Abstract
The present study aimed to analyze the antifungal, antioxidant, and irritant potential of citronella oil, both isolated and combined with caffeic acid phenethyl ester (CAPE), for topical oral candidiasis. The antioxidant potential was evaluated using two methods, the DPPH test and the reducing power test (FRAP), while the irritant potential of the solutions was assessed through the hen’s egg chorioallantoic membrane test (HET-CAM). The DPPH test (IC50) values for the CITRO III + CAPE III combination were 32 ± 9 mg/mL, and for isolated CAPE, 13 ± 3 mg/mL. The results from the FRAP method revealed a low iron-reducing power for the combination of 1.25 mg/mL of citronella and 0.0775 mg/mL of CAPE (CITRO III + CAPE III), showing no significant difference compared to the isolated solution of 0.15 mg/mL of CAPE. The antibacterial activity of CAPE and isolated citronella in vitro against microorganisms was evaluated using two methods: microdilution and biofilm assay. The results showed that the MIC and MFC values were 0.5 mg/mL for citronella at both tested times (24 h and 48 h). For CAPE, the MFC values were 0.031 mg/mL. For the biofilm assay, the isolated compounds and combinations at 1 min and 6 h showed significantly different results from the controls (p < 0.05). Furthermore, the HET-CAM results demonstrated the absence of irritability. Based on these premises, the antifungal and antioxidant actions, and absence of irritability were proven. Moreover, this work presents a natural antifungal of interest to the pharmaceutical industry.
1. Introduction
The incidence and mortality rates of fungal infections have increased over the last decade, making them a global public health concern as more epidemiological data are being published [1]. Candida spp. are commonly found in human commensal flora [2,3,4] and can cause superficial to fatal systemic infections. They are found in the oral, vaginal, and gastrointestinal mucosa, as well as on the skin and respiratory epithelium, and maintaining local homeostasis is essential to prevent the pathogenicity of these organisms [5,6].
Several species of Candida spp. have pathogenic potential, with approximately 90% of invasive diseases caused by C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei [7]. Oral candidiasis occurs when there is an imbalance between the host organism and the fungus. Under certain conditions, the fungus can transition from a benign commensal to a disease-causing agent. The most virulent strain, C. albicans, responsible for around 80% of oral lesions, grows in yeast, pseudohyphae, and hyphae forms. It invades epithelial cells, causes tissue damage, and protects itself from salivary flow [6,8,9].
The clinical treatment of infections caused by Candida spp. is routinely carried out using polyenes, azole derivatives, allylamines, thiocarbamates, fluoropyrimidines, and echinocandins. However, these drugs are associated with undesirable side effects and toxicity [10,11]. Moreover, resistance to these commonly used antifungal agents among clinical strains has been widely reported. Thus, being cost-effective and easy to use, phytotherapy stands out as a potential alternative for research, especially given the scarcity of studies in dentistry [12].
Citronella (Cymbopogon nardus) is a popular plant used to extract essential oil in Brazil. The oil is incorporated into formulations as an insect repellent, with the main phytochemicals being citronellal, citronellol, and nerol, which are antiseptics [13,14,15,16,17,18,19,20]. Furthermore, the literature demonstrates its effectiveness both in isolated and combined use, showing good antimicrobial efficacy [13,14,15,16] and no cytotoxicity or toxicity in healthy tissues [17,21,22]. Its use as a disinfectant agent for oral and maxillofacial prostheses has also been proven [18]. Therefore, citronella has antibacterial and antifungal potential, opening new perspectives for controlling human infections.
Another natural compound with various biological activities, including antibacterial, antiviral, antioxidant, anti-inflammatory, immunomodulatory, and anticancer effects, is caffeic acid phenethyl ester (CAPE), one of the main active components of propolis [19,20,23]. This compound exhibits potent anti-inflammatory and antioxidant activities [24,25]. Additionally, CAPE accelerates wound healing, reduces osteoclastogenesis, decreases tissue destruction caused by oxidative stress, and stimulates bone healing [26].
Based on this information, and considering the necessity to achieve effectiveness without negatively impacting the local microflora, with minimal adverse effects and at affordable costs, the current study aimed to assess the antifungal, antioxidant, and irritative potential of citronella oil, either alone or in combination with CAPE, for the topical treatment of oral candidiasis. This study hypothesized that citronella oil alone or combined with CAPE would exhibit antifungal effects and inhibit the growth of Candida albicans species, would not demonstrate harmful irritative potential, and would possess antioxidant properties.
2. Materials and Methods
2.1. Preparation of Solutions Containing Citronella Oil and Caffeic Acid Phenethyl Ester (CAPE)
2.1.1. Preparation of Pure Xanthan Gum Emulsion
Xanthan gum (200 mesh, 0.5 g) was dispersed in 100 mL of distilled water using a micro-controlled magnetic stirrer (Tecnal, Piracicaba, São Paulo, Brazil). After obtaining a homogeneous emulsion, the mixture was transferred into a 50 mL Falcon tube (Kasvi Produtos para Laboratório, São José dos Pinhais, Paraná, Brazil) and allowed to rest for 16 h at room temperature. Subsequently, it was sterilized using an autoclave (Prismatec Equipamentos, Itu, São Paulo, Brazil) and stored in the refrigerator.
2.1.2. Emulsion Containing Citronella Essential Oil
According to the manufacturer, citronella essential oil was added to the xanthan gum solution (Section 2.1.1) to achieve a final concentration of 8 mg/mL, considering the pure oil density of 0.88 g/mL. The mixture was dispersed using a mechanical disperser (IKA®-Werke GmbH & CO. KG, BIOVERA, Rio de Janeiro, RJ, Brazil) at 2000 rpm for 30 min at room temperature. The final emulsion was stored in 50 mL Falcon tubes and wrapped in aluminum foil at 4 °C.
2.1.3. Solution Containing CAPE
A stock solution of CAPE (0.5 mg/mL) (CAPE ≥ 97%, Sigma Aldrich®, St. Louis, MO, USA) was prepared in DMSO (Ciruvix Comercio Ltda) and used in the microdilution assay to determine the minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC).
2.1.4. Artificial Saliva
Artificial saliva was prepared as follows: 5 g of bacteriological peptone (Sigma-Aldrich), 2 g of yeast extract (Sigma-Aldrich), 2 g of glucose (Sigma-Aldrich), 1 g of porcine stomach mucin (Sigma-Aldrich), 0.35 g of NaCl (Sigma-Aldrich), 0.2 g of CaCl2 (Sigma-Aldrich), and 0.2 g of KCl (Sigma-Aldrich) were dissolved in deionized water (1 L solution).
2.2. Microdilution Assays in Broth for Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
Growth curves of C. albicans (ATCC 10231) were created to identify the phase of highest cell multiplication. MIC assays using microdilution were conducted following CLSI standards. After cultivation in RPMI-1640 broth (Sigma Aldrich®, St. Louis, MO, USA), cells were counted and adjusted to 105 cells/mL. The xanthan gum emulsions with citronella essential oil and the CAPE solution were serially diluted, and the MIC was visually determined. The MFC value was determined by plating 5 µL of cells from the MIC assay onto the surface of Petri dishes (Kasvi Produtos para Laboratório, São José dos Pinhais, Brazil) containing Sabouraud dextrose agar, incubated at 30 °C for 24 h. Subsequent biofilm assays utilized compounds at 10× the MFC, and citronella and CAPE were tested individually at concentrations of 2.5×, 5×, and 10× the MFC [17].
2.3. Biofilm Assay
2.3.1. Experimental Groups
The experimental groups were analyzed at the following time points: 2 h of pre-adhesion, 1 min of treatment, 24 h of adhesion, and 6 h of treatment, as shown in Table 1.

Table 1.
Experimental groups.
2.3.2. Strain, Growth Condition, Biofilm Formation Assay
The culture of C. albicans (ATCC 10231) was reactivated and cultivated on Sabouraud Dextrose Agar (Difco®, São Paulo, Brazil). After incubation, it was transferred to Sabouraud broth (Difco®, São Paulo, Brazil) before the experiment. Then, 96-well plates (Kasvi®, São José dos Pinhais, PR, Brazil) were pre-treated with artificial saliva for 2 h, followed by removal and filling with Sabouraud broth and diluted microbial culture. The incubation time varied according to two protocols: 2 h to simulate pre-adhesion and 24 h for biofilm formation. The assays were conducted at 37 °C, under aerobic conditions, with agitation at 120 rpm.
The wells of the plates containing C. albicans biofilms were subjected to the action of citronella oil solutions (with or without CAPE and combinations) for different durations (1 min and 6 h), simulating two different clinical treatment scenarios: a 1 min mouthwash and topical application of ointment or mucoadhesive gel with a 6 h treatment duration. After biofilm formation, the wells were washed with 0.9% saline solution and treated with solutions from Section 2.3.1. The protocols were conducted with modifications under agitation at 120–130 rpm at 37 °C. After removing the solutions, the biofilm was scraped with a microbiological inoculation loop, centrifuged, and resuspended for CFU/mL counting. The experiments were replicated twice in triplicate.
Subsequently, the antibiofilm effect was assessed by counting Colony-Forming Units (CFUs). Biofilm suspensions (20 µL of culture in 180 µL of saline solution) were vigorously agitated for 90 s, and serial decimal dilutions (in saline solution) were plated on Sabouraud Dextrose Agar (Difco®, São Paulo, Brazil) for colony counting of C. albicans. After 24 h of incubation of the plates in an incubator (Lutech, São José do Rio Preto, Brazil), the number of CFUs/mL was manually counted.
2.4. Ex Vivo Study of the Irritation Potential of the Solutions: Chorioallantoic Membrane Test of Chicken Eggs
Only the least concentrated solutions that inhibited biofilm development by 100% were used for this test. Thus, according to the CFU/mL results, the established groups were those represented in Table 2.

Table 2.
Experimental groups and respective active ingredients.
Four fertilized White Leghorn chicken eggs were used per treatment group (Table 2). On the tenth day of incubation, treatments were applied to the chorioallantoic membrane, and irritant effects were observed. After visual analysis, a thiopental solution was injected into the eggs. Each phenomenon was graded at 5 min intervals with numerical values (1, 3, 5, 7, 9) based on the time (Table 3 and Table 4). Changes in the chorioallantoic membrane were examined with a magnifying glass.

Table 3.
Numerical grading (1, 3, 5, 7, and 9) of irritative phenomena as a function of elapsed time (seconds) for their occurrence.

Table 4.
Mean of irritative phenomena and the final classification of the degree of irritation of the solutions.
2.5. Antioxidant Activity
2.5.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
The assays were conducted in 96-well microplates where 290 µL of the Fe3+-TPTZ reagent and 10 µL of the study solutions were added: (I) negative control (xanthan with DMSO), (II) CAPE (0.15 mg/mL), (III) CITRO (2.5 mg/mL), (IV) citronella (1.25 mg/mL) and CAPE (0.0775 mg/mL), and (V) 0.12% chlorhexidine. The plate was then incubated for 30 min at room temperature. The reading was performed on a spectrophotometer at 593 nm (Eon Microplate) [27].
2.5.2. DPPH-Scavenging Assay
The samples were (I) negative control (xanthan with DMSO), (II) CAPE (0.15 mg/mL), (III) CITRO (2.5 mg/mL), (IV) citronella (1.25 mg/mL) and CAPE (0.0775 mg/mL), and (V) 0.12% chlorhexidine. They were incubated for 30 min in the dark in a 100 μM ethanolic solution of DPPH (Sigma Aldrich®, St. Louis, MO, USA). Absorbance was measured at a wavelength of 515 nm using a UV-Mini 1240 spectrophotometer, with ethanol as the blank. The experiments were performed in triplicate, and the percentage of DPPH radical scavenging was calculated using the control (100 μM DPPH) without the substance as a reference in Equation (1) [28].
2.6. Statistical Analysis
The fungal count data (CFUs) from the biofilm assay were converted to Log10 (CFU/mL). These data were analyzed for homogeneity using the Shapiro–Wilk test to determine if they followed a normal distribution. Since the data distribution was parametric, analysis of variance (ANOVA) was applied, followed by the Tukey–Kramer test (α = 0.05), using IBM SPSS 20.0 (IBM, Armonk, NY, USA). For the antioxidant test data, homogeneity was analyzed using the Shapiro–Wilk test to determine if they followed a normal distribution.
3. Results
3.1. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
The MIC/MFC results for the tested Candida strains are presented in Table 5. The MIC and MFC values were 0.5 mg/mL for the citronella emulsion at both tested time points (24 h and 48 h). For CAPE, the MFC values were 0.031 mg/mL at both tested time points.

Table 5.
MIC/MFC values for citronella emulsion (citronella) and CAPE in the tested C. albicans ATCC 10231 strain at 24 h and 48 h time points.
3.2. Biofilm Assay
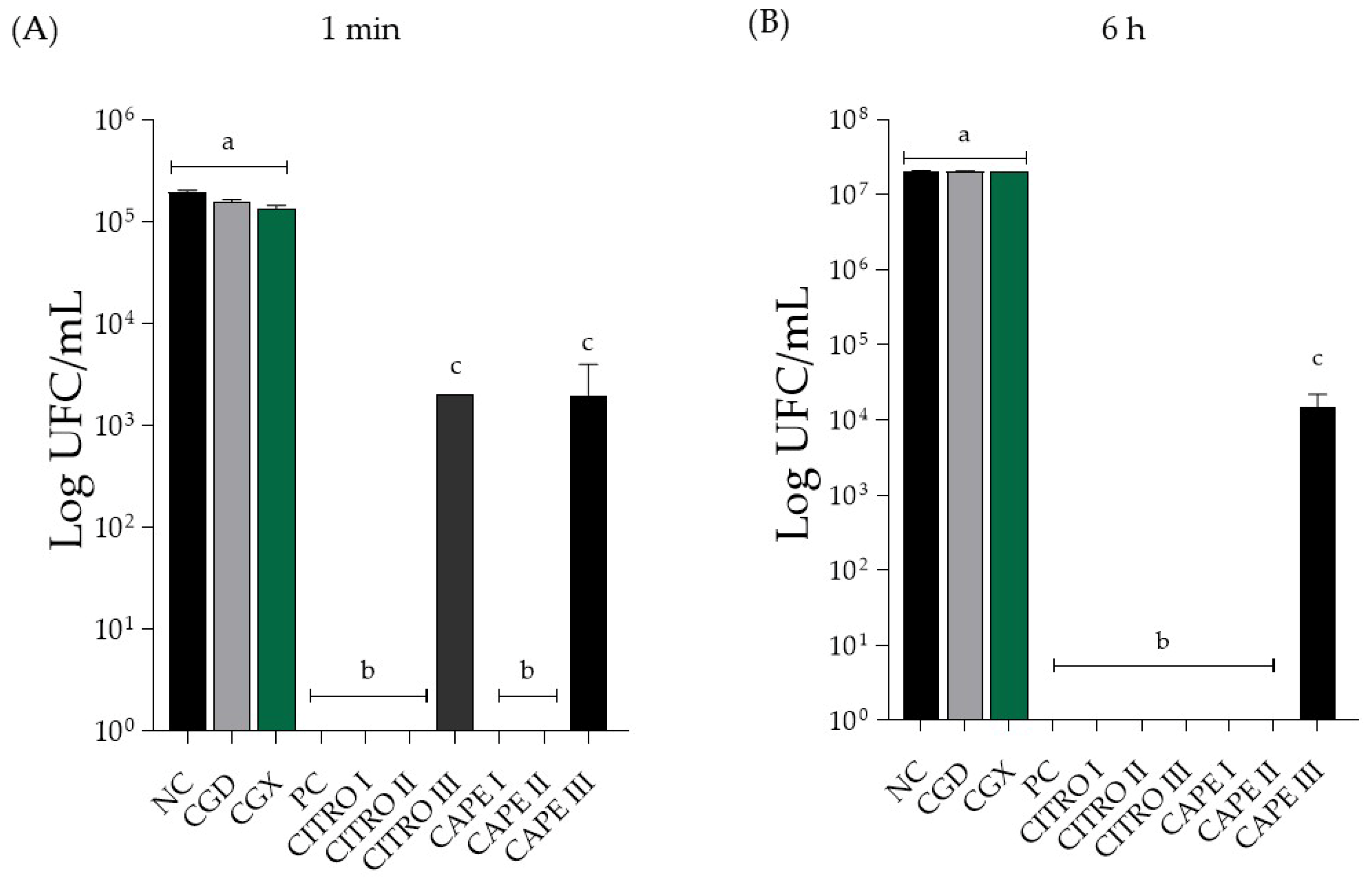
The results of the treatments of C. albicans (ATCC10231) biofilms are shown in Figure 1 and Figure 2. The results are categorized based on the use of the solutions, individually or combined, in the 1 min and 6 h treatment protocols. Figure 1 and Figure 2 display the data for the compounds used individually during the 1 min treatment times.
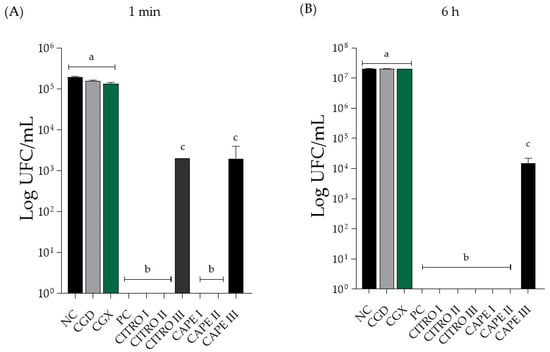
Figure 1.
Mean ± SD of C. albicans biofilm count in Log (CFU/mL) of isolated compounds, in (A) treatment for 1 min and in (B) treatment for 6 h. Groups were treated with negative control (NC; 0.9% physiological saline solution), CAPE control (CGD—xanthan gum and DMSO), xanthan gum control (CGX), positive control (PC; 0.12% Chlorhexidine), citronella (CITRO I—5 mg/mL, II—2.5 mg/mL, and III—1.25 mg/mL), CAPE (I—0.31 mg/mL, II—0.15 mg/mL, and III—0.0775 mg/mL). The letters presented after each value indicate whether there were significant differences between the different concentrations (p < 0.05). Same letter—no significant difference. Different letter—significant difference. Results are expressed as mean ± SD. One-way ANOVA followed by Tukey’s post hoc test was performed.

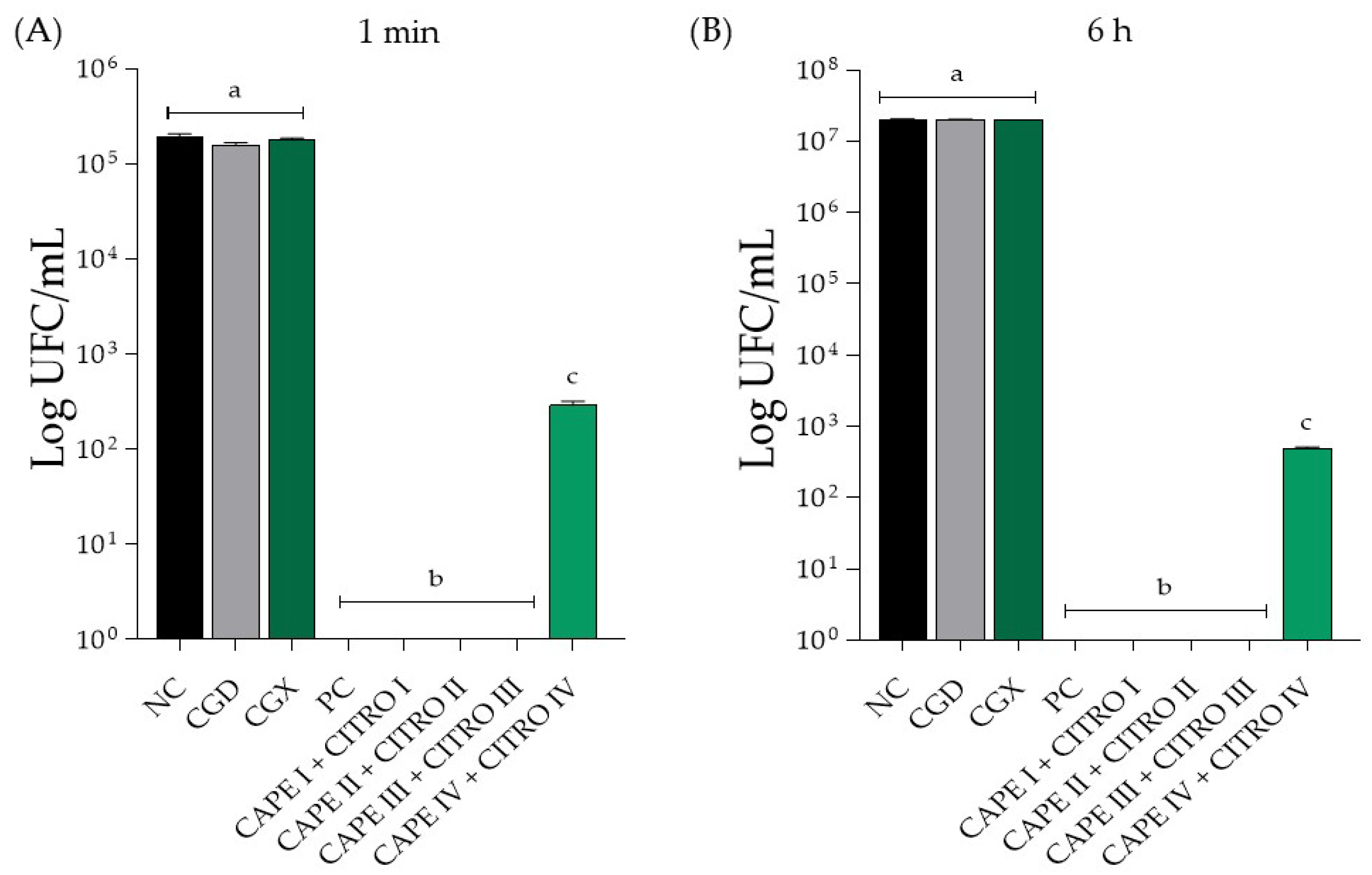
Figure 2.
Mean ± SD of C. albicans biofilm count in Log (CFU/mL) of isolated compounds, in (A) treatment for 1 min and in (B) treatment for 6 h. Groups were treated with negative control (NC; 0.9% physiological saline solution), CAPE control (CGD—xanthan gum and DMSO), xanthan gum control (CGX), positive control (PC; 0.12% Chlorhexidine), CAPE I + CITRO I (5 mg/mL Citronella + 0.31 mg/mL CAPE), CAPE II + CITRO II (2.5 mg/mL Citronella + 0.15 mg/mL CAPE), CAPE III + CITRO III (1.25 mg/mL Citronella + 0.0775 mg/mL CAPE) and CAPE IV + CITRO IV (0.625 mg/mL Citronella + 0.038 mg/mL CAPE). The letters presented after each value indicate whether there were significant differences between the different concentrations (p < 0.05). Same letter—no significant difference. Different letter—significant difference. Results are expressed as mean ± SD. One-way ANOVA followed by Tukey’s post hoc test was performed.
In Figure 2B, the antifungal activity of the compounds alone after 6 h of treatment is shown. There was a significant difference between the solutions of the isolated compounds, the negative control group, and the 0.12% chlorhexidine solution (positive control). The CAPE concentration at 0.07 mg/mL showed a significant difference from the other concentrations tested, reducing the Log (CFU/mL) value. However, it did not completely eliminate the CFU count. Additionally, there was no significant difference between the control solutions and the negative control group.
In Figure 2A, the antifungal action of the combined compounds at the 1 min treatment time is shown. There was a significant difference between the negative control and all concentrations of the evaluated compounds, indicating a substantial reduction in fungal growth for the 0.12% chlorhexidine solution (positive control). Specifically, the concentration of CAPE 0.03 mg/mL + CITRO at 0.6 mg/mL showed a statistically significant difference compared to the other tested concentrations, resulting in a reduction in the Log (CFU/mL) value but not an elimination of the CFU count. In terms of the control group of the solutions, none exhibited antifungal activity and did not present a statistically significant difference when compared to the negative control.
In Figure 2B, it is possible to observe the antifungal action of the combined compounds at the 6 h treatment time. There was a statistically significant difference between all tested concentrations compared to the negative control group and the 0.12% chlorhexidine solution (positive control). The concentration of CAPE 0.03 mg/mL + CITRO at 0.6 mg/mL showed a statistically significant difference compared to the other tested concentrations, promoting a reduction in the Log (CFU/mL) value, but did not eliminate the CFU count. Notably, there was no statistically significant difference between the control solutions and the negative control group.
3.3. Assay of the Chorioallantoic Membrane of Chicken Embryo Egg (HET-CAM)
The final classification of the treatments’ irritation level was determined by calculating the average sum of the values obtained for the four samples in each group. The results of the in vitro irritability test (Table 6) indicated a score of zero (non-irritant) for the saline solution (NC) and a score of 21 (severely irritant) for NaOH (PC2).

Table 6.
Mean grading and final classification of the HET-CAM test groups.
All other treatments were classified as non-irritant or mildly irritant, suggesting that they can safely be used on the skin.
3.4. Antioxidant Potential
3.4.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
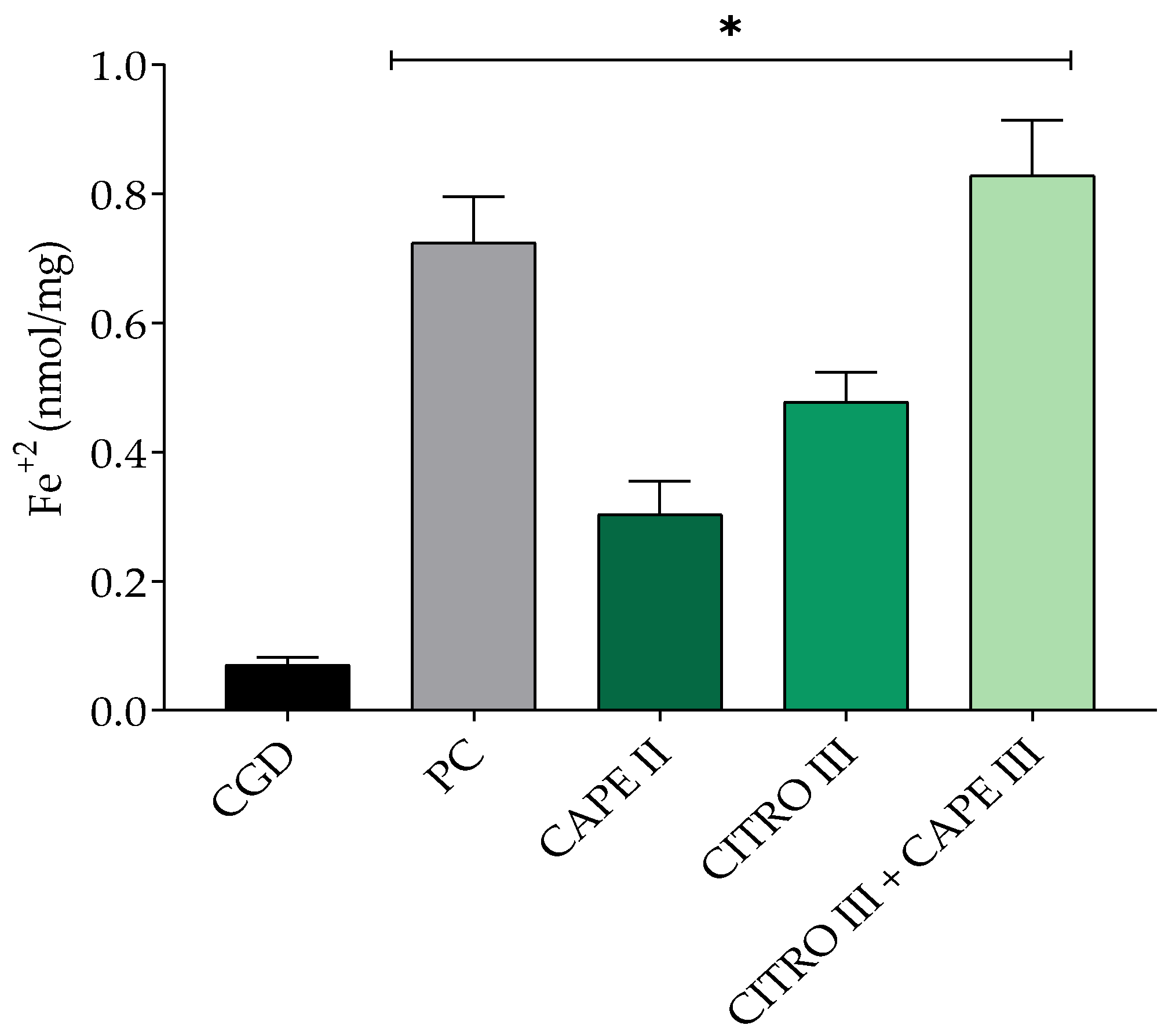
The compounds and combinations were tested for their ability to reduce Fe+3 to Fe+2 to determine their antioxidant potential. The results were measured in terms of the amount of Fe+2 produced per mass of the evaluated compound. Figure 3 illustrates the antioxidant activity as a percentage (%AA). The combination of 1.25 mg/mL citronella and 0.0775 mg/mL CAPE (CAPE III + CITRO III) showed no significant difference compared to the isolated solution of 0.15 mg/mL CAPE. Solutions of 2.5 mg/mL citronella and 0.12% chlorhexidine did not exhibit satisfactory antioxidant capacity, with values statistically similar to the control group.

Figure 3.
Mean ± SD of iron reduction, with groups treated with control xanthan gum and DMSO (CGD), positive control (PC; chlorhexidine 0.12%), CITRO III (1.25 mg/mL), CAPE II (0.15 mg/mL), and citronella and CAPE combination (CITRO III + CAPE III—1.25 mg/mL citronella + 0.0775 mg/mL CAPE). One-way ANOVA followed by Tukey’s post hoc test. The asterisk (*) indicates significant difference (p < 0.05) between groups.
3.4.2. 1,1-Diphenyl-2-picrylhydrazil Radical (DPPH) Scavenging Assay
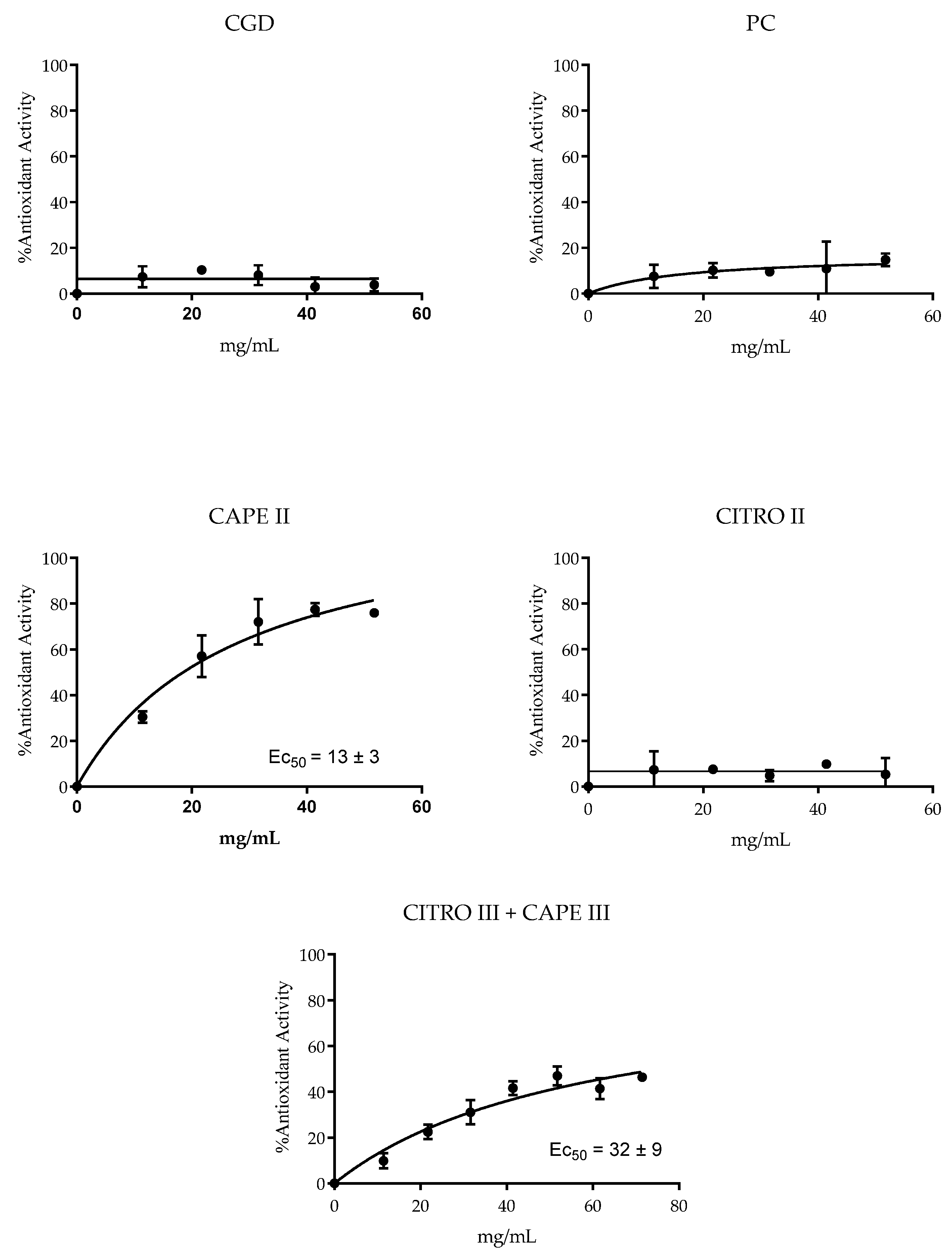
The antioxidant potential of the compounds and combinations was assessed by their ability to reduce the stable free radical DPPH. The results presented in Figure 4 indicate that CAPE, whether isolated or combined with citronella, showed antioxidant activity. The EC50 value for the CAPE/citronella combination was 32 ± 9 mg/mL (only the CAPE III + CITRO III group presented an antioxidant effect). The lowest EC50 (13 ± 3 mg/mL) for isolated CAPE indicated its higher antioxidant efficiency. In contrast, the other products evaluated showed no detectable antioxidant activity.
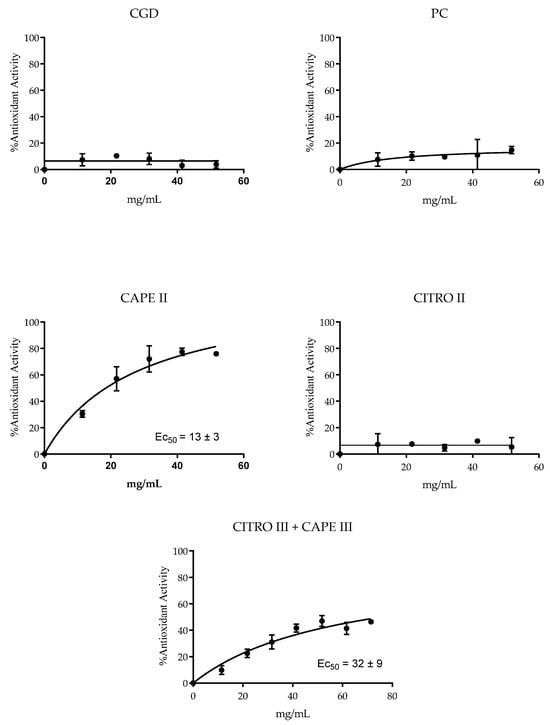
Figure 4.
Mean ± SD of DPPH reduction, with groups treated with control xanthan gum and DMSO (CGD), positive control (PC; chlorhexidine 0.12%), CITRO 2.5 mg/mL, CAPE 0.15 mg/mL, and citronella and Cape (CAPE III + CITRO III—1.25 mg/mL citronella + 0.0775 mg/mL CAPE).
4. Discussion
The hypothesis that citronella oil alone and in combination with CAPE would exhibit antifungal effects and inhibit the growth of Candida albicans was confirmed. Both compounds tested showed fungicidal action at different concentrations when used alone and in combination. According to the results obtained in the MIC/MFC assays, the solution containing citronella essential oil showed MIC/MFC values of 0.5 mg/mL. De Toledo et al. [29] found similar results in their study when they evaluated the antifungal effect of citronella essential oil (Cymbopogon nardus) against various standard and clinical strains of Candida (C. albicans, C. krusei, C. glabrata, C. tropicalis, and C. parapsilosis). They discovered a range of MIC/MFC values between 250 and 1000 μg/mL. The present study obtained even better results for CAPE, which showed MIC/MFC values approximately 10 times lower than those of citronella, resulting in MIC/MFC values of 31 μg/mL. These values are in accordance with previous published studies. Sun et al. [19] found MIC values of 32 to 64 μg/mL for CAPE against C. albicans species in their study. De Barros et al. [30] found MIC values for CAPE ranging from 16 to 64 μg/mL in C. albicans species. The values of the present study fell within the range reported in the scientific literature.
A compound’s antifungal activity can be categorized as active or inactive based on the following MIC values: 50–500 µg/mL, strong/optimal activity; 600–1500 µg/mL, moderate activity; above 1500 µg/mL, weak activity or inactive product [29,31,32]. Thus, the CAPE and citronella essential oil results suggest a strong/optimal activity. These results are in line with prior studies, confirming the antifungal properties of citronella essential oil and the CAPE on Candida fungal species [17,30,33].
The compounds have been proven to have an antibiofilm effect when used alone or in combination. The concentrations of solutions containing citronella essential oil that achieved fungicidal results were 2.5 mg/mL for the 1 min treatment protocol and 1.25 mg/mL for the 6 h protocol, resulting in complete elimination of the biofilms. These results are consistent with studies published in the literature that investigated the effectiveness and antifungal properties of the essential oil from the Cymbopogon nardus plant against Candida species, demonstrating promising results [17,34]. The active components found in citronella oil, such as citronellal, nerol, geraniol, and citronellol, have been shown in previous studies [35,36] to increase the fluidity and permeability of microorganism membranes. This can cause cellular disruptions or lysis [37]. Additionally, geraniol and citronellal have been found to be effective against C. albicans and other Candida species. This helps to explain why solutions containing citronella are highly effective, as demonstrated in the present study [38,39,40].
In this study, the solution containing citronella essential oil utilized xanthan gum, a polysaccharide produced by Xanthomonas campestris. Xanthan gum is a commonly used water-based emulsifier and stabilizer in the food industry. It demonstrates good stability across different temperatures and pH ranges [35,38,41]. The solution demonstrated satisfactory results and good antifungal and antibiofilm action, preserving its effect when used alone and when combined with CAPE.
Regarding CAPE, it was observed that the solution displayed strong antifungal action when used alone. It showed greater effectiveness at concentrations of 0.31 mg/mL and 0.15 mg/mL, completely inhibiting fungal growth. Similar results were found by Alfarrayeh et al. [42], who discovered that CAPE has a high ability to inhibit planktonic growth and biofilm formation of different Candida species tested, as well as partially inhibiting the formation of mature biofilms of these fungi. The authors noted that the effect was dose-dependent for biofilm eradication, with concentrations ranging from 50 to 100 μg/mL. Additionally, the authors pointed out that CAPE exhibits its antifungal activity by inducing cell death in Candida spp. through cell protoplasm shrinkage, abnormal cell and nuclear morphology, and distortion of cell walls and membranes, causing changes in surface micromorphology. However, it is still reported that the mechanisms of action of CAPE are not well established [19].
We observed a positive and synergistic association between the two tested compounds. At three different concentrations (CAPE 0.3 mg/mL + Citro 5 mg/mL, CAPE 0.15 mg/mL + Citro 2.5 mg/mL, and CAPE 0.07 mg/mL + Citro 1.25 mg/mL), these associations completely killed the cells of the biofilms formed at both 2 h and 24 h. In the concentration of CAPE 0.03 mg/mL + Citro 0.6 mg/mL, the C. albicans biofilm was partially inhibited for 24 h. In a study conducted by Sun et al. [19], the combined use of CAPE with caspofungin against C. albicans species was evaluated. The authors reported a synergistic effect, with the combination reducing the MIC values by 16 times compared to the individual values of each drug. This suggests that CAPE may enhance the efficacy of caspofungin in treating fungal infections caused by C. albicans. Similarly, the combined effect of CAPE and the drug fluconazole on C. albicans was evaluated and it demonstrated positive synergistic activity. This combination enhanced the effectiveness of treatment against fluconazole-resistant C. albicans, showing promise as a therapeutic option. The synergy was attributed to decreased MIC values in fluconazole-resistant clinical isolates [43].
In a study by Khan et al. [44], the antifungal activity of various essential oils, including those from the Cymbopogon genus, was evaluated against different clinical and conventional strains of C. albicans. These herbal medicines were combined with the conventional drugs Amphotericin B and fluconazole, which are commonly used to treat fungal infections caused by C. albicans. The authors concluded that combining isolated essential oils with conventional antifungals can improve the treatment of patients with candidiasis, particularly those with strains that are resistant to traditional treatments. Bioactive combinations can produce more effective results at lower concentrations by enhancing antifungal activity. This allows for a wider range of action, targeting more pathogens and reducing the likelihood of fungi developing resistance to the treatments [45]. No previous studies have reported the combination of CAPE and citronella essential oil compounds. This study is scientifically and clinically significant due to the novel results obtained. It has opened new perspectives for treatment using natural products, which could lead to the development of products such as mouth rinses and antifungal mucosal adhesive ointments or gels, especially for conditions that are resistant to conventional antifungal treatments.
The HET-CAM test is an alternative to animal testing once the egg’s CAM (chorioallantoic membrane) has functional vascularization. It provides faster results, serving as a preliminary alternative to traditional animal testing [46,47,48]. The observed effects include changes in the membrane and blood vessels. These effects consist of hemorrhage (increased bleeding from the blood vessels of the CAM), hyperemia (increased blood vessel diameter), and coagulation (intravascular or extravascular protein coagulation, which usually leads to increased CAM opacity). These effects are assessed by observing the fixation and reaction times of the solutions applied to the CAM [44]. Based on these criteria, most of the solutions studied in this research were found to be non-irritating. The only exceptions were the isolated solution of CAPE 0.15 mg/mL and the combined solution of CAPE 0.038 mg/mL + CITRO 0.625 mg/mL, which were classified as mildly irritating. This mild irritation is likely due to the presence of DMSO in the solution, which was also observed in the CAPE control solution.
This study used two methods to evaluate antioxidant effectiveness. The FRAP assay (Ferric-Reducing Antioxidant Power) involves reducing the ferric-tripyridyltriazine (Fe+3-TPZ) complex to the ferrous complex (Fe+2-TPZ) in the presence of an antioxidant under acidic conditions. The resulting complex has a deep blue color and absorbs light at 593 nm. The FRAP assay is simple, fast, and can be carried out using automated, semi-automated, or manual methods [49]. The antifungal action of CAPE may involve depriving cells of iron by forming insoluble complexes with iron ions, thus preventing their absorption [40]. In this study, the presence of CAPE in the solutions increased their antioxidant capacity. The DPPH (2,2-diphenyl-1-picrylhydrazyl) radical-scavenging test was the second antioxidant assay to assess antioxidant activity. This method is based on electron transfer [43]. The results indicated that the CAPE and CAPE + citronelle groups demonstrated a significant ability to scavenge DPPH, unlike the other groups, supporting the FRAP assays’ findings. In summary, the CAPE provided antioxidant properties to the solutions that were not present in citronelle and xanthan alone.
The FRAP and DPPH assays revealed that isolated CAPE has high antioxidant efficiency, capable of reducing Fe³⁺ to Fe²⁺ and neutralizing free radicals, with an EC50 of 13 ± 3 mg/mL. The combination of CAPE with citronella also showed significant antioxidant activity (EC50 of 32 ± 9 mg/mL), suggesting that citronella may complement the action of CAPE. Although isolated citronella and chlorhexidine solutions did not show relevant antioxidant activity, the combination of CAPE with citronella maintained good antioxidant efficacy. In summary, isolated CAPE is a potent antioxidant, and its combination with citronella retains considerable antioxidant capacity, indicating potential synergism. Noreen et al. [45] found in silico molecules with antioxidant activity where DPPH and FRAP (%) values ranged between 51 and 68%, which are good indicators of efficacy [50].
CAPE is already being used in modern medicine due to its favorable properties. Otan Ozden [51] concluded that CAPE has a beneficial effect in reducing the local oxidative state of gingival tissues in experimental models of periodontitis. This is achieved by activating cellular defense mechanisms against oxidative stress, including decreases in superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST) expression. CAPE also has good anti-inflammatory properties and can modulate the arachidonic acid cascade compared to other components of propolis [20]. Additionally, CAPE can suppress the expression of inflammatory mediators induced by H2O2. Tolba [20] reports other anti-inflammatory and antioxidant functions of CAPE, such as inhibition of immunoglobulin-mediated cutaneous passive anaphylaxis and reduction and suppression of histamine. These functions relate to neurodegenerative diseases and provide further evidence of CAPE’s potential therapeutic benefits.
The plant Cymbopogon nardus is utilized for the extraction of essential oil. Although it has no antioxidant capacity similar to CAPE, studies report [52] that citronella also has antioxidant properties. This is attributed to its high content of monoterpenes, although lower than that of gallic acid, which is used as a standard. Due to the monoterpenes found in its composition, citronella also exhibits anticancer activities, inhibiting the proliferation of LNCαP and HeLα cells [49]. Additionally, the same study reported that citronella essential oil has anti-inflammatory activities by inhibiting lipoxygenase [53].
The combination of CAPE with citronella at various concentrations showed positive results in all assays analyzed in this study. This led to decreased concentrations of the active principles, which is generally favorable as it decreases the solutions’ cytotoxic potential. Our results are consistent with those of Sun et al. [43], who reported that CAPE combined with fluconazole exhibited good synergy, suggesting it could be an alternative method for combating Candida albicans.
Our findings are important due to the increasing occurrence of oral infections, particularly Candida species such as C. tropicalis, C. glabrata, and C. guilliermondii, which are resistant to antifungal agents. Additionally, commercially available solutions like chlorhexidine can cause side effects. For these reasons, studying natural phytotherapeutic solutions shows promise for preventing denture-related stomatitis, providing an alternative method to reduce Candida colonization. While this study has focused on citronella and CAPE associations for potential future use in new formulations emphasizing their antifungal and antioxidant properties, these associations could also be investigated for many other potential uses based on these results.
5. Conclusions
Citronella and CAPE solutions, associated or not, exhibited antifungal and antibiofilm action at different concentrations. This study evidently demonstrated the superior potential of the association between CAPE and citronella solutions. The solutions did not induce irritability in the CAM membrane. The citronella solutions associated with CAPE and CAPE alone demonstrated satisfactory antioxidant activity.
Author Contributions
All authors participated in the design, interpretation of the studies, analysis of the data, and review of the manuscript; P.A.d.S.R., I.A.C., J.A.R.F., D.B.B., L.d.S., V.F.X. and A.M.G. conducted the experiments; P.A.d.S.R., I.A.C. and V.F.X. were involved with the chemical analysis of the extract and formulations, and in vitro experiments. A.M.G., D.B.B., J.A.R.F. and L.d.S. were responsible for data discussion and manuscript correction. A.M.G. was the senior researcher responsible for this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The manuscript has been read and approved by all the authors, the requirements for authorship have been met, and all authors believe that the manuscript represents honest work.
Data Availability Statement
The data that support the findings of this research are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Milho, C.; Liberal, Â.; Silva, J.; Fonseca, C.; Barbosa, A.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Antibiofilm Potential of Medicinal Plants against Candida spp. Oral Biofilms: A Review. Antibiotics 2021, 10, 1142. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Javed, F.; Samaranayake, L.P.; Romanos, G.E. Treatment of Oral Fungal Infections Using Antimicrobial Photodynamic Therapy: A Systematic Review of Currently Available Evidence. Photochem. Photobiol. Sci. 2014, 13, 726–734. [Google Scholar] [CrossRef]
- Kreulen, I.A.M.; de Jonge, W.J.; van den Wijngaard, R.M.; van Thiel, I.A.M. Candida spp. in Human Intestinal Health and Disease: More than a Gut Feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida Pathogenesis: Revisiting the Vital Drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef]
- Staniszewska, M. Virulence Factors in Candida Species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.M.; Willems, H.M.E.; Krom, B.P. Candida albicans in Multispecies Oral Communities; A Keystone Commensal? Adv. Exp. Med. Biol. 2016, 931, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Perea, S.; López-Ribot, J.L.; Kirkpatrick, W.R.; McAtee, R.K.; Santillán, R.A.; Martínez, M.; Calabrese, D.; Sanglard, D.; Patterson, T.F. Prevalence of Molecular Mechanisms of Resistance to Azole Antifungal Agents in Candida albicans Strains Displaying High-Level Fluconazole Resistance Isolated from Human Immunodeficiency Virus-Infected Patients. Antimicrob. Agents Chemother. 2001, 45, 2676–2684. [Google Scholar] [CrossRef]
- Berretta, A.A.; de Castro, P.A.; Cavalheiro, A.H.; Fortes, V.S.; Bom, V.P.; Nascimento, A.P.; Marquele-Oliveira, F.; Pedrazzi, V.; Ramalho, L.N.Z.; Goldman, G.H. Evaluation of Mucoadhesive Gels with Propolis (EPP-AF) in Preclinical Treatment of Candidiasis Vulvovaginal Infection. Evid. Based Complement. Altern. Med. 2013, 2013, 641480. [Google Scholar] [CrossRef]
- Brito, L.C.F.; Dias, L.M.F.; Pereira, G.S.S.; Alves, N.B.; Rocha, M.d.S.; Junior, J.F.d.S.; Barros, V.C.; Muratori, M.C.S. Analysis of the Chemical Composition, Antifungal Activity and Larvicidal Action against Aedes Aegypti Larvae of the Essential Oil Cymbopogon nardus. Res. Soc. Dev. 2021, 10, e543101321452. [Google Scholar] [CrossRef]
- Kusumaningrum, H.P.; Zainuri, M.; Endrawati, H.; Purbajanti, E.D. Characterization of Citronella Grass Essential Oil of Cymbopogon winterianus from Batang Region, Indonesia. J. Phys. Conf. Ser. 2020, 1524, 012057. [Google Scholar] [CrossRef]
- Nakahara, K.; Alzoreky, N.S.; Yoshihashi, T.; Nguyen, H.T.T.; Trakoontivakorn, G. Chemical Composition and Antifungal Activity of Essential Oil from Cymbopogon nardus (Citronella Grass). JARQ 2013, 37, 249–252. [Google Scholar] [CrossRef]
- Wei, L.S.; Wee, W. Chemical Composition and Antimicrobial Activity of Cymbopogon nardus Citronella Essential Oil against Systemic Bacteria of Aquatic Animals. Iran. J. Microbiol. 2013, 5, 147–152. [Google Scholar]
- Guandalini Cunha, B.; Duque, C.; Sampaio Caiaffa, K.; Massunari, L.; Araguê Catanoze, I.; Dos Santos, D.M.; de Oliveira, S.H.P.; Guiotti, A.M. Cytotoxicity and Antimicrobial Effects of Citronella Oil (Cymbopogon nardus) and Commercial Mouthwashes on S. Aureus and C. Albicans Biofilms in Prosthetic Materials. Arch. Oral Biol. 2020, 109, 104577. [Google Scholar] [CrossRef]
- Guiotti, A.M.; Cunha, B.G.; Paulini, M.B.; Goiato, M.C.; Dos Santos, D.M.; Duque, C.; Caiaffa, K.S.; Brandini, D.A.; Narciso De Oliveira, D.T.; Brizzotti, N.S.; et al. Antimicrobial Activity of Conventional and Plant-Extract Disinfectant Solutions on Microbial Biofilms on a Maxillofacial Polymer Surface. J. Prosthet. Dent. 2016, 116, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Ju, X.-C.; Li, Y.; Zeng, P.-M.; Wu, J.; Zhou, Y.-Y.; Shen, L.-B.; Dong, J.; Chen, Y.-J.; Luo, Z.-G. Generation of Vascularized Brain Organoids to Study Neurovascular Interactions. eLife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic Acid Phenethyl Ester, a Promising Component of Propolis with a Plethora of Biological Activities: A Review on Its Anti-Inflammatory, Neuroprotective, Hepatoprotective, and Cardioprotective Effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef]
- Ayenew, K.D.; Sewale, Y.; Amare, Y.E.; Ayalew, A. Acute and Subacute Toxicity Study of Essential Oil of Cymbopogon Martini in Mice. J. Toxicol. 2022, 2022, 1995578. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Gokhale, S.T.; Manjunath, S.; Al-Qahtani, S.M.; Magbol, M.A.; Nagate, R.R.; Tikare, S.; Chaturvedi, S.; Agarwal, A.; Venkataram, V. Comparative Evaluation of Locally Administered 2% Gel Fabricated from Lemongrass Polymer and 10% Doxycycline Hyclate Gel as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis-A Randomized Controlled Trial. Polymers 2022, 14, 2766. [Google Scholar] [CrossRef]
- Paracatu, L.C.; Faria, C.M.Q.G.; Quinello, C.; Rennó, C.; Palmeira, P.; Zeraik, M.L.; da Fonseca, L.M.; Ximenes, V.F. Caffeic Acid Phenethyl Ester: Consequences of Its Hydrophobicity in the Oxidative Functions and Cytokine Release by Leukocytes. Evid. -Based Complement. Altern. Med. 2014, 2014, e793629. [Google Scholar] [CrossRef] [PubMed]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory Effects of Caffeic Acid Phenethyl Ester on the Activity and Expression of Cyclooxygenase-2 in Human Oral Epithelial Cells and in a Rat Model of Inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar] [PubMed]
- Bjørklund, G.; Storchylo, O.; Peana, M.; Hangan, T.; Lysiuk, R.; Lenchyk, L.; Koshovyi, O.; Antonyak, H.; Hudz, N.; Chirumbolo, S. Caffeic Acid Phenethyl Ester: A Potential Therapeutic Cancer Agent? Curr. Med. Chem. 2024, 31, 6760–6774. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, H.O.; Bereket, M.C.; Ezirganli, S.; Aydin, M.S.; Aksakalli, S. Effects of Caffeic Acid Phenethyl Ester on Wound Healing in Calvarial Defects. Acta Odontol. Scand. 2015, 73, 21–27. [Google Scholar] [CrossRef]
- Vasconcelos, D.N.d.; Lima, A.N.; Philot, E.A.; Scott, A.L.; Boza, I.A.F.; Souza, A.R.d.; Morgon, N.H.; Ximenes, V.F. Methyl Divanillate: Redox Properties and Binding Affinity with Albumin of an Antioxidant and Potential NADPH Oxidase Inhibitor. RSC Adv. 2019, 9, 19983–19992. [Google Scholar] [CrossRef]
- Mazo, G.d.S.; Fracasso, J.A.R.; da Costa, L.T.S.; Farias Ximenes, V.; Zoppe, N.A.; Viel, A.M.; Guarnier, L.P.; Silva, B.d.C.; de Almeida, L.V.C.; dos Santos, L. Development of an Antioxidant, Anti-Aging, and Photoprotective Phytocosmetic from Discarded Agave sisalana Perrine Roots. Cosmetics 2024, 11, 104. [Google Scholar] [CrossRef]
- De Toledo, L.G.; Ramos, M.A.D.S.; Spósito, L.; Castilho, E.M.; Pavan, F.R.; Lopes, É.D.O.; Zocolo, G.J.; Silva, F.A.N.; Soares, T.H.; Dos Santos, A.G.; et al. Essential Oil of Cymbopogon nardus (L.) Rendle: A Strategy to Combat Fungal Infections Caused by Candida Species. Int. J. Mol. Sci. 2016, 17, 1252. [Google Scholar] [CrossRef]
- de Barros, P.P.; Rossoni, R.D.; Garcia, M.T.; Kaminski, V.d.L.; Loures, F.V.; Fuchs, B.B.; Mylonakis, E.; Junqueira, J.C. The Anti-Biofilm Efficacy of Caffeic Acid Phenethyl Ester (CAPE) In Vitro and a Murine Model of Oral Candidiasis. Front. Cell Infect. Microbiol. 2021, 11, 700305. [Google Scholar] [CrossRef]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Filho, B.P.D. Screening of Some Plants Used in the Brazilian Folk Medicine for the Treatment of Infectious Diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Anokwah, D.; Asante-Kwatia, E.; Asante, J.; Obeng-Mensah, D.; Danquah, C.A.; Amponsah, I.K.; Ameyaw, E.O.; Biney, R.P.; Obese, E.; Oberer, L.; et al. Antibacterial, Resistance Modulation, Anti-Biofilm Formation, and Efflux Pump Inhibition Properties of Loeseneriella africana (Willd.) N. Halle (Celastraceae) Stem Extract and Its Constituents. Microorganisms 2023, 12, 7. [Google Scholar] [CrossRef]
- Possamai Rossatto, F.C.; Tharmalingam, N.; Escobar, I.E.; d’Azevedo, P.A.; Zimmer, K.R.; Mylonakis, E. Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris. J. Fungi 2021, 7, 763. [Google Scholar] [CrossRef]
- Almeida, L.D.F.D.D.; Paula, J.F.D.; Almeida, R.V.D.D.; Williams, D.W.; Hebling, J.; Cavalcanti, Y.W. Efficacy of Citronella and Cinnamon Essential Oils on Candida albicans Biofilms. Acta Odontol. Scand. 2016, 74, 393–398. [Google Scholar] [CrossRef]
- Riquelme, N.; Robert, P.; Troncoso, E.; Arancibia, C. Influence of the Particle Size and Hydrocolloid Type on Lipid Digestion of Thickened Emulsions. Food Funct. 2020, 11, 5955–5964. [Google Scholar] [CrossRef] [PubMed]
- Espert, M.; Salvador, A.; Sanz, T. Rheological and Microstructural Behaviour of Xanthan Gum and Xanthan Gum-Tween 80 Emulsions during in vitro Digestion. Food Hydrocoll. 2019, 95, 454–461. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane Toxicity of Antimicrobial Compounds from Essential Oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fatima, Z.; Hameed, S. Citronellal-Induced Disruption of Membrane Homeostasis in Candida albicans and Attenuation of Its Virulence Attributes. Rev. Soc. Bras. Med. Trop. 2016, 49, 465–472. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Wang, Z.; Li, L.; Zheng, Y.; Häkkinen, L.; Haapasalo, M. In Vitro Cytotoxicity Evaluation of a Novel Root Repair Material. J. Endod. 2013, 39, 478–483. [Google Scholar] [CrossRef]
- Shahina, Z.; Al Homsi, R.; Price, J.D.W.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Rosemary Essential Oil and Its Components 1,8-Cineole and α-Pinene Induce ROS-Dependent Lethality and ROS-Independent Virulence Inhibition in Candida albicans. PLoS ONE 2022, 17, e0277097. [Google Scholar] [CrossRef]
- Ahmad, A.; Viljoen, A. The in Vitro Antimicrobial Activity of Cymbopogon Essential Oil (Lemon Grass) and Its Interaction with Silver Ions. Phytomedicine 2015, 22, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Alfarrayeh, I.; Pollák, E.; Czéh, Á.; Vida, A.; Das, S.; Papp, G. Antifungal and Anti-Biofilm Effects of Caffeic Acid Phenethyl Ester on Different Candida Species. Antibiotics 2021, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liao, K.; Hang, C. Caffeic Acid Phenethyl Ester Synergistically Enhances the Antifungal Activity of Fluconazole against Resistant Candida albicans. Phytomedicine 2018, 40, 55–58. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Malik, A.; Ahmad, I. Anti-Candidal Activity of Essential Oils Alone and in Combination with Amphotericin B or Fluconazole against Multi-Drug Resistant Isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar] [CrossRef]
- Noreen, S.; Sumrra, S.H.; Chohan, Z.H.; Mustafa, G.; Imran, M. Synthesis, Characterization, Molecular Docking and Network Pharmacology of Bioactive Metallic Sulfonamide-Isatin Ligands against Promising Drug Targets. J. Mol. Struct. 2023, 1277, 134780. [Google Scholar] [CrossRef]
- Rivero, M.N.; Lenze, M.; Izaguirre, M.; Pérez Damonte, S.H.; Aguilar, A.; Wikinski, S.; Gutiérrez, M.L. Comparison between HET-CAM protocols and a product use clinical study for eye irritation evaluation of personal care products including cosmetics according to their surfactant composition. Food Chem. Toxicol. 2021, 153, 112229. [Google Scholar] [CrossRef] [PubMed]
- Steiling, W.; Bracher, M.; Courtellemont, P.; de Silva, O. The HET-CAM, a Useful In Vitro Assay for Assessing the Eye Irritation Properties of Cosmetic Formulations and Ingredients. Toxicol Vitr. 1999, 13, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Bagley, D.M.; Waters, D.; Kong, B.M. Development of a 10-Day Chorioallantoic Membrane Vascular Assay as an Alternative to the Draize Rabbit Eye Irritation Test. Food Chem. Toxicol. 1994, 32, 1155–1160. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Mushtaq, F.; Ahmad, F.; Hussain, R.; Zafar, W.; Imran, M.; Zafar, M.N. Coordination Behavior, Structural, Statistical and Theoretical Investigation of Biologically Active Metal-Based Isatin Compounds. Chem. Pap. 2022, 76, 3705–3727. [Google Scholar] [CrossRef]
- Otan Özden, F.; Lütfioğlu, M.; Demir, E.; Bilgici, B. Antioxidant Effect of Caffeic Acid Phenethyl Ester in Experimentally Induced Periodontitis. Clin. Oral. Investig. 2021, 25, 4959–4966. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Coulibaly, A.Y.; Djigma, F.W.; Nagalo, B.M.; Baron, S.; Figueredo, G.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Antiproliferative Activities of the Essential Oil of Cymbopogon nardus, a Plant Used in Traditional Medicine. Biomol. Concepts 2020, 11, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-J.; Lim, H.W.; Kim, K.; Kim, K.-M.; Cho, S.; Chae, S.-W. Effect of Caffeic Acid Phenethyl Ester (CAPE) on H2O2 Induced Oxidative and Inflammatory Responses in Human Middle Ear Epithelial Cells. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 675–679. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).