Study of Formulation, Physical Properties, and Instability Process and In Vitro Evaluation of Transparent Nanoemulsions Containing Sesame Seed Oil (SO) and Raspberry Seed Oil (RO)
Abstract
1. Introduction
1.1. Sesame Seed Oil (SO)
1.2. Raspberry Seed Oil (RO)
1.3. Nanoemulsions
- An in vitro evaluation of the antioxidant activities of SO and RO;
- Formulation studies based on a ternary phase diagram;
- A study of the Ostwald ripening instability process;
- An in vitro evaluation by the HET CAM method.
2. Material and Methods
2.1. Material (INCI Name) Aqueous Phase: Purified Water Oily Phase (Lipo® Brasil)
2.2. Methods
2.2.1. In Vitro Evaluation of Antioxidant Action
Measurement of H+ Donor Activity
- Choosing the best solvent to solubilize the vegetable oils
- Inhibition (%) = the H+ donor activity of the DPPH• radical; Acontrol = the absorbance of the DPPH• solution without the sample; Asample = the absorbance of the DPPH• solution plus the sample; and A negative = the absorbance of the sample without the DPPH• solution.
- Antioxidant activity of nanoemulsions
- (a)
- The antioxidant activity of oils in the proportions contained in the formulations was controlled;
- (b)
- The antioxidant activity of the components of the formulations, in the corresponding proportions, without SO and RO and in the formulations containing BHT was determined.
Superoxide Radical Scavenging Activity Generated in Xanthine (X)/Luminol (L)/Xanthine Oxidase (XOD) System by Chemiluminescence
- (1)
- Test 1: preliminary test.
- Negative—all materials except for the sample (X + XO + L + Glycine Buffer); vegetable oils (SO and RO)/glycine buffer (1:10); Solvent: EA: IPA (1:1)
- (2)
- Test 2: solvent interference: pure SO; pure RO; RO pure + negative.
- (3)
- Test 3: turbidity interference: No L; RO; S/SO.
3. Formulation Studies
3.1. The Determination of the HLB Value for Sesame Seed Oil
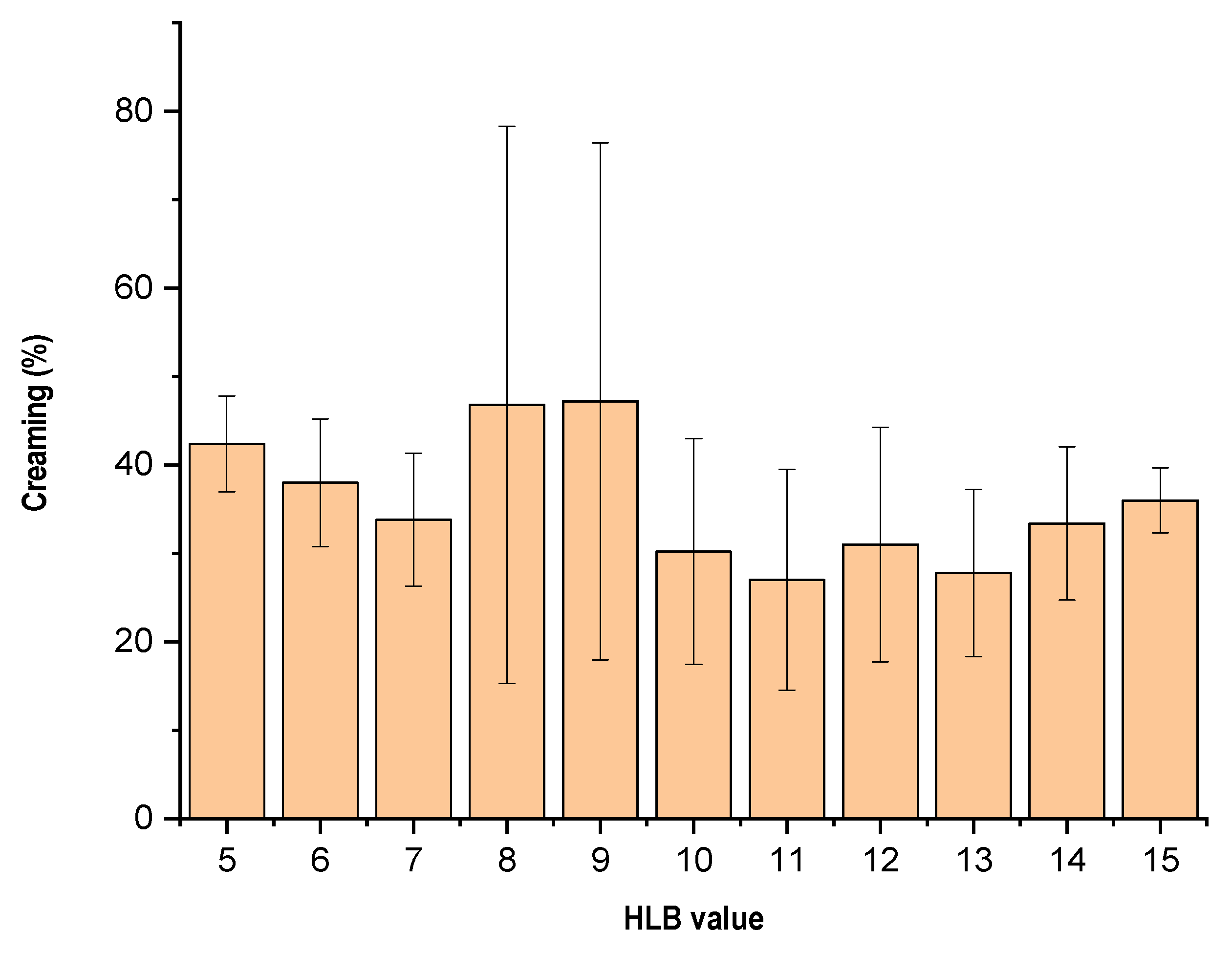
3.2. Evaluation for Creaming Emulsions
3.3. Formulation of Emulsions and Hydrophile Lipophile Balance (HLB) Study
HLBA × 0.01A + HLBB × 0.01B = HLBR
3.4. Phase Inversion Temperature (PIT) Method
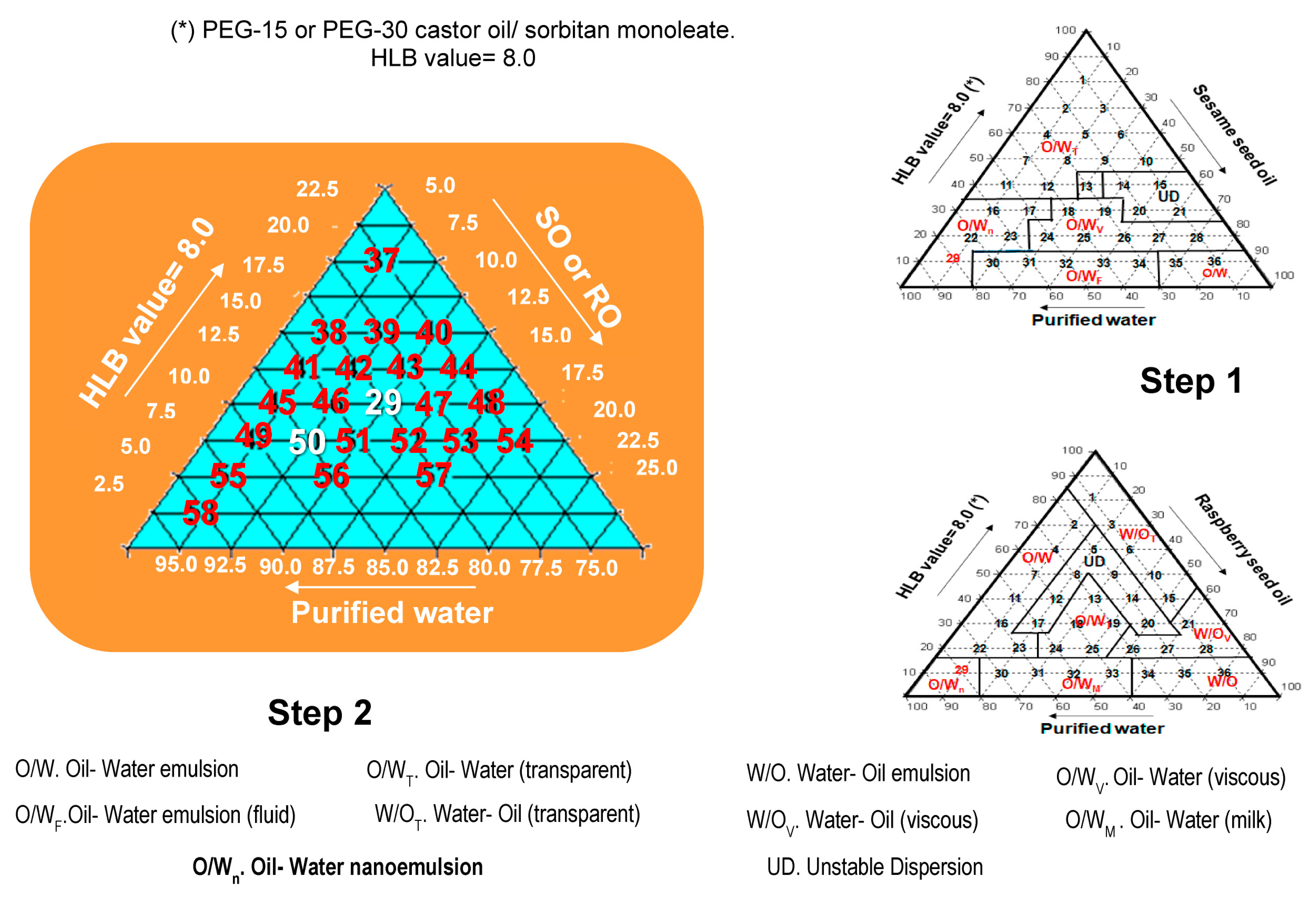
3.5. Phase Diagram for SO and RO at Required HLB Value
3.6. Stability Tests
3.6.1. Centrifugation Test
3.6.2. Thermal Stress
3.6.3. Size and Zeta Potential of Nanoemulsions
3.6.4. The Determination of the pH Value [31]
3.6.5. Analysis of Viscosity
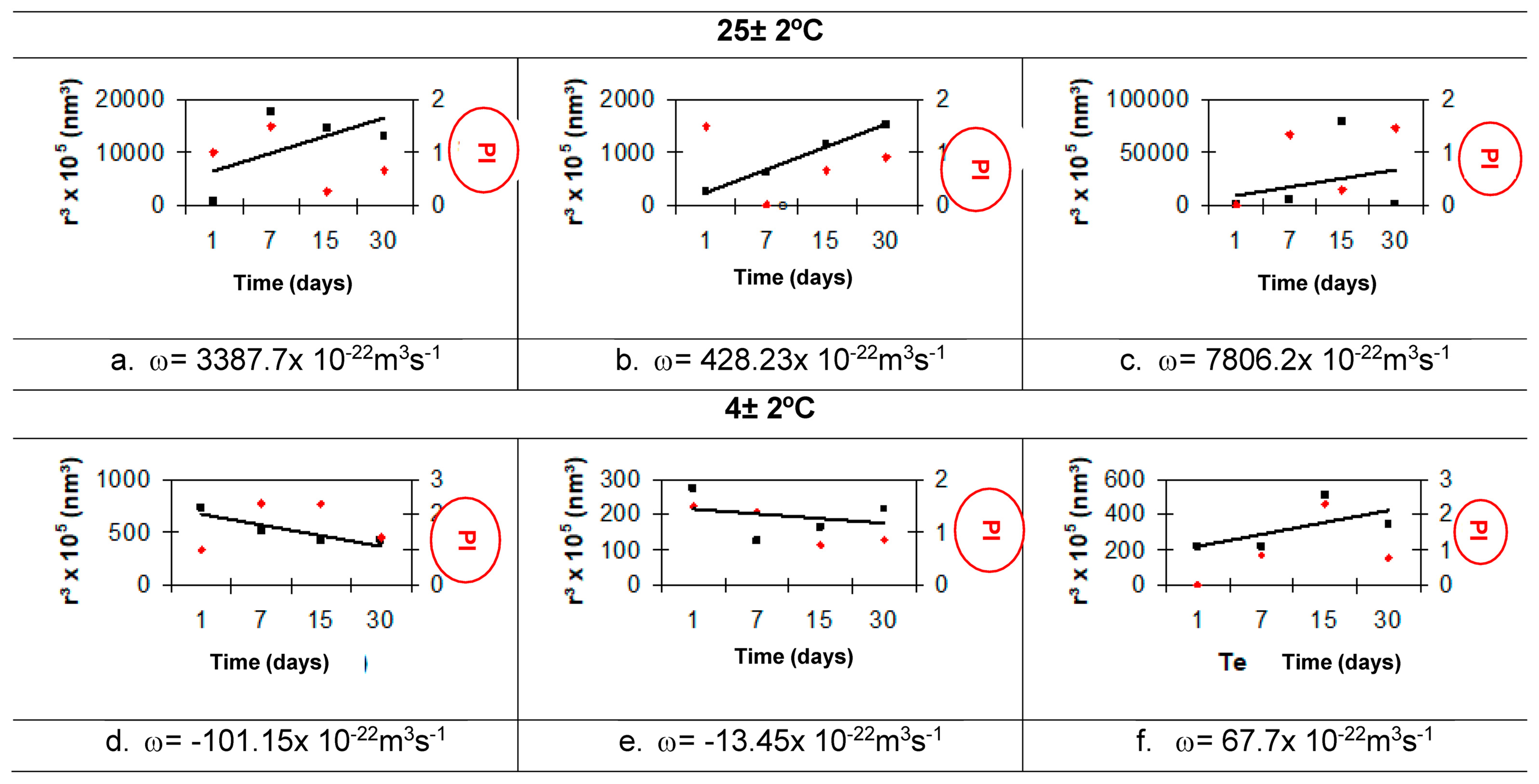
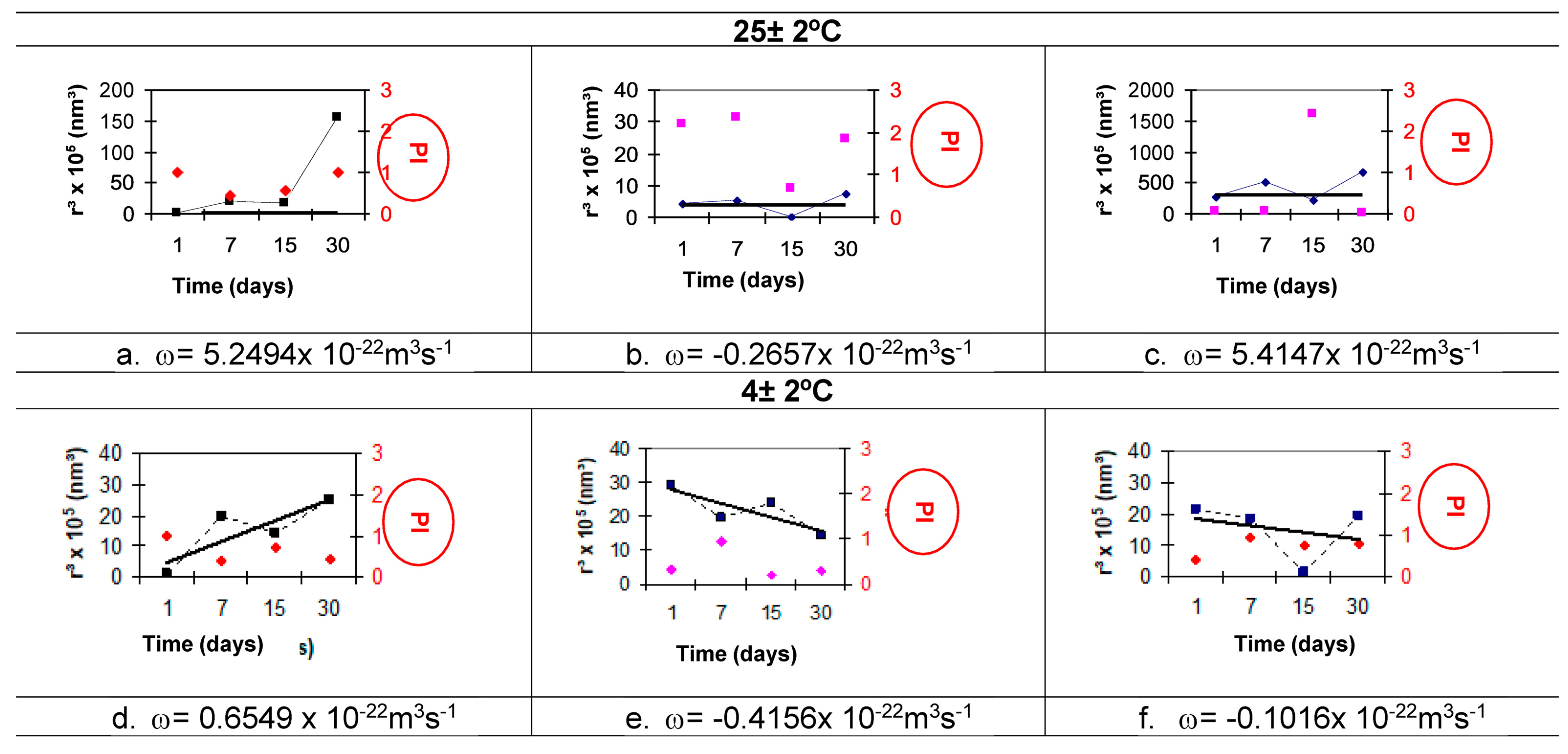
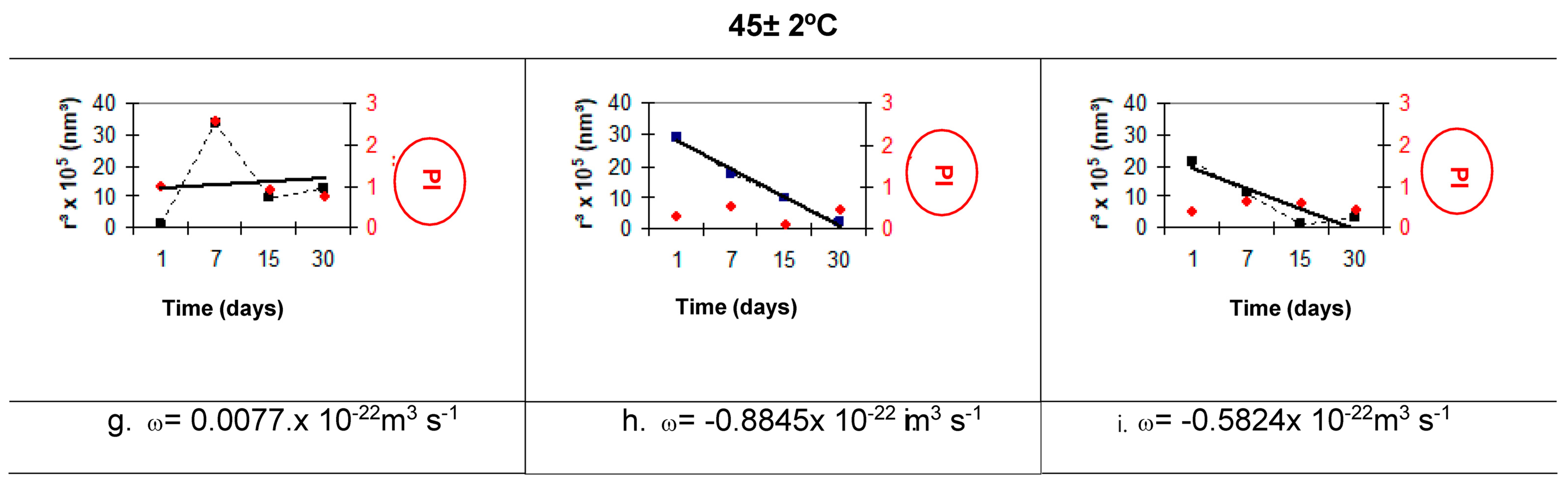
3.6.6. Ostwald Ripening Index
3.6.7. Polydispersity Index (Equation (4)) (SPAN) [34]
4. In Vitro Evaluation
4.1. Irritation Test in Organotypic Model Using Hen’s Egg Test–Chorioallantoic Membrane (HET–CAM)
4.2. Chorioallantoic Membrane Preparation
5. Results
5.1. Assessment of Antioxidant Capacity
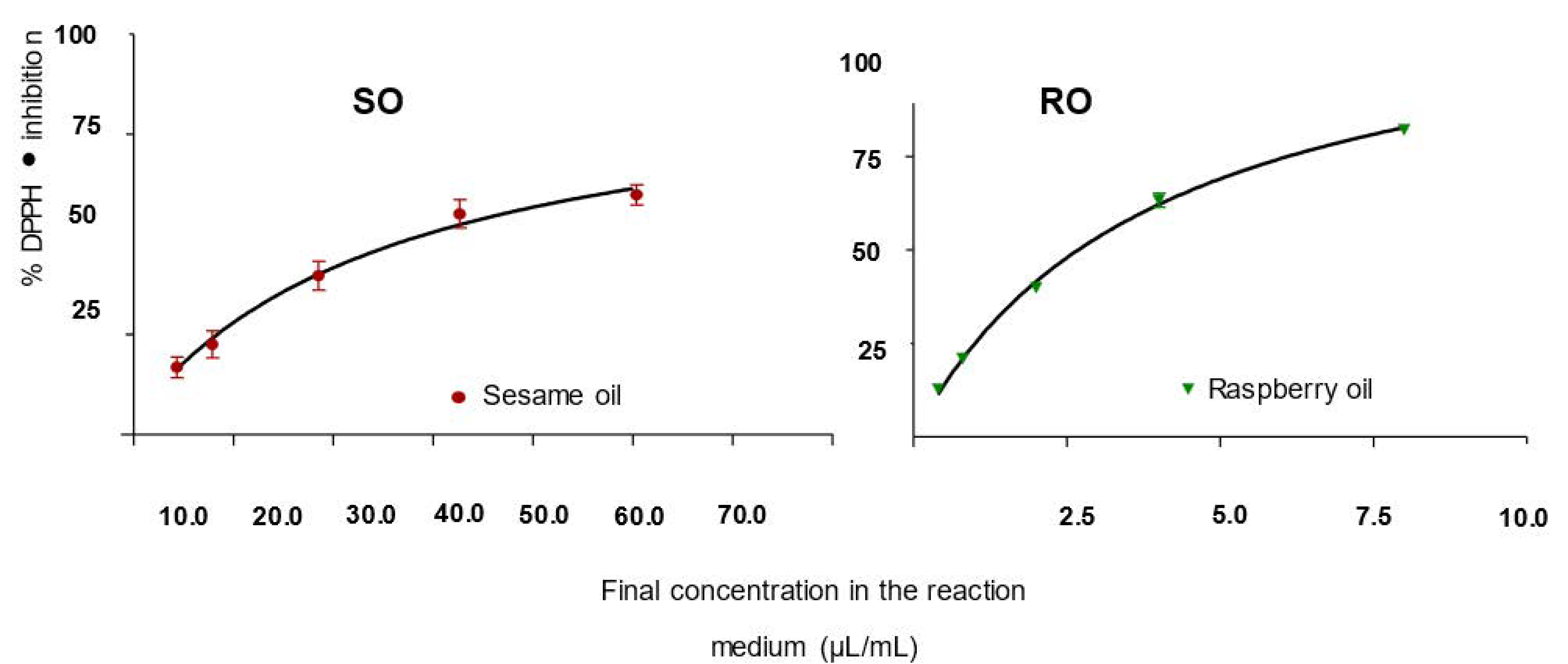
5.1.1. Inhibition of DPPH•
5.1.2. Superoxide Radical Scavenging Activity Generated in Xanthine (X)/Luminol (L)/Xanthine Oxidase (XOD) System by Chemiluminescence
6. Formulation Studies
6.1. Determination of Required HLB Value for SO and RO
6.2. Formulation Studies Based on the Ternary Diagram
6.2.1. Influence of Addition of Additives on Formulation
6.2.2. Stability Studies
Thermal Stress
Particle Size
pH and Viscosity Analysis
Ostwald Ripening
Assessment of Antioxidant Capacity
Antioxidant Activity of Nanoemulsions: Reaction with DPPH•
7. Toxicity Evaluation by HET-CAM Methodology
8. Conclusions
- The IC50 values were 310.94 μL/mL and 34 μL/mL for SO and RO, respectively. The percentages of inhibition of chemiluminescent emission for SO and RO were 20.44% and 42.08%, and these results characterize that the antioxidant activity for RO is twice as high as that of SO. The percentages of DPPH• inhibition for both pure SO and RO were relatively higher than those determined for nanoemulsions.
- The HLB value required for SO using polysorbate 80 and sorbitan monooleate was 8.0, and it was the same for RO using PEG 30 castor oil and sorbitan monooleate.
- SO nanoemulsions were obtained with PEG 15 castor oil and PEG 30 castor oil at an HLB value of 8.0; the nanoemulsions were obtained by the low-energy process (PIT), and the globule sizes of nanoemulsions A and F. 80 were 50 nm and 200 nm, respectively. The co-surfactants PEG 40 castor oil and PEG 54 castor oil contributed to the formation of Sesame Seed Oil nanoemulsions with Raspberry Seed Oil additives. The pH values of the nanoemulsions were found to be suitable for skin care products, and the lower viscosity values allow for easy application to the skin.
- The nanoemulsions A and F. 80 showed antioxidant activity (68.71% and 67.75%, respectively). The theoretical approach for evaluating antioxidant activity using the DPPH• method revealed 60% antioxidant activity for formulation A and 85% antioxidant activity for formulation F.80. An interference in the results was observed if BHT was employed in the formulations.
- The results for the Ostwald ripening index allow it to be used as a comparative indicator of nanoemulsion stability. The SO nanoemulsion with PEG 15 and PEG 30 castor oil obtained at 85 °C and 75 °C, respectively, has the lowest Ostwald ripening index (1.33 × 10−22 m3 s−1).
- The in vitro evaluation using the HET-CAM method of surfactants used to form nanoemulsions showed that they were slightly irritating. A more complete study with the application of each raw material and/or a combination of raw materials must be carried out to identify the agent responsible for this mild irritation, preventing its indication for use as a cosmetic product.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef]
- Zaid, A.; Jaradat, N.; Malkieh, N.; Al-Rimawi, S.; Hussein, F.; Isa, L.; Ali, I.; Manasra, R.; Silwadi, G.; Sadder, O.; et al. Impact of Sesame Oil Source: A Quality Assessment for Cosmeceutical and Pharmaceutical Use. FABAD J. Pharm. Sci. 2019, 44, 189–196. [Google Scholar]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Ghafoorunissa, H.S.; Rao, M.V.V. Sesame lignans enhance antioxidant activity of vitamin E in lipid peroxidation systems. Mol. Cell. Biochem. 2004, 262, 195–202. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Choe, E. Effects of sesamol, sesamin, and sesamolin extracted from roasted sesame oil on the thermal oxidation of methyl linoleate. LTW Food Sci. Technol. 2008, 41, 1871–1875. [Google Scholar] [CrossRef]
- Yaseen, G.; Ahmad, M.; Zafar, M.; Akram, A.; Sultana, S.; Ahmed, S.N.; Kilic, O. Sesame (Sesamum indicum L.). Chapter 15. In Green Sustainable Process for Chemical and Environmental Engineering and Science Plant-Derived Green Solvents: Properties and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 253–269. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Bramley, M.; Elmadfa, I.; Kafatos, A.; Kelly, J.; Manios, Y.; Roxborough, E.; Schuch, W.; Sheehy, A.; Wagner, K.-H. Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red Raspberry (Rubus idaeus L.) Seed Oil: A Review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- Wang, P.W.; Cheng, Y.-C.; Hung, Y.-C.; Lee, C.-H.; Fang, J.-Y.; Li, W.-T.; Wu, Y.-R.; Pan, T.-L. Red Raspberry Extract Protects the Skin against UVB-Induced Damage with Antioxidative and Anti-inflammatory Properties. Oxidative Med. Cell. Longev. 2019, 2019, 9529676. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Future developments in cosmetic formulations. Int. J. Cosm. Sci. 1992, 14, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications. Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–384. [Google Scholar] [CrossRef]
- Chhabra, J.; Chopra, H.; Pahwa, R.; Raina, N.; Wadhwa, K.; Saini, S.; Negi, P.; Gupta, M.; Singh, I.; Dureja, H.; et al. Potential of nanoemulsions for accelerated wound healing: Innovative strategies. Int. J. Surg. 2023, 109, 2365–2377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, A.; Eral, H.B.; Hatton, A.T.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nanoemulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Ostwald ripening in emulsions: Estimation of solution thermodinamics of the disperse phase. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Mello, L.D.; Hernandez, S.; Marrazza, G.; Mascini, M.; Kubota, L.T. Investigations of the antioxidant properties of plant extracts using a DNA-electrochemical biosensor. Biosens. Bioelectron. 2006, 21, 1374–1382. [Google Scholar] [CrossRef]
- Duan, X.J.; Zhang, W.W.; Li, X.M.; Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polusiphonia urceolata. Food Chem. 2006, 95, 37–43. [Google Scholar] [CrossRef]
- Silva, C.G.; Herdeiro, R.S.; Mathias, C.J.; Panek, A.; Silveira, C.; Rodrigues, V.; Rennó, M.; Falcão, D.; Cerqueira, D.; Minto, A.; et al. Evaluation of antioxidant activity of Brazilian plants. Pharmacol. Res. 2005, 52, 229–233. [Google Scholar] [CrossRef]
- Marquele, F.D.; Di Mambro, V.M.; Georgetti, S.R.; Casagrande, R.; Valim, Y.M.; Fonseca, M.J.V. Assessment of the antioxidant activities of Brazilian extracts of propolis alone and in topical pharmaceutical formulations. J. Pharm. Bio. Anal. 2005, 39, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, R.P.; Sheth, B.B. Influence of an optimized non-ionic emulsifier blend on properties of oil-in-water emulsions. Eur. J. Pharm. Biopharm. 1999, 48, 233–238. [Google Scholar] [CrossRef]
- Santos, O.D.H. Development and Evaluation of the Physicochemical Properties and In Vivo Cosmetic Activity of Calendula Officinalis Oil Emulsions with Liquid Crystal. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2006. [Google Scholar]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.J.; Azemar, N.; Solans, C. Formation and stability of nanoemulsions prepared using the phase inversion temperature method. Langmuir 2002, 18, 26–30. [Google Scholar] [CrossRef]
- Izquierdo, P.; Feng, J.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Garcia, M.J.; Azemar, N.; Solans, C. The influence of surfactant mixing ratio on nano- emulsion formation by the pit method. J. Colloid Interface Sci. 2005, 285, 388–394. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Khoury, E.; Gottardi, D. Validation of stability tests for cosmetic products. In Proceedings of the 12th National Congress of Cosmetology, São Paulo, Brazil, 30 June–2 July 1998; Brazilian Association of Cosmetology, ANAIS Tecnopress: São Paulo, Brazil, 1998; pp. 349–375. [Google Scholar]
- Ferrari, M. Obtaining and Application of Multiple Emulsions Containing Andiroba and Copaiba Oils. Master’s Thesis, University of São Paulo, Ribeirão Preto, SP, Brazil, 1998. [Google Scholar]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef]
- Braconi, F.L.; Oliveira, I.S.; Baroni, M.N.F.; Rocha-Filho, P.A. Cosmetic application of canola oil. In Proceedings of the XII Latin American and Iberian Congress of Cosmetic Chemists, São Paulo, Brazil, 27–31 August 1995; Brazilian Association of Cosmetology, Tecnopress: São Paulo, Brazil, 1995; pp. 6–19. [Google Scholar]
- Anvisa Brazil. National Health Surveillance Agency. In Guide to the Stability of Cosmetic Products; Thematic Series; Quality Series 1; Anvisa Brazil: Brasilia, DF, Brazil, 2004. [Google Scholar]
- Capek, I. Degradation of kinetically-stable o/w emulsions. Adv. Colloid Interface Sci. 2004, 107, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1995, 99, 175–185. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Production of sub0micron emulsions by ultrasound and microfluidization techniques. J. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Luepke, N. Hen’s egg choriallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Garcia, L. The quantitative chorioallantoic membrane test using trypan blue stain to predict the eye irritancy of liquid scintillation cocktails. Toxicol. Vitr. 2000, 14, 551–556. [Google Scholar] [CrossRef]
- Murillo, G.; Pérez, U.; Tur, E.; Vinardell, M.P.; García, G. Pascual. Estúdio comparativo de três variantes del ensayo de la membrana corioalantoidea del huevo de la galina para La evaluación de la irritación ocular. Rev. Toxicol. 2003, 20, 187–192. [Google Scholar]
- Vinardell, M.P.; Mitjans, M. The chorioallantoic membrane test as a model to predict the potential human eye irritation induced by commonly used laboratory solvents. Toxicol. Vitr. 2006, 20, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Bandoniene, D.; Murkovic, M.; Pfannhauser, W.; Venskutonis, P.; Gruzdienė, D. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and online HPLC- DPPH methods. Eur. Food Res. Technol. 2002, 214, 143–147. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Zhang, L.; Que, G. Influence of the HLB parameter of surfactants on the dispersion properties of brine in residue. Colloids Surf. A Physicochem. Eng. Asp. 2008, 320, 111–114. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and stability of paraffin oil-in-water nano- emulsions prepared by the emulsion inversion point method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; André, V.; Rieger, J.; Kühnle, A. Nano-emulsion formation by emulsion phase inversion. Colloids Surf. 2004, 251, 53–58. [Google Scholar] [CrossRef]
- Shinoda, K.; Arai, H. The effect of phase volume on the phase inversion temperature of emulsions stabilized with nonionic surfactants. J. Colloid Interface Sci. 1968, 25, 429–431. [Google Scholar] [CrossRef]
- Shinoda, K.; Saito, H. The stability of O/W type emulsions as functions of temperature and the HLB of emulsifiers: The emulsification by PIT-method. J. Colloid Interface Sci. 1969, 30, 258–263. [Google Scholar] [CrossRef]
- Ee, S.L.; Duan, X.; Liew, J.; Nguyen, Q.D. Droplet size and stability of nano- emulsions produced by the temperature phase inversion method. Chem. Eng. J. 2008, 140, 626–631. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slezov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Anton, N.; Gayet, P.; Benoit, J.-P.; Saulnier, P. Nano-emulsions and nanocapsules by the PIT method: An investigation on the role of the temperature cycling on the emulsion phase inversion. Int. J. Pharm. 2007, 344, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Gutiérrez, J.M.; García-Celma, M.J.; Solans, Y.C. A study of therelation between bicontinuous microemulsions and oil/water nanoemulsion formation. Langmuir 2003, 19, 7196–7200. [Google Scholar] [CrossRef]
- Welin-Berger, K.; Bergenståhl, B. Inhibition of Ostwald ripening in local anesthetic emulsions by using hydrophobic excipients in the disperse phase. Int. J. Pharm. 2000, 200, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; González, C.; Maestro, A.; Solè, I.; Pey, C.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interfac Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Porras, M.; Solans, C.; González, C.; Martínez, A.; Guinart, A.; Gutiérrez, J. Studies of formation of W/O nano-emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 249, 115–118. [Google Scholar] [CrossRef]
- Ostwald, W. Uber die vemeintliche Isomerie des roten und gelben quecksilberoxyds und die Oberflachen-spannung Fester Korper. Z. Für Phys. Chem. 1901, 34, 495–512. [Google Scholar] [CrossRef]
- Thomson, W.F.R.S. XLVI. Hydrokinetic solutions and observations. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 42, 362–377. [Google Scholar] [CrossRef]
- Boff, J.M.; Min, D.B. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Lee, J.H.; Koo, N.S.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Lee, J.M.; Chung, H.; Chang, P.-S.; Lee, J. Development of a method predicting the oxidative stability of edible oils using 2,2-diphenyl-1-picrylhydrazyl (DPPH). Food Chem. 2007, 103, 662–669. [Google Scholar] [CrossRef]
- Steiling, W.; Bracher, M.; Courtellemont, P.; de Silva, O. The HET-CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol. Vitr. 1999, 12, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Doucet, O.; Lanvin, M.; Zastrow, L. Comparison of three in vitro methods for the assessment of the eye irritation potential of formulated products. Vitr. Mol. Toxicol. 1999, 12, 63–76. [Google Scholar]
- Zanatta, C.F. Aplicação do Óleo de Buriti no Desenvolvimento de Emulsões e Estudo da Citotoxicidade e Potencial Foto-Protetor Em Cultivo Celular. Ph.D. Thesis, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Ribeirão Preto, Brazil, 2008. [Google Scholar] [CrossRef]

| SO or RO | 30.0% |
| Sorbitan monooleate | A% (*) |
| Polysorbate 80 | B% (*) |
| Purified water | 67.0% |
| SO or RO | 10.0% |
| Sorbitan monooleate | A% (*) |
| Polysorbate 80 | B% (*) |
| Purified water | 85.0% |
| Sample | N | Mean ± SD | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| SO 750/RO 100 | 78.03 | 75.79 | 74.67 | 67.85 | 63.55 | 62.62 | 70.42 ± 6.63 |
| SO 500/RO 50 | 71.32 | 73.93 | 74.67 | 57.76 | 52.71 | 61.50 | 65.31 ± 9.26 |
| SO 300/RO 25 | 51.21 | 59.96 | 59.59 | 42.80 | 39.44 | 39.63 | 48.77 ± 9.53 |
| SO 150/RO 10 | 38.92 | 38.36 | 37.80 | 21.12 | 22.43 | 23.55 | 30.36 ± 8.80 |
| SO 100/RO 5 | 33.97 | 29.83 | 26.03 | 20.37 | 17.20 | 18.13 | 24.26 ± 6.80 |
| Control | SO | RO | Solvent | |
|---|---|---|---|---|
| Emission (×10−8 fpm) | 6.29 | 3.54 | 3.87 | 4.13 |
| 5.30 | 3.11 | 4.06 | 4.06 | |
| Mean ± SD (×10−8 fpm) | 5.79 ± 0.70 | 3.33 ± 0.30 | 3.97 ± 0.13 | 4.10 ± 0.05 |
| (%) Inhibition | - | 42.56 | 31.50 | 29.28 |
| Control | SO | RO | |
|---|---|---|---|
| Emission (×10−8 fpm) | 2.74 | 2.04 | 1.47 |
| 3.00 | 2.40 | 1.36 | |
| 2.46 | 2.08 | 1.25 | |
| Mean ± SD (×10−8 fpm) | 2.73 ± 0.27 | 2.17 ± 0.20 | 1.36 ± 0.11 |
| Inhibition (%) | - | 20.44 | 50.26 |
| Sample | Control | RO + Luminol | RO No Luminol | RO + Buffer Solution |
|---|---|---|---|---|
| Emission (×10−8 fpm) | 1.01 | 0.51 | 9.85 × 10−4 | 11.68 × 10−4 |
| 0.93 | 0.60 | 16.72 × 10−4 | 30.44 × 10−4 | |
| 0.92 | 12.96 × 10−4 | 25.82 × 10−4 | ||
| Emission (×10−8 fpm) | 0.95 ± 0.05 | 0.55 ± 0.06 | 13.18 ± 3.44 (×10−4) | 22.65 ± 9.77 (×10−4) |
| (%) Inhibition | - | 42.08 | 99.86 | 99.76 |
| HLB Value | Creaming Profile (%) | Globule Size (nm) |
|---|---|---|
| 5.0 | 42.40 ± 5.41 | 662.67 ± 269.26 |
| 6.0 | 38.00 ± 7.21 | 503.67 ± 72.20 |
| 7.0 | 33.80 ± 7.50 | 653.3 ± 119.44 |
| 8.0 | 46.80 ± 31.48 | 405.00 ± 231.32 |
| 9.0 | 47.20 ± 29.64 | 496.33 ± 134.35 |
| 10.0 | 30.20 ± 12.76 | 730.00 ± 21.00 |
| 11.0 | 27.00 ± 12.47 | 729.67 ± 223.45 |
| 12.0 | 31.00 ± 13.27 | 701.67 ± 170.89 |
| 13.0 | 27.80 ± 9.44 | 466.33 ± 114.05 |
| 14.0 | 33.40 ± 8.65 | 620.33 ± 28.53 |
| 15.0 | 36.00 ± 3.67 | 427.33 ± 115.60 |
| HLB = 8.0 | Time | pH Value | Zeta Potential (mV) | Size (nm) |
|---|---|---|---|---|
| POE15 EO: SSM | 24 h | 5.75 | 31.8 | 20.0 |
| POE 30 EO: SSM | 24 h | 6.74 | 35.5 | 226.7 |
| POE 30 EO: SSM | 6 months | 6.03 | 49.1 | 264.0 |
| 29 | 50 | Additive PEG 54 | PEG 54 + RO | |||||
|---|---|---|---|---|---|---|---|---|
| Formula nr → Components↓ | 29 A | 50.1 B | 50.2 C | 50.3 D | 50.3.2 E | 50.3.3 F | 50.3.4 G | 50.3.5 H |
| SO | 10.00 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 |
| RO | - | - | - | - | 5.00 | 5.00 | 7.50 | 7.50 |
| PEG 30 castor oil | 5.00 | 3.75 | 3.75 | 3.75 | 5.00 | 5.00 | 5.00 | 5.00 |
| Sorbitan monoleate | 5.00 | 3.75 | 3.75 | 3.75 | 5.00 | 5.00 | 5.00 | 5.00 |
| PEG 54 castor oil | - | - | 1.00 | 0.50 | 0.50 | 1.00 | 1.00 | 5.00 |
| Purified water | 80.00 | 85.00 | 84.00 | 84.50 | 77.00 | 76.50 | 74.00 | 70.00 |
| Temperature (±2 °C) → | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 | 80 | 85 |
|---|---|---|---|---|---|---|---|---|---|---|
| Formula ↓ | ||||||||||
| 29 A | S | S | S | S | S | S | MT | MM | MM | MT |
| 50.1 * B | S | S | S | S | S | S | S | S | S | MM |
| 50.3.2 ** E | S | S | S | S | S | S | MT | ML | M | IM |
| 50.3.3 ** F | S | S | S | S | S | S | S | S | S | IM |
| 50.3.4 ** G | S | S | S | S | S | S | S | MT | IM | IM |
| 50.3.5 ** H | S | S | S | S | S | S | S | M | IM | IM |
| Components (% w/w) ↓ | F.80 | F.83 |
|---|---|---|
| SO | 7.5 | 7.5 |
| RO | 5.0 | 5.0 |
| PEG 30 castor oil | 5.0 | 5.4 |
| Sorbitan monooleate | 5.0 | 4.6 |
| PEG 54 castor oil | 1.0 | 1.0 |
| Purified water | 76.5 | 76.5 |
| Temperature (±2 °C) ↓ | Time (Days) | ||
|---|---|---|---|
| 7 | 15 | 90 | |
| 35 | 17.0 ± 2.0 | 18.0 ± 3.3 | 52.0 ± 4.8 |
| 45 | 17.0 ± 1.4 | 21.0 ± 2.7 | 33.0 ± 5.5 |
| 55 | 17.0 ± 1.4 | 16.0 ± 1.9 | 54.0 ± 4.4 |
| 65 | 17.0 ± 1.4 | 17.0 ± 1.4 | 46.0 ± 9.0 |
| 75 | 17.0 ± 1.4 | 17.0 ± 1.4 | 48.0 ± 7.6 |
| 85 | 15.0 ± 2.5 | 17.0 ± 1.4 | 61.0 ± 11.0 |
| 90 | 16.0 ± 1.9 | 17.0 ± 1.4 | 31.0 ± 3.8 |
| 25 ± 2 °C | ||||
|---|---|---|---|---|
| Days → | 1 | 7 | 15 | 30 |
| Samples ↓ | ||||
| A | 17.33 ± 3.06 | 40.0 ± 19.00 | 45.0 ± 24.75 | 42.00 ± 16.97 |
| F.80 | 88.33 ± 11.02 | 70.0 ± 11.00 | 71.00 ± 19.8 | 73.50 ± 24.75 |
| F.83 | 82.00 ± 28.79 | 49.0 ± 34.39 | 68.5 ± 12.02 | 63.50 ± 20.51 |
| E (50.3.2) | 174.00 ± 96.23 | 79.33 ± 43.50 | 76.00 ± 8.49 | 58.00 ± 5.66 |
| 4 ± 2 °C | ||||
| A | 17.33 ± 3.06 | 18.67 ± 1.53 | 15.00 ± 1.41 | 15.00 ± 1.41 |
| F.80 | 88.33 ± 11.02 | 124.33 ± 89.02 | 67.00 ± 12.73 | 77.00 ± 22.63 |
| F.83 | 82.00 ± 28.79 | 48.67 ± 31.09 | 55.50 ± 10.61 | 56.50 ± 9.19 |
| E (50.3.2) | 174.00 ± 96.23 | 49.33 ± 10.79 | 61.00 ± 0.0 | 52.00 ± 2.83 |
| 45 ± 2 °C | ||||
| A | 17.33 ± 3.05 | 25.00 ± 11.53 | - | - |
| F.80 | 88.33 ± 11.01 | 67.33 ± 17.62 | 130.00 ± 101.82 | 154.50 ± 68.59 |
| F.83 | 82.00 ± 28.79 | 58.67 ± 42.15 | 69.50 ± 14.85 | 84.00 ± 15.56 |
| E (50.3.2) | 174.00 ± 96.23 | 43.67 ± 15.31 | 79.00 ± 25.45 | 172.00 ± 39.60 |
| 25 ± 2 °C | ||||
|---|---|---|---|---|
| Days → | 1 | 7 | 15 | 30 |
| Samples ↓ | ||||
| A | 6.65 ± 0.09 | 5.96 ± 0.80 | 5.74 ± 1.00 | 6.01 ± 0.27 |
| F.80 | 6.15 ± 0.07 | 6.11 ± 0.16 | 5.83 ± 0.14 | 5.87 ± 0.26 |
| F.83 | 6.16 ± 0.10 | 6.12 ± 0.07 | 6.04 ± 0.01 | 5.79 ± 0.10 |
| E (50.3.2) | 6.21 ± 0.04 | 6.09 ± 0.16 | 6.03 ± 0.01 | 5.78 ± 0.21 |
| 4 ± 2 °C | ||||
| A | 6.65 ± 0.09 | 6.73 ± 0.05 | 6.68 ± 0.00 | 6.63 ± 0.07 |
| F.80 | 6.15 ± 0.07 | 6.17 ± 0.05 | 6.21 ± 0.01 | 6.12 ± 0.15 |
| F.83 | 6.16 ± 0.11 | 6.15 ± 0.07 | 6.19 ± 0.01 | 6.15 ± 0.04 |
| E (50.3.2) | 6.16 ± 0.11 | 6.15 ± 0.07 | 6.19 ± 0.01 | 6.15 ± 0.04 |
| 45 ± 2 °C | ||||
| A | 6.65 ± 0.09 | 6.39 ± 0.12 | - | - |
| F.80 | 6.15 ± 0.07 | 5.67 ± 0.21 | 4.78 ± 0.28 | 3.89 ± 0.18 |
| F.83 | 6.16 ± 0.11 | 5.70 ± 0.28 | 4.71 ± 0.23 | 3.84 ± 0.13 |
| E (50.3.2) | 6.21 ± 0.04 | 5.75 ± 0,24 | 4.58 ± 0.50 | 3.86 ± 0.04 |
| Time (Days) → Temperature (±2 °C) ↓ | 60 | 90 | 120 |
|---|---|---|---|
| 35 | 6.58 ± 1.63 | 6.88 ± 0.92 | 18.53 ± 0.39 ### |
| 45 | 5.98 ± 1.74 | 13.29 ± 5.13 * | 17.24 ± 8.73 ### |
| 55 | 5.84 ± 1.59 | 7.33 ± 2.18 | 21.30 ± 1.62 ### |
| 65 | 6.38 ± 2.02 | 7.24 ± 3.11 | 18.98 ± 0.95 ### |
| 75 | 5.44 ± 0.80 | 13.06 ± 11.46 * | 18.72 ± 0.55 ### |
| 85 | 5.65 ± 1.27 | 6.04 ± 1.95 | 13.87 ± 3.81 ## |
| 90 | 6.25 ± 1.02 | 4.85 ± 0.05 | 20.63 ± 0.84 ### |
| Sample A | ||
|---|---|---|
| Temperature (±2 °C) | ω (×10−22 m3s−1) | R2 (Linear Regression) |
| 45 | 4.3333 | 0.9826 |
| 55 | 5.8333 | 0.9932 |
| 65 | 0.6667 | 0.4286 |
| 75 | 6.500 | 0.4613 |
| 85 | 1.3333 | 0.9796 |
| 90 | 10.667 | 0.8745 |
| Sample F.80 | ||
| Temperature (±2 °C) | ω (×10−22 m3s−1) | R2 (Linear Regression) |
| 75 | 1.3333 | 0.9796 |
| 85 | −5.1667 | 0.316 |
| 90 | −7.6667 | 0.1159 |
| Formulas | Temperature (±2 °C) | ω (×10−22 m3 s−1) | ||
|---|---|---|---|---|
| A | 25 | A 3387.7 | b 428.23 | C 7806.2 |
| 4 | D −101.15 | e −13.45 | F 67.7 | |
| F.80 | 25 | A 5.2494 | b −0.2657 | C 5.4147 |
| 4 | D 0.6549 | e −0.4156 | F −0.1016 | |
| 45 | G 0.0077 | h −0.8845 | I −0.5824 | |
| Formulas | Oils | Oil Content (%) | μL/mL | Inhibition (%) | Total Inhibition (%) |
|---|---|---|---|---|---|
| A | SO | 10.0 | 100.0 | 20.0 | 60.0 |
| RO | 3.0 | 30.0 | 40.0 | ||
| F.80 | SO | 7.5 | 75.0 | <20.0 | 85.0 |
| RO | 5.0 | 5.0 | 65.0 |
| Sample | Inhibition (%) | Mean ± SD. | ||
|---|---|---|---|---|
| Control A | 34.92 | 37.79 | 39.89 | 37.53 ± 1.44 |
| A | 65.85 | 69.66 | 70.61 | 68.71 ± 1.45 ***£ |
| Control F.80 | 38.17 | 35.31 | 33.02 | 35.50 ± 1.49 |
| F.80 | 67.37 | 68.51 | 67.37 | 67.75 ± 0.38 ***£ |
| Sample | Inhibition (%) | Mean ± SD. | ||
|---|---|---|---|---|
| Control A | 34.92 | 37.79 | 39.89 | 37.53 ± 1.44 |
| S. A | 2.30 | 1.15 | 0.38 | 1.28 ± 0.56 |
| S. A + BHT | 22.03 | 20.69 | 22.22 | 21.6 ± 0.48 |
| Control F.80 | 38.17 | 35.31 | 33.02 | 35.50 ± 1.49 |
| S. F.80 | 16.48 | 13.03 | 9.77 | 13.1 ± 1.94 |
| S. F.80 + BHT | 20.50 | 23.56 | 22.41 | 22.2 ± 0.89 |
| Preservant | 6.51 | 6.32 | 5.17 | 6.00 ± 0.42 |
| Formula/MP | Time (s) | Score | Category |
|---|---|---|---|
| A | 93.75 ± 6.24 | 3.0 ± 0.0 | slightly irritating |
| S. A | 99.0 ± 9.20 | 3.0 ± 0.0 | slightly irritating |
| F.80 | 122.3 ± 10.14 | 2.0 ± 1.16 | slightly irritating |
| S. F.80 | 125.0 ± 7.39 | 1.5 ± 1.0 | slightly irritating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Filho, P.A.; Maruno, M. Study of Formulation, Physical Properties, and Instability Process and In Vitro Evaluation of Transparent Nanoemulsions Containing Sesame Seed Oil (SO) and Raspberry Seed Oil (RO). Cosmetics 2025, 12, 32. https://doi.org/10.3390/cosmetics12020032
Rocha-Filho PA, Maruno M. Study of Formulation, Physical Properties, and Instability Process and In Vitro Evaluation of Transparent Nanoemulsions Containing Sesame Seed Oil (SO) and Raspberry Seed Oil (RO). Cosmetics. 2025; 12(2):32. https://doi.org/10.3390/cosmetics12020032
Chicago/Turabian StyleRocha-Filho, Pedro Alves, and Monica Maruno. 2025. "Study of Formulation, Physical Properties, and Instability Process and In Vitro Evaluation of Transparent Nanoemulsions Containing Sesame Seed Oil (SO) and Raspberry Seed Oil (RO)" Cosmetics 12, no. 2: 32. https://doi.org/10.3390/cosmetics12020032
APA StyleRocha-Filho, P. A., & Maruno, M. (2025). Study of Formulation, Physical Properties, and Instability Process and In Vitro Evaluation of Transparent Nanoemulsions Containing Sesame Seed Oil (SO) and Raspberry Seed Oil (RO). Cosmetics, 12(2), 32. https://doi.org/10.3390/cosmetics12020032







