Bacteriostasis and Anti-Inflammation of Staphylococcus epidermidis Fermentation Broth to Propionibacterium acnes
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Bacterial and Cell Cultivation
2.3. Preparation of S. epidermidis Fermentation Broth
2.4. Assessment of Antibacterial Efficacy
2.5. LC-MS/MS
2.6. SEM
2.7. Cell Viability
2.8. Preparation of Thermal Inactivation of P. acnes (TI.P)
2.9. Assessment of Anti-Inflammatory Efficacy
2.10. Western Blot
3. Results and Discussion
3.1. Bacteriostasis of S. epidermidis Fermentation Broth to P. acnes
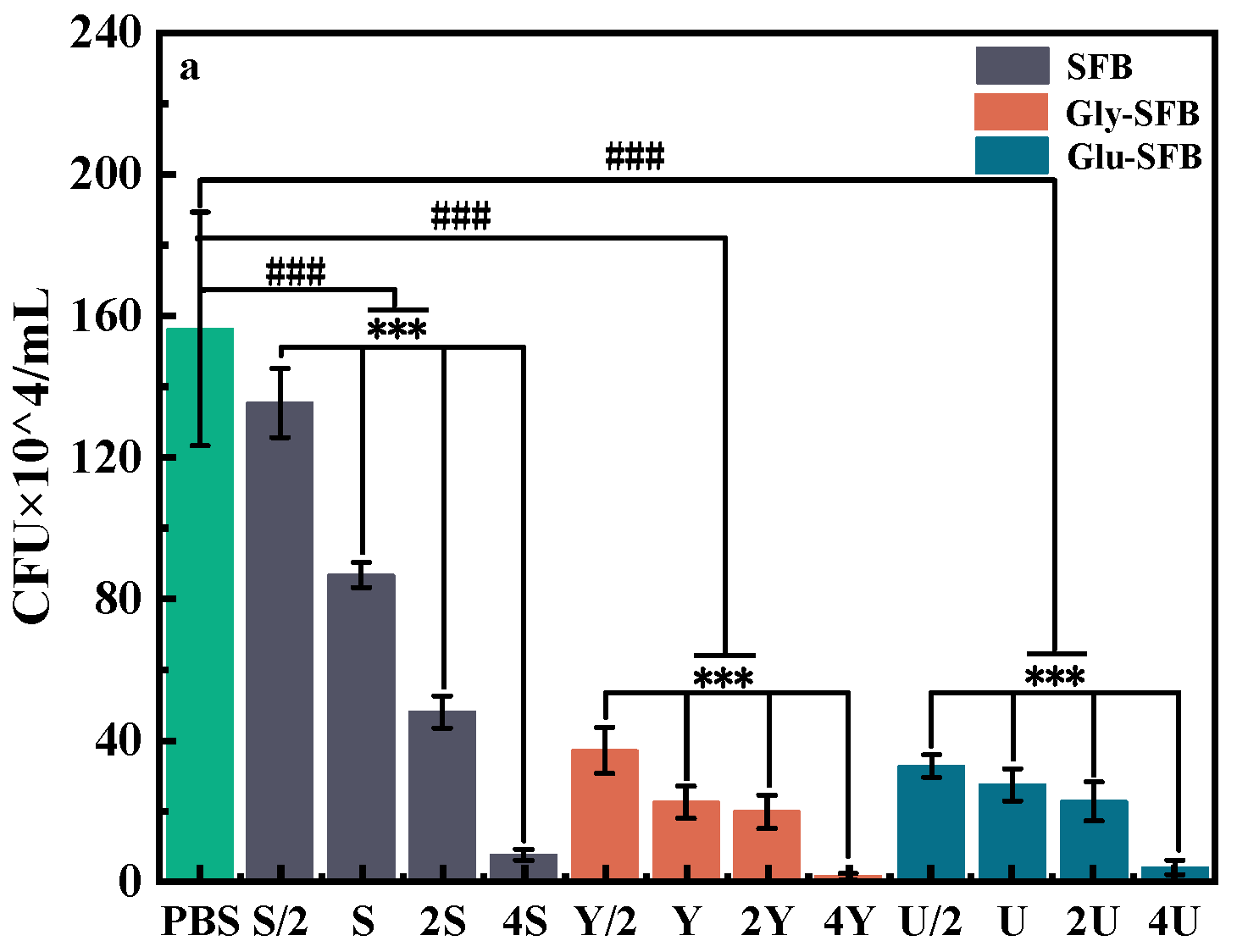
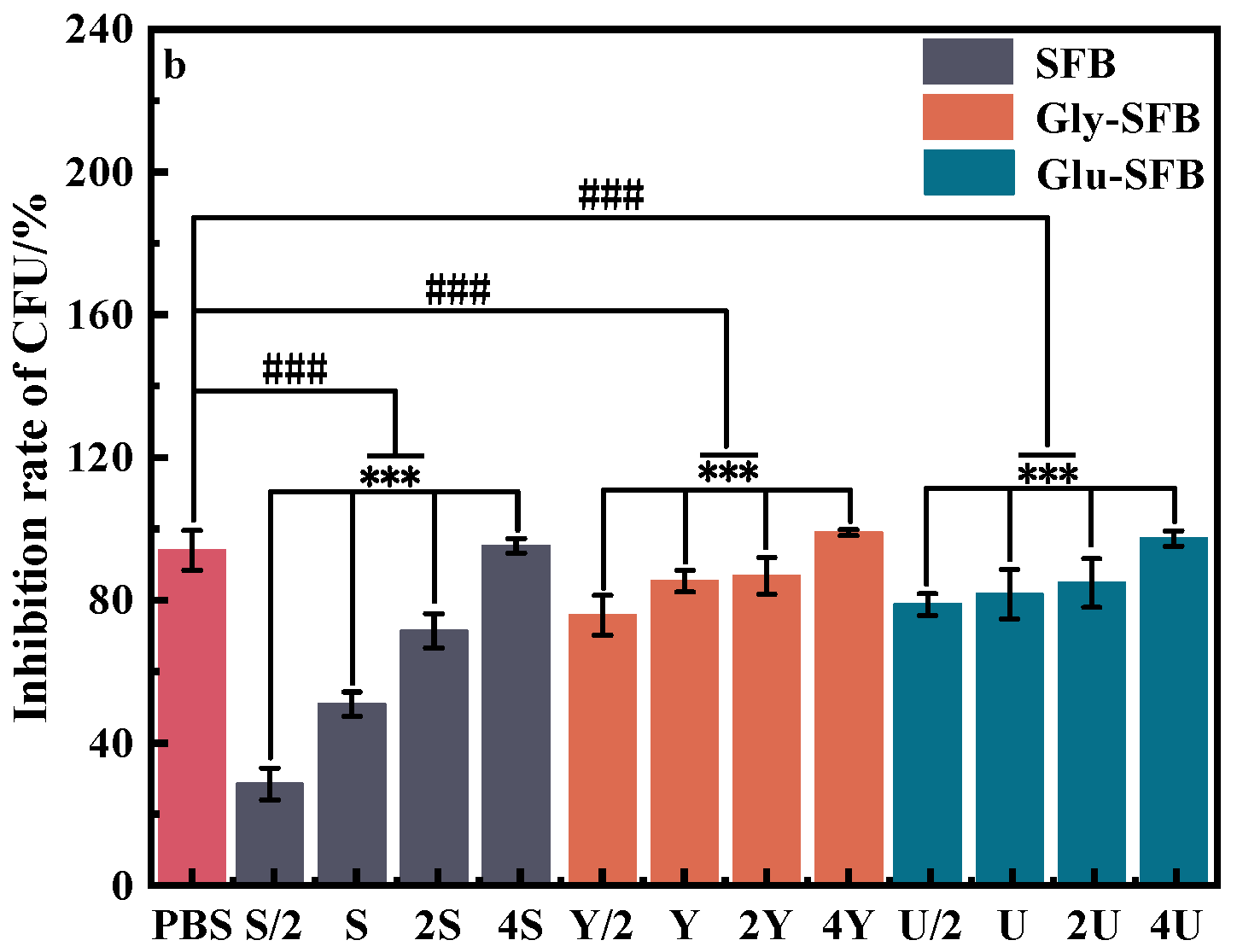
3.1.1. Inhibitory Effect of Three Kinds of S. epidermidis Fermentation Broth on P. acnes
3.1.2. pH of S. epidermidis Fermentation Broth
3.1.3. Oligopeptides Analysis with LC-MS/MS
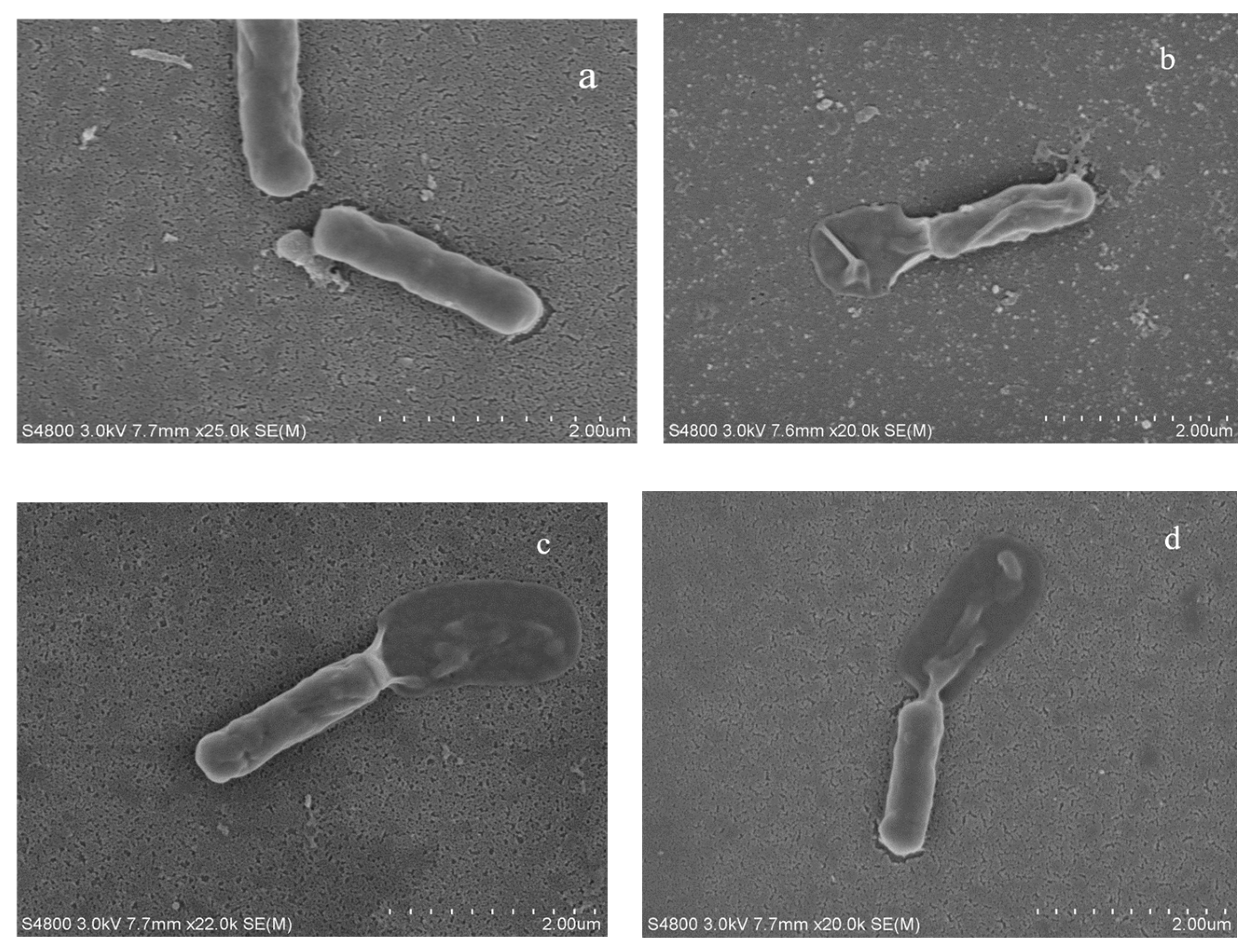
3.1.4. The Impact of Fermentation Broth on Bacterial Morphology
3.2. Anti-Inflammation of S. epidermidis Fermentation Broth
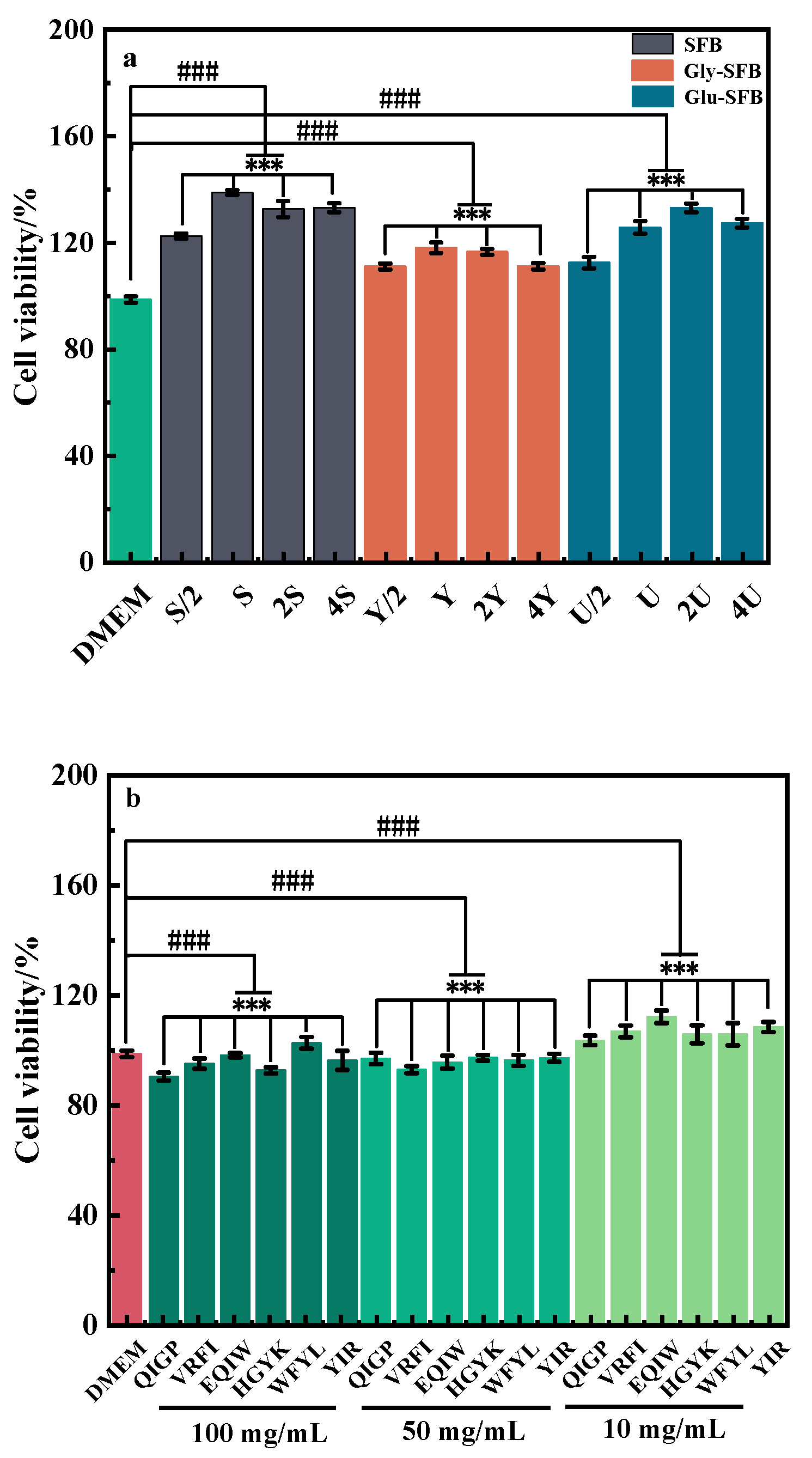
3.2.1. The Influence of Fermentation Broth or Oligopeptides on Cellular Viability
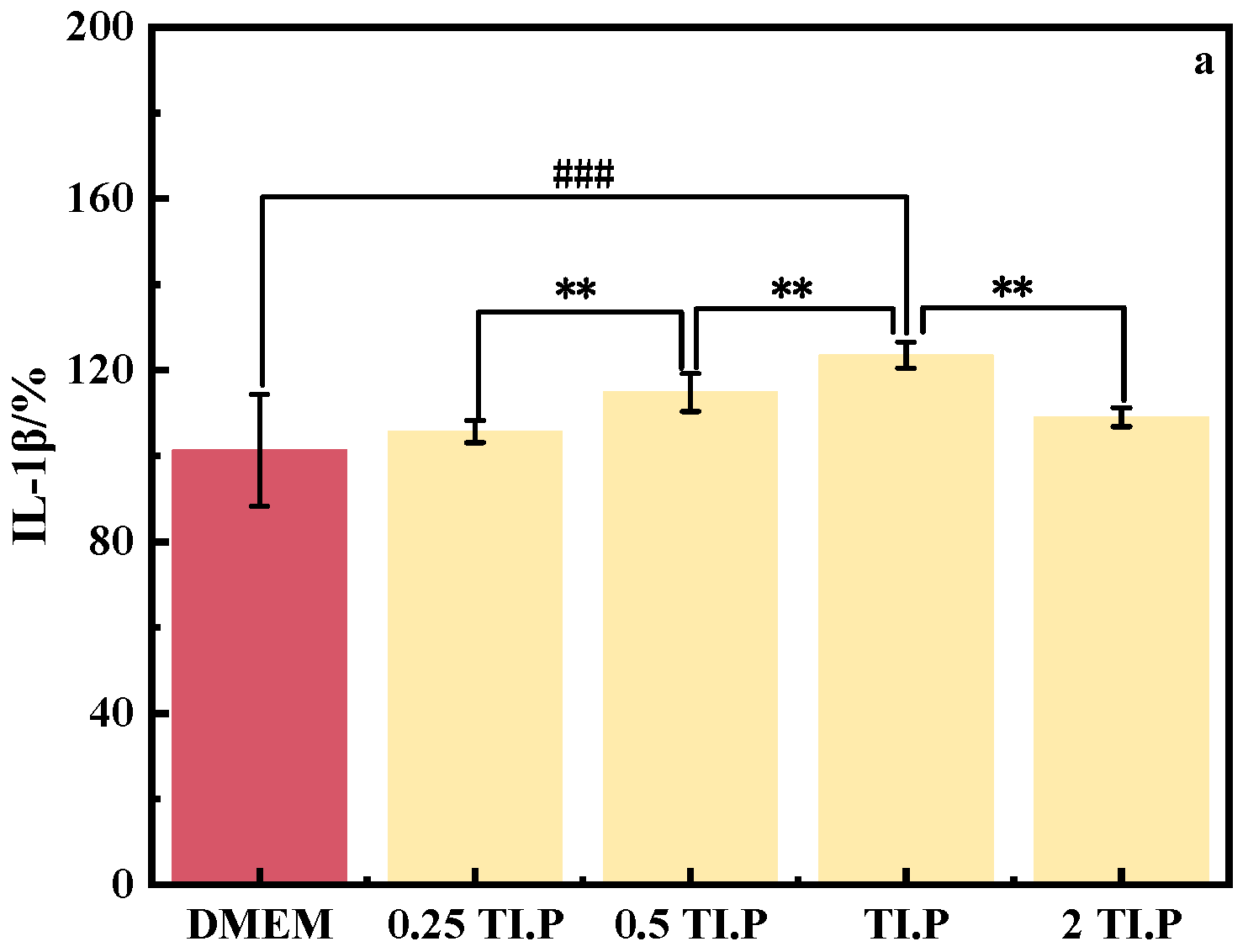
3.2.2. TI.P
3.2.3. Anti-Inflammatory Characteristics of S. epidermidis Fermentation Broth
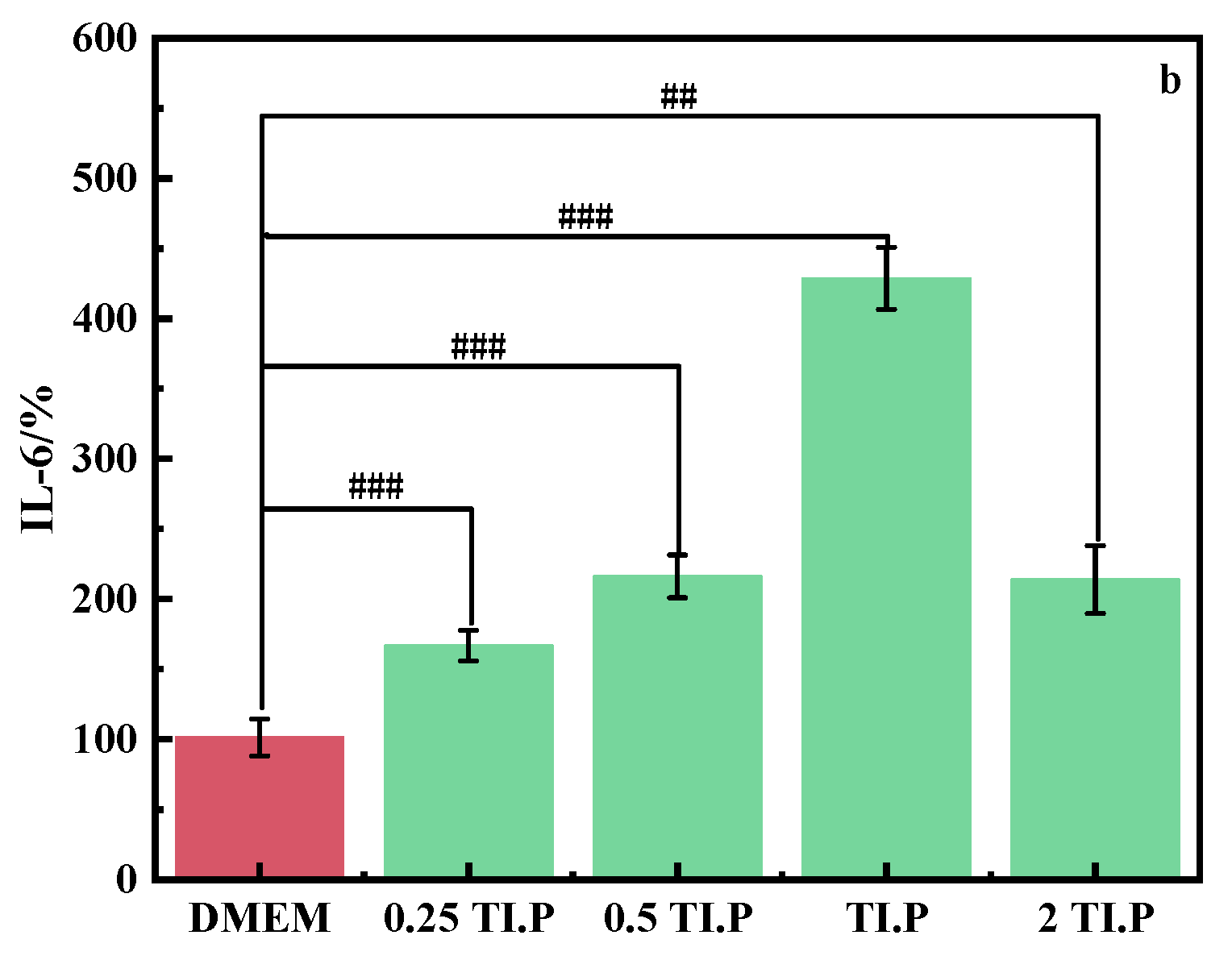
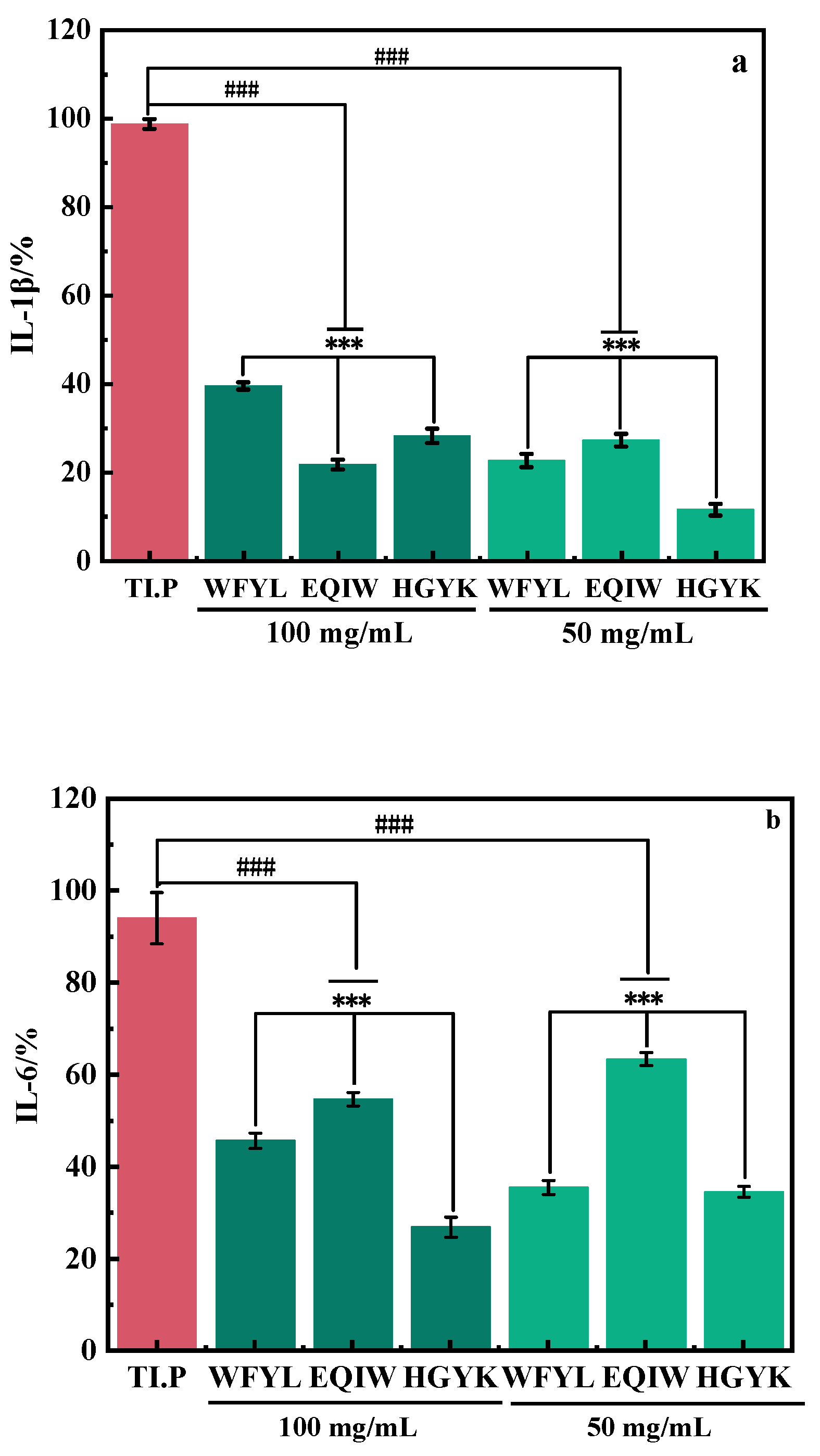
3.2.4. Anti-Inflammatory Characteristics of the Oligopeptides from S. epidermidis Fermentation Broth
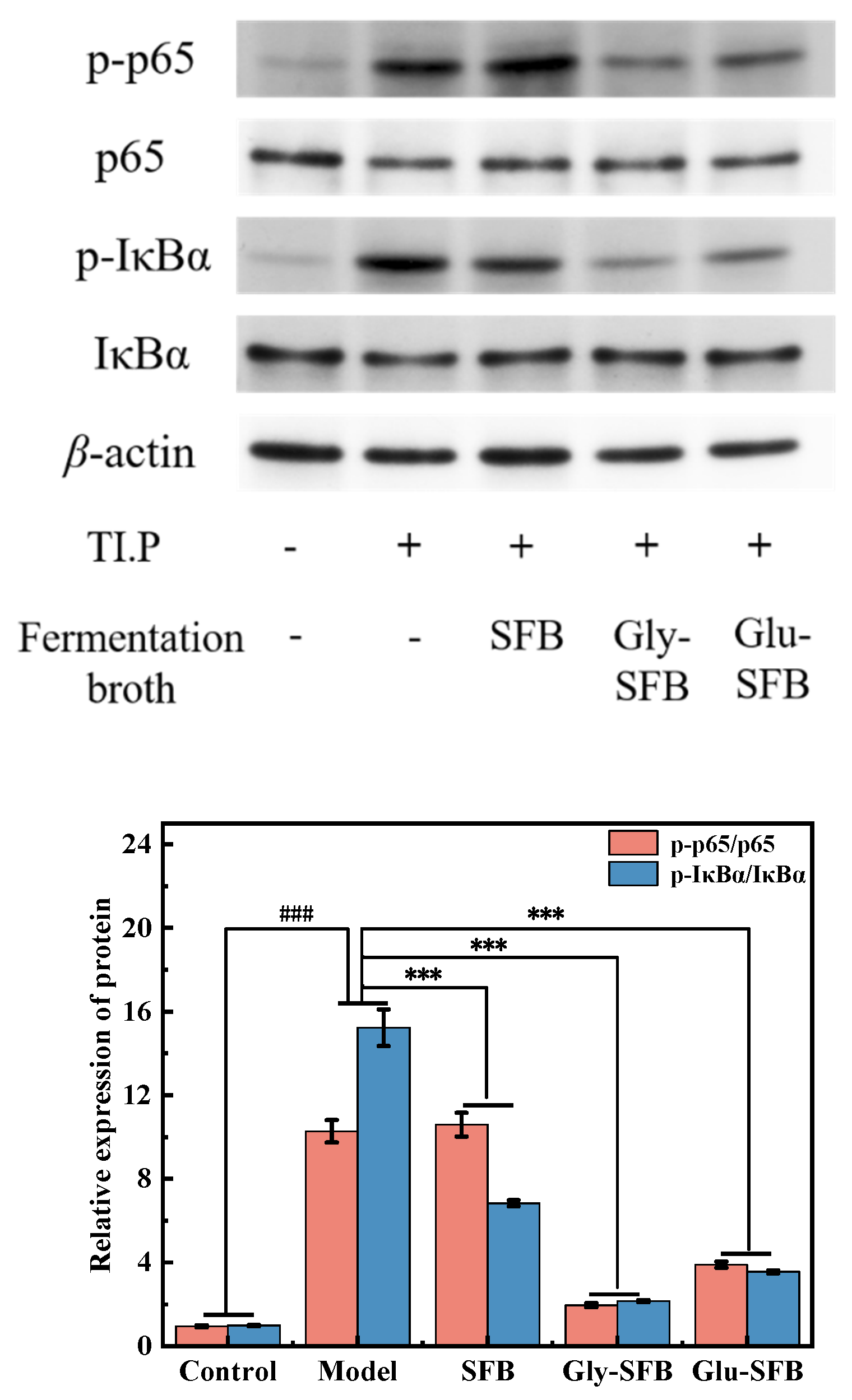
3.2.5. Investigation of Signaling Pathways Related with Inflammation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| Staphylococcus epidermidis | S. epidermidis |
| Propionibacterium acnes | P. acnes |
| Thermal inactivation of P. acnes | TI.P |
| The fermentation broth of S. epidermidis cultivated in a beef protein medium | SFB |
| The fermentation broth of S. epidermidis cultivated in a beef protein medium with adding glycerol | Gly-SFB |
| The fermentation broth of S. epidermidis cultivated in a beef protein medium with adding glucose | Glu-SFB |
| Gas-phase high-throughput time-of-flight mass spectrometer | GC-MS |
| High-performance liquid chromatography | HPLC |
| Ultra-High-performance liquid chromatography–tandem quadrupole time of flight mass spectrometer | HPLC-MS/MS |
| Field emission scanning electron microscope | SEM |
References
- Habeshian, K.A.; Cohen, B.A. Current issues in the treatment of acne vulgaris. Pediatrics 2020, 145 (Suppl. 2), 225–230. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.W.; Göbel, K.; Niessen, C.M.; Paus, R.; Van Steensel, M.A.; Lim, X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br. J. Dermatol. 2019, 181, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.S.; Taherishirazi, M.; Niazi, A.; Afsharifar, A.; Moghadam, A. Structureguided design and cloning of peptide inhibitors targeting CDK9/cyclin T1 proteinprotein interaction. Front. Pharmacol. 2024, 15, 1327820. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D.; Baldwin, H.; Cook-Bolden, F.E.; Alexis, A.F.; Gold, L.S.; Guenin, E. Effects of topical retinoids on acne and post-inflammatory hyperpigmentation in patients with skin of color: A clinical review and implications for practice. Am. J. Clin. Dermatol. 2022, 23, 69–81. [Google Scholar] [CrossRef] [PubMed]
- April, W.A.; Joshua, H.; Leon, H.K. Oral Tetracyclines and acne: A systematic review for dermatologists. J. Drugs Dermatol. 2020, 19, 6–13. [Google Scholar]
- Loomis, K.H.; Wu, S.K.; Ernlund, A.; Zudock, K.; Reno, A.; Blount, K.; Karig, D.K. A mixed community of skin microbiome representatives influences cutaneous processes more than individual members. Microbiome 2021, 9, 22. [Google Scholar] [CrossRef]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Xia, X.; Li, Z.; Liu, K.; Wu, Y.; Jiang, D.; Lai, Y. Staphylococcal LTA-Induced miR-143 Inhibits P. acnes Mediated Inflammatory Response in Skin. J. Investig. Dermatol. 2016, 136, 621–630. [Google Scholar] [CrossRef]
- Geyfman, M.; Debabov, D.; Poloso, N.; Alvandi, N. Mechanistic insight into the activity of a sulfone compound dapsone on Propionibacterium (Newly Reclassified as Cutibacterium) Acnes-mediated cytokine production. J. Exp. Dermatol. 2019, 28, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kao, M.-S.; Yu, J.; Huang, S.-H.; Marito, S.; Gallo, R.L.; Huang, C.-M. A Precision Microbiome Approach Using Sucrose for Selective Augmentation of S. epidermidis Fermentation against P. acnes. Int. J. Mol. Sci. 2016, 17, 1870. [Google Scholar] [CrossRef]
- Wei, J. Study on the Combination of Traditional Chinese Medicine Extracts for Inhibiting Acne Inflammation with Multi-Targets. Ph.D. Dissertation, Jiangnan University, Wuxi, China, 2020. [Google Scholar]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, H.G.; Bae, I.H.; Kim, W.; Park, J.; Lee, T.R.; Cho, E.-G. Propionibacterium acnes-Derived Extracellular Vesicles Promote Acne-Like Phenotypes in Human Epidermis. J. Investig. Dermatol. 2018, 138, 1371–1379. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.-L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Yu, Q.; Pan, H.; Shao, H.; Qian, C.; Han, J.; Li, Y.; Lou, Y. UPLC/MS-based untargeted metabolomics reveals the changes in muscle metabolism of electron beam irradiated Solenocera melancholy during refrigerated storage. Food Chem. 2022, 367, 130713. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, Z.P.; Yin, C.X.; Su, J.; Hu, X.; Zhu, X.R. Antifungal activity and mechanism of ε-polylysine against Geotrichum citriaurantii. Food Sci. 2020, 41, 221–229. [Google Scholar]
- Lim, H.-J.; Kang, S.-H.; Song, Y.-J.; Jeon, Y.D.; Jin, J.S. Inhibitory Effect of Quercetin on Propionibacterium acnes-induced Skin Inflammation. Int. Immunopharmacol. 2021, 96, 107557. [Google Scholar] [CrossRef]
- Ladram, A. Antimicrobial peptides from frog skin biodiversity and therapeutic promises. Front. Biosci. 2013, 21, 1341–1371. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Jeong, H.-Y.; Choi, Y.-S.; Lee, J.-K.; Lee, B.J.; Kim, W.K.; Kang, H. Anti-inflammatory activity of citric acid-treated wheat germ extract in lipopolysaccharide-stimulated macrophages. Nutrients 2017, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A. The NF-κB and IκB proteins: Discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–681. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R. Metabolomics in antimicrobial drug discovery. Expert Opin. Drug Discov. 2022, 17, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Zeki, O.C.; Eylem, C.C.; Recber, T.; Kır, S.; Nemutlu, E. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef]
- Chen, C.; Gonzalez, F.J.; Idle, J.R. LC-MS-based metabolomics in drug metabolism. Drug Metab. Rev. 2007, 39, 581–597. [Google Scholar] [CrossRef]
- Morave, J.H.; Morave, J.Z.; Yazdanparast, Z.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial peptides: Features, action, and their resistance mechanisms in bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Hu, F.X. Extraction, Purification, and Identification of Polyphenols from Chaenomeles Speciosa (Sweet)Nakai and Its Antioxidant and Anti-Inflammatory Activities. Ph.D. Dissertation, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar]
- Ji, Z.W. Study on the Preparation of Foxtail Millet Prolamins Peptide and Its Antioxidant and Anti-Inflammatory Activities. Ph.D. Dissertation, Jiangnan University, Wuxi, China, 2020. [Google Scholar]
- Ratan, Z.A.; Jeong, D.; Sung, N.Y.; Shim, Y.Y.; Cho, J.Y. Lomix, a mixture of flaxseed linusorbs, exerts anti-inflammatory effects through src and sky in the NF-κB pathway. Biomolecules 2020, 10, 843–859. [Google Scholar] [CrossRef]


| SFB | Gly-SFB | Glu-SFB | |
|---|---|---|---|
| pH | 8.04 | 4.91 | 4.49 |
| No. | Retention Time (min) | m/z | Identification | Normalized Abundance | ||
|---|---|---|---|---|---|---|
| SFB | Gly-SFB | Glu-SFB | ||||
| 1 | 5.16 | 650.2939 | TRP-PHE-TYR-LEU | 0 | 0 | 3909.597 |
| 2 | 5.22 | 414.2388 | GLN-ILE-GLY-PRO | 2951.95 | 3510.19 | 4259.72 |
| 3 | 5.72 | 556.3145 | VAL-ARG-PHE-ILE | 1665.33 | 2154.25 | 2634.44 |
| 4 | 5.86 | 783.4417 | TYR-ILE-ARG | 4696.13 | 5595.51 | 6950.33 |
| 5 | 8.01 | 1149.56 | GLU-GLN-ILE-TRP | 9097.60 | 12,044.57 | 13,739.04 |
| 6 | 8.7 | 1007.52 | HIS-GLY-TYR-LYS | 10,670.70 | 11,213.58 | 14,142.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, W.; Zhang, Y.; Cao, Y. Bacteriostasis and Anti-Inflammation of Staphylococcus epidermidis Fermentation Broth to Propionibacterium acnes. Cosmetics 2025, 12, 33. https://doi.org/10.3390/cosmetics12020033
Geng W, Zhang Y, Cao Y. Bacteriostasis and Anti-Inflammation of Staphylococcus epidermidis Fermentation Broth to Propionibacterium acnes. Cosmetics. 2025; 12(2):33. https://doi.org/10.3390/cosmetics12020033
Chicago/Turabian StyleGeng, Wenlin, Yun Zhang, and Yuhua Cao. 2025. "Bacteriostasis and Anti-Inflammation of Staphylococcus epidermidis Fermentation Broth to Propionibacterium acnes" Cosmetics 12, no. 2: 33. https://doi.org/10.3390/cosmetics12020033
APA StyleGeng, W., Zhang, Y., & Cao, Y. (2025). Bacteriostasis and Anti-Inflammation of Staphylococcus epidermidis Fermentation Broth to Propionibacterium acnes. Cosmetics, 12(2), 33. https://doi.org/10.3390/cosmetics12020033






