Abstract
Objective: Dermo-cosmetics have significantly advanced, focusing on innovative and effective products such as cosmeceuticals—cosmetics infused with bioactive ingredients for skin benefits. Synthetic peptides are prominent among these bioactive molecules, noted for their enhanced effects in cellular processes related to skin physiology. Specifically, the glycoprotein E-cadherin plays a crucial role in cellular adhesion and has shown promise in wound healing studies, although its broader cellular functions remain underexplored. Despite their widespread use, many cosmetic peptides lack genetic validation of their effects. This study focuses on the synthetic, amphiphilic acetyl hexapeptide-1, aimed to possess wound healing and anti-aging properties, with a novel exploration of its molecular mechanisms, specifically its effect on the expression of the CDH-1 gene, which encodes E-cadherin—a key protein in cellular adhesion and wound healing. Methods: In this investigation, the acetyl hexapeptide-1 was synthesized in house, followed by cell culture assessment and molecular evaluation. Human hepatocytes HepG2 were exposed to the synthetic hexapeptide to assess cytotoxic effects and examine its impact on gene expression, specifically targeting the wound healing-associated gene CDH-1, as well as apoptosis-related genes BAX, Bcl-2, Caspase-9, and Cyclin D1. Results: No cytotoxic effects were observed in cell cultures. Gene expression analysis revealed a significant increase in E-cadherin expression, along with the NO modulation of apoptosis-related genes (BAX, Bcl-2, Caspase-9) and the cell cycle-related gene Cyclin D1. These findings suggest peptide’s role in enhancing cellular adhesion, without any cytotoxic effects. Conclusions: The findings of this study provide promising insights into the potential molecular properties of synthetic acetyl hexapeptide-1, implying its applicability in cosmeceuticals. These cosmetic peptides hold enormous potential and diverse applications not only within skincare. To fully understand their benefits and expand their scope, additional investigations are warranted to comprehensively explore their molecular mechanisms across a spectrum of applications.
1. Introduction
The skin, the human body’s largest organ, comprises three main layers: the epidermis, dermis, and hypodermis. The outermost layer, the epidermis, directly interfaces with harmful elements in the environment. The dermis serves as the supporting and protective shield of the epidermis, is rich in extracellular matrix, and has high vascularity and mechanical receptors that contribute to the strength, nutrient supply, and immunity of the skin [1].
The skin functions as a physiological barrier, and its main role in the body is protection from various external factors, such as UV radiation and pathogenic microorganisms that can penetrate through the skin, while it is also an important factor of homeostasis, controlling the loss of water in the body through evaporation. The dysfunction of these mechanisms, which occur naturally over time due to a reduction in the integrity and thickness of the layers, leads to skin aging [2,3]. Also, its protective properties also vary in terms of protecting the internal organs and thus the survival of the organism. As an external organ, it is quite often subject to injury, and, therefore, its healing property is essential for survival [4].
Skin healing and repair is one of the most complex processes of the human body, involving multiple steps and the parallel action of various cell types from all layers [5]. The first response to an injury is the blockage of open blood vessels and the activation of platelets to form a fibrous clot to stop the bleeding and for the accumulation of anti-inflammatory cells at the site of the injury. Once the inflammation is dealt with, the process of angiogenesis follows, which involves the growth of endothelial cells, their metastasis, and the formation of branches so that new vessels can form. At the same time, the fibroblasts at the lesion site proliferate to form a clot and eventually a contractile fibrous tissue, while also expressing extracellular matrix (ECM) proteins, contributing to the growth phase [6]. Still, re-epithelialization, i.e., repair of the skin epithelium and thus the regeneration of skin appendages, is observed [7].
ECM proteins are a family of proteins, composed of a set of nuclear proteins to form the final ECM, where they play a catalytic role in providing the required structural support for cells and tissues. Some of these are collagen and elastin [8]. Specifically in the proliferation phase, the deposition of ECM by fibroblasts begins, forming the so-called temporary matrix, due to their migration to the site of injury. The temporary matrix allows cell separation and metastasis of the cells, to be eventually replaced by granular tissue, which is rich in fibronectin and provides the basis for the coating of collagen molecules, which is the main component of the ECM complex and is what will eventually form the wound scar. Over the years, there is deterioration and aging of this protein complex, which is directly related to the inability of adult skin to heal [9].
As the mechanisms of skin healing and the ability of cells to anchor together are analyzed, it is important to mention E-cadherin, a glycoprotein encoded by CDH-1 gene, which is found at elevated levels in regions where cell-to-cell contact occurs, the so-called adhesion junctions. These tight junctions of cells are essential for the formation of structured tissues and even allow the transmission and communication of cell membrane molecules and intracellular pathways. This glycoprotein has been shown to have a role in cell division, tissue development, organogenesis, and epithelial maintenance and to regulate other key cellular processes such as metastasis, proliferation, apoptosis, and cell differentiation, while its deficiency has been associated with malignancy and tumor development [10]. Several studies have been conducted on the expression of E-cadherin in cases of injury and how it seems to contribute to the healing process. However, its key role in healing processes such as mitosis, maturation, metastasis, and cell recruitment is undisputed [11,12].
Cosmeceuticals are hybrid formulations between cosmetics and pharmaceuticals that contain bioactive ingredients to achieve a more pronounced anti-aging, anti-wrinkle, and moisturizing effect or could even be classified as cosmetic products that are expected to exhibit a therapeutic effect and benefit the skin with continued use [2]. Despite the rapid increase in their use over the last four decades, they are not considered drugs and are not officially recognized by the U.S Food and Drug Administration (FDA) or the European Union. Their increased use is based on the theoretical benefits they provide, which, however, have only been confirmed by in vitro studies of the active ingredients. Dermo-cosmetics are now a separate subcategory between cosmetics and pharmaceuticals, and, in Europe and Japan, they fall under the subcategory of cosmetics, while, in the US, they are considered part of the pharmaceutical subcategory and are provided as over-the-counter (OTC) products [13].
A plethora of peptides have been utilized in cosmetic products due to the increased market demand for innovative and effective products that delay skin aging [14]. Initially, the incorporation of peptides in cosmetic formulations was only aimed at delivering larger molecules and thus increasing their permeability. A typical example is the synthesis of the copper tripeptide GHK-Cu, which had the potential to transport many copper molecules through the skin in order to increase skin healing and also to have an anti-inflammatory and anti-aging effect on the skin [15,16]. The scientific community has focused over the past decades on the synthesis, evaluation, and use of these peptides due to the multiple functions they exhibit such as the regulation of homeostasis molecules, growth, and reproduction, as well as antioxidant, anti-inflammatory, and anti-microbial activity [17]. In addition, factors such as their high safety in use, their hypoallergenicity, and the low-cost development of these molecules have been important parameters for their increased use, as have their proven properties through in vitro and in vivo studies, the main ones being anti-aging, moisturizing and healing, and collagen regulation [15,18].
Earlier reports emphasize that the size of cosmetic peptides must be less than 500 Da to be able to penetrate the skin, the lipophilicity coefficient must be between 1 and 3, and their melting point must be below 200 °C. Still, their aqueous solubility had to be >1 mg/mL, and their structure had to have little to no polar center. However, more recent studies have refuted the belief that only small peptides cross the skin barrier by modifying the amino acid side chains during peptide synthesis, resulting in improved characteristics such as transdermal permeability, binding to appropriate receptors, stability, and solubility [19]. Despite plenty of evidence of their beneficial effect by their addition to the formulation of cosmetic products, there are few reports on the conclusions from their use in cosmetic products. Indeed, there is a particular demand from consumers for validated studies on the safety and efficacy of products of natural and organic origin [20].
Acetyl hexapeptide is a synthetic peptide with the following amino acid sequence Ac-Ala-Arg-His-Leu-Leu-Phe-Trp-NH2 (Figure 1), and it is exploited in skin creams and treatments as an ingredient that can enhance the anti-wrinkle action of other substances, as well as smooth wrinkles and muscles.
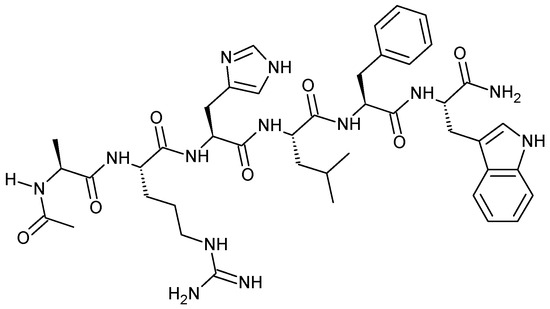
Figure 1.
Structure of synthetic acetyl hexapeptide-1 (Ac-ARHLFW-NH2).
A few studies have been conducted to confirm its properties, where differences were observed between the groups under study and the control group in terms of wrinkles, hair follicles, and skin hydration rate [21]. Another study conducted in 2023 by Ying Ye et al. examined a dermo-cosmetic product with retinol as a key ingredient. The results showed improvement in the photo-aging phenomena of women, reduction in wrinkle formation, even a reduction in pores, increase in skin elasticity, and smoothness and glow were observed [22]. Still, the effect of acetyl hexapeptide and palmitoyl pentapeptide on the formation of wrinkles and fine lines was examined and showed an improvement in wrinkle formation by 10% [23].
The above clinical studies seem to demonstrate the action of synthetic hexapeptide as an anti-wrinkle substance; however, the common thread in these studies is the lack of evaluation of its effect in genetic expression, i.e., how the peptide acts at the genetic level and whether it affects the expression of genes that help in anti-wrinkle action and wound healing. In fact, most peptides used in dermo-cosmetic formulations, although they have been tested in some clinical studies for altering skin physiology, are lacking in studies that genetically prove their activity. This fact should stimulate the scientific community for further studies so that new peptides can still be exploited in similar products [18]. The aim of the present study is to synthesize, characterize, and analyze the synthetic acetyl hexapeptide-1, followed by the evaluation of its effect on the growth rate of epithelial cells and determination of the safety of usage on a concentration of 0.6 mg/mL. Finally, this study investigates the effect of the synthetic acetyl hexapeptide-1 on the expression of the CDH-1 gene, which encodes E-cadherin—a protein integral to cellular adhesion, wound healing, and epithelial maintenance. By linking the peptide to CDH-1 expression, this research aims to elucidate the molecular basis of its purported healing and anti-wrinkle effects.
2. Materials and Methods
2.1. Materials
For peptide synthesis, microwave-assisted solid-phase peptide synthesis (SPPS) was performed using a Biotage Initiator+ SP Wave peptide synthesizer (Biotage AB, Uppsala, Sweden). The PS-PEG 2000 Fmoc Rink Amide resin (Agilent Technologies, Shropshire, UK), with a loading capacity of 0.46 mmol/g, was used as the solid support. Fmoc-protected amino acids (CBL Patras, Patras, Greece), Oxyma Pure (Merck KGaA, Darmstadt, Germany), and diisopropylcarbodiimide (DIC) (TCI Europe, Zwijndrecht, Belgium) were utilized as coupling reagents. Dichloromethane (DCM) (Merck, Germany), trifluoroacetic acid (TFA) (Merck, Germany), and acetic acid (Merck, Germany) were used for peptide cleavage and purification. The final lyophilization of the synthesized peptide was conducted using a Zirbus Vaco2 freeze dryer (Zirbus Technology GmbH, Bad Grund, Germany).
For peptide characterization, high-performance liquid chromatography (HPLC) was performed using an Alliance HPLC e2695 system (Waters Corporation, Milford, MA, USA), equipped with a PDA detector (2998, Waters, USA) controlled via Empower 3.0 software. The molecular mass of the peptide was confirmed using a QDa Aquity Mass Spectrometer (Waters, USA). Dynamic Light Scattering (DLS) analysis was conducted using a Nano ZS (Malvern Instruments Ltd., Worcestershire, UK) to determine particle size distribution and stability.
For the cell culture experiments, HepG2 human hepatocellular carcinoma cells were kindly provided by Dr. Lefteris Zacharia, Associate Professor of Pharmacology at the University of Nicosia. All culture media and reagents were obtained from established suppliers. Dulbecco’s Modified Eagle Medium (DMEM) and Phosphate-Buffered Saline (PBS, pH 7.2) were sourced from GIBCO Laboratories (Thermo Fisher Scientific, Waltham, MA, USA). The cell culture medium was supplemented with 10% (v/v) Fetal Bovine Serum (FBS) and 1% Antibiotic-Antimycotic Solution, containing 100 U/mL of penicillin, 100 µg/mL of streptomycin, and 0.25 µg/mL of amphotericin B, all supplied by Sigma-Aldrich (St. Louis, MO, USA). For cell viability assessment, 0.05% Trypsin-EDTA solution (Gibco, Thermo Fisher Scientific, USA) was used for cell detachment, and Trypan Blue dye (Sigma-Aldrich, USA) was utilized for live/dead cell discrimination. All consumables, including sterile culture plates, pipettes, and flasks, were obtained from Corning Inc. (Corning, NY, USA) to ensure optimal cell growth conditions. Cells were maintained in a humidified 5% CO2 incubator at 37°C under sterile conditions.
For gene expression analysis, TRIzol reagent (Ambion, Thermo Fisher Scientific, Waltham, MA, USA) was used for total RNA extraction, and RNA purity was assessed with the NanoPhotometer P-Class 330 (Implen, GmbH, Munich, Germany). cDNA synthesis was performed using the PrimeScript RT Reagent Kit (Perfect Real Time, Takara, Bio Inc., Shiga, Japan) with oligo-dT primers. Quantitative PCR (qPCR) was conducted using KAPA SYBR FAST qPCR Kit (KK4602-KAPABIOSYSTEMS, Biosystems, Wilmington, MA, USA). For the b-actin, CDH-1, BAX, Bcl-2, Caspase-9, and Cyclin D1 gene detection via qPCR, the primers used are shown in Table 1 and were kindly provided by the Associate Professor of Molecular Pharmacology and Pharmacogenomics, School of Pharmacy, Aristotle University of Thessaloniki, and Associate Professor of the Department of Life and Health Sciences, University of Nicosia, Dr. Ioannis Vizirianakis.

Table 1.
Primers used in qPCR analysis for the detection of b-actin, CDH-1, BAX, Bcl-2, Caspase-9, and Cyclin D1 genes.
2.2. Solid-Phase Peptide Synthesis (SPPS) of Ac-ARHLFW-NH2 Hexapeptide
The peptide was synthesized using microwave-assisted solid-phase peptide synthesis (SPPS) employing the semi-automated Biotage Initiator+ SP Wave peptide synthesizer (Biotage AB, Uppsala, Sweden). The PS-PEG 2000 Fmoc Rink Amide resin, with a loading capacity of 0.46 mmol/g, was selected as the solid support. A precise quantity of resin (0.220 g) was measured and placed into a polypropylene syringe equipped with a filter. The resin was allowed to swell in N-methyl-2-pyrrolidone (NMP) for 15 min at room temperature (RT) under continuous stirring to prepare it for peptide couplings.
Peptide synthesis commenced with the sequential addition of Fmoc-protected amino acids. Each amino acid was coupled using a mixture of the corresponding Fmoc-amino acid, Oxyma Pure, and diisopropylcarbodiimide (DIC) as the coupling reagents. The coupling reactions were facilitated by microwave irradiation. Standard amino acids were coupled at 90 °C for 2 min. For heat-sensitive residues, such as Fmoc-His(Trt)-OH and Fmoc-Arg(Pbf)-OH, the coupling was performed at lower temperatures (40 °C for 3 min and 45 °C for 7 min) to prevent side reactions. Following each coupling step, the Fmoc-protecting group was removed under microwave irradiation at 90 °C for 1 min, a process confirmed by performing a Kaiser test to verify the success of deprotection and coupling reactions. The elongation of the peptide chain continued through repeated cycles of coupling and deprotection until the full sequence was assembled.
Post-synthesis, the peptide was acetylated by adding an acetylation solution to the resin and activating the acetylation program at 75 °C for 5 min. The resin was subsequently washed and dried in the synthesizer for 10 min (2 × 5 min) to remove any residual reagents. The cleavage of the peptide from the resin was achieved by washing the resin under vacuum with dichloromethane (DCM) and treating it with the cleavage solution (TFA-TIS-H2O, 95:2.5:2.5, v/v). The solution was agitated for two and a half hours at RT to cleave the peptide and remove the sidechain-protecting groups. The cleaved peptide was collected from the liquid phase by vacuum filtration and washed with trifluoroacetic acid (TFA) to minimize losses. The peptide solution was then concentrated using a rotary evaporator (Buchi Labortechnik AG, Flawil, Switzerland) and precipitated by adding tenfold excess chilled diethyl ether (Merck, Germany), followed by overnight storage at −20 °C. The precipitated peptide was isolated by centrifugation at 13,000 rpm for 5 min, washed with chilled diethylether, and subsequently dissolved in water and in 10% acetic acid in water, frozen with liquid nitrogen, and then lyophilized in a freeze dryer (Zirbus Technology GmbH, Bad Grund, Germany) overnight.
2.3. Qualitative and Quantitative Characterization of the Synthetic Hexapeptide
The lyophilized peptide was subjected to various analytical techniques to confirm its purity and characterize its physicochemical properties. To assess the purity and molecular integrity, high-performance liquid chromatography (HPLC) was performed. Mass spectrometry (QDa Aquity Mass Spectrometry, Waters, USA) confirmed the molecular mass of the peptide. UV/VIS spectroscopy using a PDA detector (2998, Waters, USA) with Empower 3.0 software was utilized to evaluate the profile and purity of each peak by measuring the UV spectrum separately. The same software was used to control the Mass Spectrometer. Dynamic Light Scattering (DLS) was conducted using a Nano ZS (Malvern Instruments Ltd., UK) to determine particle size distribution and analyze the peptide’s interaction with light across different wavelengths. Infrared spectroscopy was employed to detect impurities and confirm the peptide structure by analyzing characteristic bond vibrations.
2.4. Cell Cultures
The human hepatocellular liver carcinoma cell line, HepG2, was chosen for this study as a well-established epithelial model, providing ease of handling and reproducibility in early experiments. While dermal cells are directly relevant for wound healing, HepG2 cells serve as an effective model for epithelial studies due to their robust growth characteristics and established use in related preliminary research. Thus, HepG2 cells were grown as monolayer culture in DMEM, supplemented with 10% v/v FBS and, 1% Gibco Antibiotic-Antimycotic and maintained in exponential growth at 37 °C, in a 5% CO2 humidified atmosphere. Before each experiment, the cells were washed with phosphate-buffered saline (PBS) pH 7.4 and then were harvested with Trypsin–EDTA solution (0.05%) and centrifuged at 1000 rpm for 5 min.
2.5. In Vitro Cell Viability and Cell Death Assays
After resuspension, 0.5 × 105 cells were seeded per well in a 24-well plate and incubated as mentioned above. The following day, 0.6 mg/mL of the Ac-ARHLFW-NH2 hexapeptide was added in cultures to assess cell viability. This concentration was selected depending on our previous studies with similar short peptides [24]. Cell growth of untreated (control) and Ac-ARHLFW-NH2 hexapeptide-treated cultures was determined at various time intervals by measuring their cell concentrations using a Neubauer hemocytometer under a light microscope. Three independent biological experiments were conducted for the measurement of cell viability for each concentration, and all data presented are the average from triplicate experiments, after 24, 48, 72, and 96 h of incubation. Complementary to cell viability assessment, the number of dead cells in cultures exposed to 0.6 mg/mL of the Ac-ARHLFW-NH2 hexapeptide mentioned above was also determined using a trypan blue solution (0.4% w/v) exclusion assay approach, as previously described [25].
2.6. Gene Expression Assessment
The time-dependent expression of CDH-1 and the expression of BAX, Bcl-2, Caspase-9, and Cyclin D1 genes, at 72 h, was evaluated via total RNA extraction from untreated/control cells and cells treated with the Ac-ARHLFW-NH2 hexapeptide, using TRIzol, according to manufacturer instructions. The purity and the integrity of the isolated RNA were evaluated using the NanoPhotometer P-Class 330.
cDNA synthesis was performed with the PrimeScript RT Reagent Kit. A measurement of 1 μg of cellular total RNA was used per reaction, using oligo-dT primers. cDNA (diluted at 1:50) served thereafter as the template for subsequent qPCR. The gene levels were quantified by qPCR analysis with KAPA SYBR FAST qPCR kit, according to the manufacturer’s instructions, and all reactions were run in duplicates. The reaction conditions were as follows: 95 °C for 3 min, 45 cycles of 95 °C for 3 s, 60 °C for 10 s, and 72 °C for 10 s. In this study, β-actin mRNA was chosen as the endogenous control. The relative levels of the IVT-mRNAs were calculated with the 2−ΔΔCt method.
2.7. Statistical Analysis
All experiments were conducted in triplicates. Statistical analysis was performed using GraphPad Prism Version 8.0 software. All data are presented as the mean ± standard error of the mean (SEM). For comparisons between different groups, an ANOVA test was executed. For comparisons of differences between two groups, a t-test was performed. p < 0.05 was considered to be significant: * p < 0.05, ** p < 0.01, *** p < 0.01, and **** p < 0.0001.
3. Results
3.1. Characterization of Ac-ARHLFW-NH2 Hexapeptide
High-performance liquid chromatographic analysis (Alliance HPLC e2695, PDA 2998, Waters, Milford, MA, USA) was performed to characterize the hexapeptide for its purity. As shown in Figure 2A, HPLC with absorbance at 220 nm was obtained with a high peak at 14.628 min. The 280 nm screening, respectively (Figure 2B), showed a high peak at 14.625, belonging to the amino acid tryptophan contained in the peptide structure. Still, a smaller peak of 14.917 min was observed, which probably belongs to the unstable amino acid histidine that possibly underwent racemization during synthesis. From the above results, the high purity of the synthesized peptide revealed to be 92%. Subsequently, after elution, the sample was forwarded to the Mass Spectrometer (Mass Spectrometry, MS), indicating the molecular mass of the peptide to be 870.57 m/z (Figure 2C). In addition, the absorption, transmission, and reflection of UV radiation from the compound were measured by UV/VIS spectroscopy. As shown in Figure 2D(I,II), the following different values indicate that the peaks 279.8 nm and 281 nm, respectively, represent the amino acid tryptophan, the 219.4 nm peak attributed to the existence of amide bonds, and the differences in spectra between the two peaks at 14.628 min and 14.919 min confirm the presence of racemization.
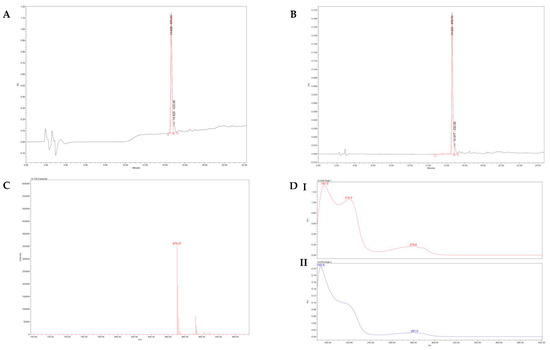
Figure 2.
(A) HPLC-PDA profile of Ac-ARHLFW-NH2 hexapeptide at 220 nm. (B) HPLC-PDA analysis of Ac-ARHLFW-NH2 hexapeptide at 280 nm. (C) HPLC-MS of Ac-ARHLFW-NH2 hexapeptide. (D) UV spectrum of the two peaks of Ac-ARHLFW-NH2 hexapeptide.
To determine the behavior of the synthetic peptide in water and the size of the molecules, an additional analysis, Dynamic Light Scattering (DLS) was conducted in a nanosizer (Nano ZS, Malvern Instruments Ltd., UK). The analysis was carried out at 37 °C in water, the Z-average (size distribution) was calculated to 211.8 nm, and the PDI was estimated (Polydispersity Index) at 0.231 (Figure 3). The peptide shows a spherical morphology in solution (water) and exhibits a degree of self-assembly, which is probably due to its structure, as it consists of amino acids with aromatic groups. Still, while performing this analysis, instability of the peptide in water over time was observed, which requires further study. The average size and the low PDI suggest that the peptide can self-assemble into nanoscale structures such as micelles or nanoparticles in an aqueous solution.
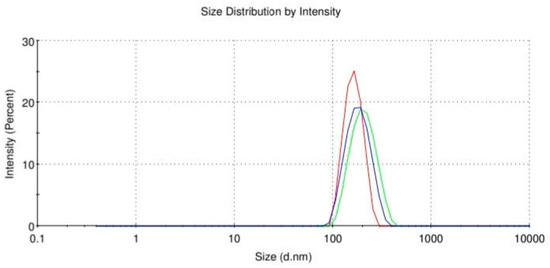
Figure 3.
DLS of Ac-ARHLFW-NH2 hexapeptide. The three lines (red, blue, green) represent the three measurements.
Self-assembled structures, such as nanospheres or fibers, improve peptide stability, penetration of the skin barrier into the dermis, and sustained release in topical applications, enhancing skin absorption and therapeutic effects. The size of 211 nm in water defines a well-structured peptide that ensures proper folding, also predicted with PEPFOLD 4.0 both in pH 5.5 and pH 7.0 (Figure 4), ensuring that the peptide retains its structure during storage, which is crucial in applications like cosmeceuticals where stability impacts shelf life and effectiveness. Additionally, the size range minimizes the risk of irritation or toxicity, making the peptide suitable for sensitive skin applications. Moreover, the uniform particle size and low PDI improve formulation aesthetics, such as texture and consistency, which are crucial in consumer products.
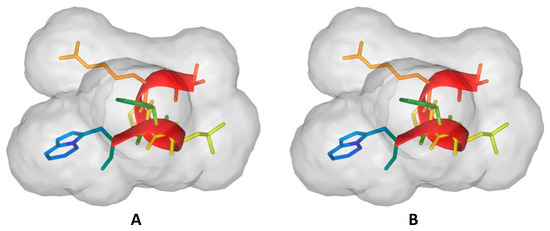
Figure 4.
Existence of an amphiphilic peptide supporting the formation of nanostructures: (A) structure at pH 7.0 and (B) structure at pH 5.5. These structures are generated using PEP-FOLD 4.0 and illustrate the solvent-accessible surface of the peptide.
3.2. Cell Growth Kinetics of HepG2 Cells
Before determining the impact of the synthetic hexapeptide, cell growth and death rate were studied. The initial concentration of cells tested was 105 cells/mL in 500 µL of the complete culture medium, and their growth was examined every 24 h for a total of 96 h. At each interval, live and dead cell counts were performed using a hemocytometer plate (Neubauer). As shown in Figure 5, no significant differences in cell growth were observed between untreated and Ac-ARHLFW-NH2-treated cells (0.6 mg/mL), indicating the absence of cytotoxic effects. Cells reached their maximum growth rate after 96 h, with no dramatic changes in cell viability or death rates observed between treated and control groups. The slight increase in cell death rate after 96 h (remaining below 0.6%) is attributed to natural cell senescence and nutrient depletion rather than any peptide-related toxicity. To further assess potential cytotoxic effects, no morphological abnormalities or significant changes in cell adherence were observed under light microscopy, indicating that the peptide did not alter cell structure at the tested concentration. While this study did not aim to determine CC50 (cytotoxic concentration 50%), IC50 (half-maximal inhibitory concentration), or SI (selectivity index) values, the viability results confirm the biocompatibility of Ac-ARHLFW-NH2, as assessed using cell counting and trypan blue exclusion assays.
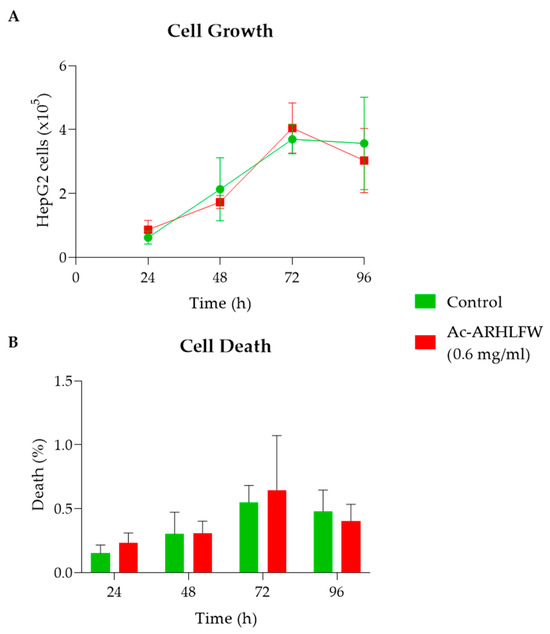
Figure 5.
Cytotoxicity evaluation of Ac-ARHLFW-NH2 hexapeptide in HepG2 cells: Effect of Ac-ARHLFW-NH2 on HepG2 cell growth (A) and cell death using a trypan blue assay (B), as assessed by cell counting with a hemocytometer plate (Neubauer) after treating with 0.6 mg/mL of the Ac-ARHLFW-NH2 hexapeptide for 24 h, 48 h, 72 h, and 96 h.
3.3. Analysis of the Effect of the Ac-ARHLFW-NH2 Hexapeptide on Gene Expression
The impact of the hexapeptide was evaluated to identify its effect on the expression of cell cycle, apoptosis and wound healing genes. For this purpose, time-course qPCR analysis was performed, using β-actin as the reference gene and CDH-1 (ranging from 24 h to 96 h) and BAX, Bcl-2, Caspase-9, and Cyclin D1 (at 72 h) as the target genes. qPCR analysis for key apoptotic and proliferative gene markers in treated cells for 72 h showed that the pro-apoptotic genes BAX and Caspase-9 as well as Bcl-2 and the cell cycle-related gene Cyclin D1 demonstrated similar expression to the control cells, confirming the absence of significant cytotoxic effects of the Ac-ARHLFW-NH2 hexapeptide in HepG2 cells (Figure 6).
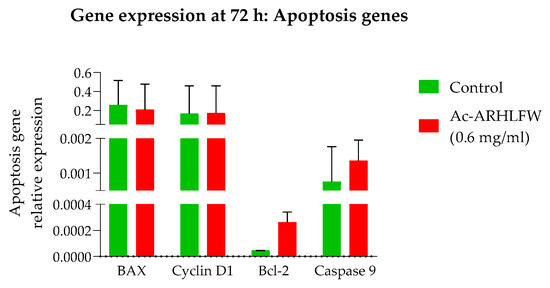
Figure 6.
Expression of BAX, Bcl-2, Caspase-9, and Cyclin D1 genes in HepG2 cells after 72 h treatment with Ac-ARHLFW-NH2 hexapeptide. HepG2 cells are treated with 0.6 mg/mL of the Ac-ARHLFW-NH2 hexapeptide for 72 h. Thereafter, total RNA is extracted from cells, and cDNA is prepared, serving as the template for qPCR analysis. No significant differences between untreated and hexapeptide-treated cells are observed.
Furthermore, the effect of hexapeptide on cells led to increased expression of the E-cadherin gene. The results show a significant increase in the CDH-1 gene in treated cells; however, there is almost equal expression of the gene in the 72 h cells. The maximum increased expression of the CDH-1 gene in the Ac-ARHLFW-NH2-treated cells is observed at 24 h, with a statistically significant increase (** p = 0.003965), which peaks at 48 h, compared to untreated cells and normalized with β-actin gene expression, as shown in Figure 7 Thereafter, the intracellular levels of CDH-1 start to decline both for untreated and Ac-ARHLFW-NH2-treated cells, still with increased CDH-1 expression in treated cells.
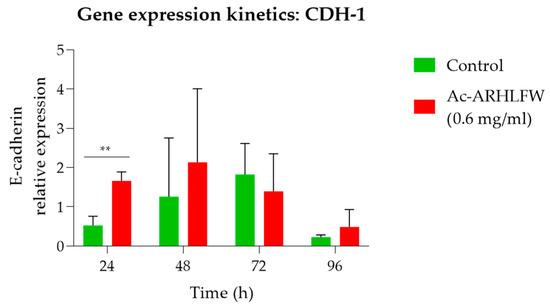
Figure 7.
Time-dependent expression of CDH-1 in HepG2 cells after treatment with Ac-ARHLFW-NH2 hexapeptide. HepG2 cells are treated with 0.6 mg/mL of the Ac-ARHLFW-NH2 hexapeptide for 24 h to 96 h. Thereafter, total RNA is extracted from cells, and cDNA is prepared, serving as the template for qPCR analysis. Based on relative quantification analysis, CDH-1 expression is increased in Ac-ARHLFW-NH2-treated cells, compared to untreated cells and normalized with β-actin gene expression [p = 0.003965 (**)].
4. Discussion
Cosmeceuticals are an emerging category of products that are particularly applicable to personal care and are a combination of cosmetic products and pharmaceutical substances. Their use is based on their anti-aging, anti-wrinkle, and moisturizing properties, and they can also have a therapeutic effect on the skin [13]. The substances that are often included in cosmetics to eventually result in the cosmeceutical product are synthetic peptides, which are chains of 2 to 50 amino acids [18,22], designed to mimic the actions of physiological peptides present in the skin [17,26]. There is a direct correlation between wound healing and the target of cosmetic peptides, as both are aimed at tissue repair and regeneration. A plethora of synthetic peptides have been recorded that find application in cosmetic products for this purpose, whose action has been validated by clinical studies. What is lacking, however, is genetic testing of their effect and thus cellular functions [18]. In particular, synthetic acetyl hexapeptide (Ac-ARHLFW-NH2) has been tested, incorporated or alone, in cosmetic creams and patches [18,22], in various concentrations for its anti-wrinkle and moisturizing activity. However, like other cosmetic peptides, no research has been recorded to study its effect at the genetic level, and this was therefore investigated in the present research work. In this study, we investigated the impact of Ac-ARHLFW-NH2 on E-cadherin (CDH-1) expression, a key adhesion protein involved in epithelial integrity and tissue regeneration.
The synthetic Ac-ARHLFW-NH2 peptide was synthesized in high purity of 92%; however, a possible racemization of histidine may have been conducted. A replacement of Fmoc-His(Trt)-OH with Fmoc-His(Boc)-OH may reduce the racemization and other possible side reactions. The hexapeptide forms interesting nanostructures and a degree of self-assembly, probably due to the amphiphilic nature of the peptide. Initially analyzing the cell growth of HepG2 cells, examined over four time intervals (24 h, 48 h, 72 h, and 96 h), it was observed that they exhibit a maximum concentration and growth rate after 96 h of incubation, and, after this time period, a slight increase in the cell death rate (but remaining below 0.64%) was observed due to the accumulation of unwanted metabolites and depletion of nutrients in their environment. While the cell death data at 72 h (Figure 5B) show a potentially higher variability, this is likely due to increased dispersion in the measurements at this time point. Further replicates and larger sample sizes may help mitigate this variability.
HepG2 cells treated with 0.6 mg/mL of the Ac-ARHLFW-NH2 hexapeptide demonstrated no significant difference in growth or death rates compared to untreated cells, underscoring the peptide’s biocompatibility and absence of cytotoxic effects. The expression of BAX, Bcl-2, Caspase-9, and Cyclin D1 genes, at 72 h, showed no significant differences at hexapeptide-treated cells, compared to untreated cells, aligning with the non-toxic nature of the peptide. Similar to the control response, this likely contributes to the peptide’s potential in promoting tissue repair and skin rejuvenation, where a balance between apoptosis and proliferation is crucial for effective healing and anti-aging outcomes [27]. Additional experiments are necessary to validate these preliminary findings and further explore the cell cycle and apoptosis molecular mechanisms underlying the peptide’s effects.
Subsequently, the effect of the peptide on gene regulation of the cells was tested by performing RT-qPCR, with the CDH-1 as the target gene. Our results demonstrated a statistically significant increase in CDH-1 expression at 24 h (p = 0.003965), in cells treated with the Ac-ARHLFW-NH2 peptide, which peaked at 48 h before stabilizing. This upregulation suggests that the peptide may play a role in reinforcing cellular adhesion and promoting tissue repair.
Several bioactive peptides have been explored for their potential to enhance skin properties by stimulating fibroblast activity and collagen synthesis. For example, Matrixyl (palmitoyl pentapeptide-4) has been shown to promote collagen production, reducing wrinkles and improving skin texture [23]. Similarly, copper peptide GHK-Cu has demonstrated wound healing properties by stimulating glycosaminoglycan synthesis and fibroblast activation, contributing to skin regeneration and firmness [28]. However, while these peptides have been widely studied in cosmetic applications, their direct genetic effects on CDH-1 expression remain largely unexamined. This highlights the importance of our study, which provides the genetic validation of Ac-ARHLFW-NH2’s role in upregulating E-cadherin, a key protein in cellular adhesion and epithelial integrity. Furthermore, the complexity of CDH-1 regulation must be considered. Research has identified alternative CDH-1 transcripts, such as CDH-1a, which may interact with canonical E-cadherin, influencing cell invasion and angiogenesis [29]. These findings suggest that targeted peptides like Ac-ARHLFW-NH2 could play a role in modulating E-cadherin-related pathways, potentially enhancing wound healing and skin barrier function.
While this study provides insight into the molecular mechanisms of acetyl hexapeptide-1, particularly its role in E-cadherin expression, it has certain limitations. This study focused on the peptide’s bioactivity and cytotoxicity but did not perform an in-depth stability assessment beyond pH stability. The heat stability, photostability, and compatibility of the peptide in emulsified or aqueous formulations remain unexplored. Given that acetyl hexapeptide-1 has been incorporated into various cosmetic formulations such as creams and patches, future studies should evaluate its long-term stability under different environmental conditions (e.g., temperature fluctuations, UV exposure, and interaction with excipients). Additionally, further formulation studies are required to assess its efficacy in real-world cosmetic applications. In addition, while this study applied rigorous statistical analysis using ANOVA and t-tests, most comparisons did not yield statistically significant differences, except for the 24 h time point, where E-cadherin (CDH-1) expression showed a significant increase (p = 0.003965). This aligns with biological variability often observed in cellular assays, suggesting that the peptide’s effect may be transient or context-dependent. To enhance statistical power and confirm reproducibility, future studies should incorporate larger sample sizes, alternative cellular models, and extended time points to provide deeper mechanistic insights. Finally, the above results also need further examination firstly as to the effect of the Ac-ARHLFW-NH2 peptide on the growth of normal skin epithelial cells for more precise results and examination of the peptide’s profile regarding skin healing and anti-wrinkle effects.
In summary, the ability of Ac-ARHLFW-NH2 peptide to upregulate CDH-1 expression positions it favorably among small peptides for therapeutic and cosmetic applications. Its structural properties, combined with genetic evidence of efficacy, suggest that it may offer advantages over other peptides lacking such validation. Further research comparing the functional outcomes of different peptides on CDH-1 expression will be instrumental in developing targeted treatments for wound healing and anti-aging.
In conclusion, this study provides genetic validation of the molecular effects of Ac-ARHLFW-NH2, demonstrating its ability to upregulate E-cadherin expression without inducing cytotoxicity. The significant increase in CDH-1 mRNA levels observed at 24 and 48 h suggests that the peptide may enhance cell–cell adhesion and contribute to epithelial integrity, which are essential for tissue rejuvenation and potential wound healing applications. Moreover, the absence of apoptosis or cell cycle modulation reinforces its safety profile, making Ac-ARHLFW-NH2 a viable candidate for incorporation into cosmeceutical formulations. By providing molecular insights into its effects, this study bridges the gap between in vitro bioactivity and real-world cosmetic applications, underscoring the need for further exploration of peptide-based interventions in skin care.
Author Contributions
Conceptualization, Y.S. and A.N.M.; methodology, Y.S., A.N.M. and N.T.; software, A.N.M. and Y.S.; validation, Y.S., A.N.M. and N.T.; formal analysis, Y.S. and A.N.M.; investigation, Y.S., A.N.M., N.T., D.N. and E.G.; resources, C.P. and E.G.; data curation Y.S., A.N.M. and E.G.; writing—original draft preparation, A.N.M.; writing—review and editing, Y.S., A.N.M., N.T., E.G. and C.P.; visualization, Y.S. and A.N.M.; supervision, Y.S. and A.N.M.; project administration, A.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
Special acknowledgements to Lefteris Zacharias for providing the human hepatocellular liver carcinoma HepG2cell lines and to Ioannis Vizirianakis for providing the primers for the qPCR analysis.
Conflicts of Interest
The authors declare that no external funding was received for the research presented in this study.
References
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In Vitro Models for Anti-Aging Efficacy Assessment: A Critical Update in Dermocosmetic Research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Yi, E.-J.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics 2024, 11, 118. [Google Scholar] [CrossRef]
- Carrillo-Norte, J.A.; García-Mir, B.; Quintana, L.; Buracchio, B.; Guerrero-Bonmatty, R. Anti-Aging Effects of Low-Molecular-Weight Collagen Peptide Supplementation on Facial Wrinkles and Skin Hydration: Outcomes from a Six-Week Randomized, Double-Blind, Placebo-Controlled Trial. Cosmetics 2024, 11, 137. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Sarandy, M.M.; de Oliveira, L.L.; da Matta, S.L.P.; Novaes, R.D.; Gonçalves, R.V. Positive Effect of Peptides Obtained from Nile Tilapia (Oreochromis niloticus) on Inflammation Regulation and Wound Healing. Cosmetics 2024, 11, 133. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Altay Benetti, A.; Tarbox, T.; Benetti, C. Current Insights into the Formulation and Delivery of Therapeutic and Cosmeceutical Agents for Aging Skin. Cosmetics 2023, 10, 54. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Corso, G.; Roviello, F. The E-Cadherin Gene Structure and Function. In Spotlight on Familial and Hereditary Gastric Cancer; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Kuwahara, M.; Hatoko, M.; Tada, H.; Tanaka, A. E-cadherin expression in wound healing of mouse skin. J. Cutan. Pathol. 2001, 28, 191–199. [Google Scholar] [CrossRef]
- Shibata-Seki, T.; Nagaoka, M.; Goto, M.; Kobatake, E.; Akaike, T. Direct visualization of the extracellular binding structure of E-cadherins in liquid. Sci. Rep. 2020, 10, 17044. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals; StatPearls Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wang, L.; Wu, Z.; Wang, X.; Wang, X.; Mao, J.; Yan, Y.; Zhang, L.; Zhang, Z. Overview of Peptides and Their Potential Roles in Skin Health and Beauty. J. Pept. Sci. 2025, 31, e3668. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Pickart, L.; Margolina, A. Skin Regenerative and Anti-Cancer Actions of Copper Peptides. Cosmetics 2018, 5, 29. [Google Scholar] [CrossRef]
- Lima, T.N.; Pedriali Moraes, C.A. Bioactive Peptides: Applications and Relevance for Cosmeceuticals. Cosmetics 2018, 5, 21. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Petrou, C.; Sarigiannis, Y. Peptide synthesis: Methods, trends, and challenges. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–21. [Google Scholar]
- Choi, J.-Y.; Oh, S.-C. The Effect of Cream containig Acetyl hexapeptide upon the Facial Skin. J. Korean Oil Chem. Soc. 2014, 31, 120–129. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Y.; Xu, C.; Wei, X. Improvement of mild photoaged facial skin in middle-aged Chinese females by a supramolecular retinol plus acetyl hexapeptide-1 containing essence. Ski. Health Dis. 2023, 3, ski2.239. [Google Scholar] [CrossRef]
- Youn, J.-H.; Kim, E.-J. Anti-wrinkle effects of Acetyl hexapeptide and Palmitoyl pentapeptide. Asian J. Beauty Cosmetol. 2006, 4, 115–128. [Google Scholar]
- Theodoroula, N.F.; Karavasili, C.; Vlasiou, M.C.; Primikyri, A.; Nicolaou, C.; Chatzikonstantinou, A.V.; Chatzitaki, A.-T.; Petrou, C.; Bouropoulos, N.; Zacharis, C.K.; et al. NGIWY-Amide: A Bioinspired Ultrashort Self-Assembled Peptide Gelator for Local Drug Delivery Applications. Pharmaceutics 2022, 14, 133. [Google Scholar] [CrossRef]
- Vizirianakis, I.S.; Tsiftsoglou, A.S. Blockade of murine erythroleukemia cell differentiation by hypomethylating agents causes accumulation of discrete small poly(A)- RNAs hybridized to 3′-end flanking sequences of beta(major) globin gene. Biochim. Biophys. Acta 2005, 1743, 101–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nikoo, M.; Habibi, M.; Naseh, M.; Akrami, S.; Choobineh, H. Therapeutic Effects of a New Topical Cream Formulation in Patients with Vitiligo. Available online: https://www.semanticscholar.org/paper/Therapeutic-effects-of-a-new-topical-cream-in-with-Nikoo-Habibi/f8a109217f5788dc1f4220c955719973ce16e4df (accessed on 4 March 2025).
- Riwaldt, S.; Corydon, T.J.; Pantalone, D.; Sahana, J.; Wise, P.; Wehland, M.; Kruger, M.; Melnik, D.; Kopp, S.; Infanger, M.; et al. Role of Apoptosis in Wound Healing and Apoptosis Alterations in Microgravity. Front. Bioeng. Biotechnol. 2021, 9, 679650. [Google Scholar] [CrossRef]
- Pickart, L.; Margolina, A. Regenerative and Protective Actions of the GHK-Cu Peptide in the Light of the New Gene Data. Int. J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, H.; Carvalho, J.; Oliveira, P.; Ferreira, D.; Pinto, M.T.; Osorio, H.; Licastro, D.; Bordeira-Carrico, R.; Jordan, P.; Lazarevic, D.; et al. Transcription initiation arising from E-cadherin/CDH1 intron2: A novel protein isoform that increases gastric cancer cell invasion and angiogenesis. Hum. Mol. Genet. 2012, 21, 4253–4269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).