Anti-Inflammatory Effects of Nephelium lappaceum Peel Extract and Geraniin on External Skin Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Extracts
2.2.1. Extraction of Nephelium lappaceum Peel
2.2.2. Preparation of Natural Deep Eutectic Solvents (NADESs)
2.2.3. Ellagitannin Quantification by HPLC
- (1)
- For geraniin content: Y = 37122X + 50,051 (with R2 = 0.999);
- (2)
- For corilagin content: Y = 32631X + 76,360 (with R2 = 0.999);
- (3)
- For gallic acid content: Y = 31305X + 121,233 (with R2 = 0.999);
- (4)
- For ellagic acid content: Y = 73567X + 138,411 (with R2 = 0.999).
2.3. In Vitro
2.3.1. Preparation and Storage of Reagents
2.3.2. Cell Culture
2.3.3. Cell Viability Assay
2.3.4. Sample Treatment
2.3.5. Quantitative Real-Time PCR (qPCR)
2.4. Clinical Trial
2.4.1. Study Design and Participants
- Healthy male or female volunteers aged 19 to 60 years without facial flushing and without wounds or scars on the forearm or upper arm.
- Volunteers without a history of photosensitivity or tape allergies.
- Individuals who have received a thorough explanation from the researcher regarding the study’s purpose, content, and potential adverse reactions, have been given sufficient time for consideration, and have voluntarily provided written informed consent for participation.
- Individuals who agree to avoid activities that may environmentally or physically affect the test area (e.g., sauna, scrubbing, swimming) during the study period.
- Individuals who can visit at designated times and are available for follow-up during the study period.
2.4.2. Induction of Skin Damage
2.4.3. Transepidermal Water Loss (TEWL) Assessment
2.4.4. Skin Redness Image Analysis (a* Value Analysis)
2.5. Statistical Analysis
3. Results
3.1. Quantification of Geraniin, Corilagin, Gallic Acid, and Ellagic Acid in Nephelium lappaceum Peel Extracts Using NADESs
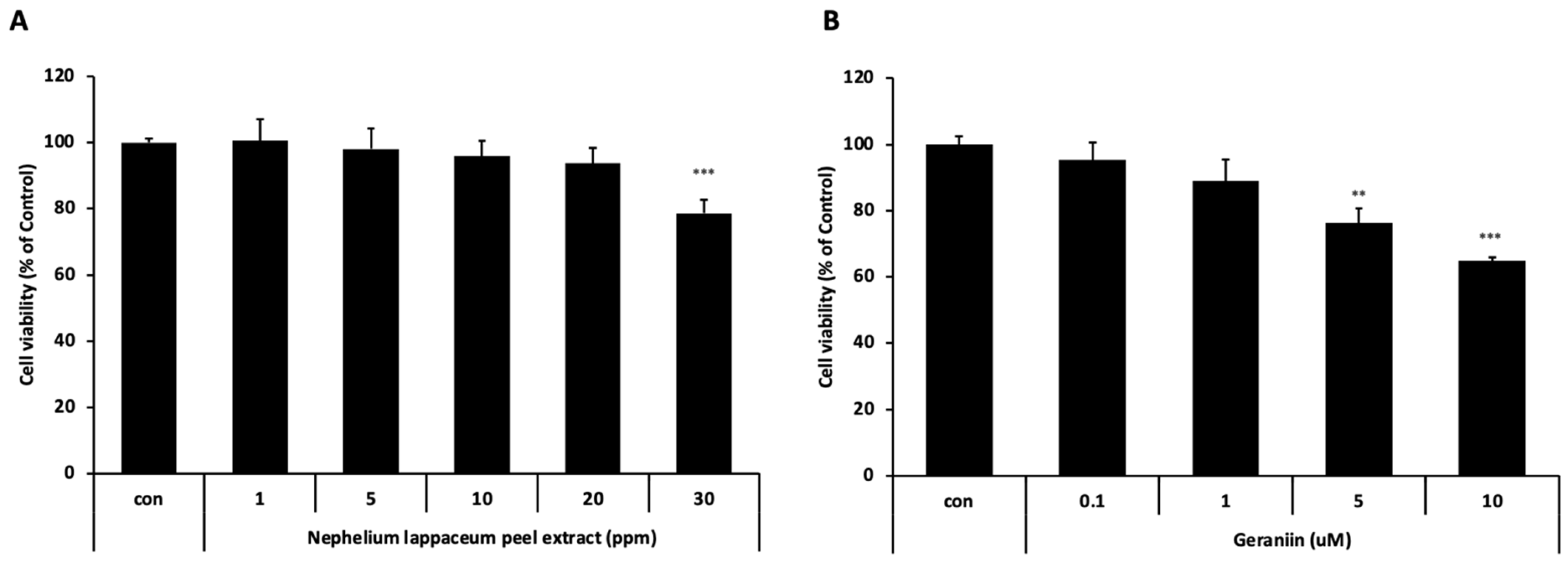
3.2. Cytotoxicity Evaluation of Nephelium lappaceum Peel Extract and Geraniin on HaCaT Cells
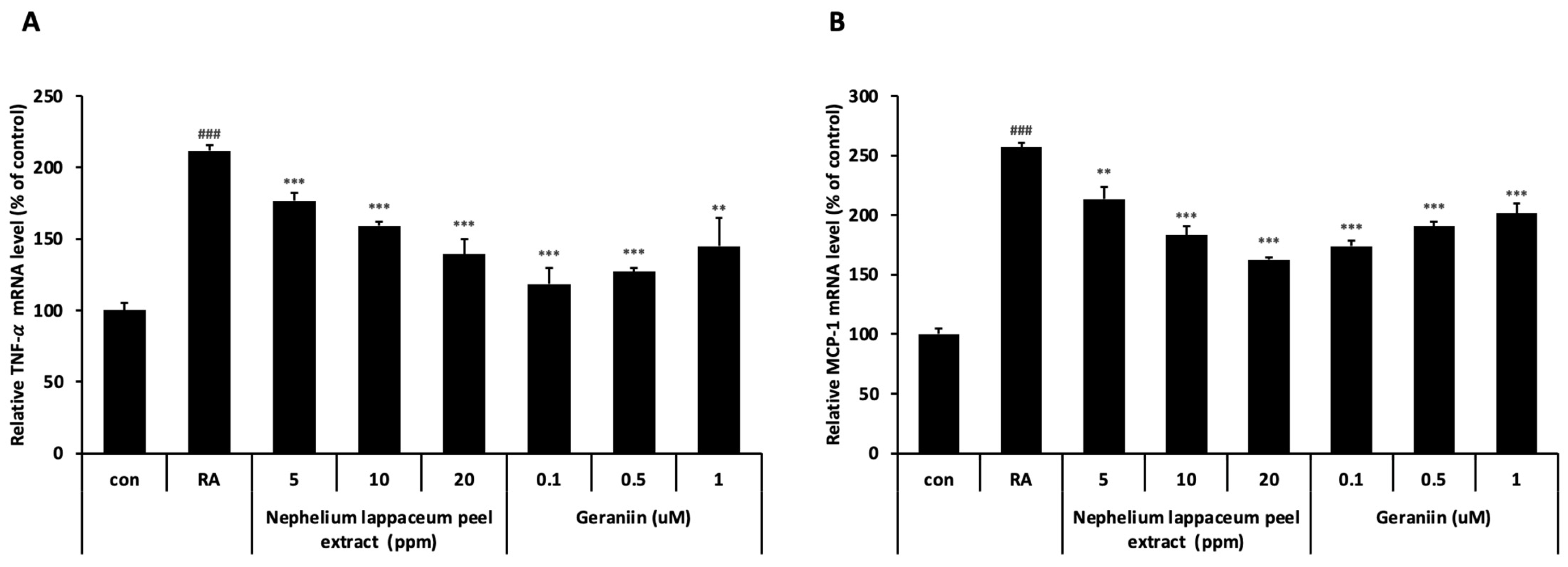
3.3. Nephelium lappaceum Peel Extract and Geraniin Inhibit RA-Induced TNF- and MCP-1 Expression
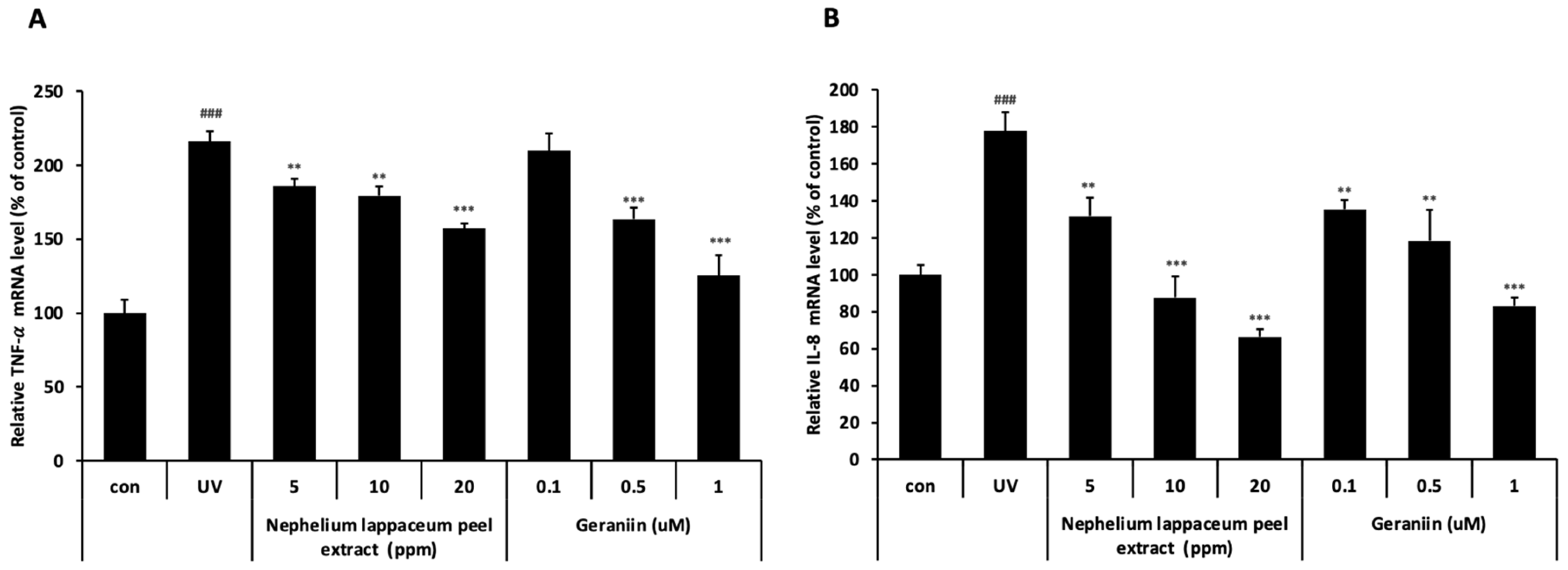
3.4. Nephelium lappaceum Peel Extract and Geraniin Inhibit UV-Induced TNF- and IL-8 Expression
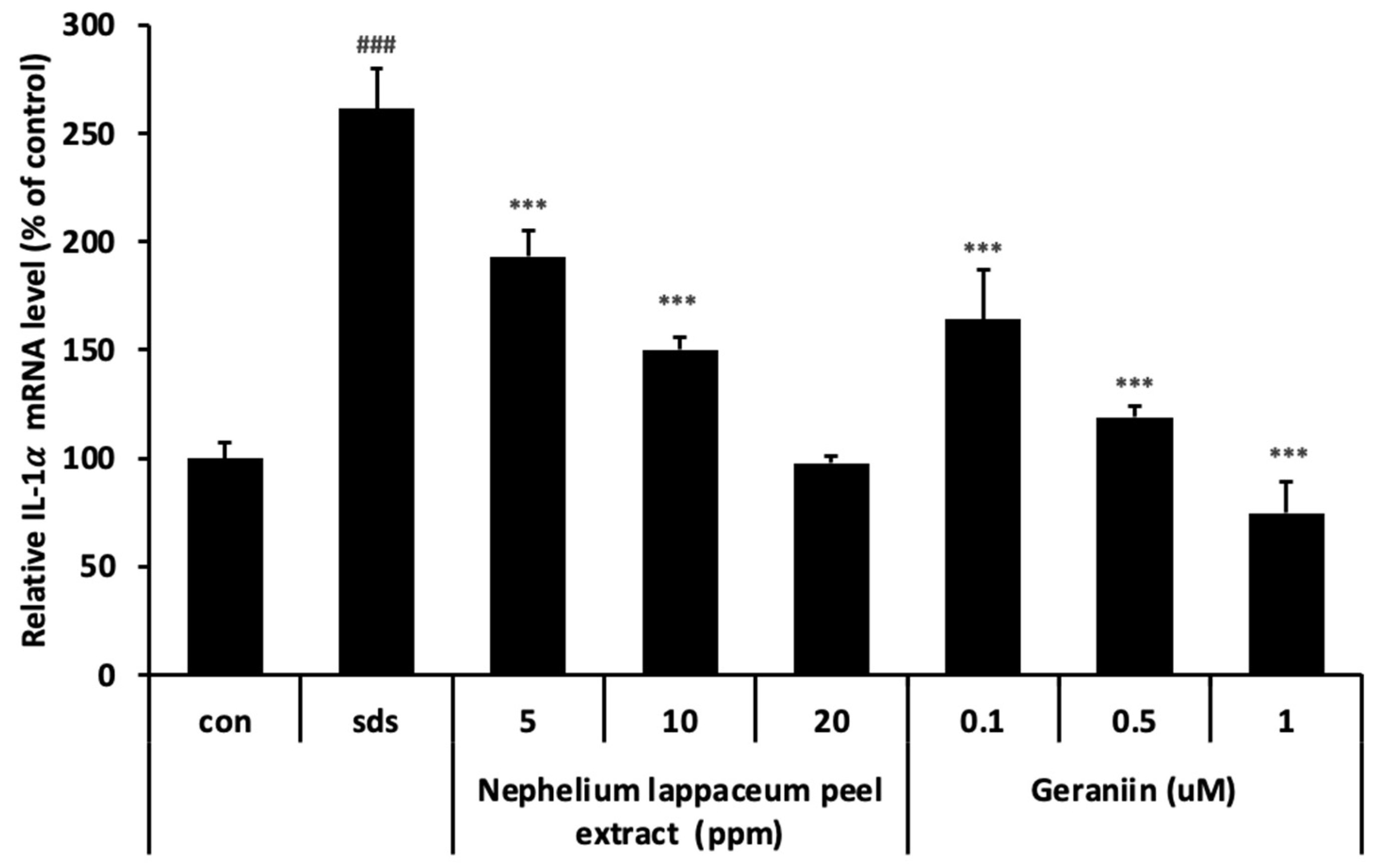
3.5. Nephelium lappaceum Peel Extract and Geraniin Inhibit SDS-Induced IL-1 Expression
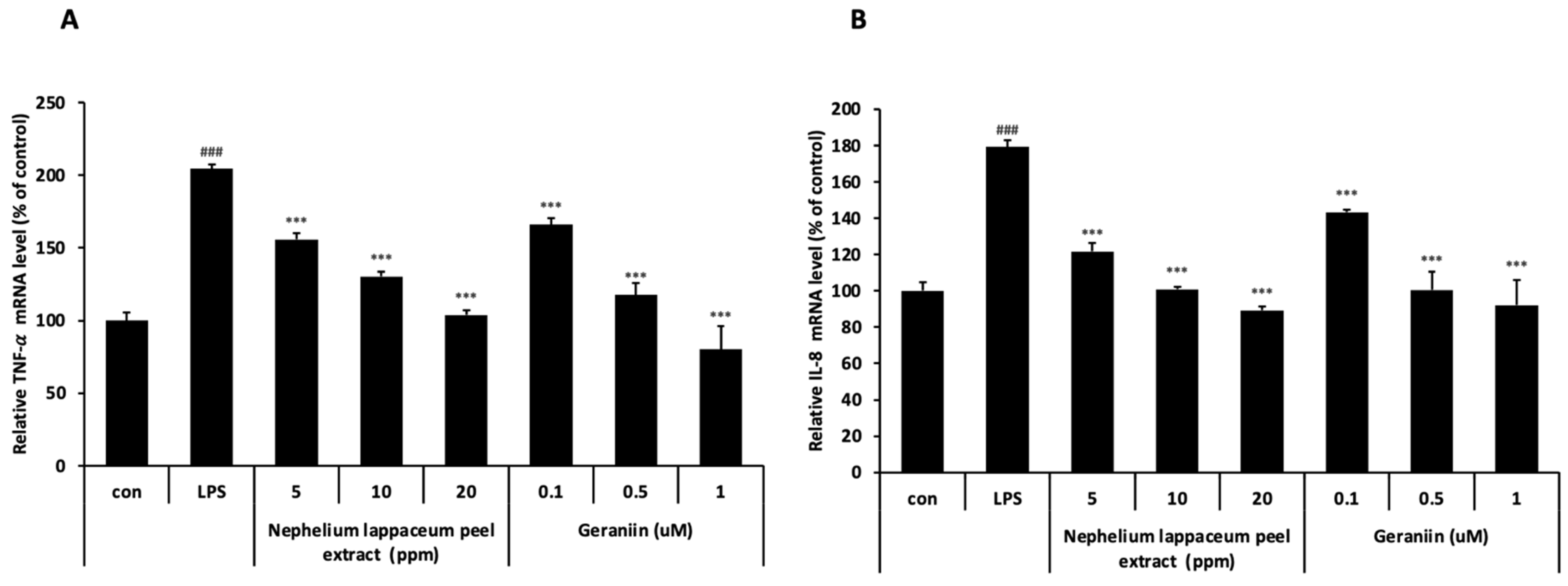
3.6. Nephelium lappaceum Peel Extract and Geraniin Inhibit LPS-Induced TNF-α and IL-8 Expression
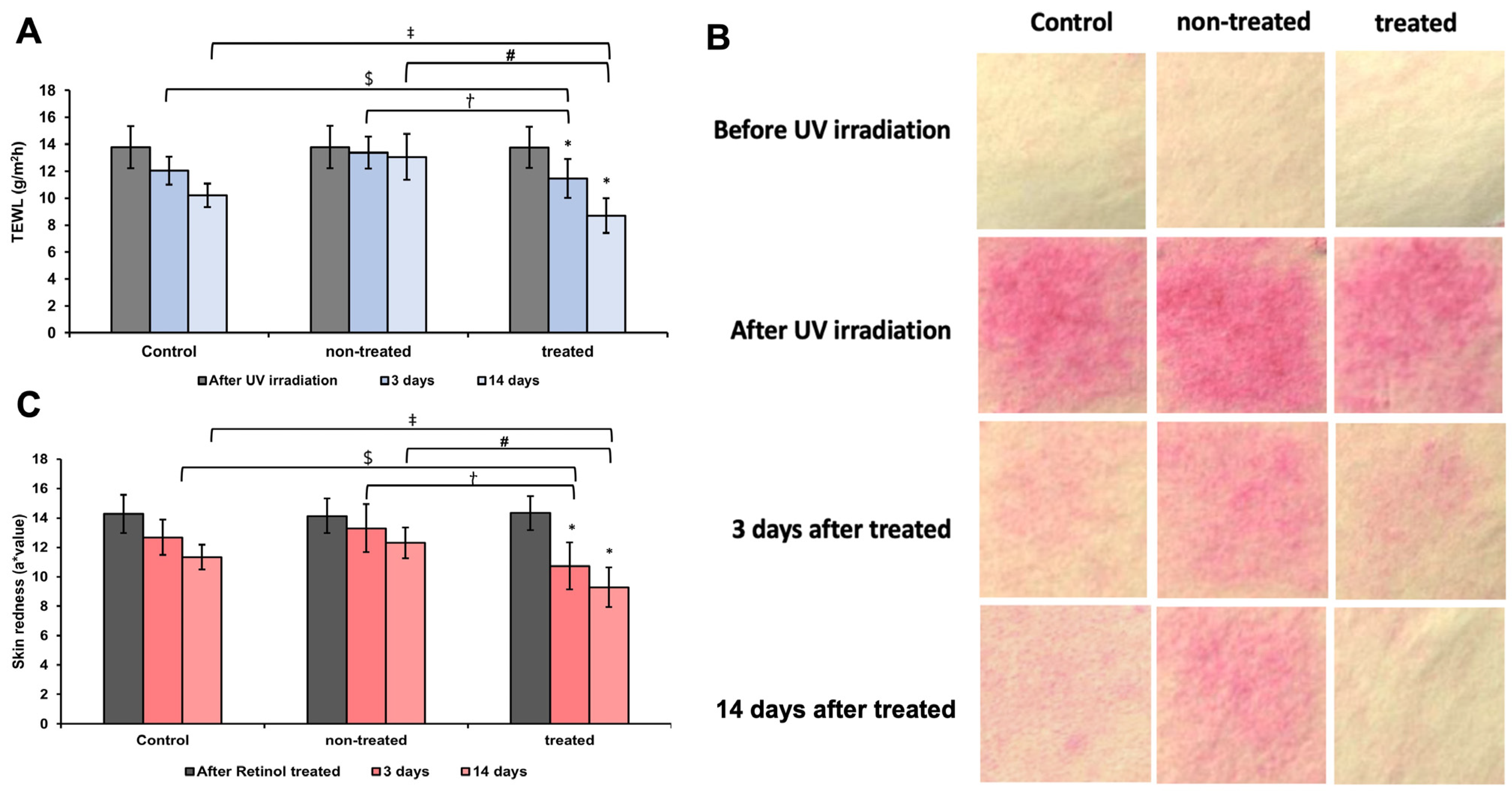
3.7. Analysis of TEWL and Redness Assessment of UV-Induced Damaged Skin Barrier
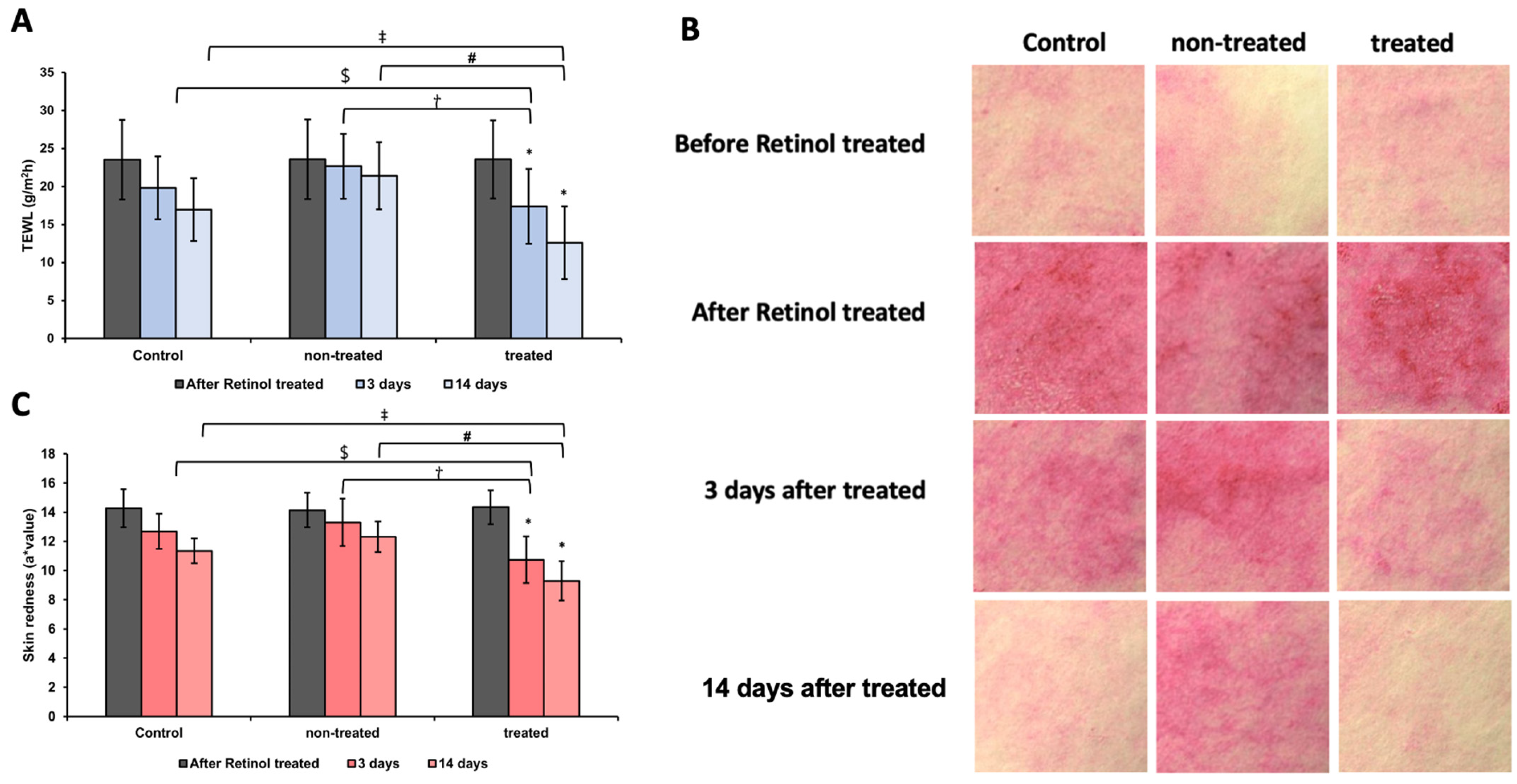
3.8. Analysis of TEWL and Skin Redness After Retinol Patch Application
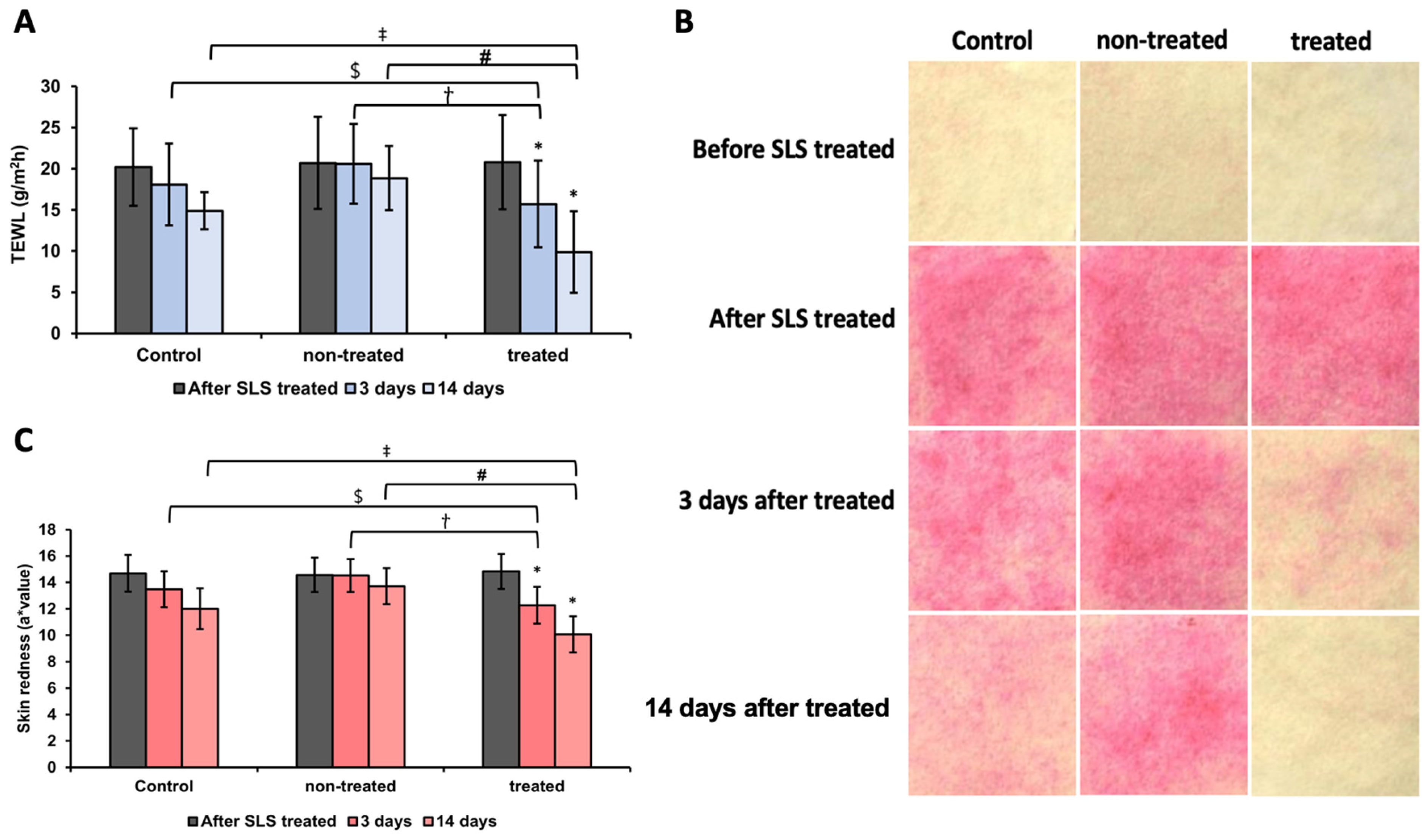
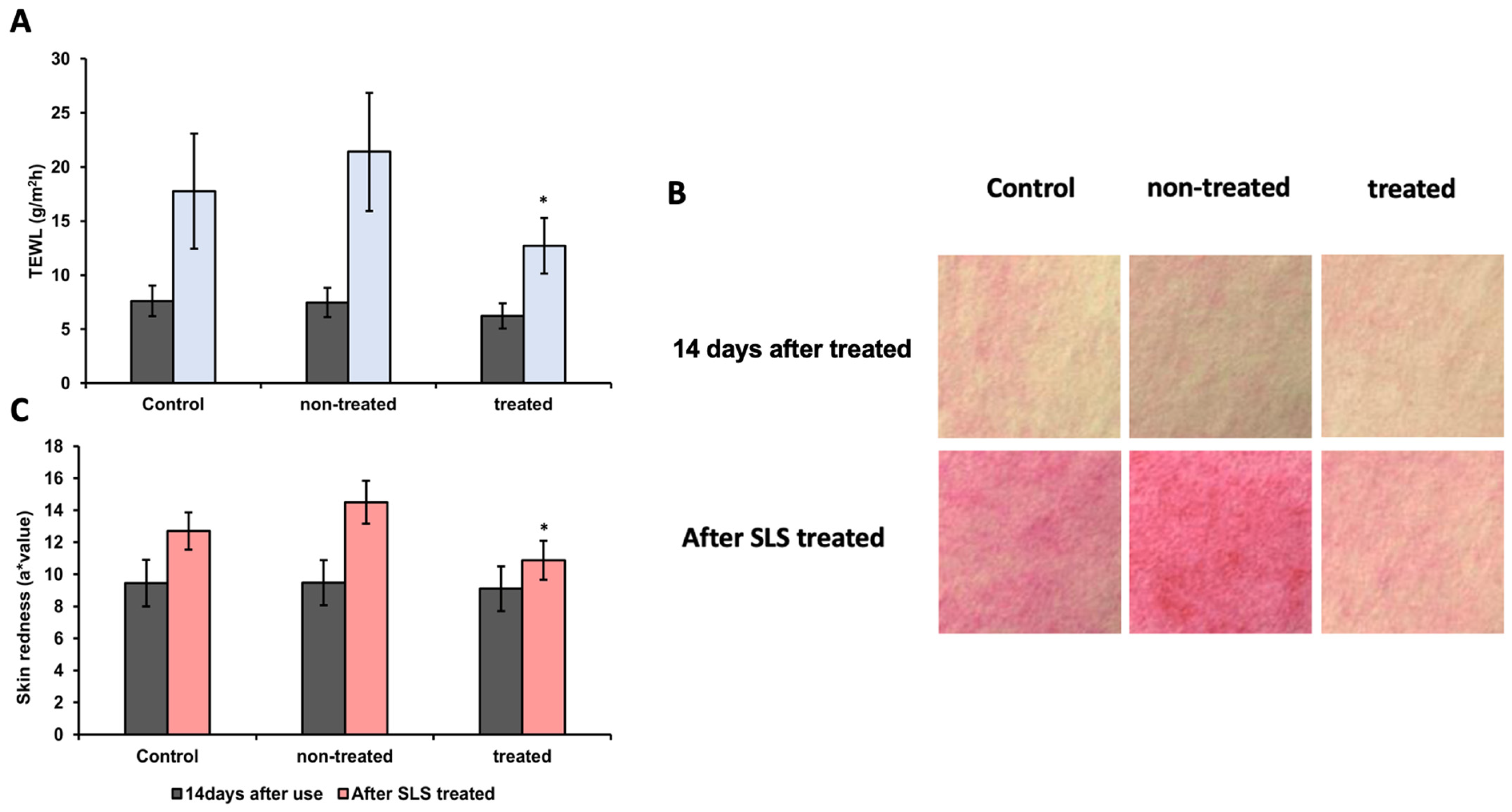
3.9. Analysis of TEWL and Skin Redness After SLS Patch Application
3.10. Analysis of Skin Damage Protective Effects and Redness Assessment Against SLS
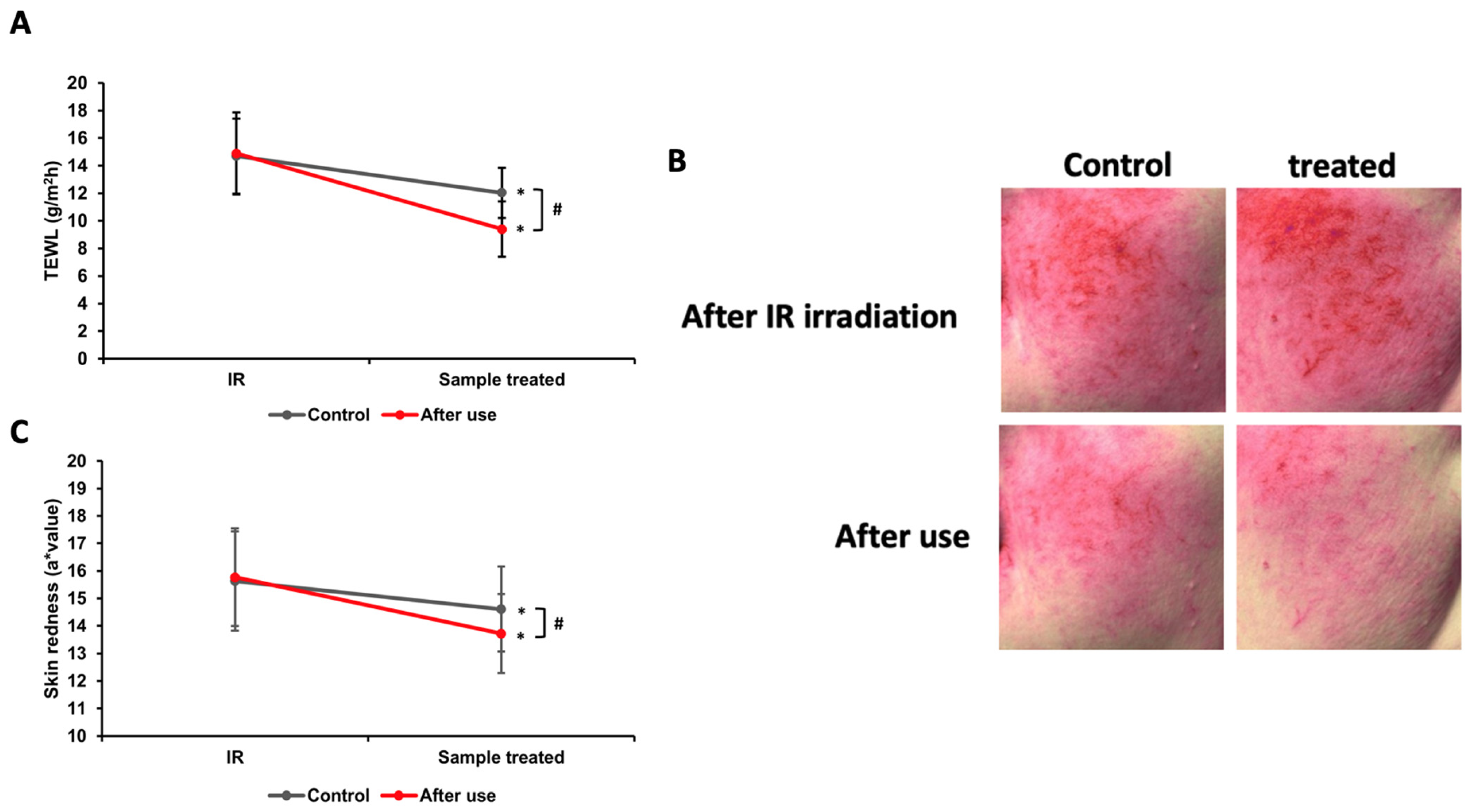
3.11. Analysis of TEWL and Skin Redness After Infrared Heating
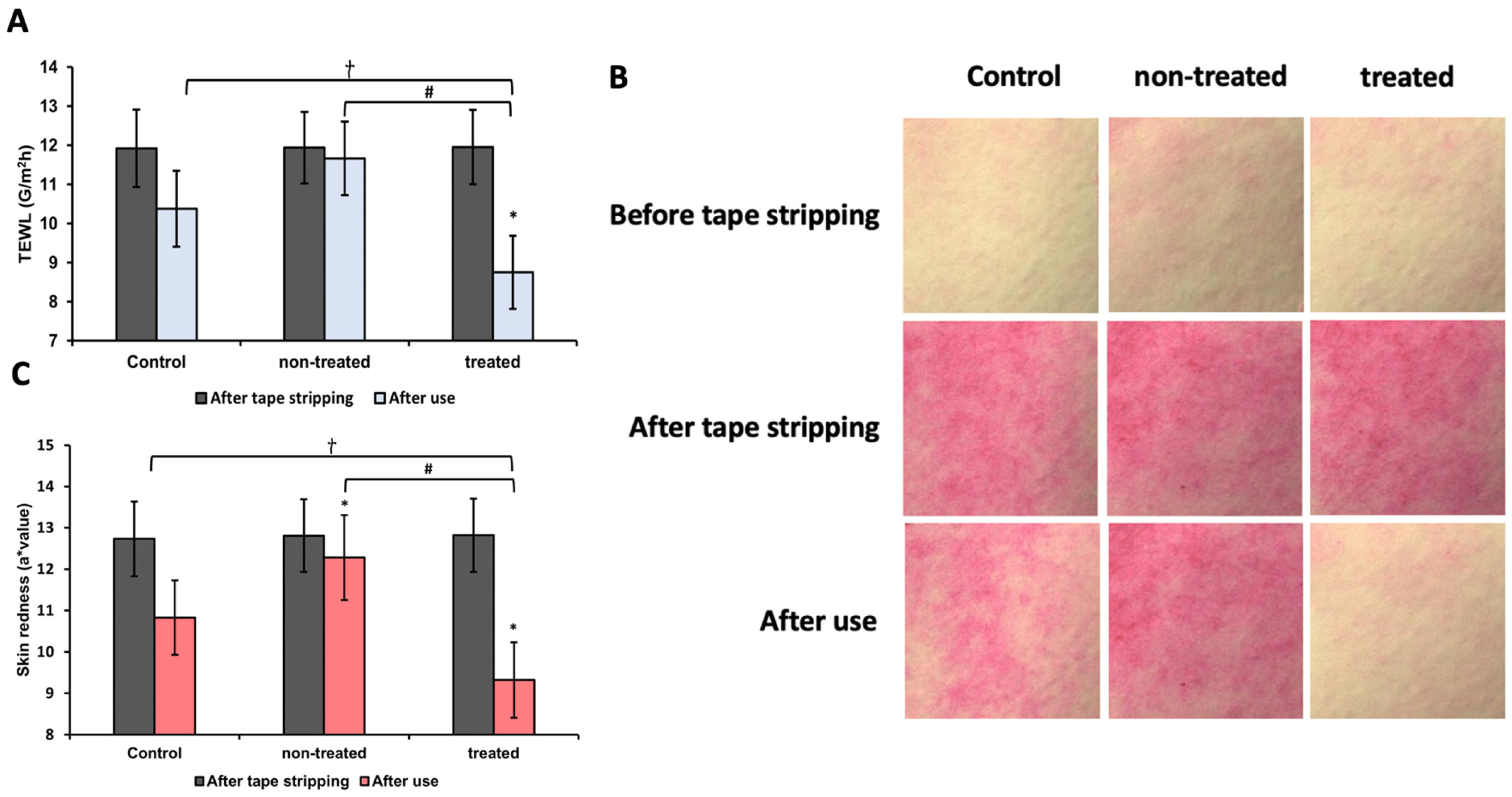
3.12. Analysis of TEWL and Skin Redness Induced by Tape Stripping
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TEWL | Transepidermal water loss |
| RA | Retinoic acid |
| SLS | Sodium lauryl sulfate |
| NF-κB | Nuclear factor kappa B |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1α | Interleukin-1 alpha |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| JNKs | Jun N-terminal kinases |
| MAPKs | Mitogen-activated protein kinases |
| AP-1 | Activator protein 1 |
| LPS | Lipopolysaccharides |
| TLR4 | Toll-like receptor 4 |
| IR | Infrared radiation |
| NADESs | Natural deep eutectic solvents |
References
- Rauma, M.; Boman, A.; Johanson, G. Predicting the absorption of chemical vapours. Adv. Drug Deliv. Rev. 2013, 65, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar]
- Dąbrowska, A.K.; Rotaru, G.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R.M. Materials used to simulate physical properties of human skin. Ski. Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar]
- Kafi, R.; Kwak, H.S.R.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J. Improvement of naturally aged skin with vitamin A (retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar]
- Quan, T. Human skin aging and the anti-aging properties of retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef]
- Geria, A.N.; Lawson, C.N.; Halder, R.M. Topical retinoids for pigmented skin. J. Drugs Dermatol. JDD 2011, 10, 483–489. [Google Scholar]
- Effendy, I.; Kwangsukstith, C.; Lee, J.Y.; Maibach, H.I. Functional changes in human stratum corneum induced by topical glycolic acid: Comparison with all-trans retinoic acid. Acta. Derm. Venereol. 1995, 75, 455–458. [Google Scholar]
- Lee, H.Y.; Stieger, M.; Yawalkar, N.; Kakeda, M. Cytokines and chemokines in irritant contact dermatitis. Mediat. Inflamm. 2013, 2013, 916497. [Google Scholar] [CrossRef]
- Matsusaka, T.; Fujikawa, K.; Nishio, Y.; Mukaida, N.; Matsushima, K.; Kishimoto, T.; Akira, S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 1993, 90, 10193–10197. [Google Scholar] [CrossRef] [PubMed]
- Rosette, C.; Karin, M. Ultraviolet light and osmotic stress: Activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 1996, 274, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, 2003, re2. [Google Scholar] [CrossRef]
- Heck, D.E.; Gerecke, D.R.; Vetrano, A.M.; Laskin, J.D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol. Appl. Pharmacol. 2004, 195, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Palacky Univ. Olomouc 2006, 150, 25. [Google Scholar] [CrossRef]
- Halliday, G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 2005, 571, 107–120. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef]
- Wang, C.; An, Q.; Zhao, D.; Li, M.; Zheng, H.; Zhang, J.; Liu, J.; Yang, L.; Su, N. Insight into the mechanism of SDS irritation on human skin keratinocytes by examination of changes in gene expression. Am. J. Biomed. Sci. 2016, 8, 311–321. [Google Scholar] [CrossRef]
- Salomon, G.; Giordano-Labadie, F. Surfactant irritations and allergies. Eur. J. Dermatol. 2022, 32, 677–681. [Google Scholar] [CrossRef]
- Tsai, S.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B Biol. 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Photoprotection of human skin beyond ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2014, 30, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeh, W.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Shi, H.; Yuan, M.; Li, J.; Song, K.; Huang, J.; Yi, P.; Shen, H.; Fu, B. Madecassoside protects against LPS-induced acute lung injury via inhibiting TLR4/NF-κB activation and blood-air barrier permeability. Front. Pharmacol. 2020, 11, 807. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Q.; Zhang, Z.; Xu, L.; Lin, C.; Gao, L.; Zhao, K.; Liang, F.; Zhang, Q. Madecassoside prevents acute liver failure in LPS/D-GalN-induced mice by inhibiting p38/NF-κB and activating Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018, 103, 1137–1145. [Google Scholar] [CrossRef]
- Cao, W.; Li, X.; Zhang, X.; Hou, Y.; Zeng, A.; Xie, Y.; Wang, S. Madecassoside suppresses LPS-induced TNF-α production in cardiomyocytes through inhibition of ERK, p38, and NF-κB activity. Int. Immunopharmacol. 2010, 10, 723–729. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in inflammatory skin diseases. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; Chavagnac, C.; Vocanson, M.; Rozieres, A.; Benetiere, J.; Pernet, I.; Denis, A.; Nicolas, J.; Hennino, A. Skin Contact Irritation Conditions the Development and Severity of Allergic Contact Dermatitis. J. Investig. Dermatol. 2007, 127, 1430–1435. [Google Scholar] [CrossRef]
- Zhang, Y.; Heinemann, N.; Rademacher, F.; Darvin, M.E.; Raab, C.; Keck, C.M.; Vollert, H.; Fluhr, J.W.; Gläser, R.; Harder, J. Skin care product rich in antioxidants and anti-inflammatory natural compounds reduces itching and inflammation in the skin of atopic dermatitis patients. Antioxidants 2022, 11, 1071. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic dogwood extracts as materials for reducing the skin irritation potential of body wash cosmetics. Molecules 2017, 22, 320. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C.; Morlett-Chávez, J.; Govea-Salas, M.; Ascacio-Valdés, J.A. Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, Y.; Tian, W.; Sun, L. In vivo acute and subacute toxicities of phenolic extract from rambutan (Nephelium lappaceum) peels by oral administration. Food Chem. 2020, 320, 126618. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.N.; Nofriyaldi, A.; Junaedi, S.A. Antipyretic Activity Test of Rambutan (Nephelium lappaceum L.) Seed in White Male Mice (Mus musculus). Recent Dev. Chem. Biochem. Res. 2023, 2720, 030003. [Google Scholar]
- Sangeeth Shyam Sundar, S.S.; Rajamanickam, C.; Saraswathy, S.; Venkatesan, K.; Balakumbahan, R.; Vijayasamundeeswari, A.; Sankar, C. Sapindaceae fruits: A comprehensive overview on phytochemicals, nutraceuticals and health benefits application. Plant Sci. Today 2024, 11, 1–14. [Google Scholar] [CrossRef]
- Nam, Y.H.; Ahn, S.M.; Seo, G.J.; Kim, N.W.; Shin, S.W.; Nuankaew, W.; Murughanantham, N.; Pandian, S.; Hwang, J.S.; Hong, B.N.; et al. Optimization of NADES-based green extraction of ellagitannins from rambutan peel with enhanced antioxidant activity. Food Chem. 2025, 475, 143308. [Google Scholar] [CrossRef]
- Okabe, S.; Suganuma, M.; Imayoshi, Y.; Taniguchi, S.; Yoshida, T.; Fujiki, H. New TNF-α releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense, Megusurino-ki. Biol. Pharm. Bull. 2001, 24, 1145–1148. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Chaiwut, P.; Saewan, N. In-vitro antioxidant potential of Nephelium lappaceum L. rind extracts and geraniin on human epidermal keratinocytes. Biocatal. Agric. Biotechnol. 2020, 23, 101482. [Google Scholar] [CrossRef]
- Siqueira, E.M.d.S.; Lima, T.L.; Boff, L.; Lima, S.G.; Lourenço, E.M.; Ferreira, É.G.; Barbosa, E.G.; Machado, P.R.; Farias, K.J.; Ferreira, L.d.S. Antiviral potential of Spondias mombin L. leaves extract against herpes simplex virus type-1 replication using in vitro and in silico approaches. Planta Med. 2020, 86, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 571, 153–173. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204. [Google Scholar] [CrossRef]
- He, Y.; Kim, B.; Kim, H.; Sun, Q.; Shi, S.; Ma, G.; Kim, Y.; Kim, O.; Kim, O. The protective role of feruloylserotonin in LPS-induced HaCaT cells. Molecules 2019, 24, 3064. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Choi, J.; Kang, S.; Lee, Y.; Park, J.; Kang, Y. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, e182–e190. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.J.; Lyngholm, A.M.; Arendt-Nielsen, L. A novel model of inflammatory pain in human skin involving topical application of sodium lauryl sulfate. Inflamm. Res. 2010, 59, 775–781. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.; Kang, K. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol. Lett. 2003, 146, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gagosian, V.S.C.; Claro, F.C.; Schwarzer, A.C.d.A.P.; Cruz, J.V.; Thá, E.L.; Trindade, E.d.S.; Magalhães, W.L.E.; Pestana, C.B.; Leme, D.M. The potential use of kraft lignins as natural ingredients for cosmetics: Evaluating their photoprotective activity and skin irritation potential. Int. J. Biol. Macromol. 2022, 222, 2535–2544. [Google Scholar] [CrossRef]
- Smith, W. Stratum corneum barrier integrity controls skin homeostasis. Int. J. Cosmet. Sci. 1999, 21, 99–106. [Google Scholar]
| Geraniin (μg/mg) | Corilagin (μg/mg) | Gallic Acid (μg/mg) | Ellagic Acid (μg/mg) |
|---|---|---|---|
| 38.06 | 5.71 | 8.38 | 3.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-J.; Ahn, S.-M.; Nam, Y.-H.; Kim, M.-D.; Jo, C.-S.; Hong, B.-N.; Cho, Y.-K.; Hwang, J.-S. Anti-Inflammatory Effects of Nephelium lappaceum Peel Extract and Geraniin on External Skin Stimulation. Cosmetics 2025, 12, 117. https://doi.org/10.3390/cosmetics12030117
Lee E-J, Ahn S-M, Nam Y-H, Kim M-D, Jo C-S, Hong B-N, Cho Y-K, Hwang J-S. Anti-Inflammatory Effects of Nephelium lappaceum Peel Extract and Geraniin on External Skin Stimulation. Cosmetics. 2025; 12(3):117. https://doi.org/10.3390/cosmetics12030117
Chicago/Turabian StyleLee, Eun-Jeong, Soo-Mi Ahn, Youn-Hee Nam, Myo-Deok Kim, Chan-Song Jo, Bin-Na Hong, Youn-Ki Cho, and Jae-Sung Hwang. 2025. "Anti-Inflammatory Effects of Nephelium lappaceum Peel Extract and Geraniin on External Skin Stimulation" Cosmetics 12, no. 3: 117. https://doi.org/10.3390/cosmetics12030117
APA StyleLee, E.-J., Ahn, S.-M., Nam, Y.-H., Kim, M.-D., Jo, C.-S., Hong, B.-N., Cho, Y.-K., & Hwang, J.-S. (2025). Anti-Inflammatory Effects of Nephelium lappaceum Peel Extract and Geraniin on External Skin Stimulation. Cosmetics, 12(3), 117. https://doi.org/10.3390/cosmetics12030117






