1. Introduction
Glycosaminoglycans (GAGs) represent some of the most intricate polysaccharides within the extracellular matrix (ECM) and play pivotal roles in tissue homeostasis [
1,
2]. Hyaluronic acid (HA) is a non-sulfated form of GAGs composed of disaccharide units of glucuronic acid and N-acetyl-glucosamine [
3]. HA plays a significant role in various biological processes within the ECM, impacting everything from tissue hydration to cellular function. Owing to their significant cosmetic efficacy, HA-based formulations are extensively used in treating skin aging, providing noticeable improvements in skin hydration, elasticity, and wrinkle reduction. In addition to cosmetic applications, HA-based biomaterials are also at the forefront of innovative therapies for tissue repair and regenerative medicine, offering promising avenues for healing and restoring tissue function through enhanced biocompatibility and scaffolding capabilities. These versatile applications underscore HA’s critical role in both aesthetic treatments and medical advancements [
4].
When HA in the ECM diminishes or its water-binding capacity weakens, there is a notable reduction in skin elasticity and microvascular support, leading to increased dryness and laxity of the skin. Consequently, most anti-aging strategies aim to reverse these effects [
5].
HA-based formulations have been extensively studied by researchers and include various delivery systems, such as serum [
6,
7], creams [
8,
9], dermal HA fillers [
10,
11], subdermal HA fillers [
12], HA gels [
13,
14], and solid-type pure HA in thread forms [
15,
16]. It is imperative to acknowledge that no singular cosmetic formulation of HA presently accessible is capable of satisfying all aesthetic demands in a comprehensive manner. Given that no single treatment modality can adequately address all facial areas, consensus guidelines on facial rejuvenation have been established. These guidelines advocate integrated approaches, employing combined techniques tailored to different regions of the face, thereby optimizing the effectiveness of aesthetic enhancements [
17].
Aesthetic medicine has continually advanced, progressively incorporating both surgical and non-surgical modalities aimed at restoring a youthful appearance for patients. Notably, the deployment of HA facial fillers and the adoption of thread lifting techniques have garnered considerable attention within the field [
18]. HA fillers excel in sculpting facial features, minimizing the appearance of wrinkles, augmenting hydration, and restoring lost volume associated with aging [
5,
19]. In contrast, thread lifts involve the subdermal placement of biodegradable sutures, which are effective in lifting sagging facial tissues [
20], thereby not only re-establishing youthful contours but also stimulating collagen production, which enhances the inherent resilience and suppleness of the skin [
21].
Given the distinct benefits of each intervention, protocols for the integrated application of lifting threads and HA facial fillers within the realm of aesthetic medicine have been suggested. However, the literature pertaining to this combined approach is relatively scarce, with few published articles and reports thoroughly investigating these methodologies [
17,
18,
22,
23,
24,
25,
26,
27].
Importantly, however, the effects of HA fillers are transient and necessitate regular maintenance treatments [
28]. These treatments are additional invasive interventions alongside the insertion of lifting threads. Nevertheless, given the biorevitalizing properties of HA and its significant role in tissue repair, augmenting thread lifts with an HA coating holds substantial potential, especially with respect to skin remodeling [
29,
30].
Despite the benefits derived from coating lifting threads with HA, merely applying the coating is inadequate for enhancing the durability of the treatment and preventing the premature degradation of HA. To overcome this shortcoming, scientific progress has facilitated the development of a new technology that involves the encapsulation of HA within a biodegradable matrix. This method enables the sustained release of HA over a prolonged period, thereby potentially increasing treatment effectiveness and longevity.
NAMICA encapsulation technology was previously mentioned in the literature as a new method for the continued delivery of HA, specifically by lifting threads [
31]. This patented technology involves creating a polysaccharide-based emulsion, followed by adding a copolymer sequentially and electrospinning to apply the resulting solution onto the surface of the lifting threads [
32]. This process forms a coating of polymer microfibers with capsules of incorporated polysaccharide. Once implanted, the encapsulated HA is released in a sequential manner, extending the localized therapeutic effect.
The gradual release of HA particles was confirmed via an analytical methodology employing high-performance liquid chromatography (HPLC). This technique was utilized to evaluate the gradual dissolution rates of capsules containing HA under simulated physiological conditions.
Although there is a broad range of biodegradable synthetic polymers suitable for the fabrication of absorbable threads, our analysis will be specifically directed toward poly(L-lactide-co-ε-caprolactone) lifting threads (P(LA/CL)). The fundamental difference between P(LA/CL) that are embedded with HA particles via NAMICA technology is the particle size: microscale (P(LA/CL)-HA-micro) [
31] and nanoscale (P(LA/CL)-HA-nano). This variance in dimensionality is a result of the distinct synthesis techniques applied in the production of micro- and nanocapsules containing sodium hyaluronate. Both variants originate from the same base materials, a copolymer and medium-molecular-weight non-crosslinked sodium hyaluronate, which is initially processed into a microparticulate powder.
For the fabrication of P(LA/CL)-HA-micro threads, this microparticulate substrate is subjected to the previously outlined technological procedures that culminate in its application to the surface of P(LA/CL) threads [
31]. Conversely, in the nanoparticle variant, the production process involves proprietary engineering modification techniques that transform these microparticulate sodium hyaluronate particles into a fluid state. This transformation is followed by their passage through an array of organic solvents. Subsequent technological interventions facilitate the formation of encapsulated nanoscale HA particles, ensuring that nanocapsules do not exceed 1000 nanometers, which can be verified by scanning electron microscopy (SEM) (
Figure 1).
The documented influence of P(LA/CL) on the synthesis of collagen, a critical component in skin biorevitalization, is strongly supported by many scientific studies [
33,
34,
35]. Additionally, research concerning the biorevitalizing properties of P(LA/CL)-HA-micro threads for skin remodeling has been published [
31]. However, the literature currently lacks data on the impact of altering the particle size of encapsulated HA from the microscale to the nanoscale on skin remodeling.
In our experimental research, we evaluated the long-term effectiveness of lifting threads produced by APTOS LLC (Tbilisi, Georgia) in an animal model. These threads are composed of P(LA/CL) integrated with nanoscale encapsulated HA particles via NAMICA technology. This new approach exploits the properties of P(LA/CL) for the gradual release of HA, thereby enhancing its therapeutic effects on skin remodeling. Through the use of NAMICA technology, the threads facilitate a sustained release profile of HA, improving its bioavailability and extending its therapeutic benefits. This study aimed to assess the efficacy of P(LA/CL)-HA-nano threads compared with that of P(LA/CL)-HA-micro threads over a six-month period, specifically in terms of skin remodeling. This study highlights the potential of nanoscale HA encapsulation to improve the performance and longevity of cosmetic and reconstructive medical implants.
2. Materials and Methods
This study was conducted in accordance with the principles outlined in the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). These guidelines were followed throughout the research to increase the reporting quality and reliability of the experiments involving animals.
The inclusion criteria for utilizing the porcine model were as follows [
31]:
Breed: Large White;
Age: Four months;
Weight: Commencing at 40 kg;
Health: The pigs must be in optimal health and devoid of any discernible diseases or abnormalities;
Gender: Female;
Housing: There should be a one-week acclimatization period prior to the commencement of the study.
The exclusion criteria for utilizing the porcine model were as follows:
Health: Any pigs demonstrating symptoms of illness, injuries, or other health complications were excluded.
Reproductive Status: Female pigs that are pregnant or currently lactating are not eligible.
Prior Research Involvement: Pigs previously utilized in experimental procedures are precluded from participation.
The study utilized five 4-month-old female Large White pigs, each weighing 40 kg at the beginning of the experiment. These animals were accommodated under conditions optimized for their health, maintaining ambient temperatures at 21 ± 2 °C, a relative humidity between 30–60%, and a light/dark cycle of 12 h each, commencing at 7 AM. Continuous access to food and water was provided. After a one-week period of acclimatization, the pigs were randomly assigned identification numbers.
The procedure for placing the threads was performed in a sterile surgical environment under both inhalational and intravenous anesthesia. The study incorporated the routine use of systemic analgesics, which are commonly administered in both veterinary and clinical settings.
The structure and sequence of the anesthesia protocol used in our study are presented in the following order [
31]:
Preparation and induction stage:
- –
Anesthesia induction: a total of 6 mL of xylazine and 0.7 mL of zoletil 100 were administered intramuscularly to standardize anesthesia induction across all subjects.
- –
Catheterization: a 22G catheter was placed into the external auricular vein to facilitate subsequent intravenous interventions.
- –
Intermittent administration: additional intravenous administration of 4 mL of propofol at 20 min intervals as needed.
Intraoperative anesthetic management:
- –
Initial maintenance: a mask was used to administer a 3.0 vol% concentration of isoflurane for three minutes.
- –
Advanced maintenance: Continuous delivery of a gaseous mixture containing 65–70% oxygen and 1.5 vol% isoflurane via a Dräger Fabius Plus anesthesia machine was employed (Berlin, Germany). Simultaneously, propofol was infused intravenously at a rate of 25 mL/hour to maintain the anesthetic plane.
Monitoring and prophylaxis:
- –
Intraoperative monitoring included continuous monitoring of heart rate, arterial pressure, and pulse oximetry to ensure stability and appropriate depth of anesthesia.
- –
Prophylactic administration: an intramuscular injection of 1.0 mL of ceftriaxone was used to prevent postoperative infections.
Recovery stage:
- –
Termination of anesthesia: gradual cessation of propofol infusion and discontinuation of inhalational anesthetics was performed.
- –
Recovery was monitored for return to consciousness, which was typically observed within 30–40 min after anesthesia was stopped.
The thread implantation procedure was performed in a sterile surgical setting. Prior to the operation, the surgical areas on the torso and limbs (including both forelimbs and hindlimbs) were shaved and cleansed with 70% alcohol. Using an 18 G straight needle, five threads of each type were implanted into the subcutaneous layer of each pig and aligned parallel to the skin surface. On the right side, access was gained through five puncture points where five units of absorbable P(LA/CL)-HA-micro threads (manufactured by APTOS LLC (Tbilisi, Georgia)) were introduced. Similarly, on the left side, five puncture points facilitated the implantation of five units of P(LA/CL)-HA-nano threads (produced by APTOS LLC (Tbilisi, Georgia)). The entry points were then securely sealed with aseptic stickers. All threads used were 15 cm in length, non-barbed, and USP 2–0 in size. The surgical interventions were executed flawlessly, with no complications arising.
Following the implantation of the lifting threads, skin and subcutaneous tissue samples were harvested for histological examination at five pre-established intervals: 7, 21, 30, 90, and 180 days post-implantation. A 15 cm segment of soft tissue was surgically excised, from which three samples were extracted for detailed histological analysis—one from each extremity and one from the median section (
Figure S1). Skin from untreated regions served as a control. Each subject underwent an identical procedural sequence at specified time points of 1 week, 21 days, 1 month, 3 months, and 6 months post-thread placement, facilitating a methodical assessment of the time-dependent effects [
31].
Following the conclusion of the experiment, euthanasia was carried out via an intravenous overdose of xylazine and zoletil 100, leading to exsanguination after the achievement of deep, drug-induced sedation [
31].
Tissue samples were preserved by immersion in 7% neutral buffered formalin for 24 h, effectively halting the biochemical activities within the tissues. Following fixation, the biopsy samples were dehydrated via a series of graded alcohols and subsequently permeated with organic solvents before being embedded in hot liquid paraffin. As the paraffin cooled and solidified, it provided essential structural support to the tissues. These prepared samples were then sectioned into full-thickness slices of 5–7 microns using a microtome. For the purpose of microscopic examination, a minimum of three sections from each sample were meticulously transferred onto glass slides. The sections were subsequently dewaxed and subjected to staining via three distinct methods: (1) hematoxylin and eosin (H&E), (2) Weigert–Van Gieson, and (3) Sirius Red staining. Each staining technique was employed to emphasize different components of the tissue, facilitating detailed histological examination. A digital microscope equipped with a 12 MP Sony sensor was used to examine the stained tissue sections through both light microscopic observation and polarized light microscopy. Throughout the course of these evaluations, the protocol for image acquisition involved capturing five images from each glass slide at 40× magnification. Additionally, ten images were taken at 100× magnification per slide—five from the dermis and five from the hypodermis. A similar approach was followed for 200× magnification, with ten images obtained from each slide, which were equally divided between the dermis and hypodermis. During these analyses, five images were captured from each sample. Morphological evaluations were conducted on a variety of parameters, including the cellular composition, dermal thickness, thickness of the fibrous sheath, and morphometric characteristics of the vessels, including the diameter of the blood vessels and the relative vascular bed area. Additionally, assessments were made of the fibrocyte count, collagen density, quantity of type I and III collagen in the dermis and hypodermis, ratio of type I/III collagen, and elastin levels in these layers. These evaluations were performed via the software programs ImageView v.3.7.7 and HistMorph v.2.3, ensuring accurate and comprehensive analysis [
31].
Statistical analyses were performed via the Statistica v.7 software package. The initial steps included computing descriptive statistics, which involved calculating mean values with standard errors (M ± m), medians, and quartiles (25% and 75%), as well as noting the minimum and maximum values for each day analyzed and cumulatively across all days (as outlined in
Tables S1 and S2). The analysis subsequently progressed to examining the day-to-day dynamics of each parameter, segmented by thread type. Data comparisons were executed via nonparametric methods, notably the Wilcoxon test for related samples. The results with a
p-value less than 0.05 were considered statistically significant, whereas values between 0.05 and 0.1 indicated a potential trend toward significance, suggesting that definitive significance might be attainable with an expanded sample size. Comparative analyses employing the Wilcoxon test were conducted to assess the differential effects of the two types of threads in relation to each other and the control, both individually and collectively across the study days.
3. Results
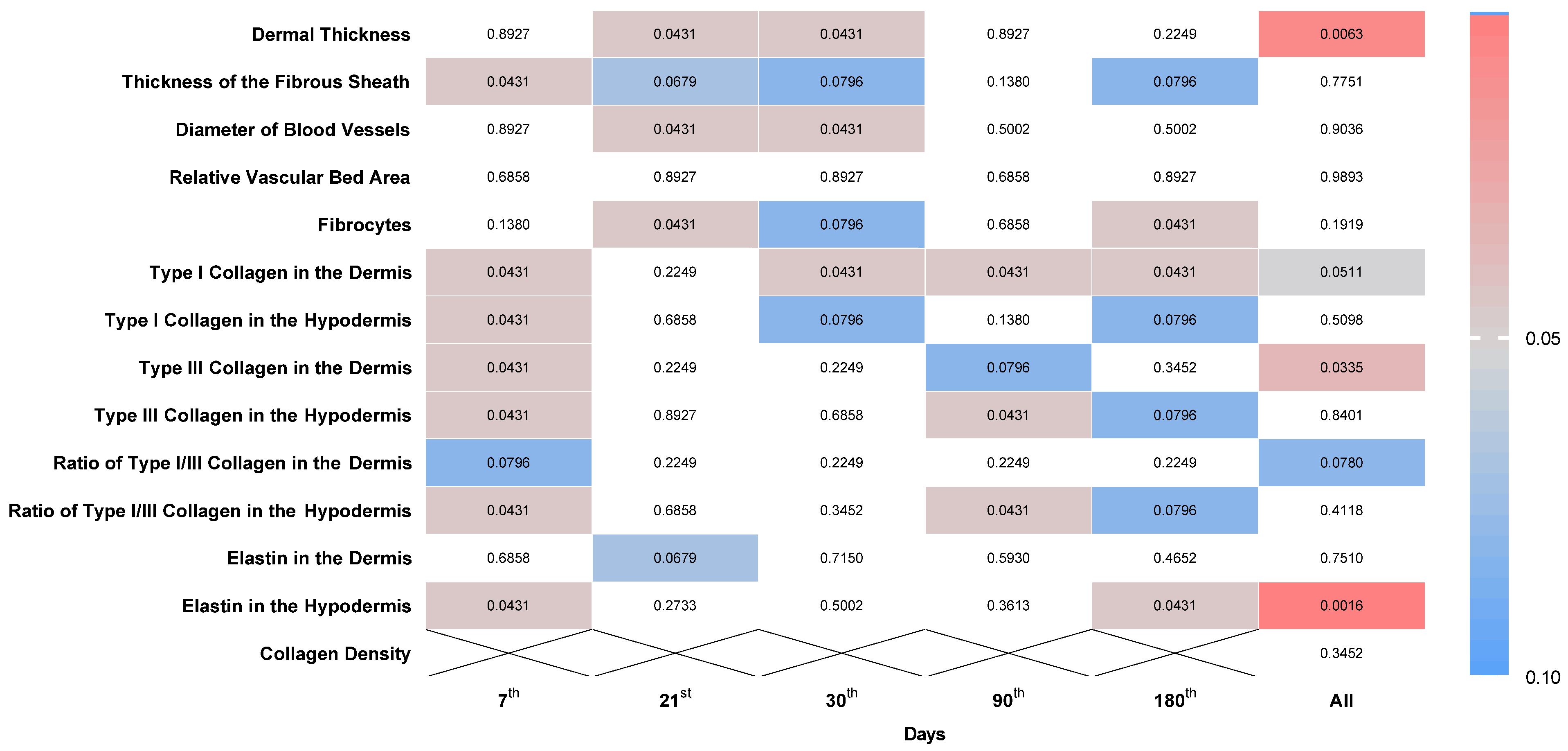
An analytical comparison employing the Wilcoxon test was executed to ascertain disparities between two distinct suture materials, P(LA/CL)-HA-micro and P(LA/CL)-HA-nano. The evaluation scrutinized the variations in the performance of these suture types on designated days and aggregated the data across the entire span of the investigation, as illustrated in
Figure 2.
According to the data obtained, when P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads were used, most histological indicators significantly differed on various days of the study. No differences were noted only in terms of the relative vascular bed area, the ratio of type I/III collagen in the dermis, the elastin levels in the dermis, or collagen density.
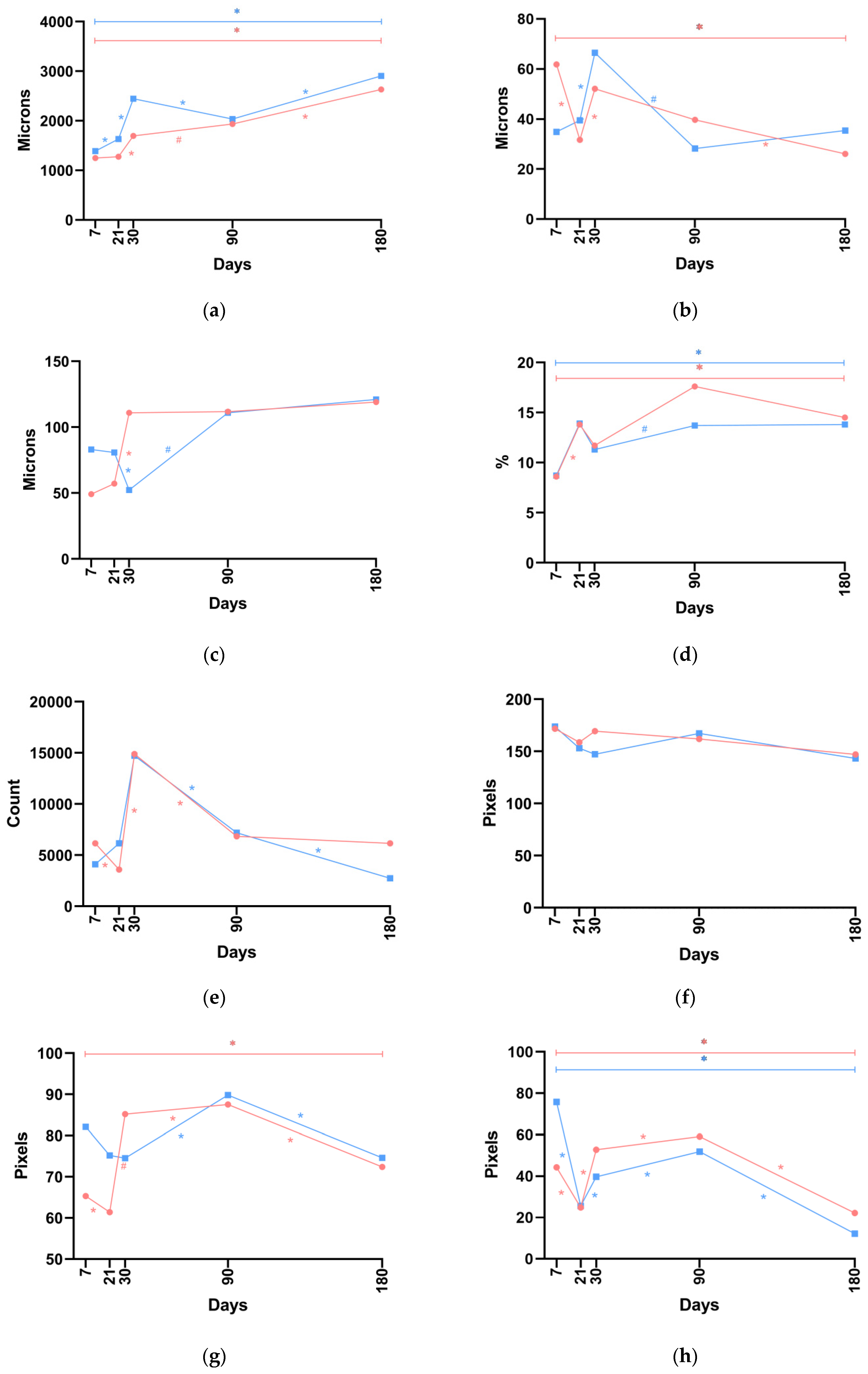
Throughout the study, the dermal thickness when P(LA/CL)-HA-nano threads were used exceeded the comparable values observed with P(LA/CL)-HA-micro threads, with the difference being statistically significant on days 21 and 30. Notably, when P(LA/CL)-HA-micro threads were used, the indicator uniformly increased throughout the study period. In contrast, with P(LA/CL)-HA-nano threads, the growth of the parameter was observed from day 7 to day 30, followed by a slight decline by day 90 and then an increase again by day 180, when it reached its maximum value (
Figure 3a and
Figure S2).
The thickness of the fibrous sheath when P(LA/CL)-HA-nano threads were used exceeded the comparable values observed with PLA-HA-micro on days 21, 30, and 180, with a trend toward a statistically significant difference. Conversely, on days 7 and 90, the values for P(LA/CL)-HA-micro threads were greater than those for P(LA/CL)-HA-nano threads, with the difference being statistically significant on day 7. Notably, when P(LA/CL)-HA-micro threads were used, the maximum value of the indicator was recorded on day 7; it decreased on day 21, increased again on day 30, began to decline on days 90 and 180, and reached its minimum value on day 180. The dynamics of this indicator when P(LA/CL)-HA-nano threads were used differed: from days 7 to 30, the parameter increased and reached its maximum value on day 30, after which it decreased to its lowest level by day 90 and rose again by day 180 (
Figure 3b and
Figure S3).
Statistically significant differences in the diameter of blood vessels were observed on days 21 and 30. The values of this parameter were greater on day 21 with P(LA/CL)-HA-nano threads and on day 30 with P(LA/CL)-HA-micro threads. Notably, with P(LA/CL)-HA-micro threads, the indicator increased from day 7 to day 30 and then maintained its values at nearly the same level, whereas with P(LA/CL)-HA-nano threads, this parameter decreased from day 7 to day 30, subsequently increased by day 90, and remained at almost the same level by day 180 (
Figure 3c and
Figure S4).
As noted earlier, no statistically significant difference was observed in the relative area of the vascular bed. Importantly, when both P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads were used, nearly identical values of this parameter were detected on days 7 and 21. Both decreased on day 30, increased on day 90, and then began to decrease again by day 180. Additionally, on days 30, 90, and 180, the values for P(LA/CL)-HA-micro threads were higher (although not significantly so) than those observed for P(LA/CL)-HA-nano threads (
Figure 3d and
Figure S5).
Significant differences in fibrocyte counts when P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads were used were noted on days 21 and 180. On day 21, the values for P(LA/CL)-HA-nano threads significantly exceeded those for P(LA/CL)-HA-micro, whereas on day 180, the values were greater for P(LA/CL)-HA-micro. Notably, on day 30, a significant increase in counts was observed for both types of threads compared with day 21, followed by a sharp decrease by day 90 compared with day 30, although P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads did not significantly differ from each other on these days (although a trend toward a significant difference was noted). On days 7, 30, and 180, fibrocyte counts for P(LA/CL)-HA-micro threads were greater than those for P(LA/CL)-HA-nano threads. Conversely, on days 21 and 90, P(LA/CL)-HA-nano threads showed a slight advantage over P(LA/CL)-HA-micro threads (see
Figure 3e and
Figure S6).
The values for collagen density when P(LA/CL)-HA-micro threads were used were greater than those observed with P(LA/CL)-HA-nano threads, but the difference was not statistically significant (
Figure 3f and
Figure S7).
The levels of type I collagen in the dermis were significantly greater on days 7, 90, and 180 when P(LA/CL)-HA-nano threads were used compared to those observed with P(LA/CL)-HA-micro threads. Conversely, on days 21 and 30, the levels associated with P(LA/CL)-HA-micro threads were greater than those with P(LA/CL)-HA-nano threads, achieving statistical significance on day 30. Notably, there was a sharp decrease in this parameter when P(LA/CL)-HA-micro threads were used on day 180. In the case of P(LA/CL)-HA-nano threads, a decrease was also observed by day 180 compared with day 90, although the reduction was not as substantial (
Figure 3g and
Figure S8).
Type I collagen values in the hypodermis, when P(LA/CL)-HA-nano threads were used, significantly exceeded the values observed with P(LA/CL)-HA-micro threads on day 7. There was also a non-significant increase in this parameter with P(LA/CL)-HA-nano threads on day 21. On days 30, 90, and 180, the values of this parameter were greater when P(LA/CL)-HA-micro threads were used, with a trend toward a significant difference noted on days 30 and 180. Notably, the maximum density of type I collagen in the hypodermis when P(LA/CL)-HA-nano threads were used was observed on day 7, whereas with P(LA/CL)-HA-micro threads, it reached its maximum value on day 90. The minimum values in both cases were observed on day 180 (
Figure 3h and
Figure S9).
When P(LA/CL)-HA-micro threads were used, the levels of type III collagen in the dermis exceeded those observed with P(LA/CL)-HA-nano threads on days 7, 21, 30, and 180, with the difference reaching statistical significance on day 7. Conversely, on day 90, the values with P(LA/CL)-HA-nano threads exceeded those with P(LA/CL)-HA-micro threads; although the difference was not statistically significant, a trend toward significance was noted. Notably, this parameter decreased from day 7 to day 90 when both P(LA/CL)-HA-micro threads and P(LA/CL)-HA-nano threads were used. However, an increase was observed by day 180; this was statistically significant with P(LA/CL)-HA-micro threads and not significant with P(LA/CL)-HA-nano threads (see
Figure 3i and
Figure S10).
The level of type III collagen in the hypodermis was greater when P(LA/CL)-HA-micro threads were used compared to P(LA/CL)-HA-nano threads on days 7 and 21, with the difference reaching statistical significance on day 7. Conversely, on days 30, 90, and 180, the values were greater when P(LA/CL)-HA-nano threads were used, with the difference being statistically significant on day 90 and a trend toward a significant difference noted on day 180. When both types of threads were used, the density of type III collagen in the hypodermis increased by day 21 (reaching its maximum value with P(LA/CL)-HA-micro), then decreased on days 30 and 90, and increased again on day 180 (reaching its maximum value with P(LA/CL)-HA-nano) (
Figure 3j and
Figure S11).
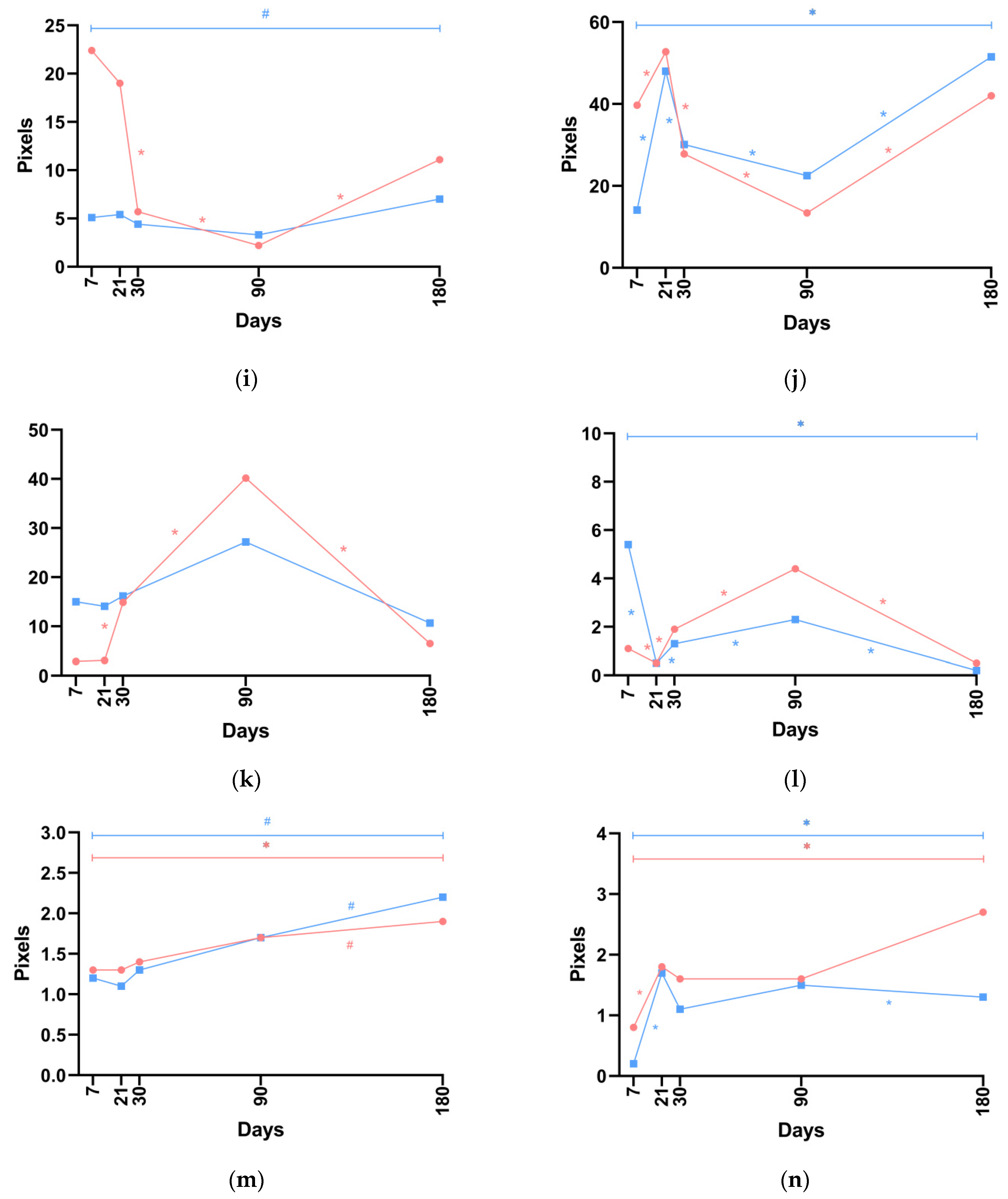
No statistically significant differences in the ratio of type I/III collagen in the dermis were noted between P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads, except on day 7, when a trend toward a significant difference was detected. When P(LA/CL)-HA-nano threads were used, the values of this parameter were greater than those with P(LA/CL)-HA-micro on days 7, 21, 30, and 180. Conversely, on day 90, there was a non-significant excess in the values of this parameter when P(LA/CL)-HA-micro threads were used (
Figure 3k and
Figure S12).
When P(LA/CL)-HA-nano threads were used, the ratio of type I/III collagen in the hypodermis on days 7 and 21 exceeded the corresponding values observed with P(LA/CL)-HA-micro, with the difference being statistically significant on day 7. Conversely, on days 30, 90, and 180, the values were greater when P(LA/CL)-HA-micro threads were used, with the difference being statistically significant on day 90, and a trend toward a significant difference was noted on day 180. Notably, the maximum value of this parameter was observed on day 7 with P(LA/CL)-HA-nano threads, while the maximum level occurred on day 90 with P(LA/CL)-HA-micro threads. By day 180, this parameter had decreased for both P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads (
Figure 3l and
Figure S13).
Throughout the study period, no statistically significant differences in elastin levels in the dermis were observed. However, a trend toward a significant difference was noted between the values for P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads on day 21. When P(LA/CL)-HA-micro threads were used, the values increased uniformly across all days. With P(LA/CL)-HA-nano threads, a decrease in the parameter was noted from day 7 to day 21, followed by an increase up to day 180. On day 90, the values of the parameter when both types of threads were used were virtually identical; on days 7, 21, and 30, the values for P(LA/CL)-HA-micro were greater than those for P(LA/CL)-HA-nano threads, whereas on day 180, the opposite was true, with higher values observed for P(LA/CL)-HA-nano (
Figure 3m and
Figure S14).
Elastin levels in the hypodermis were greater when P(LA/CL)-HA-micro threads were used, with a statistically significant difference observed on days 7 and 180. Notably, the maximum value of this parameter for P(LA/CL)-HA-micro threads was observed on day 180, whereas for P(LA/CL)-HA-nano threads, it peaked on day 21 (
Figure 3n and
Figure S15).
4. Discussion
4.1. Dermal Thickness
This study examined the changes in dermal thickness over a period of six months after employing two different types of resorbable threads: P(LA/CL)-HA-micro and P(LA/CL)-HA-nano. Notably, the comparison yielded insights into the differential behavior of these thread formulations over a six-month period.
Initially, measurements taken on day 7 indicated no significant difference in dermal thickness between the two types of threads, implying comparable early tissue reactions to both. However, the subsequent data revealed distinct trajectories in their influence on dermal thickness. Notably, on days 21 and 30, the nano-modified threads induced a statistically significant increase in dermal thickness relative to the P(LA/CL)-HA-micro threads, as demonstrated by p-values of 0.0431 at these time points.
By day 90, the increase in dermal thickness observed with the P(LA/CL)-HA-nano threads had noticeably diminished, with the thickness only rebounding by day 180 (p = 0.0431). In contrast, the P(LA/CL)-HA-micro threads exhibited a progressive, uninterrupted increase in dermal thickness throughout the study, culminating in a substantial difference by day 180 (p = 0.0431).
The analysis over the entire study period, confirmed by a p-value of 0.0063, suggests that the P(LA/CL)-HA-nano threads were generally more effective at promoting significant dermal thickening. Nonetheless, the observed fluctuations in response underscore the complex and dynamic nature of biological interactions with these biomaterials, particularly at varying dimensional scales.
The findings derived from our animal model substantiate the hypothesis that nano-scale modifications to the P(LA/CL)-HA structure can lead to a more marked increase in dermal thickness. This occurs even though both thread types notably enhance dermal thickening according to the conclusion of the study. However, the impact of the nano-enhanced threads tends to show variability over time compared to their micro-scale counterparts.
4.2. Thickness of the Fibrous Sheath
Our experimental design aimed to compare the in vivo response to these biomaterials, focusing on their ability to influence fibrous sheath formation.
Notably, on day 7, the thickness associated with P(LA/CL)-HA-micro threads was significantly greater (p < 0.05) than that associated with P(LA/CL)-HA-nano threads, suggesting an initial heightened fibrotic response to the micro-sized formulation. Additionally, observations revealed a significant reduction in fibrous sheath thickness from day 7 to day 21 for P(LA/CL)-HA-micro threads (p = 0.0431), followed by a statistically significant increase by day 30 (p = 0.0431). This fluctuation suggests a dynamic interaction between the biomaterial and the host’s tissue, potentially influenced by an initial expressed immune response that stabilizes and then shifts toward a regenerative or remodeling phase as the material integrates into the surrounding tissue.
For P(LA/CL)-HA-nano threads, the thickness of the fibrous sheath increased significantly from day 21 to day 30 (p = 0.0431). This trend indicates a progressive fibrotic response, which may be attributed to the nano-scale size of the HA particles, with peak fibrous tissue deposition occurring on day 30.
Interestingly, despite the significant changes noted at the initial time point, the overall analysis from day 7 to day 180 did not reveal a significant difference between the two thread types (p = 0.7751), suggesting that while the initial responses vary, long-term fibrous encapsulation may be similar.
4.3. Morphometric Characteristics of Vasculature
This study assessed the effects of two different thread types, P(LA/CL)-HA-micro and P(LA/CL)-HA-nano, on vascular remodeling over a six-month period. Focusing on changes in the diameter of blood vessels and the relative vascular bed area, our goal was to understand how these biomaterials influence angiogenesis and the stabilization of blood vessels, which are key processes for successful tissue regeneration and the integration of implanted materials.
Statistical analysis via the Wilcoxon signed-rank test initially revealed no significant differences in vessel diameter on day 7. However, as the experiment progressed, distinct patterns began to emerge, providing insight into the temporal dynamics of the vascular response to each thread type. By day 21, P(LA/CL)-HA-nano threads demonstrated a significant increase in vessel diameter (p = 0.0431), suggesting a potential reduction in active neoangiogenesis compared to micro-sized HA particles formulation. Conversely, by day 30, P(LA/CL)-HA-micro threads exhibited a significant increase in vessel diameter (p = 0.0431), indicating an ongoing active neoangiogenesis phase, likely leading to vessel maturation.
This reversal in trends suggests that while P(LA/CL)-HA-micro threads may support earlier stages of vessel formation characterized by smaller diameters, P(LA/CL)-HA-nano threads may enhance later stages of neoangiogenesis, promoting more significant histological changes and subsequent vessel maturation and stabilization. By day 180, both thread types effectively supported the formation and stabilization of newly formed vascular structures.
Concurrently, our analyses of the relative vascular bed area revealed no statistically significant differences between the two thread types on most assessment days (day 7, day 21, day 30, day 90, and day 180), with p-values indicating comparable performance in supporting vascularization. This uniformity suggests that both micro- and nano-formulations contribute similarly to vascular development.
Descriptive statistics provided further insight into the vascular dynamic patterns throughout the experiment. Initially, both thread types displayed nearly identical median values for the vascular bed area on day 7, indicating a similar initial response to the biomaterials. By day 21, an increase in this parameter for both thread types suggested an increase in early vascular development. However, a decrease observed on day 30 in both thread types indicated possible transient regression or remodeling of vascular networks, which was then followed by a notable increase by day 90, which was particularly pronounced with P(LA/CL)-HA-micro threads. This pattern may reflect better support by the micro-formulation for the maturation of stable vascular networks compared to that of P(LA/CL)-HA-nano, although this difference was not statistically significant.
By day 180, a slight decrease in the vascular bed area was noted, likely representing stabilization or natural regression of vascularization as the tissue became more fully integrated with the implanted materials. The overall pattern of changes in the vascular bed area throughout the study period suggests that both P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads equally support the long-term formation and stabilization of vascular networks, which is essential for the success of tissue-engineered constructs.
4.4. Fibrocytes
Fibrocytes play crucial roles in tissue regeneration and wound healing, as they are pivotal in the deposition of the extracellular matrix and the modulation of inflammatory responses [
36]. Understanding the behavior of these cells in response to different biomaterials is vital for optimizing tissue engineering strategies.
On day 7, the initial counts were greater for the P(LA/CL)-HA-micro threads than for the P(LA/CL)-HA-nano threads, although this difference was not statistically significant (p = 0.1380). These findings suggest early activation of the wound healing process by the micro-sized formulation.
Significant changes in fibrocyte counts were observed on day 21, where P(LA/CL)-HA-nano threads presented significantly higher counts than P(LA/CL)-HA-micro threads did (p = 0.0431). This reversal could indicate differential temporal activation of fibrocytes by the nano-sized formulation, which may enhance cellular recruitment or proliferation at this stage of healing.
By day 30, both thread types presented substantial increases in fibrocyte counts, with almost the same cell counts. This peak suggests robust engagement of fibrocytes in tissue remodeling and matrix deposition. The presence of a trend toward significance (p = 0.0796) indicates a continued active response, albeit without clear superiority of one thread type over the other.
Interestingly, by day 90, the fibrocyte counts significantly decreased for both thread types, aligning with the expected transition from a proliferative phase to a more mature phase of tissue remodeling. The counts for P(LA/CL)-HA-micro threads remained relatively stable from day 90 to day 180, suggesting a sustained level of engagement in the later stages of tissue repair. In contrast, P(LA/CL)-HA-nano threads significantly decreased by day 180 (p = 0.0431), with counts decreasing below the initial levels observed on day 7. This sharp decline could reflect a more rapid resolution of the healing process or a diminishing role of fibrocytes in the context of the P(LA/CL)-HA-nano threads as the tissue approaches a more finalized state of repair.
4.5. Collagenogenesis
The extracellular matrix (ECM) of the skin predominantly contains type I and type III collagens, with type I collagens constituting 80–85% and type III collagens making up approximately 8–11% of the dermal ECM; type I collagens, rigid fibrillar proteins, impart tensile strength, whereas type III collagens form an elastic network [
37,
38].
Statistical analysis conducted via the Wilcoxon signed-rank test highlighted significant fluctuations in collagen density at various time points, demonstrating the dynamic interaction between these biomaterials and the host tissue.
Initially, on day 7, both the dermis and hypodermis presented significantly higher levels of type I collagen with P(LA/CL)-HA-nano threads than with P(LA/CL)-HA-micro threads (p = 0.0431). This early increase in collagen density suggests that the nano-formulation might be more effective at promoting rapid type I collagen synthesis, potentially because of enhanced molecular interactions that facilitate an immediate tissue response.
However, the pattern shifted by day 30. In the dermis, collagen levels were significantly greater for P(LA/CL)-HA-micro threads (p = 0.0431), indicating a response delayed by the slower release of bioactive molecules or different interactions with dermal fibroblasts. In the hypodermis, a similar trend was observed, albeit with a trend toward significance (p = 0.0796), suggesting that micro threads also promote a sustained response in deeper tissue layers, albeit at a slower pace.
By day 90, a notable reversal occurred in the dermis, where P(LA/CL)-HA-nano threads again presented significantly greater collagen densities (p = 0.0431). This trend continued until day 180, although a general decline in collagen density was noted for both thread types. The decline was particularly sharp and significant for P(LA/CL)-HA-micro threads (p = 0.0431) in the dermis, whereas in the hypodermis, the collagen density remained consistently greater for P(LA/CL)-HA-micro threads, with a trend toward significance on day 180 (p = 0.0796). This pattern underscores the differences in how micro- and nano-formulations influence type I collagen stability and degradation over time.
Intriguingly, the peak density of type I collagen varied between the two tissue layers. In the dermis, the maximum collagen density was achieved identically for both thread types by day 90. Conversely, in the hypodermis, the maximum collagen density occurred on day 7 for the P(LA/CL)-HA-nano threads and on day 90 for the P(LA/CL)-HA-micro threads. This disparity highlights the different kinetics of collagen production induced by the thread formulations, reflecting their varying bioactivity profiles in distinct tissue environments.
By day 180, both thread types presented the minimum collagen densities observed during the study period in the hypodermis, suggesting the possible culmination of the tissue remodeling process. At this stage, the demand for collagen synthesis decreases as tissues reach structural maturity and stability.
This study also provides an analysis of the dynamic changes in type III collagen density in both the dermis and hypodermis, which are influenced by the implantation of P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads over a six-month period.
Initial assessments revealed significant disparities in type III collagen density as early as day 7, with P(LA/CL)-HA-micro threads exhibiting significantly greater levels than P(LA/CL)-HA-nano threads (p = 0.0431) in both tissue layers. This early prominence of type III collagen around the P(LA/CL)-HA-micro threads may suggest their enhanced ability to stimulate the early phase of wound healing and collagen matrix formation, likely due to a more favorable interaction with cellular components or a distinct profile of bioactive molecule release.
However, the pattern evolved distinctly in the dermis and hypodermis as the study progressed. In the dermis, the initial high levels of type III collagen associated with P(LA/CL)-HA-micro threads decreased by day 30, reaching a minimum on day 90. This notable decline likely reflects a physiological shift from type III to type I collagen synthesis, which is a natural progression in mature scar formation.
Conversely, in the hypodermis, while type III collagen density was initially greater for P(LA/CL)-HA-micro threads on day 21, the difference between the thread types was not statistically significant, suggesting a convergence in their effects on the collagen response. However, by day 90, the pattern reversed, with P(LA/CL)-HA-nano threads showing significantly greater collagen density (p = 0.0431). This trend continued into day 180, albeit less markedly, with a notable trend toward significance (p = 0.0796). This sustained increase in collagen density with P(LA/CL)-HA-nano threads could be attributed to a slower, more gradual interaction with hypodermal components, perhaps due to continuous stimulation of fibroblasts or delayed degradation of P(LA/CL)-HA-nano threads.
In the dermis, P(LA/CL)-HA-nano threads, despite starting with significantly lower levels of type III collagen on day 7, did not exhibit such a pronounced decline. By day 90, the levels of type III collagen around the P(LA/CL)-HA-nano threads were greater than those around the P(LA/CL)-HA-micro threads, suggesting that the nano-formulation may support a more sustained, albeit lower intensity, collagen response.
Interestingly, by day 180, an increase in type III collagen density was observed for both thread types in both the dermis and hypodermis. This late-stage increase was more pronounced and statistically significant for P(LA/CL)-HA-micro threads in the dermis (p = 0.0431) and for P(LA/CL)-HA-nano threads in the hypodermis, with a trend toward significance (p = 0.0796). This resurgence may indicate a secondary wave of collagen remodeling or reactivation of regenerative activities, potentially driven by environmental or biochemical cues related to the ongoing presence of the threads.
Overall, the study demonstrated that while temporal fluctuations in type III collagen density are evident, the long-term effects differ between the dermis and hypodermis. The significant difference in average collagen density over all days in the dermis (p = 0.0431) highlights that P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads can differentially influence the structural and functional remodeling of tissue-specific matrices.
The ratio of type I to type III collagen is a critical indicator of tissue maturation and remodeling dynamics and is essential for understanding the healing efficacy and integration of biomaterials used in tissue treatment.
Early in the trial, particularly on day 7, P(LA/CL)-HA-nano threads presented a greater ratio of type I to type III collagen compared to P(LA/CL)-HA-micro threads in both the dermis (with a trend toward significance, p = 0.0796) and the hypodermis (statistically significant, p = 0.0431). This initial higher ratio in the P(LA/CL)-HA-nano thread-treated areas suggests that these threads may accelerate the deposition of type I collagen relative to type III collagen. This effect is likely due to finer scale interactions of the nano-formulation with cellular components, promoting a rapid transition toward a more mature collagen network.
Throughout the remainder of the study, encompassing days 21, 30, 90, and 180, the differences in the collagen ratio in the dermis became non-significant, indicating a convergence in the patterns elicited by both thread types. Despite the lack of statistically significant differences during these times, descriptive statistics revealed that P(LA/CL)-HA-nano threads generally maintained a higher median ratio than P(LA/CL)-HA-micro threads did, suggesting a sustained influence of the nano-formulation on the relative abundance or stability of type I collagen.
In contrast, in the hypodermis, the collagen ratio was significantly greater in areas treated with P(LA/CL)-HA-micro threads by day 90 (p = 0.0431). This finding indicates a delayed but robust maturation of the collagen matrix facilitated by the (LA/CL)-HA-micro threads, suggesting that these threads support a more gradual promotion of type I collagen synthesis or stabilization over time.
By day 180, both thread types demonstrated a decrease in the collagen ratio in both the dermis and hypodermis. This decrease, particularly noted with a trend toward significance in the hypodermis for P(LA/CL)-HA-micro threads (p = 0.0796), suggests a regression in the relative abundance of type I collagen compared with type III collagen. This pattern aligns with the natural progression of tissue healing, where initial rapid collagen deposition shifts toward stabilization and maturation of the tissue matrix.
The overall analysis across all days revealed a trend toward a greater ratio of type I/III collagen in tissues treated with P(LA/CL)-HA-nano threads in the dermis (p = 0.0780). These findings suggest that the long-term effects of the nano-formulation may be more conducive to promoting a type I collagen-rich environment, which is beneficial for scenarios requiring rapid and robust tissue repair and maturation.
In conclusion, this investigation elucidates the intricate dynamics of collagen density modulation within dermal and hypodermal matrices under the influence of different biomaterial formulations. Through statistical analysis, we observed that P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads distinctly impact collagen types I and III, reflecting differential bioactivity and interactions with tissue-specific cellular environments. Notably, the fluctuating collagen densities over the six-month period underscore the complex interplay of biomaterial degradation, collagen synthesis, and tissue remodeling.
4.6. Elastogenesis
This study investigated the dynamics of elastin levels in the dermis and hypodermis over a six-month period following the application of P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads. Elastin, which is crucial for providing skin stretch, recoil, and elasticity, plays an integral role in the aesthetic and functional outcomes of biomaterial integration in tissue treatment [
39,
40].
In the dermis, elastin levels exhibited only minor fluctuations throughout the study period, with a slight trend toward significance on day 21 (p = 0.0679). This finding suggests that while both types of threads influence elastin production, the effects are generally subtle and do not result in long-term statistically significant alterations. By day 21, a noticeable trend suggested lower elastin levels in areas treated with P(LA/CL)-HA-nano threads than in those treated with P(LA/CL)-HA-micro threads, suggesting that different interactions with the cellular mechanisms responsible for elastin synthesis occur. However, by day 30, and continuing through day 90, the elastin levels between the thread types converged, indicating stabilization of elastin production across both biomaterials. Interestingly, by day 180, P(LA/CL)-HA-nano threads presented a non-significant increase in elastin levels compared with P(LA/CL)-HA-micro threads (p = 0.4652), suggesting variability in the long-term elastin response to the P(LA/CL)-HA-nano threads.
Conversely, in the hypodermis, significant disparities were observed early in the study on day 7, with P(LA/CL)-HA-micro threads showing significantly higher elastin levels than P(LA/CL)-HA-nano threads (p = 0.0431). This initial difference suggests a more robust activation of elastin synthesis by the micro-formulation, potentially due to its structure or interaction dynamics with hypodermal components. Throughout the middle of the study period (days 21 to 90), the differences in elastin levels between the thread types decreased, reflecting a phase where the initial rapid synthesis of elastin subsided as the tissue matured and the healing processes stabilized. Remarkably, by day 180, a statistically significant increase in elastin levels was observed for P(LA/CL)-HA-micro threads (p = 0.0431), indicating a delayed but sustained response, likely reflecting a secondary remodeling phase where elastin becomes critical again to support the ongoing restructuring of the hypodermal matrix.
The overall analysis across all days confirms a statistically significant difference in the hypodermis (p = 0.0016), indicating that P(LA/CL)-HA-micro threads generally maintained higher elastin levels throughout the study compared to P(LA/CL)-HA-nano threads. This sustained increase in the level of elastin in the hypodermis suggests that a micro-formulation is particularly effective in promoting and maintaining elastin synthesis, which is vital for long-term tissue functionality and integrity.
In summary, while both thread types are capable of influencing elastin levels, P(LA/CL)-HA-micro threads exhibit a more pronounced and lasting impact in the hypodermis.
5. Conclusions
The investigation delineates the variable impacts of biodegradable sutures, specifically P(LA/CL)-HA-micro and P(LA/CL)-HA-nano threads, on a spectrum of histological indicators related to cutaneous remodeling within an animal model over a duration of six months. Our findings substantiate that modifications incorporating nano-scale HA particles on the surface of P(LA/CL)-HA treads induce more pronounced variations in dermal thickness and vascular response compared to modifications incorporating micro-scale HA particles.
Notably, initially, the fibrous sheath response was more vigorous with the P(LA/CL)-HA-micro threads. However, both suture types eventually converged toward a similar long-term effect, indicative of equivalent efficacy in promoting fibrous encapsulation. The activity of fibrocytes, pivotal for tissue regeneration, was preferentially enhanced by the P(LA/CL)-HA-nano threads in the initial phases. Nevertheless, by the end of the observation period, both suture types displayed analogous levels of cellular engagement.
The dynamics of collagen synthesis revealed in this study are particularly remarkable. The P(LA/CL)-HA-nano sutures initially accelerated the swift production of type I collagen, which is essential for the early stages of wound healing. In contrast, the P(LA/CL)-HA-micro threads facilitated a more delayed yet consistent and extensive collagen response throughout the dermal and subdermal strata, highlighting their potential suitability for applications necessitating prolonged tissue remodeling.
Moreover, our analysis of elastogenesis revealed a definitive advantage of P(LA/CL)-HA-micro threads in promoting and maintaining elastin levels within the hypodermis, an essential factor for long-term tissue functionality and structural integrity.
In light of the gathered data, the concept of integrating both micro- and nano-formulations of HA particles via NAMICA technology has emerged as a compelling proposition. This hybrid approach could theoretically address the observed limitations of each thread type while synergistically amplifying the overall biorevitalizing effects on skin remodeling.
Importantly, these observations are derived from a pig model, which serves as a surrogate to predict potential human outcomes but is not fully representative of human physiological processes. The distinct behaviors noted between the P(LA/CL)-HA-nano and P(LA/CL)-HA-micro threads underscore the complexity of interactions between tissue and biomaterials, suggesting that scale-dependent modifications may have significant therapeutic implications.
The extrapolation from animal models to human clinical applications requires consideration and stringent validation. To elucidate the ramifications of these threads in clinical medicine, further investigations utilizing human models are imperative. Such studies should aim to confirm the noted effects and adjust for the complexities of human tissue properties and immune responses. Only through thorough and targeted research can we hope to accurately predict and enhance the efficacy of these biomaterials in human tissue applications, thereby ensuring the development of optimized therapeutic strategies that are both efficacious and safe in clinical settings.