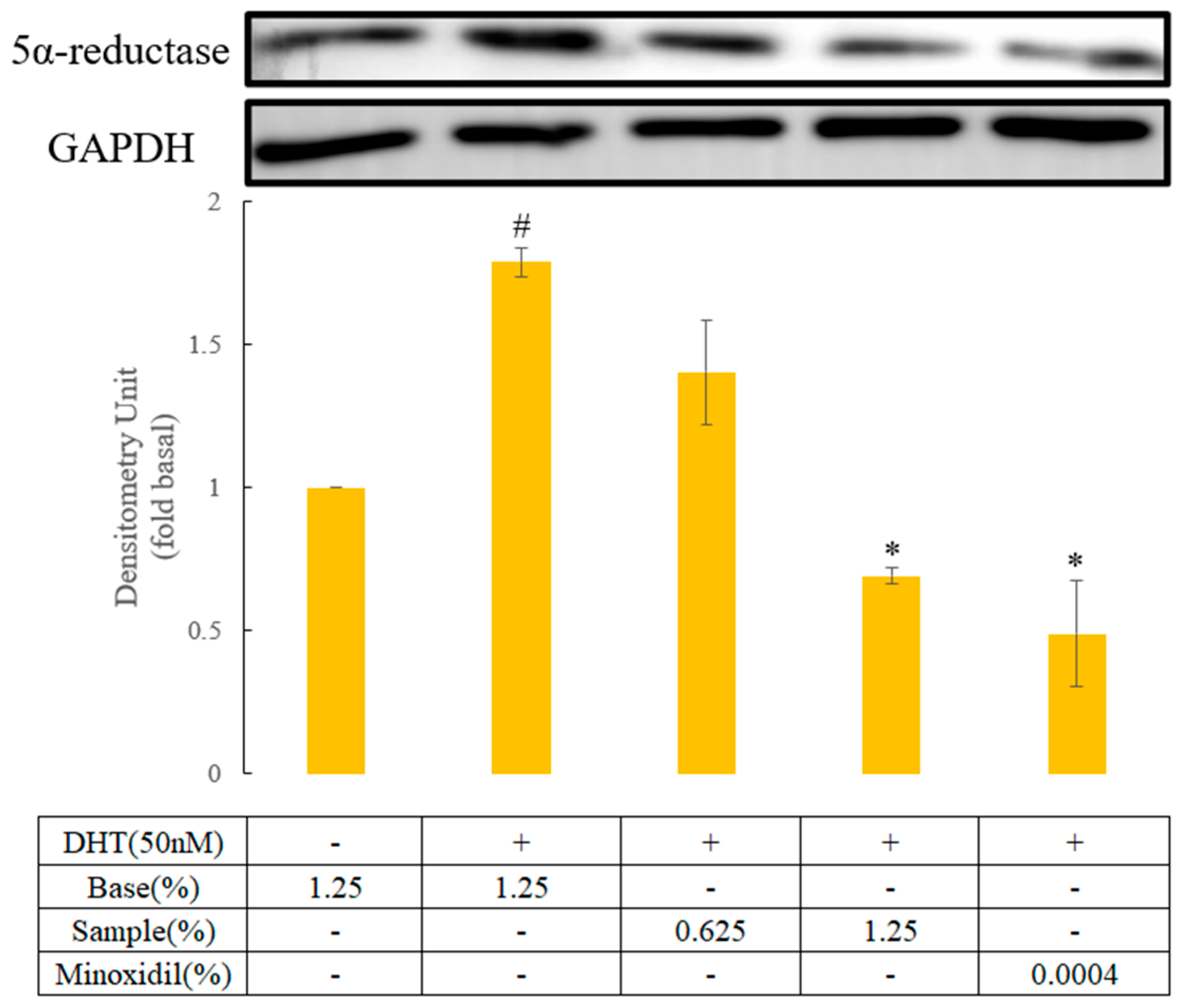
3.1.4. In Vitro Hair Growth Activity
3.2. Safety Profile and Efficacy of Biosea® Revive Serum for Hair Growth
3.2.1. Safety Profile
3.2.2. Hair Growth Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clinic, A.; Asper, A.; Mittal, A.; Shome, D.; Parbhoo, D.; Thanzama, J.; Doshi, K.; Sachde, N.; Gaunkar, R.; Kapoor, R.; et al. Evaluation of the Safety and Effectiveness of Intradermal Administration of QR678 Neo® Hair Growth Factor Formulation: A Phase-IV, Open-Label, Single-Arm Multi-Ethnicity Clinical Trial. J. Cosmet. Dermatol. 2022, 21, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.Y.; Jung, W.H.; Shin, S.L.; Kim, T.R.; Sohn, M.; Suk, J.; Jung, I.; Lee, Y.I.; Lee, J.H. Heat-Treated Limosilactobacillus Fermentum LM1020 with Menthol, Salicylic Acid, and Panthenol Promotes Hair Growth and Regulates Hair Scalp Microbiome Balance in Androgenetic Alopecia: A Double-Blind, Randomized and Placebo-Controlled Clinical Trial. J. Cosmet. Dermatol. 2024, 23, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment Options for Androgenetic Alopecia: Efficacy, Side Effects, Compliance, Financial Considerations, and Ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Ledwoń, P.; Errante, F.; Papini, A.M.; Rovero, P.; Latajka, R. Peptides as Active Ingredients: A Challenge for Cosmeceutical Industry. Chem. Biodivers. 2021, 18, e2000833. [Google Scholar] [CrossRef]
- Loing, E.; Lachance, R.; Ollier, V.; Hocquaux, M. A New Strategy to Modulate Alopecia Using A Combination of Two Specific and Unique Ingredients. J. Cosmet. Sci. 2013, 64, 45–58. [Google Scholar]
- Wongrakpanich, A.; Leanpolchareanchai, J.; Morakul, B.; Parichatikanond, W.; Teeranachaideekul, V. Phyllanthus emblica Extract-loaded Transfersomes for Hair Follicle Targeting: Phytoconstituents, Characterization, and Hair Growth Promotion. J. Oleo Sci. 2022, 71, 1085–1096. [Google Scholar] [CrossRef]
- Okunishi, I. Improvement of Cognitive Function by Wasabi Component “Hexaraphane”. In Herbs and Spices-New Perspectives in Human. Health and Food Industry: New Perspectives in Human. Health and Food Industry; IntechOpen: London, UK, 2024; p. 227. [Google Scholar]
- Singh, S.; Sonia; Sindhu, R.K.; Alsayegh, A.A.; Batiha, G.E.; Alotaibi, S.S.; Albogami, S.M.; Conte-Junior, C.A. Formulation Development and Investigations on Therapeutic Potential of Nanogel from Beta vulgaris L. Extract in Testosterone-Induced Alopecia. Biomed. Res. Int. 2023, 2023, 1777631. [Google Scholar] [CrossRef]
- Naeini, A.H.; Mahdavipour, K.; Rastegari, A.; Aghsami, M.; Montazeri, H.; Faghihi, H.; Mohammadi, Z. Chitosan and Its Amphiphilic Derivative Nanoparticles Loaded with Minoxidil for Induction of Hair Growth: In vitro and in vivo Evaluation. Int. J. Biol. Macromol. 2024, 259, 129122. [Google Scholar] [CrossRef]
- Meymandi, S.S.; Amiri, R.; Aflatunian, M.; Pardakhti, A. Comparison of the Efficacy of Niosomal Minoxidil with Conventional Minoxidil in the Treatment of Androgenetic Alopecia: A Randomized, Controlled, Double-Blind Clinical Trial. J. Dermatol. Cosmet. 2014, 5, 53–60. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kang, J.I.; Yoon, H.S.; Kim, S.M.; Park, J.E.; Hyun, Y.J.; Ko, A.; Ahn, Y.S.; Koh, Y.S.; Hyun, J.W.; Yoo, E.S.; et al. Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. Int. J. Mol. Sci. 2018, 19, 2770. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Lee, J.H.; Chang, S.J.; Chae, Y.; Lee, M.H.; Kim, S.H.; Han, K.I.; Kim, T.J. Heat-Killed Enterococcus faecalis EF-2001 Induces Human Dermal Papilla Cell Proliferation and Hair Regrowth in C57BL/6 Mice. Int. J. Mol. Sci. 2022, 23, 5413. [Google Scholar] [CrossRef]
- Wen, T.-C.; Li, Y.-S.; Rajamani, K.; Harn, H.-J.; Lin, S.-Z.; Chiou, T.-W. Effect of Cinnamomum osmophloeum Kanehira Leaf Aqueous Extract on Dermal Papilla Cell Proliferation and Hair Growth. Cell. Transplant. 2018, 27, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Sadick, N.S.; Callender, V.D.; Kircik, L.H.; Kogan, S. New Insight into the Pathophysiology of Hair Loss Trigger A Paradigm Shift in the Treatment Approach. J. Drugs. Dermatol. 2017, 16, s135–s140. [Google Scholar] [PubMed]
- Garre, A.; Piquero, J.; Trullas, C.; Martinez, G. Efficacy and Safety of A New Topical Hair Loss-Lotion Containing Oleanolic Acid, Apigenin, Biotinyl Tripeptide-1, Diaminopyrimidine Oxide, Adenosine, Biotin and Ginkgo Biloba in Patients with Androgenetic Alopecia and Telogen Effluvium: A Six-Month Open-Label Prospective Clinical Study. J. Cosmetol. Trichol. 2018, 4, 1000132. [Google Scholar] [CrossRef]
- Inui, S.; Fukuzato, Y.; Nakajima, T.; Yoshikawa, K.; Itami, S. Androgen-Inducible TGF-Beta1 from Balding Dermal Papilla Cells Inhibits Epithelial Cell Growth: A Clue to Understand Paradoxical Effects of Androgen on Human Hair Growth. FASEB J. 2002, 16, 1967–1969. [Google Scholar] [CrossRef]
- Shin, H.; Yoo, H.G.; Inui, S.; Itami, S.; Kim, I.G.; Cho, A.R.; Lee, D.H.; Park, W.S.; Kwon, O.; Cho, K.H.; et al. Induction of Transforming Growth Factor-Beta 1 by Androgen Is Mediated by Reactive Oxygen Species in Hair Follicle Dermal Papilla Cells. BMB Rep. 2013, 46, 460–464. [Google Scholar] [CrossRef]
- Rajput, R. A Scientific Hypothesis on the Role of Nutritional Supplements for Effective Management of Hair Loss and Promoting Hair Regrowth. J. Nutrition. Health. Food. Sci. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin Hydration: A Review on Its Molecular Mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Kim, K.S.; Shin, M.K.; Kim, J.H.; Kim, M.H.; Haw, C.R.; Park, H.K. Effects of Atopic Dermatitis on the Morphology and Water Content of Scalp Hair. Microsc. Res. Tech. 2012, 75, 620–625. [Google Scholar] [CrossRef]
- Girdwichai, N.; Chanprapaph, K.; Vachiramon, V. Behaviors and Attitudes Toward Cosmetic Treatments Among Men. J. Clin. Aesthet. Dermatol. 2018, 11, 42–48. [Google Scholar] [PubMed]
- Basaria, S.; Jasuja, R.; Huang, G.; Wharton, W.; Pan, H.; Pencina, K.; Li, Z.; Travison, T.G.; Bhawan, J.; Gonthier, R.; et al. Characteristics of Men Who Report Persistent Sexual Symptoms After Finasteride Use for Hair Loss. J. Clin. Endocrinol. Metab. 2016, 101, 4669–4680. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, J. TrichoScan®: A Useful Method For Measuring Hair Growth Parameters and Enhancing Patient Selection for Hair Restoration Surgery. Hair Transplant. Forum Int. 2009, 19, 56–59. [Google Scholar] [CrossRef]
- Bayer, M.; Gahrtz, M.; Voss, W.; Schlippe, G.; Whitfield, T. The Effect of A Food Supplement and A Hair Lotion on the Progression of Androgenetic Alopecia. J. Cosmet. Dermatol. Sci. Appl. 2019, 9, 292–304. [Google Scholar] [CrossRef]
- Sasaki, G.H. The Effects of Lower vs. Higher Cell Number of Platelet-Rich Plasma (PRP) on Hair Density and Diameter in Androgenetic Alopecia (AGA): A Randomized, Double-Blinded, Placebo, Parallel-Group Half-Scalp IRB-Approved Study. Aesthet. Surg. J. 2021, 41, NP1659–NP1672. [Google Scholar] [CrossRef]
| Particle Size (nm) | PDI |
|---|---|
| 102.63 ± 64.87 | 0.19 ± 0.05 |
| Sample | Inhibitory Percentage (%) & |
|---|---|
| 0.625% BRS | 43.66 ± 2.98 |
| 1.250% BRS | 28.12 ± 7.13 |
| Minoxidil 0.0004% | 31.46 ± 3.53 |
| Sample | Inhibition Percentage (%) & |
|---|---|
| 0.625% BRS | 50.16 ± 19.71 * |
| 1.250% BRS | 139.36 ± 1.01 |
| Minoxidil 0.0004% | 163.83 ± 19.57 |
| Male | Female | |||
|---|---|---|---|---|
| Day | Application Site | Control Area | Application Site | Control Area |
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 15 | 110.7 ± 81.9 | 130.6 ± 80 | 86.9 ± 54.3 | 113.4 ± 67.5 |
| 30 | 113 ± 88 | 113.4 ± 48.5 | 103.2 ± 93.1 | 132.8 ± 107.9 |
| 45 | 98.9 ± 52.5 | 125.2 ± 56.3 | 85.8 ± 55.1 | 123.2 ± 64.7 |
| 90 | 111.6 ± 74.1 | 122.2 ± 54.1 | 70.2 ± 41.9 * | 100.4 ± 68.6 |
| Day | Hair Density $ (%) | Hair Mass # (%) | Median Hair Thickness a (%) | Mean Hair Thickness b (%) |
|---|---|---|---|---|
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 15 | 109.7 ± 22.4 | 110.5 ± 22.6 * | 101.8 ± 17.2 | 101.3 ± 10.7 |
| 30 | 110.1 ± 16.7 * | 111.8 ± 16.9 * | 102.2 ± 14.3 | 101.8 ± 6.8 |
| 45 | 114.6 ± 21.5 * | 114.3 ± 18.2 * | 101.9 ± 18.6 | 100.5 ± 8.4 |
| 90 | 107.2 ± 20.5 | 105.7 ± 20.2 | 97.9 ± 15.6 | 99.2 ± 8.6 |
| Day | Vellus Hair Density@ (%) | Terminal Hair Density& (%) | Vellus Hair Ratioc (%) | Terminal Hair Ratiod (%) |
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 15 | 124.1 ± 85.9 | 110.9 ± 21.4 | 107.9 ± 56.6 | 102.1 ± 14.4 |
| 30 | 120 ± 52.4 | 109.1 ± 17.8 | 108.1 ± 37.3 | 99.3 ± 8.9 |
| 45 | 146.3 ± 102.8 | 111.8 ± 20.4 | 121.4 ± 63.7 | 98.6 ± 14.1 |
| 90 | 129.3 ± 63.2 | 100.7 ± 24.4 | 118.8 ± 45.8 | 94.2 ± 14.7 |
| Day | Hair Density $ (%) | Hair Mass # (%) | Median Hair Thickness a (%) | Mean Hair Thickness b (%) |
|---|---|---|---|---|
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 15 | 116.1 ± 34.9 * | 114.5 ± 35.9 | 99 ± 13.9 | 98.5 ± 8.8 |
| 30 | 119.5 ± 45.6 | 117.1 ± 43.3 | 99 ± 7.6 | 98.6 ± 6.9 |
| 45 | 117.9 ± 41 | 113.5 ± 37.7 | 98.6 ± 12.2 | 96.8 ± 9.9 |
| 90 | 97 ± 29.9 | 96.2 ± 32.4 | 99.3 ± 6.8 | 98.3 ± 11.5 |
| Day | Vellus Hair Density @ (%) | Terminal Hair Density & (%) | Vellus Hair Ratio c (%) | Terminal Hair Ratio d (%) |
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 15 | 144.3 ± 111.8 | 114.5 ± 33.5 | 116 ± 56.3 | 99 ± 12.6 |
| 30 | 146.4 ± 98.1 * | 118.2 ± 45.2 | 123.5 ± 84.8 | 99.4 ± 10 |
| 45 | 187.4 ± 216.5 | 115.1 ± 39.7 | 143.9 ± 107.1 | 98.4 ± 19 |
| 90 | 167.7 ± 212.4 | 93.6 ± 31.5 | 163.4 ± 178.5 | 95.9 ± 17.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-J.; Yang, C.-Y.; So, P.B.; Hu, H.-Y.; Yang, S.-H.; Hsueh, H.-M.; Wu, T.-H.; Yen, F.-L. Safety Profile and Efficacy of Biosea® Revive Serum for Hair Growth Through In Vitro Assessment and Clinical Evaluation. Cosmetics 2025, 12, 139. https://doi.org/10.3390/cosmetics12040139
Wu C-J, Yang C-Y, So PB, Hu H-Y, Yang S-H, Hsueh H-M, Wu T-H, Yen F-L. Safety Profile and Efficacy of Biosea® Revive Serum for Hair Growth Through In Vitro Assessment and Clinical Evaluation. Cosmetics. 2025; 12(4):139. https://doi.org/10.3390/cosmetics12040139
Chicago/Turabian StyleWu, Chi-Ju, Chun-Yin Yang, Pamela Berilyn So, Hui-Yu Hu, Shang-Hsuan Yang, Hsiang-Ming Hsueh, Tzu-Hui Wu, and Feng-Lin Yen. 2025. "Safety Profile and Efficacy of Biosea® Revive Serum for Hair Growth Through In Vitro Assessment and Clinical Evaluation" Cosmetics 12, no. 4: 139. https://doi.org/10.3390/cosmetics12040139
APA StyleWu, C.-J., Yang, C.-Y., So, P. B., Hu, H.-Y., Yang, S.-H., Hsueh, H.-M., Wu, T.-H., & Yen, F.-L. (2025). Safety Profile and Efficacy of Biosea® Revive Serum for Hair Growth Through In Vitro Assessment and Clinical Evaluation. Cosmetics, 12(4), 139. https://doi.org/10.3390/cosmetics12040139






