Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation
Abstract
1. Introduction
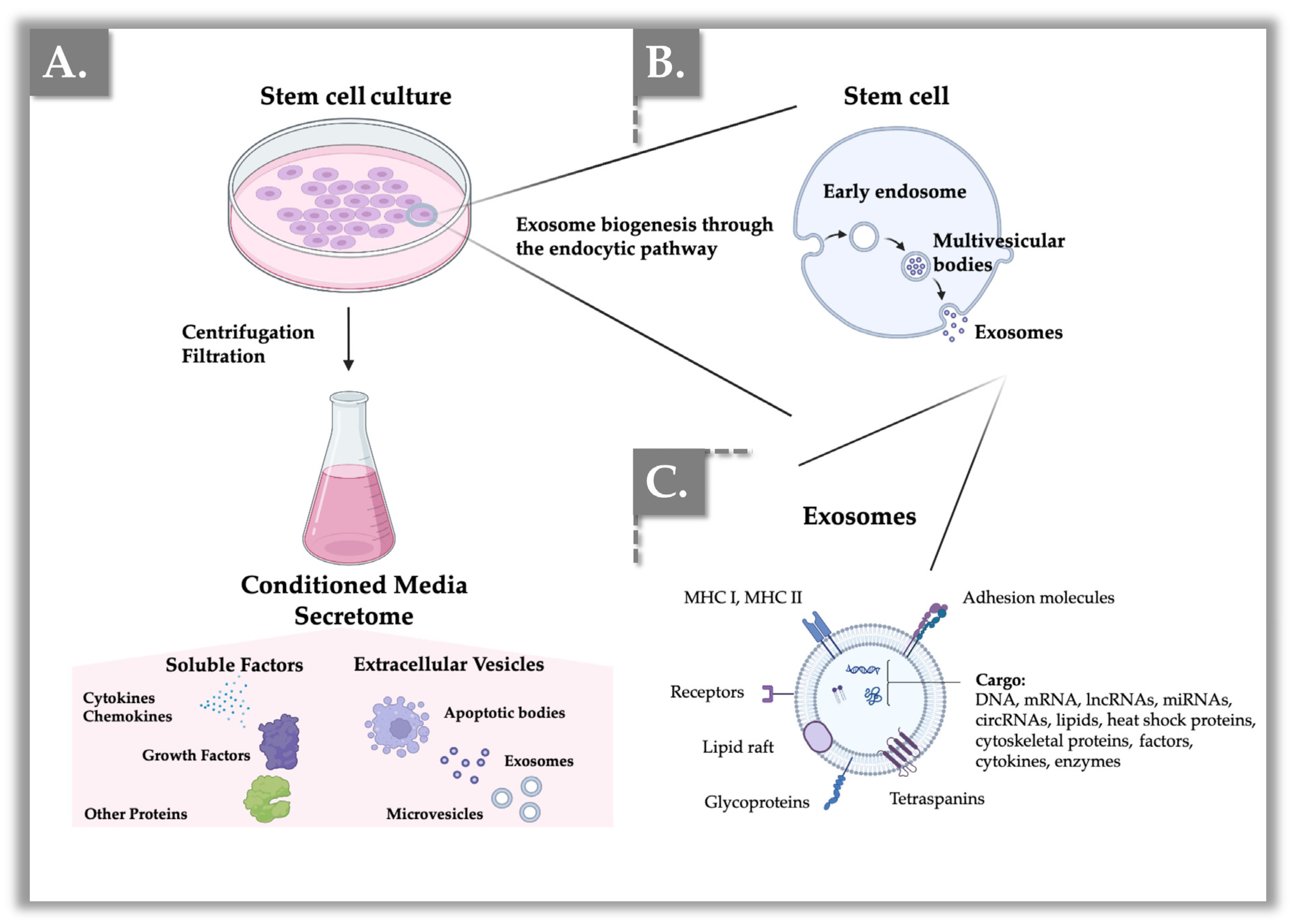
2. General Biological Characteristics of Exosomes
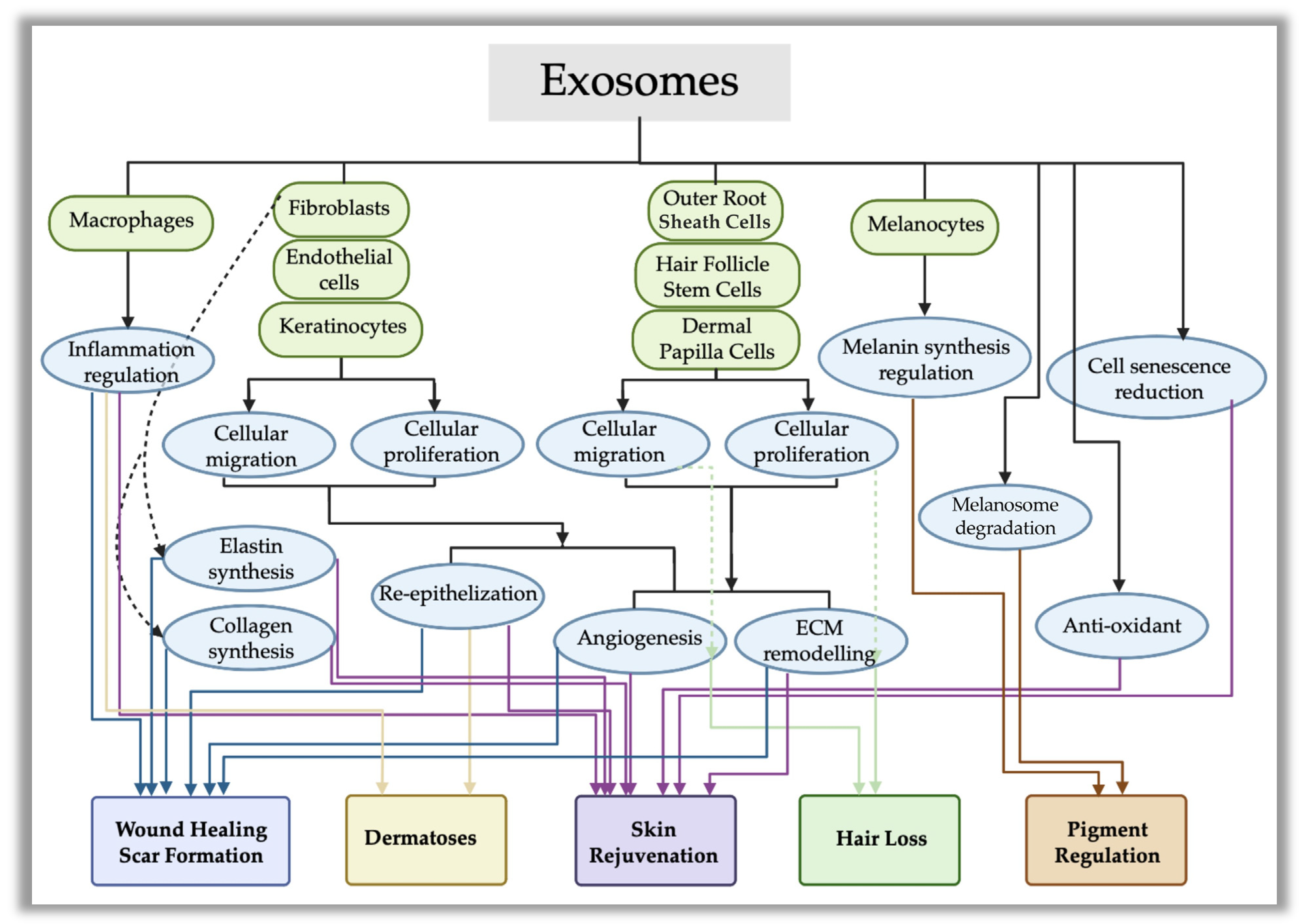
3. Modes of Action of Exosomes in Cutaneous Applications
3.1. Roles of Exosomes in Cutaneous Wound Healing
3.2. Roles of Exosomes in Scar Formation and Cutaneous Pigment Regulation
3.3. Use of Exosomes for Managing Dermatoses
3.4. Use of Exosomes for Skin Rejuvenation
3.5. Use of Exosomes for Managing Hair Loss
4. Clinical Studies on the Topical Cutaneous Use of Exosomes
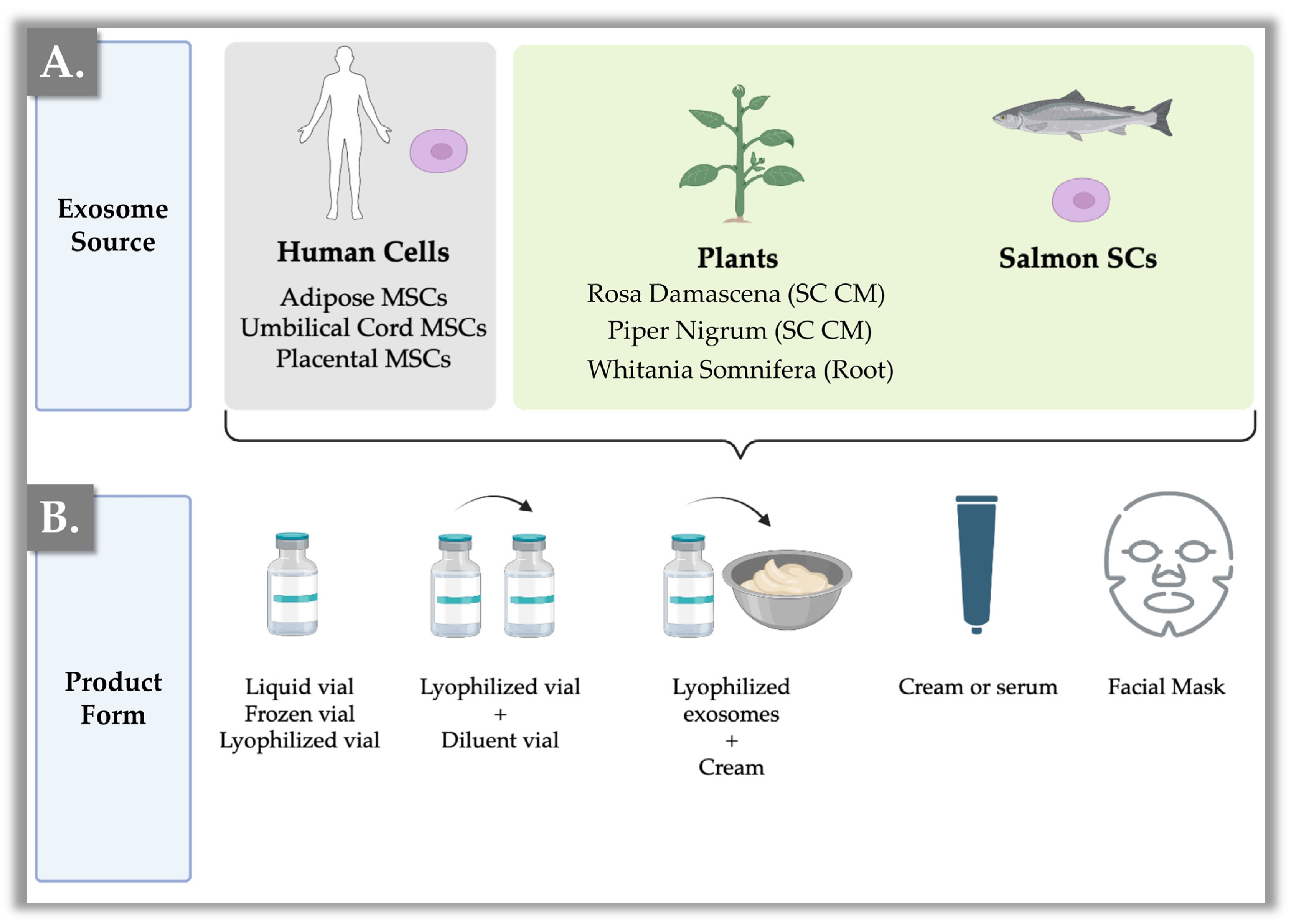
5. Commercialized Exosome Products
5.1. Commercial Exosome Product Formula Analyses
5.2. Exosome Ingredient and Product Sourcing Considerations
5.3. Secretome Products for Potential Technical Rationalization
5.4. Current Best Practices in Commercial Exosome Isolation
6. Analysis of EU and Global Regulatory Frameworks for Exosome-Based Products
6.1. Technical Hurdles in the Registration and Market Implementation of Exosome Products
6.2. Off-Label Uses and Illicit Commercialization of Exosome Products
6.3. Dangers of Off-Label Uses for Commercial Exosome Products
6.4. Harmonization of Regulations for Exosomes and EVs
7. Exosome Use in Cosmetic Products and Aesthetic Medicine Protocols
8. Galenic Form and Storage of Exosome-Based Products
8.1. Topical Formulation Possibilities for Exosome Products
8.2. Storage Conditions and Stability of Exosome/Secretome Products
8.2.1. Frozen Storage for Exosome Products
8.2.2. Lyophilized Storage for Exosome Products
8.3. Stabilization Processing of Exosome Products
8.3.1. Formulation with Cryoprotectants/Lyoprotectants
8.3.2. Quality Considerations: Ingredient Resuspension after Lyophilization
9. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | adipose stem cells |
| ATMP | advanced therapy medicinal product |
| BMSCs | bone marrow stem cells |
| CD | cluster of differentiation |
| circRNA | circular RNA |
| CM | conditioned medium |
| CaHA | calcium hydroxylapatite |
| cGMPs | current good manufacturing practices |
| CHUV | Lausanne University Hospital |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| ECM | extracellular matrix |
| EV | extracellular vesicle |
| EMA | European Medicines Agency |
| EPSCs | expanded potential stem cells |
| ESCRT | endosomal sorting complex required for transport |
| EU | European Union |
| FD-MSCs | fetal dermal mesenchymal stem cells |
| FDA | US Food and Drug Administration |
| HA | hyaluronic acid |
| hAFSCs | human amniotic fluid stem cells |
| hAMSCs | human amniotic mesenchymal stem cells |
| hCMSCs | human cord mesenchymal stem cells |
| hESCs | human embryonic stem cells |
| hUC-MSCs | human umbilical cord mesenchymal stem cells |
| HUVECs | human umbilical vein endothelial cells |
| INCI | international nomenclature of cosmetic ingredients |
| IND | investigational new drug |
| iPSCs | induced pluripotent stem cells |
| LED | light-emitting diode |
| lncRNA | long non-coding RNA |
| MenSCs | menstrual blood-derived stem cells |
| MHC | major histocompatibility complex |
| miRNA | microRNA |
| MoA | mechanism of action |
| mRNA | messenger ribonucleic acid |
| MSCs | mesenchymal stem cells |
| NLF | nuclear localization factor |
| NTA | nanoparticle tracking analysis |
| OMLP-PCs | oral mucosa lamina propria-progenitor cells |
| PRP | platelet-rich plasma |
| PBS | phosphate-buffered saline |
| PAGE | polyacrylamide gel electrophoresis |
| QA | quality assurance |
| QC | quality control |
| Rabs | GTPases families |
| RNA | ribonucleic acid |
| SCs | stem cells |
| TNF | tumor necrosis factor |
| WJ-MSCs | Wharton-jelly mesenchymal stem cells |
| UCMSCs | umbilical cord mesenchymal stromal cells |
| USA | United States of America |
| USCs | urine-derived stem cells |
| UV | ultraviolet |
References
- Zhang, B.; Gong, J.; He, L.; Khan, A.; Xiong, T.; Shen, H.; Li, Z. Exosomes based advancements for application in medical aesthetics. Front. Bioeng. Biotechnol. 2022, 10, 1083640. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomat. Res. 2021, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, J.; Yan, Y.; Xu, Y.; Wang, X.; Wu, H.; Liu, Y.; Li, L.; Zhuo, F. Efficacy of microneedling combined with local application of human umbilical cord-derived mesenchymal stem cells conditioned media in skin brightness and rejuvenation: A randomized controlled split-face study. Front. Med. 2022, 9, 837332. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Moftah, N.H.; Nasif, G.A.; Ameen, S.W.; Ibrahim, M.R.; Ragaie, M.H. Facial rejuvenation using stem cell conditioned media combined with skin needling: A split-face comparative study. J. Cosmet. Dermatol. 2020, 19, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Behrangi, E.; Feizollahi, M.; Zare, S.; Goodarzi, A.; Ghasemi, M.R.; Sadeghzadeh-Bazargan, A.; Dehghani, A.; Nouri, M.; Zeinali, R.; Roohaninasab, M.; et al. Evaluation of the efficacy of mesenchymal stem cells derived conditioned medium in the treatment of striae distensae: A double blind randomized clinical trial. Stem Cell Res. Ther. 2024, 15, 62. [Google Scholar] [CrossRef]
- Minoretti, P.; Emanuele, E. Clinically actionable topical strategies for addressing the hallmarks of skin aging: A primer for aesthetic medicine practitioners. Cureus 2024, 16, e52548. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Yu, Y.; Wang, L.; Zhao, M. Umbilical cord-derived mesenchymal stem cell secretome promotes skin regeneration and rejuvenation: From mechanism to therapeutics. Cell Prolif. 2024, 57, e13586. [Google Scholar] [CrossRef]
- Hani, R.; Khayat, L.; Rahman, A.A.; Alaaeddine, N. Effect of stem cell secretome in skin rejuvenation: A narrative review. Mol. Biol. Rep. 2023, 50, 7745–7758. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H. Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regen. Ther. 2022, 21, 73–80. [Google Scholar] [CrossRef]
- Available online: https://metacelltech.com/mct-exosomes/ (accessed on 26 June 2024).
- Yi, K.H.; Winayanuwattikun, W.; Kim, S.Y.; Wan, J.; Vachatimanont, V.; Putri, A.I.; Hidajat, I.J.; Yogya, Y.; Pamela, R. Skin boosters: Definitions and varied classifications. Skin Res. Technol. 2024, 30, e13627. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Dhurat, R.; Sukesh, M. Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, D.; Wang, M.; Xu, Z.; Chen, X.; Liu, Q.; Sun, W.; Li, J.; Gong, Y.; Liu, D.; et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 358. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The machinery of exosomes: Biogenesis, release, and uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Lau, N.C.H.; Yam, J.W.P. From exosome biogenesis to absorption: Key takeaways for cancer research. Cancers 2023, 15, 1992. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Ves. 2024, 13, e12404. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Bojmar, L.; Chen, H.; Li, Z.; Tobias, G.C.; Hu, M.; Homan, E.A.; Lucotti, S.; Zhao, F.; et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature 2023, 618, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Ves. 2015, 4, 27066. [Google Scholar] [CrossRef]
- Pelissier Vatter, F.A.; Cioffi, M.; Hanna, S.J.; Castarede, I.; Caielli, S.; Pascual, V.; Matei, I.; Lyden, D. Extracellular vesicle- and particle-mediated communication shapes innate and adaptive immune responses. J. Exp. Med. 2021, 218, e20202579. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Q.; Jiang, L. Current knowledge on exosome biogenesis, cargo-sorting mechanism and therapeutic implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Prot. Cell Biol. 2006, 3, 3.22. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- White, M.J.; Roife, D.; Gomer, R.H. Galectin-3 binding protein secreted by breast cancer cells inhibits monocyte-derived fibrocyte differentiation. J. Immunol. 2015, 195, 1858–1867. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating extracellular vesicles in human disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Benmoussa, A.; Lee, C.H.; Laffont, B.; Savard, P.; Laugier, J.; Boilard, E.; Gilbert, C.; Fliss, I.; Provost, P. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal tract conditions. J. Nutr. 2016, 146, 2206–2215. [Google Scholar] [CrossRef]
- Shandilya, S.; Rani, P.; Onteru, S.K.; Singh, D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the intestinal barrier in vitro. J. Agri. Food Chem. 2017, 65, 9506–9513. [Google Scholar] [CrossRef]
- Lv, H.; Liu, H.; Sun, T.; Wang, H.; Zhang, X.; Xu, W. Exosome derived from stem cell: A promising therapeutics for wound healing. Front. Pharmacol. 2022, 13, 957771. [Google Scholar] [CrossRef]
- Li, D.; Wu, N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 187, 109882. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med. 2024, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.X.; Chang, T.; Lin, X. Secretomes as an emerging class of bioactive ingredients for enhanced cosmeceutical applications. Exp. Dermatol. 2022, 31, 674–688. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of exosomes in dermal wound healing: A systematic review. J. Investig. Dermatol. 2022, 142, 662–678. [Google Scholar] [CrossRef]
- Qin, X.; He, J.; Wang, X.; Wang, J.; Yang, R.; Chen, X. The functions and clinical application potential of exosomes derived from mesenchymal stem cells on wound repair: A review of recent research advances. Front. Immunol. 2023, 14, 1256687. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Vu, D.M.; Nguyen, V.T.; Nguyen, T.H.; Do, P.T.X.; Dao, H.H.; Hai, D.X.; Le, N.T.; Nguyen, X.H.; Than, U.T.T. Effects of extracellular vesicles secreted by TGFβ-stimulated umbilical cord mesenchymal stem cells on skin fibroblasts by promoting fibroblast migration and ECM protein production. Biomedicines 2022, 10, 1810. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Chinnici, C.M.; Iannolo, G.; Cittadini, E.; Carreca, A.P.; Nascari, D.; Timoneri, F.; Bella, M.D.; Cuscino, N.; Amico, G.; Carcione, C.; et al. Extracellular vesicle-derived microRNAs of human Wharton’s jelly mesenchymal stromal cells may activate endogenous VEGF-A to promote angiogenesis. Int. J. Mol. Sci. 2021, 22, 2045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnol. 2021, 19, 202. [Google Scholar] [CrossRef]
- He, L.; Zhu, C.; Jia, J.; Hao, X.Y.; Yu, X.Y.; Liu, X.Y.; Shu, M.G. ADSC-Exos containing MALAT1 promotes wound healing by targeting miR-124 through activating Wnt/β-catenin pathway. Biosci. Rep. 2020, 40, BSR20192549. [Google Scholar] [CrossRef]
- Yuan, R.; Dai, X.; Li, Y.; Li, C.; Liu, L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol. Med. Rep. 2021, 24, 758. [Google Scholar] [CrossRef] [PubMed]
- Al-Masawa, M.E.; Alshawsh, M.A.; Ng, C.Y.; Ng, A.M.H.; Foo, J.B.; Vijakumaran, U.; Subramaniam, R.; Ghani, N.A.A.; Witwer, K.W.; Law, J.X. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: A systematic review and meta-analysis of animal studies. Theranostics 2022, 12, 6455–6508. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, X.; Zhao, H.; Deng, Y.; Zeng, W.; Yang, K.; Chen, H.; Yan, Q.; Li, C.; Wu, J.; et al. The effectiveness of cell-derived exosome therapy for diabetic wound: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 85, 101858. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, W.; Xiao, C.; Su, N.; Han, Y.; Zhai, L.; Hou, C. Exosome from adipose-derived mesenchymal stem cells attenuates scar formation through microRNA-181a/SIRT1 axis. Arch. Biochem. Biophys. 2023, 746, 109733. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Kee, L.T.; Ng, C.Y.; Al-Masawa, M.E.; Foo, J.B.; How, C.W.; Ng, M.H.; Law, J.X. Extracellular vesicles in facial aesthetics: A review. Int. J. Mol. Sci. 2022, 23, 6742. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Z.; Zhang, Y.; Zhao, Z.; Zhu, H.; Yue, C. Extracellular vesicle: A magic lamp to treat skin aging, refractory wound, and pigmented dermatosis? Front. Bioeng. Biotechnol. 2022, 10, 1043320. [Google Scholar] [CrossRef]
- Dehghani, P.; Varshosaz, J.; Mirian, M.; Minaiyan, M.; Kazemi, M.; Bodaghi, M. Keratinocyte exosomes for topical delivery of tofacitinib in treatment of psoriasis: An in vitro/ in vivo study in animal model of psoriasis. Pharm. Res. 2024, 41, 263–279. [Google Scholar] [CrossRef]
- Kim, J.; Kim, E.H.; Lee, H.; Sung, J.H.; Bang, O.Y. Clinical-scale mesenchymal stem cell-derived extracellular vesicle therapy for wound healing. Int. J. Mol. Sci. 2023, 24, 4273. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, T.T.; Sim, W.K.; Zhang, B.; Lim, S.K. A roadmap from research to clinical testing of mesenchymal stromal cell exosomes in the treatment of psoriasis. Cytotherapy 2023, 25, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, J.; Li, Z.; Zheng, J.; Sun, Q. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate psoriasis-like skin inflammation. J. Interferon Cytokine Res. 2022, 42, 8–18. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, S.B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine mesenchymal-stem-cell-derived extracellular vesicles attenuate atopic dermatitis. Animals 2023, 13, 2215. [Google Scholar] [CrossRef]
- Han, H.S.; Koh, Y.G.; Hong, J.K.; Roh, Y.J.; Seo, S.J.; Park, K.Y. Adipose-derived stem cell exosomes for treatment of dupilumab-related facial redness in patients with atopic dermatitis. J. Dermatol. Treat. 2023, 34, 2220444. [Google Scholar] [CrossRef]
- Jang, Y.N.; Lee, J.O.; Lee, J.M.; Park, A.Y.; Kim, Y.J.; Kim, S.Y.; Seok, J.; Yoo, K.H.; Kim, B.J. Exosomes derived from human dermal fibroblasts (HDFn-Ex) alleviate DNCB-induced atopic dermatitis (AD) via PPARα. Exp. Dermatol. 2024, 33, e14970. [Google Scholar] [CrossRef]
- Cai, C.S.; He, G.J.; Xu, F.W. Advances in the applications of extracellular vesicle for the treatment of skin photoaging: A comprehensive review. Int. J. Nanomed. 2023, 18, 6411–6423. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Huang, L.; Yan, Y.; Zhong, Y.; Xie, H.; Wang, X. Bone marrow mesenchymal stem cell-derived exosome miR-29b-3p alleviates UV irradiation-induced photoaging in skin fibroblast. Photodermatol. Photoimmunol. Photomed. 2023, 39, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jin, S.; Wang, P.; He, Q.; Yang, Y.; Gao, Z.; Wang, X. Microneedle based adipose derived stem cells-derived extracellular vesicles therapy ameliorates UV-induced photoaging in SKH-1 mice. J. Biomed. Mat. Res. 2021, 109, 1849–1857. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.U.; Cheng, K. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.A.; Kim, S.Y.; Park, J.H.; Jo, D.G.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Ves. 2019, 8, 1565885. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xin, Y.; Zhang, Z.; Zou, X.; Xue, K.; Zhang, H.; Zhang, W.; Liu, K. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res. Ther. 2020, 11, 264. [Google Scholar] [CrossRef]
- Chou, Y.; Alfarafisa, N.M.; Ikezawa, M.; Khairani, A.F. Progress in the development of stem cell-derived cell-free therapies for skin aging. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3383–3406. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Kwack, M.H.; Jeyaraman, M.; Hong, C.M.; Sung, Y.K.; Ahn, B.C. Application of cell-derived extracellular vesicles and engineered nanovesicles for hair growth: From mechanisms to therapeutics. Front. Cell Dev. Biol. 2022, 10, 963278. [Google Scholar] [CrossRef]
- Yan, H.; Gao, Y.; Ding, Q.; Liu, J.; Li, Y.; Jin, M.; Xu, H.; Ma, S.; Wang, X.; Zeng, W.; et al. Exosomal micro RNAs derived from dermal papilla cells mediate hair follicle stem cell proliferation and differentiation. Int. J. Biol. Sci. 2019, 15, 1368–1382. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J.; Zhang, X.; Dai, Y.; Yang, N.; Bao, Z.; Chen, Y.; Wu, X. Exosomal miRNA-181a-5p from the cells of the hair follicle dermal papilla promotes the hair follicle growth and development via the Wnt/β-catenin signaling pathway. Int. J. Biol. Macromol. 2022, 207, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wang, Q.; Zhang, Y.; Cui, L.; Huang, X. Exosomes secreted from adipose-derived stem cells are a potential treatment agent for immune-mediated alopecia. J. Immunol. Res. 2022, 2022, 7471246. [Google Scholar] [CrossRef] [PubMed]
- Lueangarun, S.; Cho, B.S.; Tempark, T. Hair repigmentation of poliosis circumscripta in androgenetic alopecia patient treated with exosomes and fractional picosecond laser. J. Cosmet. Dermatol. 2024, 23, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Lee, J.; Won, Y.; Duncan, D.I.; Jin, R.C.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Park, B.C.; et al. Skin brightening efficacy of exosomes derived from human adipose tissue-derived stem/stromal cells: A prospective, split-face, randomized placebo-controlled study. Cosmetics 2020, 7, 90. [Google Scholar] [CrossRef]
- Kwon, H.H.; Yang, S.H.; Lee, J.; Park, B.C.; Park, K.Y.; Jung, J.Y.; Bae, Y.; Park, G.H. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: A 12-week prospective, double-blind, randomized, split-face study. Acta. Dermatol. Venerol. 2020, 100, adv00310. [Google Scholar] [CrossRef]
- Chernoff, G. Combining topical dermal infused exosomes with injected calcium hydroxylapatite for enhanced tissue biostimulation. J. Cosmet. Dermatol. 2023, 22, 15–27. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Wang, D.; Zhang, C.; Hu, K.; Zhang, H.; Lin, J.; Chen, X. Stem cell-derived exosomes in the treatment of melasma and its percutaneous penetration. Lasers Surg. Med. 2023, 55, 178–189. [Google Scholar] [CrossRef]
- Proffer, S.L.; Paradise, C.R.; DeGrazia, E.; Halaas, Y.; Durairaj, K.K.; Somenek, M.; Sivly, A.; Boon, A.J.; Behfar, A.; Wyles, S.P. Efficacy and tolerability of topical platelet exosomes for skin rejuvenation: Six-week results. Aesthet. Surg. J. 2022, 42, 1185–1193. [Google Scholar] [CrossRef]
- Park, K.Y.; Han, H.S.; Park, J.W.; Kwon, H.H.; Park, G.H.; Seo, S.J. Exosomes derived from human adipose tissue-derived mesenchymal stem cells for the treatment of dupilumab-related facial redness in patients with atopic dermatitis: A report of two cases. J. Cosmet. Dermatol. 2022, 21, 844–849. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, Y.; Su, Z.; Wu, S.; Li, Y.; Yi, J.; Lai, W.; Chen, J.; Zheng, Y. hMSC exosomes as a novel treatment for female sensitive skin: An in vivo study. Front. Bioeng. Biotechnol. 2022, 10, 1053679. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Chung, H.; Jung, H.; Song, H.K.; Park, E.; Choi, H.S.; Jung, K.; Choe, H.; Yang, S.; Oh, E.S. Extracellular vesicles from Korean Codium fragile and Sargassum fusiforme negatively regulate melanin synthesis. Molecules Cells 2021, 44, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, E.G.; Kang, S.; Sung, J.H.; Chung, H.M.; Kim, D.H. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: A randomized, controlled, blinded split-face study. Ann. Dermatol. 2014, 26, 584–591. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y.; Cho, A.R.; Kim, Y.S. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl. Med. 2020, 9, 839–849. [Google Scholar] [CrossRef]
- Zhou, B.R.; Zhang, T.; Bin Jameel, A.A.; Xu, Y.; Xu, Y.; Guo, S.L.; Wang, Y.; Permatasari, F.; Luo, D. The efficacy of conditioned media of adipose-derived stem cells combined with ablative carbon dioxide fractional resurfacing for atrophic acne scars and skin rejuvenation. J. Cosmet. Laser Ther. 2016, 18, 138–148. [Google Scholar] [CrossRef]
- Wang, X.; Shu, X.; Huo, W.; Zou, L.; Li, L. Efficacy of protein extracts from medium of adipose-derived stem cells via microneedles on Asian skin. J. Cosmet. Laser Ther. 2018, 20, 237–244. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Pratiwi, F.D.; Herwanto, N.; Citrashanty, I.; Indramaya, D.M.; Murtiastutik, D.; Sukanto, H.; Rantam, F.A. The effects of amniotic membrane stem cell-conditioned medium on photoaging. J. Dermatol. Treat. 2019, 30, 478–482. [Google Scholar] [CrossRef]
- Available online: https://www.dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=bb0c73a8-daa0-49ee-b2b9-cfa3d78416d2 (accessed on 28 June 2024).
- Available online: https://www.fda.report/DailyMed/a11bc2f2-93db-49e7-8c89-86acd1dd08f3#google_vignette (accessed on 28 June 2024).
- Available online: https://www.7dermacenter.com/deals/ngf-574h-stem-cells-hair-tonic/ (accessed on 28 June 2024).
- Available online: https://www.fda.report/DailyMed/6b1dab05-16b0-4947-875d-48f8b4a3b1d9#google_vignette (accessed on 28 June 2024).
- Asadpour, A.; Yahaya, B.H.; Bicknell, K.; Cottrell, G.S.; Widera, D. Uncovering the gray zone: Mapping the global landscape of direct-to-consumer businesses offering interventions based on secretomes, extracellular vesicles, and exosomes. Stem Cell Res. Ther. 2023, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Brembilla, N.C.; Vuagnat, H.; Boehncke, W.H.; Krause, K.H.; Preynat-Seauve, O. Adipose-derived stromal cells for chronic wounds: Scientific evidence and roadmap toward clinical practice. Stem Cells Transl. Med. 2023, 12, 17–25. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.; Laurencin, C.T. Emergence of the stem cell secretome in regenerative engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Kumar, P.L.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Kupcova Skalnikova, H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013, 95, 2196–2211. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, R.H.; Rusdiana, T.; Wathoni, N. Mesenchymal stem cell secretome for dermatology application: A review. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Yuan, T.; Zhang, C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef]
- Lin, T.J.; Huang, Y.L.; Kang, Y.N.; Chen, C. Effectiveness of topical conditioned medium of stem cells in facial skin nonsurgical resurfacing modalities for antiaging: Systematic review and meta-analysis of randomized controlled trials. Aesthet. Plast. Surg. 2023, 47, 799–807. [Google Scholar] [CrossRef]
- Zimber, M.P.; Mansbridge, J.N.; Taylor, M.; Stockton, T.; Hubka, M.; Baumgartner, M.; Rheins, L.; Hubka, K.; Brandt, E.N.; Kellar, R.; et al. Human cell-conditioned media produced under embryonic-like conditions result in improved healing time after laser resurfacing. Aesthet. Plast. Surg. 2012, 36, 431–437. [Google Scholar] [CrossRef]
- Takahashi, H.; Ohnishi, S.; Yamamoto, Y.; Hayashi, T.; Murao, N.; Osawa, M.; Maeda, T.; Ishikawa, K.; Sakamoto, N.; Funayama, E. Topical application of conditioned medium from hypoxically cultured amnion-derived mesenchymal stem cells promotes wound healing in diabetic mice. Plast. Reconstruct. Surg. 2021, 147, 1342–1352. [Google Scholar] [CrossRef]
- Guo, S.; Wang, T.; Zhang, S.; Chen, P.; Cao, Z.; Lian, W.; Guo, J.; Kang, Y. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol. Cell. Biochem. 2020, 463, 67–78. [Google Scholar] [CrossRef]
- Zheng, Y.; Campbell, E.C.; Lucocq, J.; Riches, A.; Powis, S.J. Monitoring the Rab27 associated exosome pathway using nanoparticle tracking analysis. Exp. Cell Res. 2013, 319, 1706–1713. [Google Scholar] [CrossRef]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. 2017, 96, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mat. 2022, 25, 732–747. [Google Scholar] [CrossRef]
- Davies, O.G.; Williams, S.; Goldie, K. The therapeutic and commercial landscape of stem cell vesicles in regenerative dermatology. J. Contr. Rel. 2023, 353, 1096–1106. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Pinto, H.; Sánchez-Vizcaíno Mengual, E. Exosomes in the real world of medical aesthetics: A review. Aesthet. Plast. Surg. 2024, 48, 2513–2527. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/kimera-labs-inc-649343-09012023 (accessed on 28 June 2024).
- Available online: https://www.kimeralabs.com/kimera-labs-receives-fda-phase-i-iia-ind-approval-for-its-msc-exosomes-human-study/ (accessed on 28 June 2024).
- Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/cosmetics-us-law (accessed on 28 June 2024).
- Available online: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf (accessed on 28 June 2024).
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic values of exosomes in cosmetics, skin care, tissue regeneration, and dermatological diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, S.D.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Lee, J.H.; Park, S.R.; Youn, J.; et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 2020, 115, 104686. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.; Bae, S. Whitening and moisturizing enhancing effects of three-dimensional human adipose-derived mesenchymal stem cell-conditioned medium-containing cream. J. Cosmet. Dermatol. 2023, 22, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, B.; Kim, S.; Lee, Y.I.; Kim, J.; Lee, J.H. The effect of human umbilical cord blood-derived mesenchymal stem cell media containing serum on recovery after laser treatment: A double-blinded, randomized, split-face controlled study. J. Cosmet. Dermatol. 2020, 19, 651–656. [Google Scholar] [CrossRef]
- Umar, A.K. Stem cell’s secretome delivery systems. Adv. Pharm. Bull. 2023, 13, 244–258. [Google Scholar] [CrossRef]
- Jeong, S.H. Analytical methods and formulation factors to enhance protein stability in solution. Arch. Pharm. Res. 2012, 35, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef]
- Available online: https://patents.google.com/patent/US20160158291A1/en (accessed on 28 June 2024).
- Szcześ, A.; Jurak, M.; Chibowski, E. Stability of binary model membranes--prediction of the liposome stability by the Langmuir monolayer study. J. Colloid Interface Sci. 2012, 372, 212–216. [Google Scholar] [CrossRef]
- Prasadani, M.; Kodithuwakku, S.; Pennarossa, G.; Fazeli, A.; Brevini, T.A.L. Therapeutic potential of bovine milk-derived extracellular vesicles. Int. J. Mol. Sci. 2024, 25, 5543. [Google Scholar] [CrossRef] [PubMed]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Ves. 2022, 11, e12238. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Ves. 2022, 11, e12162. [Google Scholar] [CrossRef]
- Rogulska, O.; Vackova, I.; Prazak, S.; Turnovcova, K.; Kubinova, S.; Bacakova, L.; Jendelova, P.; Petrenko, Y. Storage conditions affect the composition of the lyophilized secretome of multipotent mesenchymal stromal cells. Sci. Rep. 2024, 14, 10243. [Google Scholar] [CrossRef]
- Driscoll, J.; Yan, I.K.; Patel, T. Development of a lyophilized off-the-shelf mesenchymal stem cell-derived acellular therapeutic. Pharmaceutics 2022, 14, 849. [Google Scholar] [CrossRef]
- Lőrincz, Á.M.; Timár, C.I.; Marosvári, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, Á.; Ligeti, E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell. Ves. 2014, 3, 25465. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Adv. Healthc. Mater. 2022, 11, 2100538. [Google Scholar] [CrossRef]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Yazdanpanah, G.; Kanu, L.N.; Chen, E.; Kang, K.; Anwar, K.N.; Ghassemi, M.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R. Therapeutic effects of lyophilized conditioned-medium derived from corneal mesenchymal stromal cells on corneal epithelial wound healing. Curr. Eye Res. 2020, 45, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Richter, M.; de Rossi, C.; Lehr, C.M.; Fuhrmann, K.; Fuhrmann, G. Author correction: Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci. Rep. 2019, 9, 15702. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, W.; Klinke, D.J. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef]
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163. [Google Scholar] [CrossRef]
- Sowemimo-Coker, S.O.; Goodrich, R.P.; Zerez, C.R.; Tanaka, K.R. Refrigerated storage of lyophilized and rehydrated, lyophilized human red cells. Transfusion 1993, 33, 322–329. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhang, Y.; Hong, S.; Li, L.; Liu, Z. Effects of lyophilization and rehydration on membrane surface antigens of human red blood cells. Cryo Let. 2016, 37, 53–58. [Google Scholar]
- Sane, P.; Bogner, R.H.; Bhatnagar, B.; Tchessalov, S. Reconstitution of highly concentrated lyophilized proteins: Part 1 Amorphous formulations. J. Pharm. Sci. 2020, 109, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Patel, S.M.; Bogner, R.H. Reconstitution time for highly concentrated lyophilized proteins: Role of formulation and protein. J. Pharm. Sci. 2020, 109, 2975–2985. [Google Scholar] [CrossRef]
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davagnino, J.; Rambhatla Gupta, S. Lyophilized drug product cake appearance: What is acceptable? J. Pharm. Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
| Study Reference | Clinical Indication | Active Ingredients/ Concomitant Treatment | Clinical Results | Product/Protocol Details |
|---|---|---|---|---|
| Cho et al. [86] | Skin brightening | Exosomes from human adipose tissue-derived SC CM | Significant reduction in melanin levels; improvement in skin brightness | Topical formulation 1 with glycerin, 1,2-hexanediol, L-arginine, xanthan gum, carbopol, water for injection |
| Jang et al. [93] | Skin brightening | EVs from Codium fragile and Sargassum fusiforme | Improvement in skin brightness | Cream containing Codium fragile EVs, 5 µg/mL |
| Wang et al. [97] | Skin rejuvenation | Protein extracts from ADSCs/Microneedles | Improvement in melanin index, luminosity, brightness, elasticity, and wrinkles | Protein extracts from ADSCs |
| Proffer et al. [90] | Skin rejuvenation | Topical platelet exosomes for skin rejuvenation | Improvement in skin health; reduction in redness, wrinkles, and melanin production; improvement in luminosity and color evenness | Intensive Repair Serum from Rion containing human leukocyte-reduced apheresed platelet extracts |
| Lee et al. [94] | Skin rejuvenation | Human embryonic SC CM/0.25 mm microneedle roller | Significant improvement in pigmentation and wrinkles | CM secretory factors of endothelial precursor cells from human embryonic SCs |
| Chernoff et al. [88] | Tissue biostimulation | Exosomes from placental MSCs/Injected with CaHA/Cavitating ultrasound/LED therapy | Enhanced tissue biostimulation | Exosomes from Kimera Labs, 1 mL containing 106 exosomes, botulinum toxin, HA, and CaHA |
| Prakoeswa et al. [98] | Photoaging | Amniotic membrane SC CM/Microneedling | Significant improvement in photoaging 2 | Amniotic membrane SC CM |
| Lueangarun et al. [85] | Androgenetic alopecia, hair repigmentation | Exosomes from human adipose-derived MSCs/Fractional picosecond laser | Hair regrowth; repigmentation of gray hair and poliosis circumscripta | ASCE + HRLV-S 1: 20 mg of lyophilized exosomes with 1010 exosome particles |
| Tak et al. [95] | Androgenetic alopecia | Adipose-derived SC extract for androgenetic alopecia | Increase in hair count and hair diameter | T-Stem product: 1% ADSCE-CE in distilled water/Gentle massage |
| Han et al. [71] | Dupilumab-related facial redness in patients with atopic dermatitis | Exosomes from human adipose-derived MSCs | Decreased erythema; reduced expression of inflammatory molecules; increased expression of angiogenesis proteins | ASCE SRLV-S 1: 20 mg of lyophilized exosomes applied with prism sonophoresis |
| Park et al. [91] | Dupilumab-related facial redness in patients with atopic dermatitis | Human adipose-derived MSC exosomes for dupilumab-related facial redness | Improvement in erythematous facial lesions | ExoCoBio technology 1, 2 × 109 particles/mL/Electroporation |
| Ye et al. [92] | Female sensitive skin | Human MSC exosomes for female sensitive skin | Improved roughness, scaling, erythema, tension, burning, and itching symptoms | Exosomes from human MSCs |
| Kwon et al. [87] | Acne scars | Exosomes from human adipose tissue-derived SC CM/Fractional CO2 laser | Significant improvement in acne scars; less erythema; reduced post-treatment downtime | ASCE gel 1, 1,2-hexanediol, glycerin, ammonium acryloyldimethyltaurate/VP copolymer, L-arginine, water for injection |
| Zhou et al. [96] | Atrophic acne scars and skin rejuvenation | ADSC-CM/Fractional CO2 laser resurfacing | Improvement in skin hydration, elasticity, collagen, and elastin density | ADSC-CM |
| Wang et al. [89] | Melasma | hUCMSC-exosomes/Non-ablative fractional laser | Improvement in melasma symptoms | hUCMSC-exosomes with various non-ablative treatments |
| Company (Headquarters) | Product | Formulation Presentation | Active Ingredient Source | Uses/Applications | Storage Information 2 |
|---|---|---|---|---|---|
| ExoCoBio/Benev (Seoul, South Korea) | ASCEplus SRLV/HRLV/IRLV | 20 mg lyophilizate + 5.0 mL solution | Plant/Rosa damascena | Topical/Skin rejuvenation | 2–8 °C |
| ExoCoBio/Benev (Seoul, South Korea) | ExoBalm | 20 mg lyophilizate capsule + 20 mL cream | Plant/Rosa damascena | Topical/Skin rejuvenation | 4–8 °C during 28 days after reconstitution |
| ExoCoBio/Benev (Seoul, South Korea) | Soothing Gel Mask | Gel mask | Plant/Rosa damascena | Topical/Calming, cooling, recovery, hydration | Ambient |
| Croma Pharma (Leobendorf, Austria) | EXO/E Serum | Solution | Plant/Ustilago cynodontis, Piper nigrum L SC, Withania somnifera root SCs | Topical/Skin revitalizing complex | Ambient |
| Stemica Labs (Beirut, Lebanon) | Secretome from UCMSCs | Solution | UCMSCs | Mesotherapy/Skin rejuvenation and hair restoration | Ambient |
| Medipost (Seongnam City, South Korea) | NGF-574H Hair Serum | Solution | CM of hUCBMSCs | Topical/Hair growth in androgenic alopecia | Ambient |
| Medipost (Seongnam City, South Korea) | NGF-574H Solution | Solution (mesotherapy; 3 to 6 sessions every 2 weeks) | CM of hUCBMSCs | Mesotherapy | Ambient |
| Rion Aesthetics (Rochester, MN, USA) | Intense Serum | Serum | Human platelet extract | Topical/Anti-aging skin care, skin rejuvenation | Ambient |
| Exocel Bio (San Diego, CA, USA) | Exovex Revive | 5.0 mL cryopreserved solution | Placental MSCs | Topical, microinfusion after microneedling | −80 °C to −20 °C |
| Exoqure, Resilielle (Los Angeles, CA, USA) | Resilielle Age Zero Exosomes | 5.0 mL cryopreserved solution | WJ-MSC | Topical with microneedling, laser | −80 °C, 15-month shelf life; −20 °C, 6-month shelf life; refrigerator, 3-month shelf life |
| JuveXo (Miami, FL, USA) | JuveXO Skin | 5.0 mL solution | Umbilical MSCs | Topical with microneedling, dermabrasion, laser therapy | Ambient |
| PrimaCure (Incheon, South Korea) | E-50 Skin: Dry Ampoule | Lyophilizate | Salmon-tested cells cultivated in salmon embryonic SC media | Topical/Skin rejuvenation, skin inflammation, hair loss, hair growth | Ambient |
| AnteAge MDX (Irvine, CA, USA) | Exosome Solution | Lyophilizate + 6 mL HA solution | hBMSCs and hUCSCs | Topical with microneedling, radiofrequency, laser, and other ablative treatments | 2–8 °C |
| DP Derm (North Miami Beach, FL, USA) | MG-Exo-skin Serum | 5 mL solution (5×) | MSCs | Topical with microneedling | Ambient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, C.; Porcello, A.; Chemali, M.; Raffoul, W.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Applegate, L.A.; Pelissier Vatter, F.; et al. Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation. Cosmetics 2024, 11, 154. https://doi.org/10.3390/cosmetics11050154
Rodriguez C, Porcello A, Chemali M, Raffoul W, Marques C, Scaletta C, Lourenço K, Abdel-Sayed P, Applegate LA, Pelissier Vatter F, et al. Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation. Cosmetics. 2024; 11(5):154. https://doi.org/10.3390/cosmetics11050154
Chicago/Turabian StyleRodriguez, Clara, Alexandre Porcello, Michèle Chemali, Wassim Raffoul, Cíntia Marques, Corinne Scaletta, Kelly Lourenço, Philippe Abdel-Sayed, Lee Ann Applegate, Fanny Pelissier Vatter, and et al. 2024. "Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation" Cosmetics 11, no. 5: 154. https://doi.org/10.3390/cosmetics11050154
APA StyleRodriguez, C., Porcello, A., Chemali, M., Raffoul, W., Marques, C., Scaletta, C., Lourenço, K., Abdel-Sayed, P., Applegate, L. A., Pelissier Vatter, F., & Laurent, A. (2024). Medicalized Aesthetic Uses of Exosomes and Cell Culture-Conditioned Media: Opening an Advanced Care Era for Biologically Inspired Cutaneous Prejuvenation and Rejuvenation. Cosmetics, 11(5), 154. https://doi.org/10.3390/cosmetics11050154









