Abstract
Green soybean (Glycine max L.) seed contains a high procyanidin content and high antioxidant activity. Moreover, ultrasound-assisted extraction (UAE) has proved to be advantageous in providing high extraction efficiency. Hence, this study aimed to extract procyanidins from green soybean seeds (GSSs) using UAE. This study also evaluated the inhibitory activities of tyrosinase and the cytotoxic effects of crude procyanidin extract. The extract exhibited maximum levels of bioactive components and antioxidant capacity when subjected to a temperature of 15 °C and an extraction time of 20 min. The crude procyanidin extract at a concentration of 10 mg/mL inhibited the tyrosinase enzyme by more than 60%, and the half-maximal inhibitory concentration (IC50) value obtained for the extract was 6.85 ± 0.81 mg/mL. This result was much greater than the IC50 value obtained for kojic acid (0.089 ± 0.08 mg/mL), which was used as a positive control. For the cytotoxicity assessment, the results indicated that the crude procyanidin extract showed no cytotoxicity and actually stimulated the growth of human skin fibroblast cells. More than 80% of the bioactive compounds (total phenolic content (TPC), total flavonoid content (TFC), procyanidin content (PC)) and antioxidant activities (DPPH and FRAP) of the crude extract powder were retained at 38.68 ± 0.01 mg GAE/g, 16.07 ± 0.01 mg CAE/g, 9.24 ± 0.01 mg PC/g, 359.8 ± 0.72 μM Trolox eq/g, and 1640 ± 2.86 μM Trolox eq/g, respectively, after 12 weeks of storage at 25 °C. The crude procyanidin extract powder was then included in a facial serum formulation and tested for pH value and physical evaluation. The stability of the crude procyanidin extract facial serum was shown to be greater for bioactive compounds and antioxidant activity when stored at a temperature of 4 °C than when stored at a temperature of 25 °C. These results suggest that the GSS extracts obtained via ultrasonication show promise for use in cosmeceutical formulations for whitening skincare products.
Keywords:
procyanidin; ultrasonic extraction; tyrosinase; cell toxicity; green soybean; facial serum 1. Introduction
Green soybean (Glycine max L.) is a legume and an excellent source of both nutritional and non-nutrient components that are useful to the human body. Generally, the strong antioxidant capacity of GSSs is due to their high phenolic content [1]. Peiretti et al. [2] also reported that black and green soybeans exhibit higher ferric-reducing antioxidant power (FRAP) than yellow soybeans. Hence, green soybean has been used as an alternative supplement source for bioactive compounds in food products such as butter cake [3] and cookies [4]. The most abundant phytochemicals in GSS, as reported in our previous research, are procyanidins (contents higher than 3 mg/100 g) [5]. According to our previous published paper of Khonchaisri et al. [1], the highest procyanidin content of 21.4 ± 0.37 mg/g was attained via ultrasound-assisted extraction and spray-dried green soybean extract under optimum conditions.
The ultrasonic approach is an established environmentally friendly bio-refining technology, primarily because it may decrease costs, shorten operation time, consume less energy, and yield higher quantities. Furthermore, ultrasonic technology is frequently employed in the food industry due to its efficacy in expediting chemical reactions through cavitation [6]. The application of ultrasonic waves has the potential to induce alterations in the internal structure of food matrices [7]. Ultrasonic waves cause the formation of cavitation bubbles through a series of compression and rarefaction cycles when they pass through solids, liquids, or gaseous media. These bubbles may enlarge in size due to coalescence and collapse during the compression phase, creating a hot spot. The collapsing cavitation bubbles and sound waves result in fragmentation, pore formation, and shearing in the cellular matrix of plant material, leading to the solubilization of the bioactive components into the extraction liquid [8]. Sun et al. [9] optimized the conditions for the UAE of procyanidins from grape seed. The extraction yield tended to increase significantly between 0.64 and 2.53 during 0 to 30 min of ultrasonication (p < 0.05). Therefore, this procedure enables the liberation of biologically active substances from plant structures following the destruction of cells [10].
Procyanidins belong to the class of flavonoids known as proanthocyanidins, also referred to as condensed tannins. Procyanidins represent a significant class of bioactive compounds that have garnered attention for their potential therapeutic applications in the treatment of chronic metabolic disorders, including cancer, diabetes, and cardiovascular disease. These compounds exhibit promising properties by mitigating cellular harm associated with oxidative stress [11]. Furthermore, procyanidin exhibits inhibitory effects on the human tyrosinase enzyme, demonstrating potent inhibition of both monophenolase and diphenolase enzymes. The biosynthesis of melanin occurs through a series of oxidative reactions involving the amino acid tyrosine in the presence of the enzyme tyrosinase. Hence, the overactivity of this enzyme leads to dermatological disorders such as age spots, melanoma, and sites of actinic damage [12]. Kojic acid and procyanidins, including procyanidin B2, competitively inhibit tyrosinase as they are able to bind to the active site of the tyrosinase enzyme via hydrogen and electrovalent bonds [13,14]. Procyanidin B2 has been found to be highly accumulated in the seed coat of soybean [15], pea [16], lentil [17], and green soybean [5].
Tyrosinase (Enzyme Commission number (EC) 1.14.18.1) plays a crucial role in the synthesis of melanin in the skin. Melanin is a pigment that serves a crucial function in protecting the skin from ultraviolet radiation (UV) damage and acts as a vital defensive mechanism against dangerous elements [18]. The generation of reactive oxygen species (ROS) under UV radiation is one mechanism through which UV light can manifest its possible detrimental effects on health. When an imbalance develops due to ROS generation exceeding the body’s antioxidant defense mechanisms, oxidative stress can develop. Oxidative stress can lead to cellular damage (e.g., lipid peroxidation and deoxyribonucleic acid (DNA) fragmentation), apoptosis, and cell death [19]. Although melanin has some advantages, it is also implicated in the development of aberrant pigmentation and melanoma. Multiple skin conditions, such as age spots, freckles, and melisma, are characterized by the excessive synthesis and buildup of melanin [20].
The term “anti-tyrosinase activity” applies to the capacity of a compound to interfere with the functioning of tyrosinase [21]. Numerous natural substances have demonstrated an ability to inhibit tyrosinase. Various phenolic substances, such as simple phenols, polyphenols, flavonoids (including flavones, isoflavones, flavanones, flavanoles, dihydroflavones, and anthocyanidins), and tannins, have been identified as common inhibitors with tyrosinase inhibitory properties [13,22]. Plant extracts have found extensive applications in the cosmetics industry and serve various functions, including, but not limited to, their use as whiteners, moisturizers, sunscreens, anti-wrinkle products, coloring cosmetics, anti-acne products, preservatives, antioxidants, and thickeners. The utilization of natural plant extracts in skin-lightening products has gained significant popularity due to their efficacy, safety, and non-toxic nature [23]. The inclusion of antioxidants in anti-aging cosmetics has gained significant popularity owing to their ability to scavenge free radicals, thereby mitigating or preventing oxidative stress in the skin and potentially decelerating the process of skin aging [24].
Extract from French maritime pine (Pinus pinaster Ait.) bark contains a rather high procyanidin content of 70%. This procyanidin extract showed effects on human skin by improving skin conditions and skin inflammation. Moreover, this extract has attracted special attention in the field of dermatology regarding its application in cosmetic formulations. Some studies found evidence that skin products supplemented with P. pinaster extract benefit human skin by increasing skin hydration and skin elasticity; they also showed that these effects were most likely due to the increased synthesis of extracellular matrix molecules such as hyaluronic acid and possibly collagen [25,26]. Therefore, procyanidins might be a good resource for further development as antioxidants and anti-tyrosinase agents in the pharmaceutical, cosmetic, food, and agricultural industries [27]. According to Leksawasdi et al. [5], procyanidins represent a subclass of flavonoids that are present in frequently consumed food products, including fruits, vegetables, legumes, grains, and nuts. These compounds are receiving growing interest due to their possible positive effects on human health.
The main purpose of this research was to assess the anti-tyrosinase activity of crude procyanidin extract from GSS by utilizing UAE under optimal conditions. The in vitro cytotoxic effects of the extract were assessed on human cell lines to ensure the safety of the natural product, and the storage stability of the crude procyanidin extract powder was determined. A crude procyanidin extract facial serum was formulated to assess its stability. Here, the bioactive components of green soybean seed (Glycine max L.) extract were examined to determine their potential as a natural source of bioactive agents for skincare products.
2. Materials and Methods
2.1. Materials
Whole green soybeans (Glycine max L.) were received from By Love and Hope Company Limited, Bangkok, Thailand. The pods were removed from the seeds by hand. The sample was soaked in tap water for a duration of 1 min and oven-dried at a temperature of 60 °C for a period of 48 h in a hot-air oven (Memmert UF 110, Schwabach, Germany) until the moisture content decreased to less than 10% [1]. A commercial mill (HR2602, Philips, Ningbo, China) with a 40-mesh sieve was used to grind the dry seeds into a fine powder. The seed powder was enclosed in a vacuum-sealed aluminum foil bag and kept at 3–5 °C until it was ready for further examination. The analytical quality of all substances was assessed.
2.2. Ultrasound-Assisted Extraction of Procyanidins
The UAE technique was conducted in accordance with the methodology outlined by Leksawasdi et al. [5]. In brief, 10 g of GSS dried powder was mixed with 200 mL of distilled water in a 500 mL beaker. The UAE protocol was conducted by using a sonication power of 500 W, a frequency of 20 kHz, and an amplitude of 50%. The effects of different temperatures (15 and 25 °C) and durations (10, 15, and 20 min) of the ultrasonic probe system (Büchi, Straubenhardt, Germany) on the bioactive compound extraction process were evaluated. The mixtures were subjected to centrifugation at 5000 rpm for 15 min at 4 °C (Nüve NF400R, Ankara City, Turkey) [1]. The supernatant was separated using filter paper (Whatman No. 1, Wallingford, UK) and stored at 4 °C for the subsequent investigation of the total phenolic content (TPC), total flavonoid content (TFC), procyanidin content (PC), and antioxidant activity.
2.3. Determination of Total Phenolic Content
The TPC of the extracts was assessed using the Folin–Ciocâlteu test, following Abozed et al. [28], with minor adjustments to the mixture volume of sodium carbonate solution to eliminate turbidity. The experimental procedure involved diluting 0.25 mL of either the standard solution or extract sample with distilled water to a total volume of 12.5 mL in a 25 mL volumetric flask. Then, 1.25 mL of Folin–Ciocâlteu reagent and 5 mL of a 20% (w/v) aqueous sodium carbonate solution were introduced. The solution was then adjusted by adding distilled water until it reached the desired volume. The solution was agitated manually for a duration of 30 s and maintained at room temperature for a period of 30 min. A UV–Vis spectrophotometer (Agilent, Penang, Malaysia) was used to measure the absorbance of the solution at a wavelength of 765 nm, employing a quartz cuvette. The development of the standard curve involved the utilization of different concentrations of gallic acid in ethanol (10–100 μg/mL), and the outcomes are expressed in mg of gallic acid equivalent per g of dry weight of the sample (mg GAE/g). The equation representing the calibration curve equation was Y = 0.0006X + 0.0013, with a determination coefficient of R2 = 0.994.
2.4. Determination of Total Flavonoid Content
The colorimetric assay method was employed to measure the TFC [29]. However, in this research, the method was slightly modified by increasing the dilution ratio of the extract samples, where 0.1 mL of the extract sample was diluted to 1.25 mL with distilled water. Next, 0.15 mL of 10% (w/v) aluminum chloride and 0.075 mL of 5% (w/v) sodium nitrate were also introduced. After a duration of 6 min, the mixture was supplemented with 0.5 mL of 4% (w/v) sodium hydroxide solution. Subsequently, the absorbance was measured using a UV–Vis spectrophotometer (Agilent, Penang, Malaysia) operated at a specific wavelength of 510 nm. Catechin was utilized in different concentrations of 10–100 μg/mL to generate the standard curve, and the total flavonoid content was quantified in mg of catechin equivalents per g of dry weight of the sample (mg CAE/g). The equation representing the calibration curve equation was Y = 0.111X + 0.0452, with the determination coefficient being R2 = 0.991.
2.5. Quantification of Procyanidin Content via HPLC
The procyanidin extract from the UAE extraction process described above was analyzed via high-performance liquid chromatography (HPLC). The quantification was carried out as previously reported by Zhou et al. [30], with some modifications. Briefly, an Agilent HPLC system (Agilent Technologies, Santa Clara, CA, USA), consisting of a binary pump and a photodiode-array detector equipped with an Agilent Zorbax C18 (4.6 × 250 mm, 3.5 μm) column, was employed. The mobile phase consisted of solution A (0.1% v/v trifluoroacetic acid) and solution B (pure methanol), which were used to create gradients according to the following program: 0 min, 15% B; 5 min, 25% B; 9 min, 55% B; 12 min, 75% B; 15 min, 75% B; 18 min, 15% B. The flow rate was 0.8 mL/min, and the injection volume was 5.0 μL. The detection wavelength was set at 260 nm. The reference standard (procyanidin B2) was weighed exactly into appropriate volumetric flasks and dissolved in a hydrolysis solution of acetone/acetic acid/distilled water (140:1:59 volume ratio) to obtain a standard solution with 100 μg procyanidin B2/mL. Subsequently, this standard solution was diluted with the hydrolysis solution to obtain an additional standard solution with 10 μg procyanidin B2/mL. The contents of procyanidin in the extract were determined according to the calibration curve Y = 0.66915X (R2 = 0.998). The results are expressed as mg of procyanidins per g of seed extract (mg PC/g).
2.6. Antioxidant Analysis
2.6.1. DPPH Radical Scavenging Capacity Assay
The seed extract was tested for its ability to scavenge free radicals against 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radicals, following a method described by Baliyan et al. [31]. Briefly, a mixture was prepared by mixing 0.15 mL of extract solution with 3 mL of a DPPH solution (0.06 mM) in methanol. Subsequently, the reaction mixture was subjected to a 30 min period of room temperature and darkness. The absorbance of each extract was determined at a wavelength of 517 nm using a UV–Vis spectrophotometer (Agilent, Penang, Malaysia). The calibration curve was established using Trolox, and the measurements are expressed as μmol of Trolox equivalents per g of dry weight of the sample (μM Trolox/g). The calibration curve equation was Y = 0.0386X − 0.0059, for which the determination coefficient was determined to be R2 = 0.996. In this equation, Y represents light absorbance, and X represents the compound concentration.
2.6.2. Ferric-Reducing Antioxidant Potential (FRAP) Assay
The FRAP assay was conducted following the methodology outlined by Fernandes et al. [32]. The ferric ion (Fe3+) can be reduced to the ferrous ion (Fe2+) by a potential antioxidant, resulting in the formation of a blue complex (Fe2+/2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ)). The FRAP reagent comprised a mixture of acetate buffer (300 mM, pH 3.6), a solution of 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3 in a volumetric ratio of 10:1:1. A thorough mixture was prepared by mixing the reagent (2.85 mL) with the sample solution (0.15 mL). The absorbance was measured at a wavelength of 593 nm using a UV–Vis spectrophotometer (Agilent, Penang, Malaysia) following a 30 min period in a dark environment at ambient temperature. A calibration curve was constructed using Trolox, and the results are expressed as μmol of Trolox equivalents per g of dry weight of the sample (μM Trolox eq/g). The calibration curve equation can be expressed as Y = 0.0061X + 0.0094, with an R2 value of 0.996. In this equation, Y represents the light absorbance, and X represents the compound concentration.
2.7. Determination of Tyrosinase Inhibition Activity
The crude procyanidin extract previously obtained under optimal UAE conditions was investigated for its tyrosinase inhibition activity. The samples were evenly distributed to a thickness of approximately 1 cm on an aluminum tray and stored at a temperature of −20 °C in a deep freezer for 24 h. The samples were subjected to lyophilization using a freeze dryer (Labconco, KS, USA) for a duration of 42 h, maintaining a consistent weight. During the drying process, the pressure in the vacuum chamber and the temperature of the condenser were maintained at 0.133 mbar and −40 °C, respectively. Once the samples were dried, they were placed into vacuum-sealed aluminum foil bags and stored in the refrigerator until they were required.
The modified dopachrome method was employed to ascertain the tyrosinase inhibition activity of the extract or procyanidin B2 standard, with tyrosine serving as the substrate, according to Masuda et al. [33]. Each well of a 96-well plate contained 50 μL of eight serial concentrations of the extracts (ranging from 0.0625 to 10 mg/mL) dissolved in ethanol, 50 μL of 100-unit mushroom tyrosinase solution in 0.1 M phosphate buffer, 50 μL of 1 mg/mL tyrosine solution in 0.1 M phosphate buffer, and 50 μL of 0.1 M phosphate buffer. The sample was maintained at a temperature of 37 ± 2 °C for 20 min. The absorbance at a wavelength of 490 nm was subsequently measured using a microplate reader (BioTek 800TS, Agilent, Winooski, VT, USA). The absorbance was quantified prior to and subsequent to the incubation period. Kojic acid within a concentration range of 0.001–10 mg/mL was employed as a positive control. An extract-free solution served as a negative control. The percentage of tyrosinase inhibition was calculated using the following equation:
where A was the absorbance of the control with the enzyme, B was the absorbance of the control without the enzyme, C was the absorbance of the test sample with the enzyme, and D was the absorbance of the test sample without the enzyme. The half-maximal inhibitory concentration (IC50) value, which represents the concentration at which 50% inhibition is achieved, was determined by analyzing the relationship between the percentage of tyrosinase inhibitory activity and the concentration of the sample.
2.8. Cytotoxicity in Human Skin Fibroblasts
A human skin fibroblast (BJ) cell line was grown in a 5% CO2 incubator at 37 °C using Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cytotoxic effect of crude procyanidin extract on the BJ cell line was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, as previously published, with minor adjustments [34]. Following Ayazoglu Demir et al. [35], BJ cells were cultured at a density of 1 × 105 cells per well. Subsequently, the cells were treated with various concentrations of the extract (0, 0.125, 0.25, 0.5, 1, and 2 mg/mL) within a 5% CO2 incubator at 37 °C for 24 h. After the designated time period, the contents of the plate were removed, and 15 μL of MTT dye (5.0 mg/mL) in phosphate buffer (pH 7.4) was introduced into the wells. Subsequently, the cells were incubated with this dye in a 5% CO2 incubator for 4 h at 37 °C, as described by Turan et al. [36]. After the incubation period, the formazan crystals were dissolved in 200 μL of dimethyl sulfoxide (DMSO). The absorbance was then measured at 570 nm using a microplate reader (Accu Reader M965 Mate, Bangkok, Thailand). A negative control was employed using untreated cells. All experiments were performed in triplicate. The following equation was used to calculate the cell viability (%):
2.9. Storage Stability Test on Crude Procyanidin Extract Powder
A freeze-drying process was employed to prepare the crude procyanidin extract powder, as described in Section 2.7. The extract powder was carefully placed into aluminum foil bags that were subsequently sealed using a vacuum. The bags were then stored at controlled temperatures of 25, 35, and 45 °C. The stability of the extract powder was evaluated at regular weekly intervals for a duration of 12 weeks (weeks 0, 2, 4, 8, and 12). This assessment involved measuring many parameters, including the moisture content, water activity, color, TPC, TFC, procyanidins, and antioxidant activity. All experiments were performed in triplicate. The optimal storage conditions for crude procyanidin extract powder were evaluated based on the retention of up to 80% of the bioactive compounds and antioxidants at the end of storage (12 weeks).
2.10. Moisture Content and Water Activity
The moisture content of the samples was determined by submitting them to a drying process in an air oven (FED 53, Binder, Germany) at 110 °C for 10 h, following the instructions established in the AOAC method [37]. A water activity meter (Aqua Lab, Queensland, Australia) was utilized to measure the water activity at a temperature of 25 °C.
2.11. Formulation of Facial Serum Containing Crude Procyanidin Extract
A formulation for 40 mL of facial serum was developed with crude procyanidin extract using several compositions through a cool process, according to Marlina et al. [38]. Deionized water, ascorbic acid, ferulic acid, L-arginine, sodium hyaluronate, glycerine, polysorbate 80, glyceryl undecylenate, glyceryl caprylate, and 5% crude procyanidin extract powder were mixed (Table 1). At concentrations of 5%, ascorbic acid was used as an antioxidant agent in the formulation to retard the degradation of procyanidins, which, in turn, could retard the degradation of certain active ingredients [39]. This concentration level was slightly modified by Prieto [40], who mentioned that the optimal concentration should always be greater than 8% and less than 20%; higher concentrations were irritating, failing to offer an increase in their activity, and may be formulated as associated with other active ingredients.

Table 1.
Ingredients of the facial serum product formulation.
The formulations of facial serum were subjected to various evaluation tests. The pH of the serum was determined by a pH meter that had been calibrated. The pH of the combination was determined by accurately measuring and blending almost 1 mL of the face serum with 10 mL of clean water. The viscosity of the serum formulation was determined by using a Brookfield viscometer at 100 rpm and a spindle-type model DV-II+. After dipping the spindle in 5 mL of the serum in a beaker for approximately 5 min, readings were obtained [41]. Two grams of the serum were placed on the surface. A slide was attached to a pan to which 20 g weight was added. The time (s) required to separate the upper slide from the surface was taken as a measure of spreadability. Visual observation was made of the formulation’s color, homogeneity, and odor based on their appearance [42].
The physicochemical properties, including pH and viscosity, were observed at two different controlled temperatures (4 and 25 °C) in a refrigerator or incubator for 6 weeks. Moreover, stability of bioactive compounds and antioxidant activity studies were also performed at two different storage temperatures. The physicochemical properties, TPC, TFC, procyanidins, and antioxidant activity of the finished product were measured in three replicates. The facial serum products that retained up to 70% of their bioactive compounds and antioxidant activity at the end of the storage period (6 weeks) were considered for the selection of optimal storage conditions.
2.12. Statistical Analysis
All data are expressed as the mean ± standard deviation (SD). An analysis of variance (ANOVA) was conducted on the data, followed by Tukey’s post hoc test for multiple comparisons using Statgraphics 5.1 Software. Statistical significance was determined when the p-value was less than 0.05.
3. Results and Discussion
3.1. Effects of Extraction Conditions on Bioactive Compounds and Antioxidant Activity from GSS
The results of UAE, presented in Table 2, demonstrate that the GSS extract exhibited the greatest amounts of bioactive compounds and antioxidant capacity when subjected to a temperature of 15 °C and an extraction time of 20 min. This study provides evidence that the extraction temperature plays a significant role in the outcomes of UAE. The TPC, TFC, and procyanidin content exhibited a small increase when the extraction temperature was increased from 15 to 25 °C for both the 10 min and 15 min extraction durations. According to Biswas et al. [43], the acceleration due to heat throughout the procedure results in an increased diffusion coefficient for the compounds under investigation, improving their solubility. Ilmu et al. [44] found that increasing temperatures lead to a weakening of the cell wall network of solution particles, which accelerates the extraction process and facilitates the transfer of solvent to solids.

Table 2.
Effects of extraction temperature and time on bioactive compounds and antioxidant capacity of GSS extract.
However, the production of these bioactive compounds declined with a longer extraction time of 20 min at a high extraction temperature of 25 °C. This phenomenon could plausibly be attributed to the alteration of the structures of flavonoids and procyanidins under elevated temperatures and extended extraction durations. The main degradation reaction includes the oxidation of phenols, flavonoids, and glycosides via thermal treatment [45]. Haslina et al. [46] emphasized the significance of considering the temperature during extraction. Extractions conducted at excessively high temperatures or for prolonged durations may result in oxidation, leading to the loss of compounds in the solution. Two variables that could potentially have an impact are the temperature and the duration of the extraction process. If the extraction temperature is too low and the extraction duration is too short, the bioactive components extracted from the material will not be optimal, leading to a low yield of bioactive components [46]. In this research, it was found that a higher extraction of bioactive compounds was achieved at lower temperatures and prolonged the extraction times. Similar trends were observed in the DPPH and FRAP values, where a temperature of 15 °C and an extraction time of 20 min resulted in the highest (p < 0.05) antioxidant capacity (447 ± 1.00 and 1812 ± 4.50 μM Trolox eq/g, respectively) for the GSS extract. The antioxidant activity of the GSS extract is related to its chemical composition and is primarily attributed to its richness in terms of its TPC, TFC, and procyanidin content. These results are in accordance with those from Zhang et al. [47], who observed that the procyanidin B1 contents of macadamia green peel extract obtained via UAE showed a significant positive correlation with DPPH antioxidant capacity and FRAP.
The findings of this study underscore the importance of optimizing both extraction temperature and duration for maximizing the yield of bioactive compounds and antioxidant capacity in GSS extracts. An extraction temperature of 15 °C for a duration of 20 min was determined to be the most favorable. The most suitable conditions in this study agree with those reported by Lv et al. [48], who used ultrasound (20 kHz, 50% amplitude, 30 °C) for the extraction of proanthocyanidin from kiwifruit leaves. The yield of proanthocyanidin was increased significantly (p < 0.05) with a sonication time of up to 20 min (107.51 ± 4.28 mg PC/g) compared to the control (55.82 ± 4.32 mg PC/g). Then, a slight decrease was observed over longer durations (25 and 30 min). This reinforces the notion that both temperature and extraction time must be carefully controlled to avoid the degradation of bioactive compounds at higher temperatures and prolonged extraction durations, as observed in the current research.
3.2. Tyrosinase Inhibition Activity
Tyrosinase is a crucial enzyme that facilitates the oxidation of tyrosine to produce melanin and other pigments. The present study aimed to assess the potential of crude procyanidin extract as a skin-whitening agent by examining its inhibitory effects on tyrosinase enzyme activity. The results regarding the tyrosinase inhibition activity of the crude procyanidin extract at different concentrations (0.0625–10 mg/mL) are presented in Figure 1. The percentage of tyrosinase inhibition rose in a dose-dependent manner as the concentration of the extract increased. At a dosage level of 10 mg/mL, the extract exhibited a tyrosinase enzyme inhibition rate above 60%. This was confirmed by the results for the procyanidin standard, which showed a similar trend to those for the procyanidin extract sample. The enzyme’s inhibitory impact can likely be attributed to the existence of secondary metabolites, including phenolic compounds and flavonoids. Polymerized forms of flavanols, a subclass of flavonoids known as procyanidins, are frequently encountered in plant-derived sources. These compounds possess the capacity to form chelates with metals such as iron, copper, and silver [49].
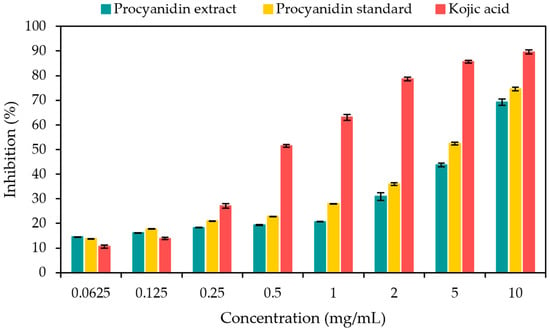
Figure 1.
The tyrosinase inhibition effects of aqueous crude procyanidin extract, procyanidin standard, and kojic acid. The data are shown as the mean ± standard deviation (SD) derived from three independent experiments (n = 3) (p < 0.05).
In addition, our previous study [5] quantified the phytochemical composition (procyanidins, quercetin, glycitein, daidzein, genistin, and linalool) of GSS using HPLC. The ranking order of phytochemicals in GSS was as follows: procyanidins (3.27 ± 0.01 mg/100 g), linalool (1.93 ± 0.03 mg/100 g), glycitein (1.13 ± 0.01 mg/100 g), quercetin (0.64 ±0.01 mg/100 g), daidzein (0.02 ± 0.00 mg/100 g), and genistein (0.01 ± 0.00 mg/100 g). Our previous report on the bioactive compounds found in GSS showed that procyanidins were present in the highest concentration; thus, they may play an important role in tyrosinase inhibition.
In this research, it was also observed that the extract exhibited a lower level of activity in comparison to the standard. The IC50 value of the crude procyanidin extract (6.85 ± 0.81 mg/mL) in the present study was higher than those of the positive control (kojic acid) (0.089 ± 0.08 mg/mL) and the procyanidin standard (4.78 ± 0.57 mg/mL), suggesting a poorer capacity for inhibiting tyrosinase. This is in line with the findings of Momtaz et al. [50], who observed that procyanidin B2 purified from the bark of Sideroxylon inerme L. (stem bark) inhibited tyrosinase activity by 54% at a concentration of 200 μg/mL. Procyanidin B2 obtained from the stem bark of S. inerme demonstrated lower anti-tyrosinase activity when compared to kojic acid (positive control).
Furthermore, the findings of Bi et al. [51] indicated that flavonoids with a C-3′ hydroxyl group in the B ring, a C-3 hydroxyl group, and a C-4 carbonyl group in the C ring have enhanced efficacy as tyrosinase inhibitors. Nguyen et al. [52] found that the inclusion of methoxyl and hydroxyl groups in the structural framework of flavonoids significantly contributes to their tyrosinase inhibition activity. Zolghadri et al. [53] also found that the majority of phenolic acids and flavonoids, such as flavanoles, flavan-3,4-diols, and flavanones, could have the ability to effectively block the tyrosinase enzyme. The green soybean is recognized as a rich source of antioxidants, as evidenced by the results presented in Table 1. The crude procyanidin extract possesses distinctive antioxidant components that are beneficial in protecting skin macromolecules and fighting the reactive quinones produced during the degradation of 3,4-dihydroxy-L-phenylalanine (L-DOPA). Hence, the enhanced inhibitory ability against tyrosinase of crude procyanidin extract at concentrations greater than 1.0 mg/mL could translate to its potential as a skin-whitening agent with an inhibition level higher than 20%. The other antioxidants found in green soybeans are flavonols (quercetin and kaempferol) and isoflavones (daidzein and genistein) [54]. Flavonols possess inhibition ability against tyrosinase due to their similarity to the structure of kojic acid’s 3-hydroxy-4-keto moiety, while daidzein and genistein show no inhibitory effect on tyrosinase [55].
3.3. Cytotoxicity of Crude Procyanidin Extract
An MTT assay was conducted to evaluate the cytotoxicity of the crude procyanidin extract on cultured human skin fibroblasts treated with different concentrations ranging from 0.125 to 2 mg/mL. The percentage of viability was calculated by defining the absorption of cells without crude procyanidin extract treatment as 100%. The results are the average of three independent experiments. As shown in Figure 2, the crude procyanidin extract enhanced cellular viability at all concentration levels, with cell viability values higher than 108%. The application of a 2 mg/mL crude procyanidin extract for a duration of 48 h resulted in a relative cell viability exceeding 129% in comparison to the control group, which did not receive any treatment. Moreover, 48 h after exposure, the samples at all concentration levels showed a higher percentage of living cells (121.2%) compared to those at 24 and 72 h (111.9 and 117.4%, respectively).
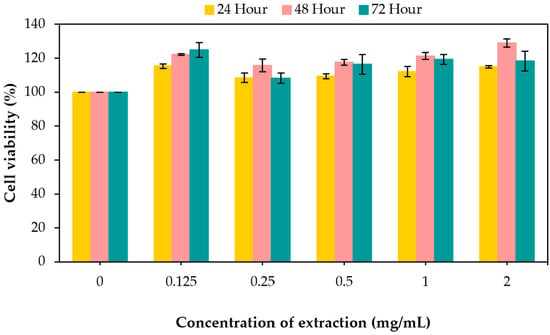
Figure 2.
Cytotoxicity of the crude procyanidin extract at various concentrations against human skin fibroblasts. BJ cells were treated with crude procyanidin extract at a final concentration ranging from 0.125 to 2 mg/mL for 24, 48, and 72 h. Cell viability was determined via the MTT assay. The percentage of cell survival was calculated by defining the absorption of cells without crude procyanidin extract treatment as 100%. The data are expressed as the mean ± SD.
This is similar to the results of an experiment by Kim et al. [56], in which oligomeric procyanidins from grape (Vitis vinifera) seed extracts stimulated the proliferation of human fibroblast cells in a dose- (0–20 ug/mL) and time-dependent (24, 48, or 72 h) manner. Oligomeric procyanidins are well known for their antioxidant, anti-bacterial, and anti-viral properties, and recent studies suggest that oligomeric procyanidins can also regulate the wound healing process. Mortensen et al. [57] reported that oligomeric procyanidins inhibit cell migration and modulate the proliferation of human umbilical vascular endothelial cells by inducing significant gene expression changes. They also reported that oligomeric procyanidins have a regulatory effect on the proliferation and migration of endothelial cells [58].
An increase in cell viability was observed with all treatments. It has been described that complex procyanidin has a rather high polymerization degree. Hence, it is expected that these compounds do not pass the plasmatic membrane and could protect cell membranes against lipid peroxidation, a well-known mechanism that causes severe membrane damage and potential cell death [59]. Therefore, treatments with procyanidin displayed higher cell viability than the control sample.
This is also in accordance with results from Shruti Taparia and Aparna Khanna [60], in which cocoa procyanidin-rich extract treatment was found to be nontoxic to normal human dermal fibroblasts. On the contrary, the extract caused an increase in the viability of human dermal fibroblasts by virtue of the antioxidant properties of its polyphenolic constituents. Treatment with cocoa procyanidin-rich extract demonstrated selective cytotoxicity of the extract toward cancer cells, leaving normal cells unharmed. Thus, these results suggest that the crude procyanidin extract does not exhibit cytotoxicity; rather, it promotes the growth of human skin cells. The tyrosinase inhibitory activities of the crude procyanidin extract at 2.0 mg/mL suggest that it could be used as a 5% (w/v) ingredient in a 40 mL facial serum product. At this concentration, tyrosinase activity is inhibited by more than 30%, and the extract is non-toxic to human cells.
3.4. Effects of Time and Temperature on the Stability of Crude Procyanidin Extract Powder during Storage
Table 3 presents the variations in the moisture content and water activity of the crude procyanidin extract powder as a function of the storage temperature and duration. There was a statistically significant increase in the moisture levels and water activity after storage at temperatures of 25, 35, and 45 °C. The storage condition of 45 °C showed a lower moisture content and water activity (4.43–5.70% and 0.35–0.39, respectively) when compared to 25 °C (5.06–6.50% and 0.36–0.49, respectively) and 35 °C (4.82–6.40% and 0.36–0.42, respectively). According to Kim et al. [61], the equilibrium moisture content is significantly influenced by temperature and water activity. The equilibrium moisture content of the powder decreased as the temperature increased, while the water activity remained constant. This is related to the excitation states exhibited by water molecules. The surface tension of water decreases as the temperature increases, weakening the cohesive forces between water molecules. An increase in temperature thus results in a decrease in the level of water absorption at a specific water activity level. A hysteretic effect was also observed [62]. Due to the aforementioned factors, the moisture content and water activity of the crude procyanidin extract powder decreased as the temperature increased.

Table 3.
Moisture content, water activity, bioactive compounds, and antioxidant activities of crude procyanidin extract powder during storage.
It is important to understand how long-term storage affects the stability of bioactive molecules, the moisture content, and the water activity in order to exploit crude procyanidin extract powder as a functional ingredient in industry. This study analyzed crude procyanidin extract powder during a 12-week storage period in vacuum-sealed aluminum foil bags at 25, 35, and 45 °C. The TPC, TFC, procyanidins, and antioxidant activity were stable during 8 weeks at 25 and 35 °C, maintaining 80% stability. However, the largest decrease in the mentioned compounds occurred at a higher temperature of 45 °C, with a reduction of up to 26% by the completion of the storage period. TPC at 45 °C exhibited a faster decrease, from 37.42 to 30.29 mg GAE/g, after 12 weeks of storage: a reduction of 29% compared to the first day of storage (p < 0.05). The TPC reduction at 45 °C was greater than the reductions observed at storage temperatures of 25 °C (9%) and 35 °C (17%) after 12 weeks of storage. A study conducted by Deng et al. [63] yielded comparable findings, indicating a 16% reduction in the TPC of vacuum-packed apricots following a 6-month storage period at 25 °C in conditions of darkness, while the TPC of the vacuum-packed apricots decreased by 25% after storage at 35 °C for 8 weeks. Fang and Bhandari [64] also observed that the decline in the TPC of bayberries was positively correlated with the storage temperature. Specifically, the TPC decreased by 8%, 9%, and 37% after storage at 4, 25, and 40 °C for a duration of 6 months. According to Cao et al. [65], the polyphenol content may be altered by storage conditions, mostly as a result of hydrolysis, oxidation, and challenges associated with storage at elevated temperatures. These factors have been found to have an adverse effect on the stability of polyphenols. Polyphenols exhibit excellent stability at low temperatures, with their structures being reasonably stable for extended durations. This stability may be attributed to a reduction in phenol oxidase activity at low temperatures, resulting in reduced oxidation, condensation, and degradation [65].
The TFC followed similar trends to the TPC under the same storage temperatures. The phenol and flavonoid contents diminished as the temperature increased. A study conducted by Ferreira et al. [66] demonstrated that the phenol and flavonoid contents in extracts are influenced by various storage conditions. Furthermore, their study found that the concentrations of all bioactive components exhibited a substantial reduction with time. Hence, the stability of phenolics and flavonoids is significantly affected by the temperature and duration of storage [63].
The results showed that procyanidins were influenced by both the storage temperature and time. With an increase in the storage temperature above 35 °C, the content of procyanidins decreased to less than 6 mg/g after 8 weeks. It is worth noting that the procyanidin levels were only lowered by 17% when the extracts were stored at 25 °C for the whole duration of the study. Rodríguez-Pérez et al. [67] also demonstrated the instability of procyanidin. The procyanidin content remained stable at 25 °C for a period of 3 months, but after 6 months of storage at 40 °C, the procyanidin content in the samples decreased by approximately 80%.
The scavenging of DPPH and FRAP radicals at 25, 35, and 45 °C exhibited a consistent decrease with time and with variations in the storage temperature, as indicated in Table 3. The DPPH radical scavenging activity of the crude procyanidin extract powder exhibited comparable trends under storage conditions of 25 and 35 °C until 8 weeks, with retention percentages of over 80. However, a retention percentage below 70 was detected at 45 °C after 4 weeks of storage. The storage settings of 45 °C for a duration of 12 weeks resulted in the lowest DPPH radical scavenging activity, measuring 267.7 μM Trolox eq/g. The FRAP radical scavenging activity exhibited a retention percentage exceeding 90% at temperatures of 25 and 35 °C throughout the storage period of 12 weeks. The FRAP radical scavenging activity exhibited the highest reduction, whereas the antioxidant activity of DPPH was the lowest, measuring 1333 μM Trolox eq/g under storage conditions of 45 °C for 12 weeks.
The observed decline in antioxidant activity may be related to the oxidation of the TPC and TFC structures, resulting from prolonged storage at elevated temperatures. After 8 weeks of storage, the total phenols, flavonoids, and procyanidins decreased drastically. In addition, a high temperature of 45 °C was the main factor that decreased the amounts of bioactive compounds and antioxidant activity. Therefore, 25 °C resulted in the greatest stability, conserving up to 80% of the bioactive compounds and antioxidant activities; the results for 25 °C were better than those for 35 and 45 °C throughout the storage period.
3.5. Evaluation of Formulated Facial Serum Products
The pH of the formulation was found to be 6.2. This pH was suitable for the skin because the skin has an acidic pH of around 4.5 to 6.5 [68]. The pH of the skin serum must be between 5 and 9 [68,69] or 4 and 7 [70], which should be similar to the pH of normal skin. Chemical inertness refers to the fact that a skin serum should not be excessively acidic or too alkaline [69]. Similar results were observed by Purva Rajdev et al. [71]. The pH of the polyherbal extract face serum formulation was found to be acidic, at 6.4. Viscosity is a critical parameter for tropical formulations, as solutions with low viscosity have faster clearance than viscous solutions, so the viscosity of the face serum was found to be 12.6 pascal seconds and the spreadability of the face serum was 6 cm. Yeskar et al. [69] reviewed spreadability up to 5 to 6 cm, which provided a very soothing feeling, as well as emollient and moisturizing action.
Facial serum containing crude procyanidin extract formulation was a translucent pale yellow viscous liquid preparation with a smooth homogenous texture and glossy appearance.
3.6. Stability Test of Facial Serum Products
Evaluations of the stability of bioactive compounds and antioxidant activity in a face serum containing crude procyanidin extract were performed during 6 weeks of storage at 4 and 25 °C (Table 4). The remaining TPC, TFC, DPPH, and FRAP were determined using a spectrophotometer, while the procyanidins were determined via HPLC for the different storage temperatures at the initial time point, and at 2, 4, and 6 weeks. The protocol for the measurement of the serum’s bioactive compounds and antioxidant activity involved collecting 0.1 mL samples at the various time points for further analysis through the methods mentioned in Section 2.4, Section 2.5 and Section 2.6. The stability test results suggested that the serum was more stable at 4 °C than at 25 °C under the storage conditions. Moreover, the stability studies revealed more than 76% retention of bioactive compounds and antioxidant activities under the 4 °C storage conditions throughout the 6 weeks of stability testing, and there was no significant (p ˃ 0.05) change in the TFC or DPPH compared to the control. This is similar to the result obtained by Gyawali et al. [70], who examined extracts of the medicinal plants Cinnamomum zeylanicum Blume, Glycyrrhiza glabra L., and Azadirachta indica A. A cream prepared from this herbal composition was found to have stable DPPH antioxidant activity after 3 months of storage at both 25 °C (95.22%) and 40 °C (95.77%). In addition, Parwanto et al. [72] determined the stability of quercetin from Lantana camara Linn. leaf extract during 24 weeks of storage to evaluate its use as a natural ingredient in creams. It was observed that the 4% L. camara Linn. leaf extract cream was stable when stored at 45 °C for 180 days.

Table 4.
Stability testing results for facial serum containing crude procyanidin extract under two different controlled temperatures (4 and 25 °C) over 6 weeks.
The polyphenol concentration of a product can be influenced by its storage conditions, as indicated by the findings of Cao et al. [65]. Furthermore, it has been observed that the process of epimerization of procyanidin B2 takes place at elevated temperatures [73]. Moreover, there was degradation of the TPC in the facial serum products throughout the storage period. García-Villegas et al. [74] investigated the use of cherry stem extract as a bioactive ingredient in a cosmetic gel formulation and subjected it to further stability evaluations at 20 °C over a three-month period. The TPC of the gels decreased slightly during the first two months of storage. However, from the second month onward, the values remained constant until the end of three months of storage. This reduction could primarily be linked to the degradation of bioactive compounds due to factors such as storage time or temperature.
The physicochemical properties of facial serum, such as pH and viscosity, were determined for 6 weeks of storage at two different controlled temperatures (4 and 25 °C). A very slight change in pH and viscosity was observed in the serum. The stability of the procyanidins contained in the facial serum product exhibited a similar trend to that in crude procyanidin extract powder due to the storage effects of time and temperature. In particular, the stored extract powder showed the best stability at 25 °C, with more than 80% retention of procyanidins after 12 weeks, while approximately 73% of the content in the serum product was maintained after 6 weeks of storage. Therefore, the most stable storage temperatures with procyanidin retention percentages higher than 80% for the dry extract powder and serum products were 25 and 4 °C, respectively. Panontin et al. [75] stated that antioxidant agents in skin care formulations are an important ingredient for product stabilization over the anticipated shelf life under specified storage conditions. A phytochemical extract in the formulation serves not only as a topically administered antioxidant to effectively prevent skin aging, but also to stabilize the formulation and allow it to remain active until it reaches the target in long-term use.
4. Conclusions
An extract from GSS, obtained using UAE, demonstrated significant antioxidant properties and the ability to inhibit the tyrosinase enzymes responsible for skin aging. The crude procyanidin extract also showed no cytotoxicity and, in fact, increased cell proliferation. During storage under ambient conditions (25 °C), up to 80% of the bioactive compounds and antioxidant activities of the crude procyanidin extract powder were maintained throughout the storage time. In addition, the stability of the facial serum containing the crude procyanidin extract was at its best under storage conditions of 4 °C for 4 weeks. It can be concluded that the crude procyanidin extract exhibits potential efficacy as a component for cosmeceuticals, particularly with regard to anti-aging, skin-whitening, and antioxidant applications. This study displays the potential for the preparation of crude procyanidin extract powder from GSS via UAE under optimal conditions, which could have extensive applications in the cosmetics industry. This process may be readily feasible for commercial-scale serum production using a lower temperature, consuming less energy, and without the use of organic solvents. Further research should characterize and investigate the efficacy of facial serum product formulation, as well as the application of microencapsulation techniques to enhance the stability and extend the shelf life of the product.
Author Contributions
Conceptualization, K.P., S.D. and J.K.; formal analysis, S.D., N.L. and R.N.; funding acquisition, P.R.; Investigation, K.P., S.D., M.O. and J.K.; methodology, S.D., M.O. and J.K.; project administration, S.D. and J.K.; resources, N.L., C.T., S.R.S. and J.K.; supervision, N.L., C.T., N.S., S.R.S., P.R. and R.N.; writing—original draft, N.S. and J.K.; writing—review and editing, N.L., C.T., N.S., S.R.S., P.R., R.N. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by the Industrial Research and Technology Capacity Development Program: IRTC 2022, Chiang Mai University, Chiang Mai, Thailand: 87/2565. This research work was partially supported by Center of Excellence—Agro Bio–Circular–Green Industry (Agro-BCG) (CoE66-P001) and the Thailand Research Fund (TRF) Research Team Promotion Grant, RTA, Senior Research Scholar (N42A671052).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge By Love and Hope Company Limited for the supply of green soybeans for this project. The authors would like to thank Science and Technology Park (STeP), Chiang Mai University (CMU), and Center of Excellence—Agro Bio–Circular–Green Industry (Agro-BCG) (CoE66-P001) for their assistance. The present study was partially supported by the Thailand Research Fund (TRF) Research Team Promotion Grant, RTA, Senior Research Scholar (N42A671052).
Conflicts of Interest
The authors declare no conflicts of interest. The authors alone were responsible for the content and writing of the paper.
Abbreviations
| AOAC | Association of Official Agricultural Chemists |
| BJ | human skin fibroblasts |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| DPPH | 2, 2-diphenyl-1-picryl-hydrazyl |
| EC | Enzyme Commission number |
| FRAP | ferric-reducing antioxidant power |
| GSS | green soybean seeds |
| HPLC | high-performance liquid chromatography |
| IC50 | half-maximal inhibitory concentration |
| L-DOPA | 3,4-Dihydroxy-L-phenylalanine |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| PC | procyanidin content |
| ROS | reactive oxygen species |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| TPTZ | 2,4,6-Tris(2-pyridyl)-1,3,5-triazine |
| UAE | ultrasound-assisted extraction |
| UV | ultraviolet radiation |
References
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of ultrasonic-assisted bioactive compound extraction from green soybean (Glycine max L.) and the effect of drying methods and storage conditions on procyanidin extract. Foods 2022, 11, 1775. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Karamać, M.; Janiak, M.; Longato, E.; Meineri, G.; Amarowicz, R.; Gai, F. Phenolic composition and antioxidant activities of soybean (Glycine max (L.) Merr.) plant during growth cycle. Agronomy 2019, 9, 153. [Google Scholar] [CrossRef]
- Mai, H.N.D.; Lan, K.P.T.; Techapun, C.; Leksawasdi, N.; Taesuwan, S.; Hanprom, N.; Sompakdee, N.; Nunta, R.; Khemacheewakul, J. Quality evaluation of butter cake prepared by substitution of wheat flour with green soybean (Glycine max L.) okara. J. Culin. Sci. Technol. 2021, 21, 606–619. [Google Scholar]
- Yusufu, M.I.; Obiegbuna, J.E. Studies on the utilization of green bean as raw material in cookies produced from wheat flour. Agric. Sci. Res. J. 2015, 5, 92–97. [Google Scholar]
- Leksawasdi, N.; Taesuwan, S.; Prommajak, T.; Techapun, C.; Khonchaisri, R.; Sittilop, N.; Halee, A.; Jantanasakulwong, K.; Phongthai, S.; Nunta, R.; et al. Ultrasonic extraction of bioactive compounds from green soybean pods and application in green soybean milk antioxidants fortification. Foods 2022, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Adamou, P.; Harkou, E.; Hafeez, S.; Manos, G.; Villa, A.; Al-Salem, S.M.; Constantinou, A.; Dimitratos, N. Recent progress on sonochemical production for the synthesis of efficient photocatalysts and the impact of reactor design. Ultrason. Sonochem. 2023, 100, 106610. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019, 58, 104643. [Google Scholar] [CrossRef]
- Mehta, N.; S, J.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-assisted extraction and the encapsulation of bioactive components for food applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Du, J.; Wang, T.; Yu, D. Ultrasonic-assisted extraction of grape seed procyanidins, preparation of liposomes, and evaluation of their antioxidant capacity. Ultrason. Sonochem. 2024, 105, 106856. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pagan, J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 2020, 309, 125786. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From agro-industrial waste to food as bioactive molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef] [PubMed]
- Mapunya, M.B.; Vassileva Nikolova, R.; Lall, N. Melanogenesis and antityrosinase activity of selected south african plants. Evid. Based Complement. Alternat. Med. 2012, 2013, 374017. [Google Scholar] [CrossRef]
- Kim, H.D.; Choi, H.; Abekura, F.; Park, J.Y.; Yang, W.S.; Yang, S.H.; Kim, C.H. Naturally-occurring tyrosinase inhibitors classified by enzyme kinetics and copper chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; Valente, R.; Souza da Costa, C.H.; da S. Gonçalves Vianez, J.L., Jr.; Santana da Costa, K.; de Molfetta, F.A.; Nahum Alves, C. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: A molecular modeling approach. Molecules 2021, 26, 2875. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszyńska, A. Antioxidant activity of extract of pea and its fractions of low molecular phenolics and tannins. Polish J. Food Nutr. Sci. 2003, 53, 10–15. [Google Scholar]
- Contreras-López, E.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; del Socorro Cruz-Cansino, N.; González-Olivares, L.G.; Rodríguez-Martínez, J.S.; Ramírez-Godínez, J. Release of antioxidant compounds of Zingiber officinale by ultrasound-assisted aqueous extraction and evaluation of their in vitro bioaccessibility. Appl. Sci. 2020, 10, 4987. [Google Scholar] [CrossRef]
- Chool Boo, Y. Emerging strategies to protect the skin from ultraviolet rays using plant-derived materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar]
- Skoczyńska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and lipofuscin as hallmarks of skin aging. Adv. Dermatol. Allergol. Dermatologii Alergol. 2017, 34, 97. [Google Scholar] [CrossRef]
- Jin, Y.H.; Jeon, A.R.; Mah, J.H. Tyrosinase inhibitory activity of soybeans fermented with Bacillus subtilis capable of producing a phenolic glycoside, arbutin. Antioxidants 2020, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, D.; Lister, I.N.E.; Girsang, E.; Nasution, A.N.; Widowati, W. Comparison of antioxidant and anti-tyrosinase activity between black soybean (Glycine max (L.) merr.) and daidzein. Bul. Farmatera 2020, 5, 163–171. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.E.; Chaves, K.S.; Gebara, C.; Infante, F.N.S.; Grosso, C.R.F.; Gigante, M.L. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Marini, A.; Grether-Beck, S.; Jaenicke, T.; Weber, M.; Burki, C.; Formann, P.; Brenden, H.; Schönlau, F.; Krutmann, J. Pycnogenol effects on skin elasticity and hydration coincide with increased gene expressions of collagen type I and hyaluronic acid synthase in women. Skin Pharmacol. Physiol. 2012, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Thuy, P.T.; Quan, P.M.; Duc, D.X.; Son, N.T. The antioxidative potential of procyanidin B1: DFT (density functional theory) and docking approaches. J. Mol. Model. 2022, 28, 356. [Google Scholar] [CrossRef]
- Abozed, S.S.; El-kalyoubi, M.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Benyahia, S.; Cutler, S.J. Total Phenolic and flavonoid content and biological activities of extracts and isolated compounds of Cytisus villosus Pourr. Biomolecules 2019, 9, 732. [Google Scholar] [CrossRef]
- Zhou, R.; Cai, W.; Xu, B. Phytochemical profiles of black and yellow soybeans as affected by roasting. Int. J. Food Prop. 2017, 20, 3179–3190. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Article, O.; Vichit, W.; Saewan, N. Antioxidant activities and cytotoxicity of thai pigmented rice. Int. J. Pharm. Pharm. Sci. 2015, 7, 329–334. [Google Scholar]
- Ayazoglu Demir, E.; Demir, S.; Turan, I. Investigation of the cytotoxic effect of ethyl pyruvate on various cancer cell lines. KSU J. Agric. Nat. 2021, 24, 49–56. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Kilinc, K.; Yaman, S.O.; Misir, S.; Kara, H.; Genc, B.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression. J. Pharm. Anal. 2018, 8, 394–399. [Google Scholar] [CrossRef]
- AOAC (2000) Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; Methods 925.10, 65.17, 974.24, 992.16.
- Marlina, M.; Beandrade, M.U.; Nathalia, D.D.; Anindita, R. View of formulation test of facial serum mangosteen rind extract (Garcinia mangostana) and niacinamide. In Proceedings of the 3rd International Allied Health Students Conference (IAHSC), Singapore, 1–5 November 2023; pp. 58–62. [Google Scholar]
- Sheraz, M.A.; Ahmed, S.; Ahmad, I.; Shaikh, R.H.; Vaid, F.H.M.; Iqbal, K. Formulation and stability of ascorbic acid in topical preparations. Syst. Rev. Pharm. 2011, 2, 86–90. [Google Scholar] [CrossRef]
- Prieto, E.L. Cosmetic topical use of vitamin C. In Ascorbic Acid-Biochemistry and Functions; IntechOpen: London, UK, 2023; pp. 1–21. [Google Scholar]
- Kombathethil Ali, A.; Varghese, J. Formulation and evaluation of polyherbal face serum: Research article. Int. J. Creat. Res. Thoughts 2023, 11, 2320–2882. [Google Scholar]
- Gite, A.V.; Udapurkar, P.P.; Sanap, A.S. Formulation and development of face serum. Int. J. Creat. Res. Thoughts 2023, 11, 2320–2882. [Google Scholar]
- Biswas, A.; Dey, S.; Xiao, A.; Deng, Y.; Birhanie, Z.M.; Roy, R.; Akhter, D.; Liu, L.; Li, D. Ultrasound-assisted extraction (UAE) of antioxidant phenolics from Corchorus olitorius leaves: A response surface optimization. Chem. Biol. Technol. Agric. 2023, 10, 64. [Google Scholar] [CrossRef]
- Ilmu, J.; Pangan, T.; Sekarsari, S.; Rai Widarta, W.; Agung, A.; Ngurah, G.; Jambe, A.; Program, M.; Ilmu, S.; Pertanian, T.; et al. Pengaruh suhu dan waktu ekstraksi dengan gelombang ultrasonik terhadap aktivitas antioksidan ekstrak daun jambu biji (Psidium guajava L.). J. Ilmu Teknol. Pangan 2019, 8, 267–277. [Google Scholar]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Haslina, H.; Larasati, D.; Sani, E.Y.; Nazir, N. Sudjatinah black mangrove powder extracts with variation of temperature and length of time using ultrasound-assisted extraction (UAE). IOP Conf. Ser. Earth Environ. Sci. 2023, 1177, 012040. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Shuai, X.X.; Qiao, J.; Wei, C.B.; Ma, F.Y.; Zhang, Y.H.; Du, L.Q. Ultrasound-assisted extraction of phenolic compounds from macadamia (Macadamia integrifolia) green peel: Purification, identification and antioxidant activities. LWT 2023, 189, 115552. [Google Scholar] [CrossRef]
- Lv, J.M.; Gouda, M.; Zhu, Y.Y.; Ye, X.Q.; Chen, J.C. Ultrasound-assisted extraction optimization of proanthocyanidins from kiwi (Actinidia chinensis) leaves and evaluation of its antioxidant activity. Antioxidants 2021, 10, 1317. [Google Scholar] [CrossRef]
- Benouchenne, D.; Bellil, I.; Tachour, S.H.; Akkal, S.; Djeghim, H.; Kebaili, F.F.; Nieto, G.; Khelifi, D. Tyrosinase inhibitory ability and in vitro, in vivo acute oral and in silico toxicity evaluation of extracts obtained from Algerian Fir Needles. Plants 2022, 11, 2389. [Google Scholar] [CrossRef]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef]
- Bi, Y.; Lu, Y.; Yu, H.; Luo, L. Optimization of ultrasonic-assisted extraction of bioactive compounds from Sargassum henslowianum using response surface methodology. Pharmacogn. Mag. 2019, 15, 156–163. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Pham, N.M.Q.; Van Vuong, Q.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Phytochemical retention and antioxidant capacity of xao tam phan (Paramignya trimera) root as prepared by different drying methods. Drying Tech. 2016, 34, 324–334. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Human nutrition and metabolism dietary intakes of flavonols, flavones and isoflavones by japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration 1. J. Nutr 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, J.K.; Kim, B.K.; Park, S.J.; Kim, M.K.; won Lee, C.; Choi, L.M.; Hur, J.A.; Kim, S.H.; Beom, J.; et al. Oligomeric procyanidins (OPCs) inhibit procollagen type I secretion of fibroblasts. Tissue Eng. Regen. Med. 2017, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Kulling, S.E.; Schwartz, H.; Rowland, I.; Ruefer, C.E.; Rimbach, G.; Cassidy, A.; Magee, P.; Millar, J.; Hall, W.L.; et al. Oligomeric procyanidins inhibit cell migration and modulate the expression of migration and proliferation associated genes in human umbilical vascular endothelial cells. Mol. Nutr. Food Res. 2009, 53, 266–309. [Google Scholar]
- Oak, M.H.; El Bedoui, J.; Schini-Kerth, V.B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J. Nutr. Biochem. 2005, 16, 1–8. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C.; De Freitas, V.; Mateus, N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agric. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef]
- Taparia, S.S.; Khanna, A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016, 83, 130–140. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Han, H.J.; Lee, K.Y.; Kim, A.N.; Kim, J.C.; Choi, S.G.; Heo, H.J. Effect of storage temperature on the antioxidant activity and catechins stability of Matcha (Camellia sinensis). Food Sci. Biotechnol. 2020, 29, 1261–1271. [Google Scholar] [CrossRef]
- Zeymer, J.S.; Corrêa, P.C.; de Oliveira, G.H.H.; de Araujo, M.E.V.; Guzzo, F.; Baptestini, F.M. Moisture sorption isotherms and hysteresis of soybean grains. Acta Sci. Agron. 2022, 45, e56615. [Google Scholar] [CrossRef]
- Deng, L.Z.; Xiong, C.H.; Pei, Y.P.; Zhu, Z.Q.; Zheng, X.; Zhang, Y.; Yang, X.H.; Liu, Z.L.; Xiao, H.W. Effects of various storage conditions on total phenolic, carotenoids, antioxidant capacity, and color of dried apricots. Food Control 2022, 136, 108846. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, C.; Nunes, F.M. Effect of storage conditions on phenolic composition, vitamin C and antioxidant activity of “Golden delicious” and “Red delicious” apples. Postharvest Biol. Technol. 2024, 210, 112754. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Khanna, T.; Joshi, S. Formulation and evaluation of anti acne face serum. J. Med. Plants Stud. 2024, 12, 166–170. [Google Scholar]
- Yeskar, H.; Makde, P.; Tiware, S.A.; Shirbhate, T.M.; Thakre, S.V.; Darne, C.S.; Sable, J.B.; Warghane, K.K.; Baheti, J.R. Formulation and evaluation of a face serum containing fenugreek extract. Int. J. Basic Clin. Pharmacol. 2023, 12, 799–804. [Google Scholar] [CrossRef]
- Gyawali, R.; Gupta, R.K.; Shrestha, S.; Joshi, R.; Paudel, P.N. Formulation and evaluation of polyherbal cream containing cinnamomum Zeylanicum Blume, Glycyrrhiza glabra L and Azadirachta indica A. Juss. extracts to topical use. J. Inst. Sci. Technol. 2020, 25, 61–71. [Google Scholar] [CrossRef]
- Purva Rajdev, S.; Gaikwad, S.D.; Somvanshi, A.A.; Gunjal, S.S. Formulation and evaluation of face serum. Int. J. Adv. Res. Sci. Commun. Technol. 2022, 2, 255–259. [Google Scholar] [CrossRef]
- Lambertus, M.; Parwanto, E.; Tjahyadi, D.; Edy, H.J.; Wratsangka, R.; Guyansyah, A. Stability of Lantana camara Linn. leaf extract cream base on the level of Fe, Mg, Zn and quercetin equivalent of flavonoid. Int. J. Pharm. Res. 2021, 13, 1–18. [Google Scholar]
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature influences epimerization and composition of flavanol monomers, dimers and trimers during cocoa bean roasting. Food Chem. 2013, 141, 3656–3663. [Google Scholar] [CrossRef]
- García-Villegas, A.; Fernández-Ochoa, Á.; Alañón, M.E.; Rojas-García, A.; Arráez-Román, D.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Bioactive compounds and potential health benefits through cosmetic applications of cherry stem extract. Int. J. Mol. Sci. 2024, 25, 3723. [Google Scholar] [CrossRef] [PubMed]
- Panontin, J.F.; Barbosa, R.d.S.; Isaac, V.; Seibert, C.S.; Scapin, E. Chemical composition, antioxidant activity and development of a facial serum formulation from the extract of Hancornia speciosa. Nat. Prod. Res. 2022, 36, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).