1. Introduction
Reactive oxygen species, called free radicals, induced by UV radiation, can damage the structure of the dermis layer, causing the skin to lose its elasticity and leading to wrinkle formation. With the increasing incidence of skin damage, it is necessary to develop prevention strategies and therapies. One strategy to prevent and reduce aging levels is topical formulations with antioxidant effects [
1,
2]. Antioxidants are compounds that can donate one or two electrons to free radicals, inhibiting oxidation reactions in cells and minimizing cell damage. Antioxidants are available in various forms, including vitamins, minerals, and secondary metabolites [
1,
3].
Astaxanthin and zeaxanthin are secondary metabolites belonging to the group of xanthophylls, characterized by their lipophilic properties and strong antioxidant activity. The DPPH radical scavenging assay demonstrated that astaxanthin exhibited significant antioxidant potential with an IC50 value of 17.5 ± 3.6 ppm, while zeaxanthin displayed even stronger antioxidant potential, with an IC50 value of 10 ppm. Both compounds are classified as very strong antioxidants, as their IC
50 values are below the 50 ppm threshold [
4,
5,
6].
Abd El-Baky (2004) reported that the combined use of these carotenoids results in a mutual enhancement of their free radical scavenging activities, providing greater protection against oxidative damage than when used individually. This enhanced efficacy may be due to the differing mechanisms of action of the two compounds, allowing them to more effectively address and neutralize a broader spectrum of reactive oxygen species (ROS). Recent findings have also shown that astaxanthin and zeaxanthin offer the most effective protection against UV-B radiation and inhibit radical peroxidation processes more efficiently than β-carotene [
7].
In recent years, carotenoids such as astaxanthin and zeaxanthin have gained visibility and attracted attention for cosmetic and dermatological applications. Their antioxidant properties are much stronger than those of tocopherol, with positive effects on skin health and protection against UV radiation, which may have potential applications in anti-aging products [
8,
9]. In addition, topical astaxanthin and zeaxanthin applications have been reported in several clinical studies on skincare, including antioxidants, anti-aging, protection against UV irradiation, anti-wrinkle, hydration, and wound healing [
4,
5,
9,
10,
11,
12,
13,
14,
15].
Astaxanthin and zeaxanthin have more potent antioxidant activity than β-carotene, as their polar ionone rings on both ends of their structures can quench free radicals and other reactive oxygen species (ROS), and the thirteen conjugated double, polyunsaturated bonds can remove high-energy electrons. Their amphipathic structure with polar-nonpolar-polar characteristics allows astaxanthin to be inserted into the bilayers of cell membranes, confines lipoperoxidation promoters to penetrate across the lipid bilayer, and thus reduces peroxidation-caused damage [
16,
17].
The synergistic combination of astaxanthin and zeaxanthin enhances the efficacy of preventing aging and improving skin brightening, surpassing the effectiveness of cosmetic therapies that rely solely on a single bioactive ingredient. However, the low bioavailability and solubility of carotenoids (astaxanthin and zeaxanthin) limit their use in topical formulations. In recent years, considerable research has been dedicated to investigating various delivery systems aimed at enhancing the properties of carotenoids. These systems, which have been employed to improve the functionality of lipophilic bioactive compounds, can be broadly categorized into lipid-based and polymer-based delivery systems. In particular, lipid-based nano-delivery systems, such as liposomes, solid lipid nanoparticles, nanostructured lipid carriers (NLCs), and nanoemulsions, have been shown to significantly enhance the solubility and stability of specific carotenoid classes. In a previous study, astaxanthin showed notable potential with an IC50 value of 8.62 ppm, while zeaxanthin nanoemulsions exhibited antioxidant activity with an IC50 value of 9.44 ppm, and their combination achieved an IC50 value of 5.85 ppm. Considering these findings, the formulation of innovative delivery systems for astaxanthin and zeaxanthin to enhance their solubility, stability, and consequently, penetration, is highly desirable [
4,
18,
19].
Nanoemulsions are an innovative delivery system widely used in the cosmetic industry for encapsulating bioactive compounds with poor water solubility. These systems have been proven to improve the stability, textural properties, solubility, skin penetration, and efficacy of active ingredients in cosmetic formulations. Nanoemulsions are composed of extremely small droplets, which provide a larger surface area, allowing for better absorption of active compounds into the skin, making them highly effective for topical applications [
20,
21,
22]. In cosmetic applications, nanoemulsions have been used to deliver active ingredients that target anti-aging, skin hydration, UV protection, and skin brightening. Furthermore, nanoemulsions provide the benefit of controlled release, enabling sustained delivery of active ingredients over time, which can improve long-term skin benefits [
22,
23,
24].
A previous study developed a nanoemulsion system to enhance the stability, solubility, and skin penetration of carotenoids (astaxanthin and zeaxanthin) for topical application. The results demonstrated an improvement in the physicochemical stability and skin permeability of the functionalized nanoemulsion compared to the pure forms of carotenoids [
25,
26,
27,
28]. Accordingly, the objective of this research is to evaluate the stability, ex vivo penetration, and in vivo efficacy of a radiance serum containing a astaxanthin–zeaxanthin nanoemulsion, designed as an anti-wrinkle agent for topical administration.
4. Discussion
Astaxanthin and zeaxanthin are synthesized by the alga species
Haematococcus pluvialis [
39]. This alga belongs to the division Chlorophyta, class Chlorophyceae, order Volvocales, family Haematococcaceae, and genus
Haematococcus. The secondary metabolites produced by this alga are carotenoids, specifically astaxanthin and zeaxanthin [
10]. Astaxanthin and zeaxanthin are natural compounds projected to gain popularity in the coming years due to their potent antioxidant effects, which can prevent deoxyribonucleic acid (DNA) damage and enhance mitochondrial function [
17,
40]. They are effective in anti-aging and could reduce skin damage caused by UV radiation [
11,
12,
13,
14]. Additionally, they can activate the nuclear factor erythroid 2-related factor (Nrf2) pathway to stimulate the production of other antioxidants, promote skin regeneration by controlling inflammation and enhancing collagen synthesis, inhibit matrix metalloproteinases (MMPs), and aid in wound healing [
15,
16]. These carotenoids are being developed to maximize their potential as cosmetic raw materials with anti-wrinkle effects. The process begins with the production of astaxanthin–zeaxanthin nanoemulsions (AZ-NE), followed by serum formulation, stability testing, ex vivo penetration, irritancy testing, and histopathological and in vivo efficacy testing of the AZ-NE radiance serum.
The self-nanoemulsifying drug delivery system (SNEDDS) is a homogeneous anhydrous liquid mixture composed of oil, surfactant, co-surfactant, and active ingredients [
39]. In this study, sunflower oil was used as the oil phase, polyoxy-35-castor oil served as the surfactant, PEG 400 functioned as the co-surfactant, and astaxanthin–zeaxanthin acted as the active ingredient. The appropriate type and ratio of the oil phase, surfactant, and co-surfactant are critical parameters for the formation of nanoemulsions. The ratio of oil, surfactant, and co-surfactant was 1:8:1, following the guidelines and methods for nanoemulsion preparation from previous research [
29]. The resulting nanoemulsion was a thick, orange liquid with a distinct aroma, a zeta potential of −28.4 mV, and a particle size of 20.5 nm (see
Figure 1). The zeta potential indicated the surface charge characteristics of the nanoparticle system. This zeta potential value suggested good stability, implying that the particles were dispersed by the repulsive forces between similarly charged particles. The AZ-NE radiance serum had a zeta potential of approximately −28.4 mV, indicating a moderately negative charge [
26,
41].
The solubility results for free astaxanthin, free zeaxanthin, and astaxanthin–zeaxanthin nanoemulsions (AZ-NE) are presented in
Table 4. Based on the data, the solubility values for free astaxanthin (0.06 ± 0.02 mg/mL), free zeaxanthin (0.08 ± 0.01 mg/mL), astaxanthin nanoemulsions (25.00 ± 0.21 mg/mL), and zeaxanthin nanoemulsions (30.21 ± 0.26 mg/mL) were determined. The solubility of astaxanthin in the nanoemulsion is nearly 416 times higher than in its pure form, while the solubility of zeaxanthin in the nanoemulsion is approximately 377 times higher compared to its free form. These findings suggest that the astaxanthin–zeaxanthin nanoemulsion can effectively enhance both penetration and bioavailability.
The purity levels of free astaxanthin, free zeaxanthin, and astaxanthin–zeaxanthin nanoemulsions (AZ-NE) are presented in
Table 5. Based on the data, the purity of free astaxanthin is measured at 1.13 ± 0.02%, while free zeaxanthin has a purity of 40.30 ± 0.18%. The purity levels of astaxanthin and zeaxanthin in the nanoemulsions are determined to be 0.94 ± 0.67% and 34.5 ± 0.53%, respectively. The lower purity of astaxanthin and zeaxanthin in the nanoemulsions compared to their free forms indicates the presence of additional components such as surfactants and emulsifiers, which are essential for stabilizing the formulations and enhancing their solubility in aqueous environments. Although the purity of astaxanthin in the nanoemulsion is only 0.94 ± 0.67% and that of zeaxanthin is 34.5 ± 0.53%, these formulations may provide improved bioavailability and absorption compared to their free forms, thereby illustrating the effectiveness of nanoemulsion technology in maximizing the functional benefits of these carotenoids despite the trade-off in purity.
The nanoemulsion was then formulated into a cosmetic serum, which primarily consisted of an aqueous phase. The base included suitable emollients, emulsifiers, solvents, preservatives, and fragrances. The formula for the prototype of the developed radiance serum is shown in
Table 1. The formulation results indicated that the produced serum was in liquid form, orange in color, and odorless (see
Figure 2). The quality parameters of Radiance Serum AZ-NE were evaluated through stability tests, irritation tests, and anti-aging effectiveness tests. The parameters for the stability tests of Radiance Serum AZ-NE included organoleptic properties, pH, viscosity, and microbial and heavy metal contamination. Organoleptic testing of the AZ-NE radiance serum involved observations of its appearance, color, and odor. Based on the observations presented in
Table 6,
Table 7,
Table 8,
Table 9 and
Table 10, it was evident that the AZ-NE radiance serum exhibited stable organoleptic properties over a three-month storage period under room temperature conditions (25 ± 2 °C/75 ± 5% RH), climatic chamber conditions (40 ± 2 °C/75 ± 5% RH), and refrigerator conditions (5 ± 3 °C), with a liquid consistency, orange color, and odorless quality.
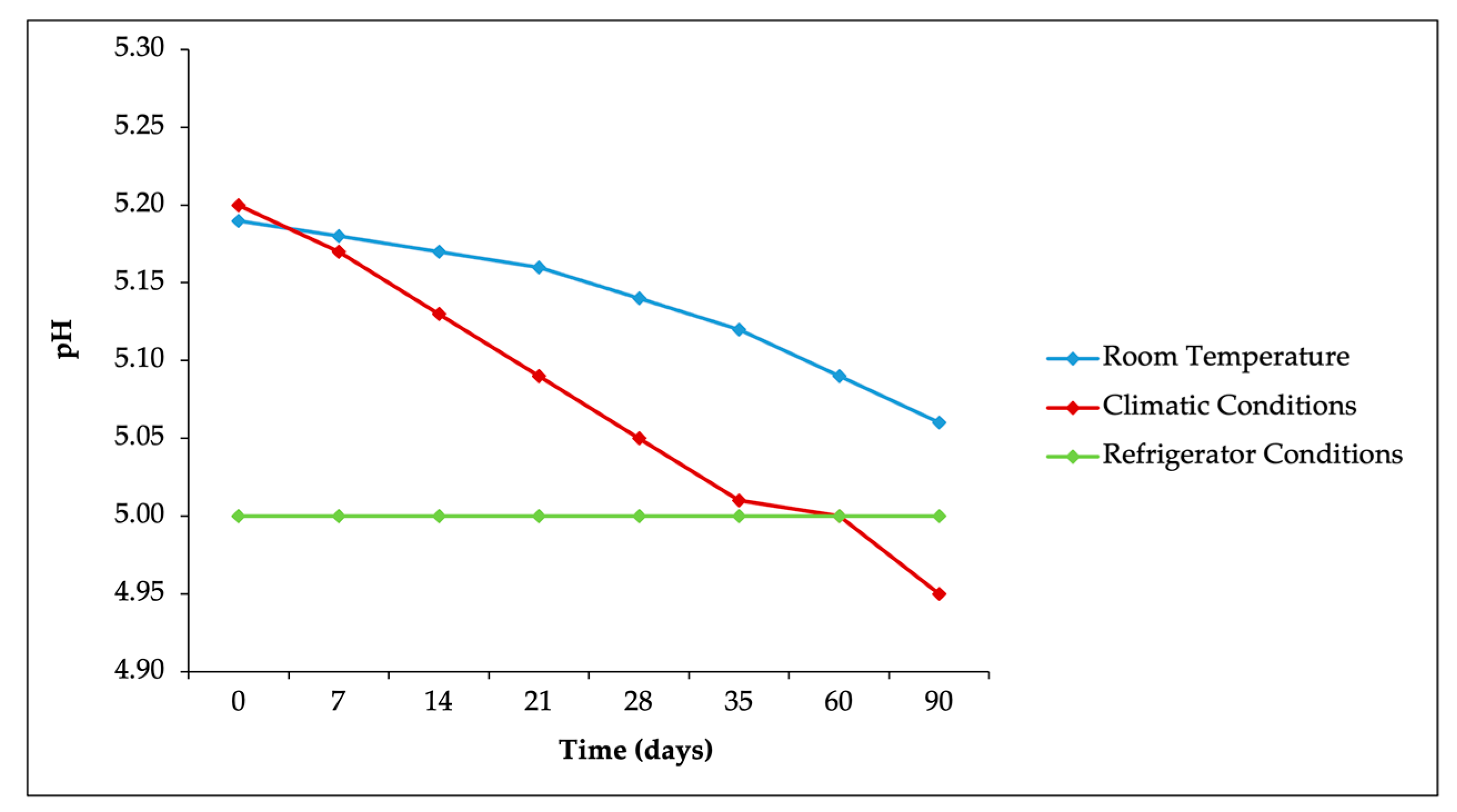
Topical products such as serums are recommended to maintain a pH within the range of 4–6. A reference pH range of 4.5 to 5.5 is considered normal for women, and 4 to 5.5 is considered normal for men. Formulating facial serums within this pH range is beneficial as it supports the skin barrier function [
42]. The pH measurements of the radiance serum are detailed in
Figure 3. According to
Figure 3, during storage at room temperature (25 ± 2 °C/75 ± 5% RH), climatic chamber conditions (40 ± 2 °C/75 ± 5% RH), and refrigerator conditions (5 ± 3 °C), the pH of the radiance serum decreased from 5.19 ± 0.04 to 5.06 ± 0.03 and from 5.20 ± 0.02 to 4.95 ± 0.03, respectively. This pH decrease might be attributed to the influence of CO
2 in the formulation, where atmospheric CO
2 reacted with the aqueous phase of the serum, leading to acid formation. However, despite the pH decrease, the formulated AZ-NE radiance serum remained within the acceptable pH range, specifically around pH 5, in accordance with the standards specifying a pH range of 4–8 [
43]. In particular, there were no significant differences in pH values across the three storage temperature variations, indicating that the formulation maintained a consistent level of acidity under varying conditions (
p > 0.05).
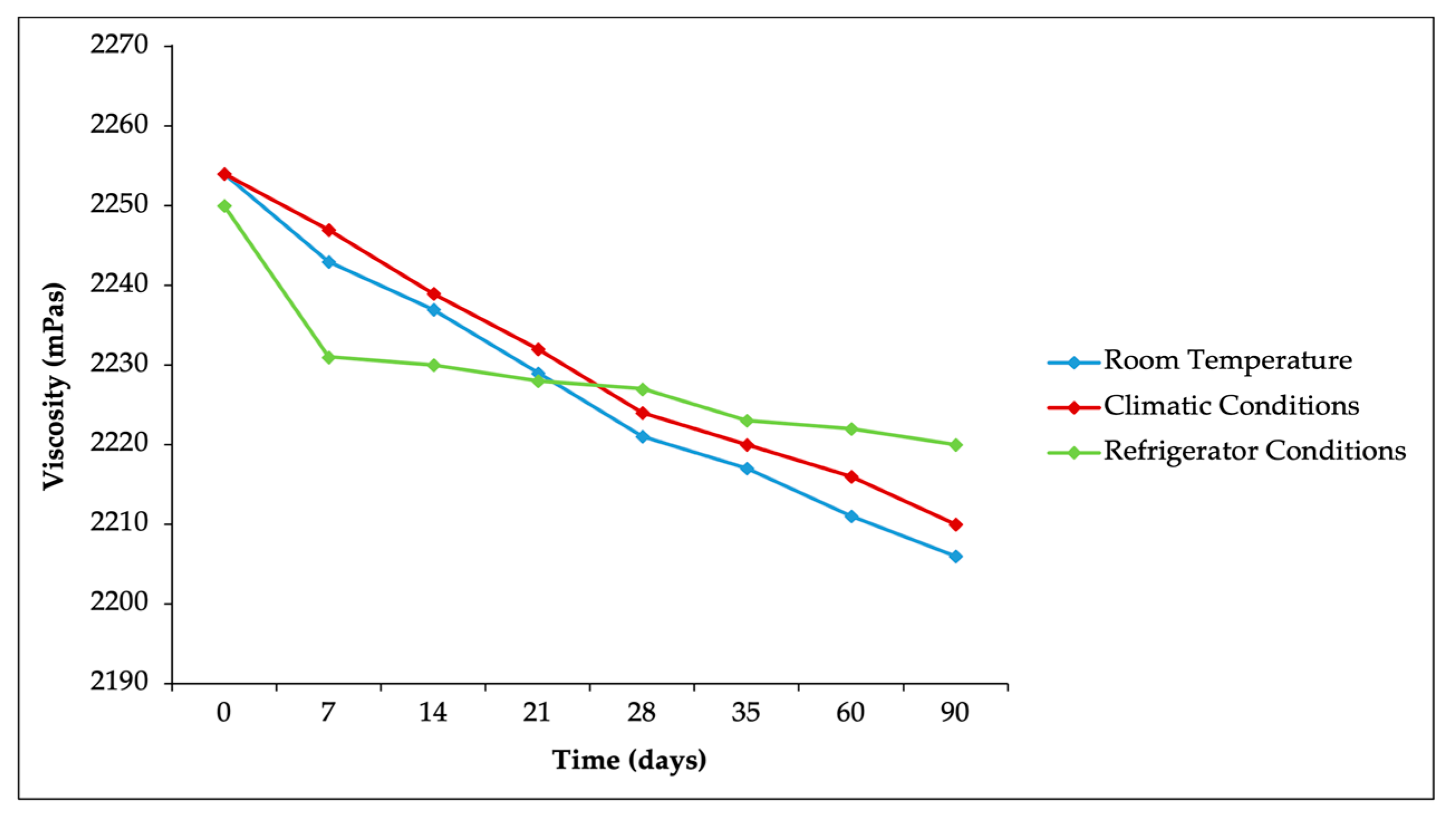
Viscosity measurements were conducted to observe changes in consistency during the storage of the formulation. The viscosity measurements are presented in
Figure 4. Based on
Figure 4, a decrease in the viscosity of the formulation was observed during storage at room temperature (25 ± 2 °C/75 ± 5% RH), climatic chamber conditions (40 ± 2 °C/75 ± 5% RH), and refrigerator conditions (5 ± 3 °C) (
p > 0.05). This reduction in viscosity could be attributed to increased water absorption due to higher temperatures and humidity, making the formulation more reactive to air. Despite this decrease, the viscosity values remained within the required standard range of 800 to 2000 mPas [
44]. In particular, there were no significant differences in viscosity across the three storage conditions, suggesting that the formulation maintained its consistency regardless of the temperature environment (
p > 0.05).
In this study, microbial and heavy metal contamination were investigated in AZ-NE radiance serum. Measurements of microbial contamination included total plate count, yeast and mold count,
Staphylococcus aureus,
Pseudomonas aeruginosa, and
Candida albicans. The objective of these tests was to ensure that the cosmetic serums produced during the formulation processes met the contamination limits specified for cosmetic products. Microbial contamination poses health risks due to the presence of pathogenic bacteria. The results of microbial contamination testing are presented in
Table 9, demonstrating compliance with the requirements. Furthermore, the results of heavy metal contamination testing are found in
Table 10. The tests showed no presence of heavy metals such as arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg). Heavy metal contamination refers to pollution by metallic and metalloid elements with high atomic and specific weights, which are toxic to living organisms. Evaluating microbial and heavy metal contamination is a critical step in ensuring the safety of cosmetic products [
32,
33].
Numerous animal models have been employed as alternatives to human skin for evaluating percutaneous permeation of substances, including pigs, rats, guinea pigs, and snakes. The use of shed snakeskin as a barrier membrane in in vitro permeation studies was first proposed by Higuchi and Kans. Shed snakeskin is non-living tissue that can be harvested without harming the animal, lacks hair follicles, and presents less variability compared to human or animal skin. Various permeation studies have utilized shed skins from different snake species, including
Elaphe obsoleta,
Python reticulatus, and
Ophiophagus hannah. Haigh et al. (1998) examined the effects of species, anatomical sites, and body regions of the shed snakeskin on permeability measurements and their correlation with human skin performance. They reported a significant correlation with human skin, supporting the potential use of shed snakeskin as a model membrane for permeation studies despite anatomical and chemical differences [
45,
46,
47,
48].
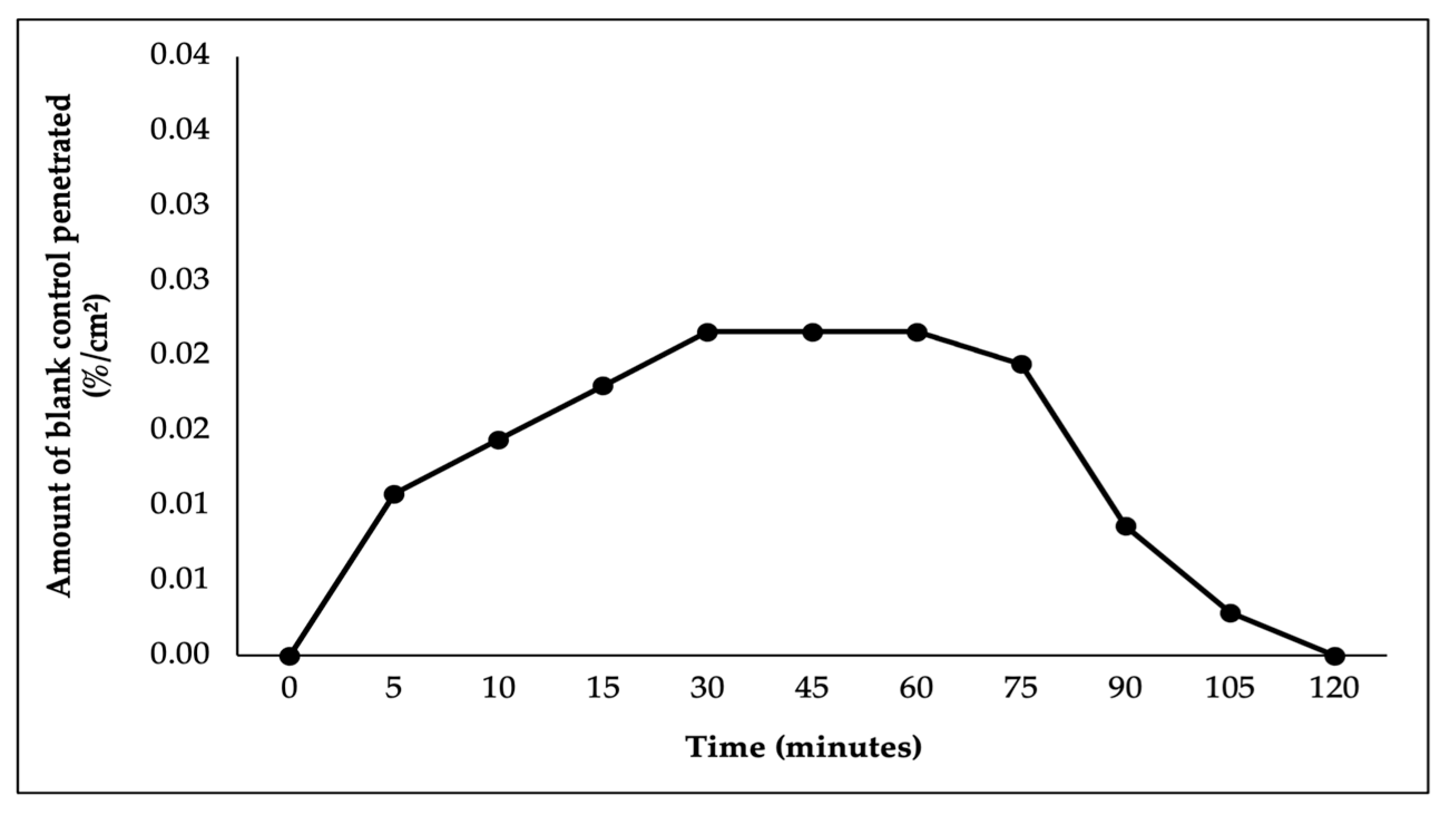
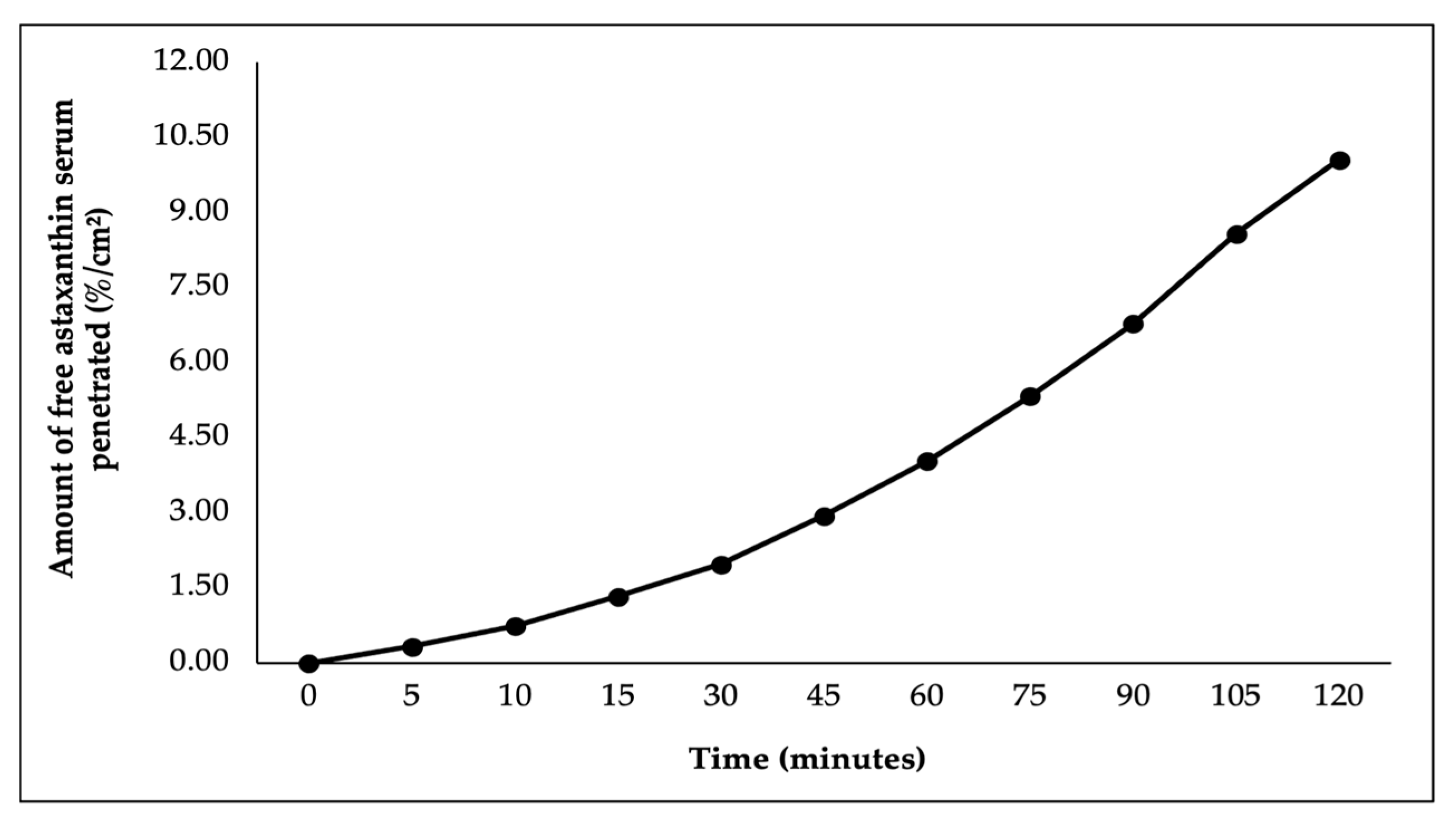
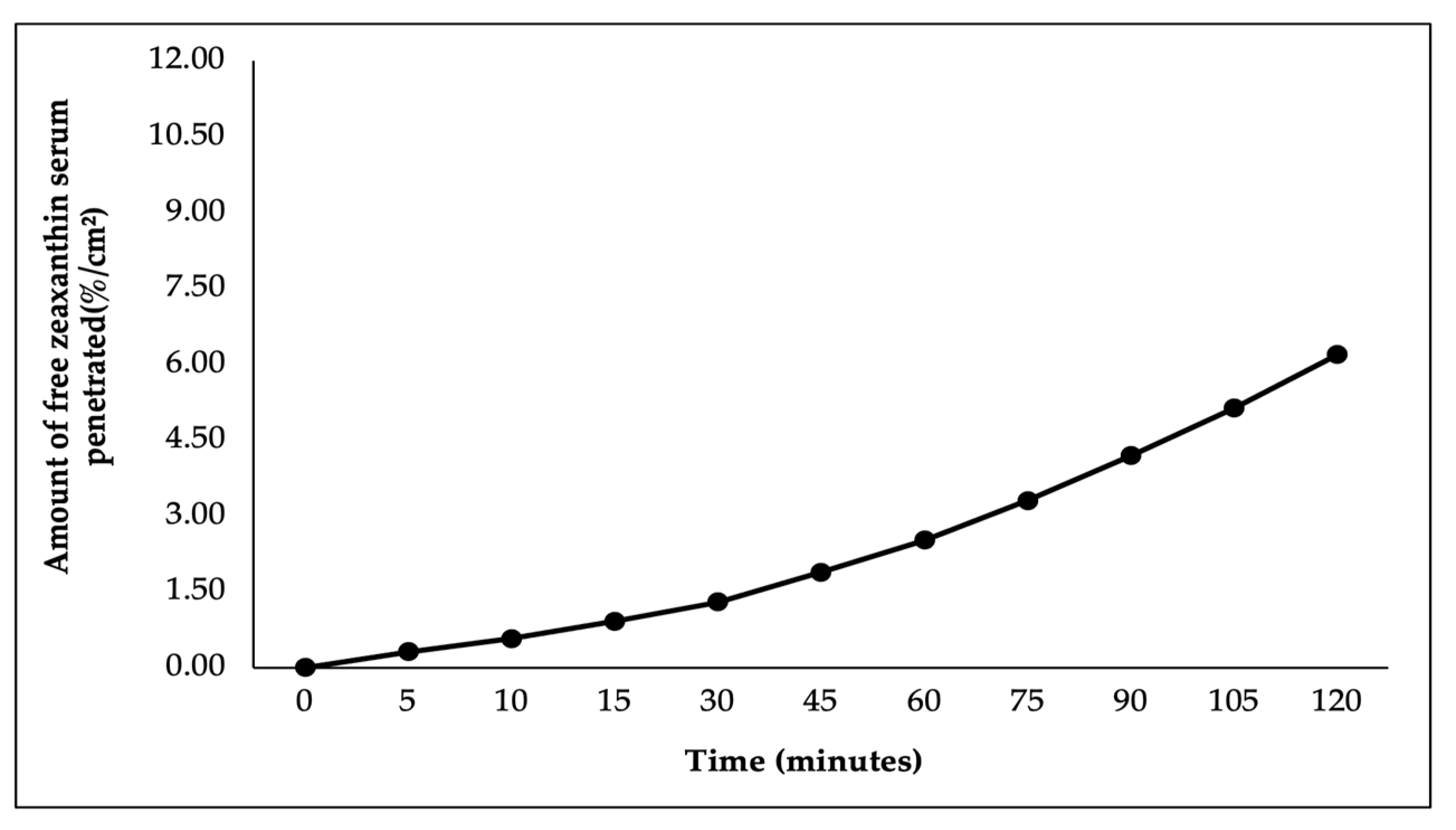
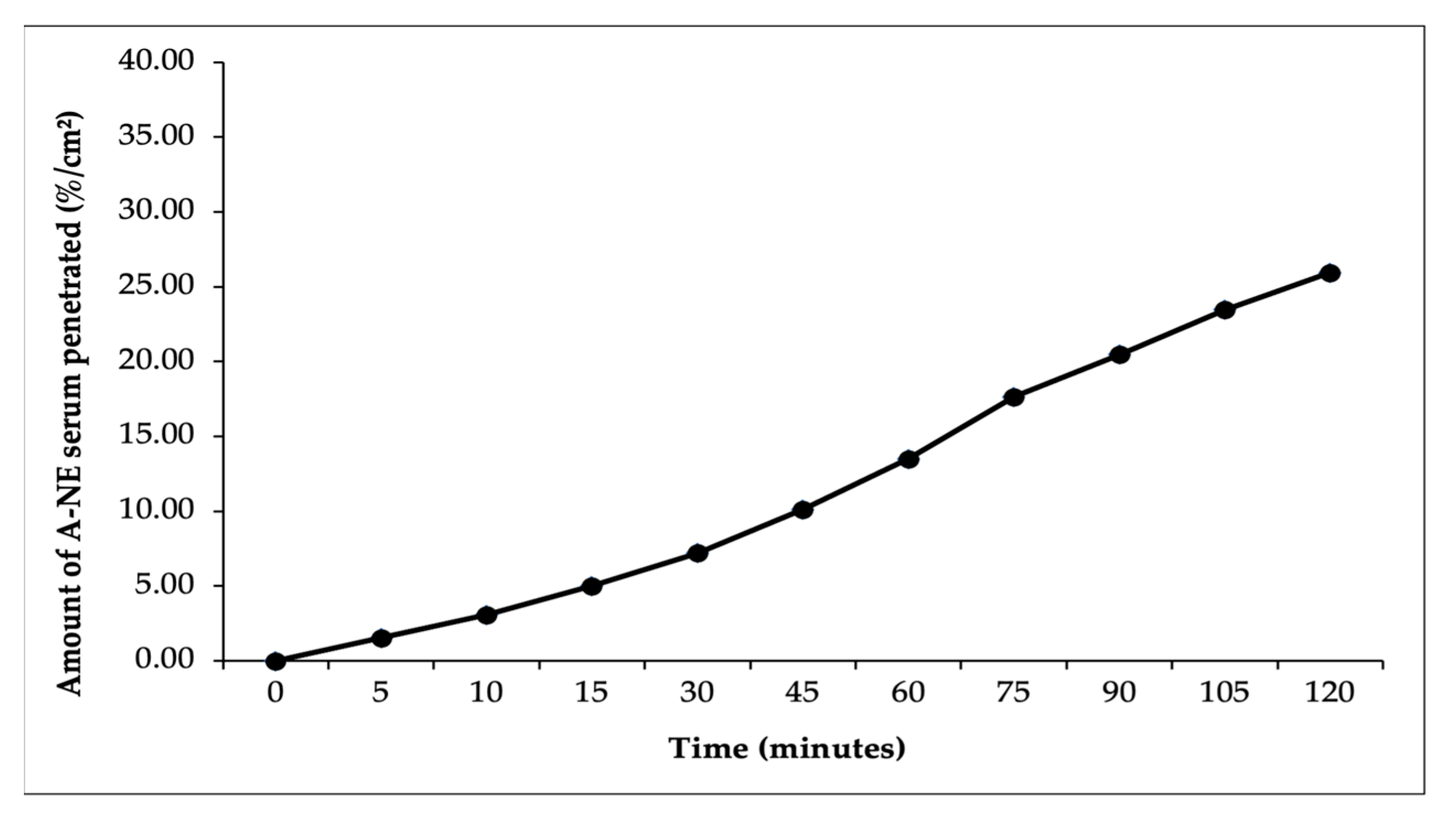
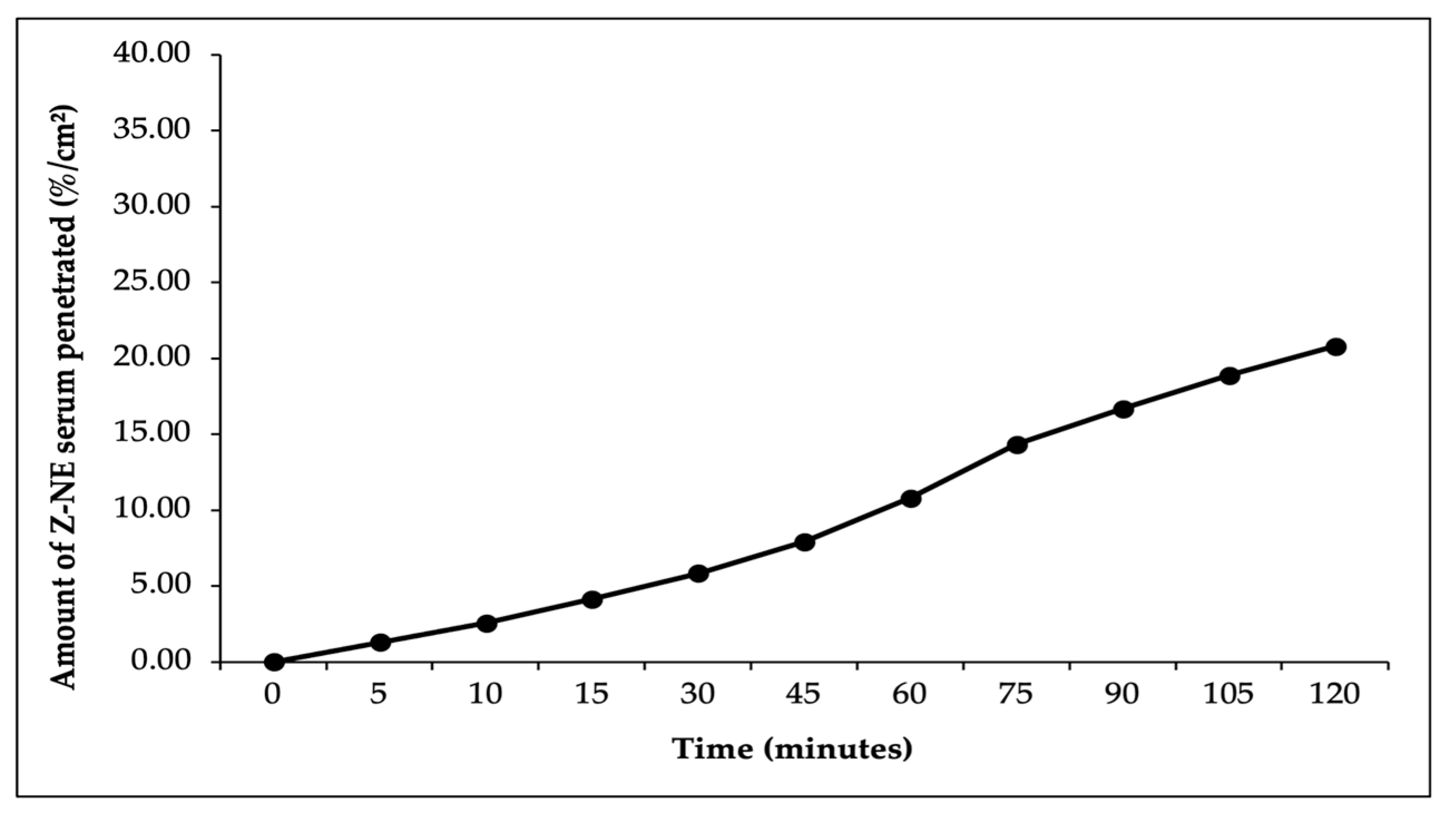
In this study, ex vivo penetration testing was conducted to simulate and measure the ability of astaxanthin–zeaxanthin nanoemulsions (AZ-NE) in serum to penetrate skin layers using a controlled model system with shed skin from
Python reticulatus. The penetration test results, presented in
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9, indicated that AZ-NE from the serum gradually diffused into the phosphate buffer medium at pH 7.4. After 120 min, the penetration of astaxanthin was measured at 25.95%/cm
2, and zeaxanthin at 20.80%/cm
2. Over the 120 min period, both astaxanthin and zeaxanthin showed a steady increase in penetration, with astaxanthin reaching 1.53%/cm
2 at 5 min and gradually rising to 25.95%/cm
2, while zeaxanthin started at 1.27%/cm
2 and increased to 20.79%/cm
2. In contrast, free astaxanthin demonstrated a penetration rate of 10.06%, and free zeaxanthin exhibited a penetration rate of 6.19%, both of which were significantly lower than their nanoemulsified counterparts. The comparison between free forms and nanoemulsified forms indicates that the nanoemulsion formulation of astaxanthin and zeaxanthin (AZ-NE) significantly enhances the penetration and diffusion of active ingredients through the skin layers compared to their free forms. These results suggest that nanoemulsions not only improve the solubility and stability of active ingredients but also enhance their bioavailability, making them more effective for dermatological applications aimed at improving skin health and combating oxidative stress. Therefore, the use of nanoemulsion technology is a promising approach for developing more effective formulations in skincare products [
49,
50,
51].
The human irritation study aimed to evaluate the potential irritation caused by the AZ-NE radiance serum on human skin. Assessing skin irritation is crucial for determining the safety of cosmetic products prior to their broader application among the human population. This study included 20 women (mean age 38 years) with varying skin types, such as dry, normal, oily, and combination skin. The AZ-NE radiance serum was applied to the subjects’ backs, and signs of irritation, including erythema, oedema, or a burning sensation, were monitored. Observations were recorded over a 48-h period at 0, 24, and 48 h post-application [
34]. The results of this irritation study on human subjects are presented in
Table 11. The study findings indicated that the AZ-NE radiance serum did not induce irritation on human skin. This conclusion was supported by observations made during the application period, revealing no erythema, oedema, or other signs of skin irritation among the study subjects, with a Primary Irritation Index (PII) value of 0. This study demonstrated the safety of using the AZ-NE radiance serum.
The efficacy of an anti-wrinkle product was evaluated through a clinical study involving human subjects. This study aimed to determine the product’s ability to reduce the appearance of wrinkles and improve skin texture [
17,
52,
53]. The objective of this anti-wrinkle efficacy study was to evaluate the performance of the AZ-NE radiance serum in reducing signs of aging in humans. The testing focused on reducing wrinkle levels in 15 women (mean age 42 years) who regularly used the serum for 28 days. The results of this study are presented in
Table 12. The reduction in the number of wrinkles from day 0 to day 28 showed positive results. A significant decrease in wrinkles was experienced by all subjects, with a percentage reduction ranging from 80% to 93% and an average reduction of 84%. The most substantial reduction in wrinkles was observed during the first week of use, with more than a 50% reduction experienced by some subjects. The number of wrinkles on day 0 ranged from 19% to 43%, while on day 28, it decreased to between 2% and 8%. Significant wrinkle reduction was observed in both younger and older subjects, proving the effectiveness of this serum across various age ranges.
In this study, the mechanism of astaxanthin–zeaxanthin nanoemulsions as anti-wrinkle agents was also investigated. These nanoemulsions enhance the delivery of carotenoids, providing potent antioxidant protection against free radicals, which damage collagen and elastin. Furthermore, by improving hydration and reducing moisture loss, the nanoemulsions help maintain skin structure, firmness, and elasticity, thereby reducing the appearance of wrinkles and enhancing overall skin elasticity [
52,
53,
54]. These results support the claim that the radiance serum is effective in reducing wrinkles and improving skin condition. The serum was deemed safe for use on various skin types and ages, offering hope for those seeking an effective anti-wrinkle solution.