1. Introduction
In recent years, there has been a significant increase in the number of esthetic facial rejuvenation procedures. Notably, the application of dermal fillers stands out due to their less invasive nature and lower cost compared to traditional surgical procedures. Hyaluronic acid is the most commonly used dermal filler worldwide. It is a biodegradable glycosaminoglycan that is widely distributed in the extracellular matrix. More recently introduced to the market, collagen biostimulators are considered the latest generation of dermal fillers. Poly-L-lactic acid, calcium hydroxyapatite, and polycaprolactone (PCL) are biodegradable materials capable of stimulating new collagen synthesis [
1,
2,
3].
PCL, which is the most recent among the collagen biostimulators, is a soft, biodegradable, and bioabsorbable polymer [
4]. PCL received European Conformity (CE) certification as a dermal filler in 2009 and was approved by the National Health Surveillance Agency (ANVISA) in Brazil in 2018. In the United States, polycaprolactone has been registered as a medical device since 2001. The PCL-based collagen biostimulator, Ellansé
® (Sinclair Pharma, London, United Kingdom), is commercially available. It comprises 30% PCL microspheres with a diameter of 25–50 μm and 70% carboxymethylcellulose gel (CMC) [
4,
5,
6]. The Ellansé dermal filler exhibits an immediate volumizing effect, which is attributed to the CMC gel. Over time, the CMC gel is reabsorbed and gradually replaced by the newly formed collagen. The maintenance of volume is facilitated by PCL microspheres, which stimulate the formation of collagen. The microspheres, which are protected from phagocytosis, degrade into non-toxic and bioabsorbable products. These products are metabolized into CO
2 and H
2O, and are excreted through normal physiological pathways. The total bioresorption time depends on the length of the PCL polymer chain, that is, the longer the chain, the longer it lasts in the body. The volumizing effect is enhanced by the collagen that is produced and the formation of a 3D framework [
1,
5,
6]. Among the advantages of this treatment, we can highlight its extended biodegradation time, which translates to longer-lasting results. Additionally, its high capacity to stimulate collagen production sets PCL apart from conventional hyaluronic acid injectables [
1,
6].
PCL dermal filler is used to reduce signs of aging such as sagging, wrinkles, and loss of volume. The recommended areas for the application of PCL include the frontal, temporal, malar, submalar, and nasal regions; the oral commissures; the chin; and the lateral areas of the eyebrow. Nevertheless, it is not advised to use it on the lip, glabella, or periorbital area. These regions have little subcutaneous fat, which increases the risk of product visibility and nodule formation [
1,
2]. At locations where PCL is injected, the most common early reactions are hematomas, erythema, edema, and ecchymosis. These reactions typically resolve spontaneously in a short time [
7,
8]. The occurrence of nodules is less common and is associated with the method of filler application [
1]. Philiberte et al. [
9] and Skrzypek et al. [
10] reported clinical cases of multiple nodules on the face of patients, months after the application of the product. The diagnosis, obtained through an anatomopathological examination, revealed the presence of foreign-body granulomas.
PCL has been widely used as a collagen biostimulator in esthetic procedures on the face. However, there are still a limited number of scientific studies investigating tissue responses in the orofacial region. The objective of the present study was to evaluate, through macroscopic and histopathological analysis, early and late reactions resulting from the submucosal and subcutaneous application of polycaprolactone in the orofacial region of rats.
2. Materials and Methods
2.1. Sample
This study was approved by the Scientific Committee of the School of Health and Life Sciences and by the Ethics Committee on the Use of Animals (CEUA), both from the Pontifical Catholic University of Rio Grande do Sul (PUCRS), under protocol 11121. The sample consisted of 48 female Wistar rats, approximately 90 days old and weighing between 200 and 300 g at the beginning of the experiment. The animals were obtained from the Center for Experimental Biological Models (CeMBE/PUCRS). The sample size calculation was conducted using IBM SPSS Statistics for Windows version 22.0. Fisher’s exact test was employed, considering the comparison of six subgroups, at a significance level of 5%. A total of 42 rats were estimated, 7 in each subgroup. Due to the risk of losing animals during the study, the sample size was increased by approximately 15%. This adjustment involved adding 3 animals per group, resulting in a total of 48 rats, with 8 rats per subgroup.
The animals were kept in micro-isolators equipped with air inlet and outlet filters, which had a controlled humidity, a temperature of 23 °C, and 12 h light–dark lighting cycles. The animals were housed in cages, with four rats per box, receiving a standard diet of irradiated pelleted feed Nuvilab-Cr1
® (Nuvilab, Colombo, PR, Brazil) and filtered water
ad libitum. After 10 days of acclimation, the animals were weighed and randomly divided into two groups—the polycaprolactone (PCL) group, which received the application of Ellansé
® S, and the control group, which received saline solution. Each group was divided into three subgroups—24 h, 30 days, and 90 days (
Figure 1). This study was conducted in accordance with ARRIVE 2.0 guidelines for experiments on animals.
2.2. Procedures
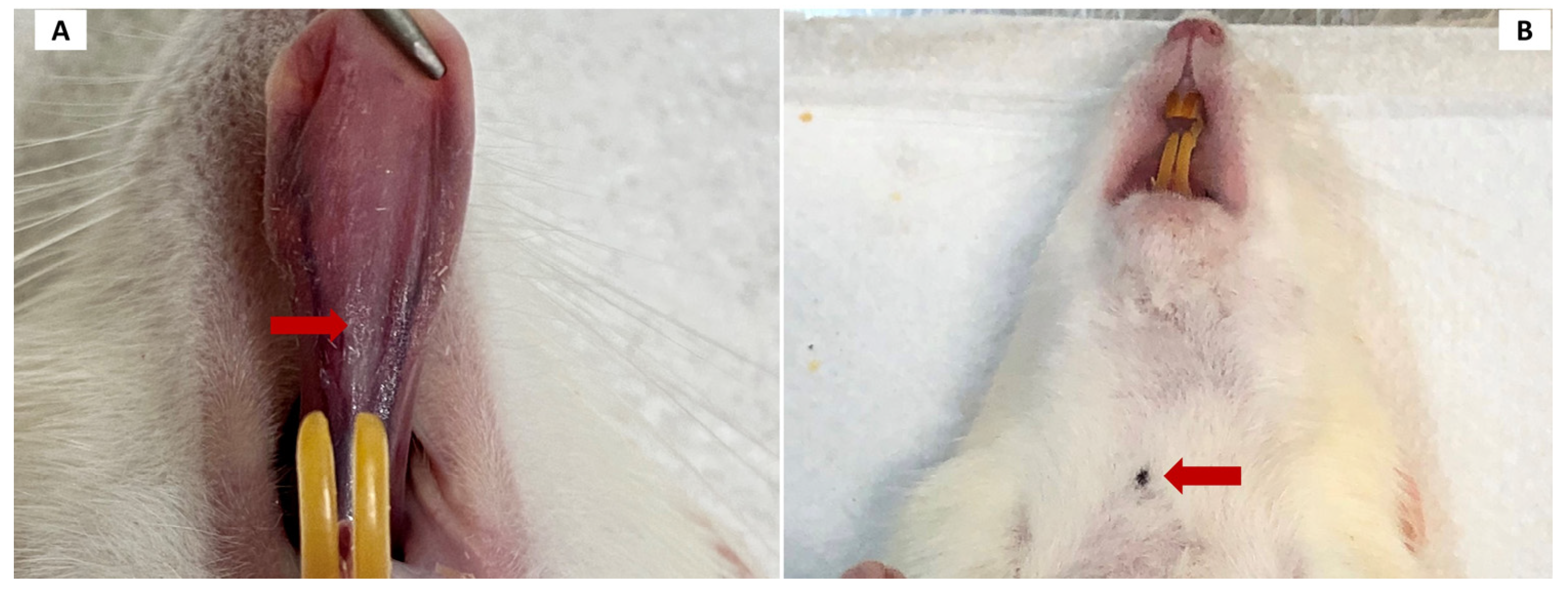
Before the application of PCL, the animals were weighed and anesthetized with 10% ketamine hydrochloride (100 mg/kg) and 2% xylazine hydrochloride (10 mg/kg) until the loss of protective reflexes. The material was applied at two points—in the middle third of the ventral tongue, 2 mm to the left of the midline (7 mm in front of the frenulum), and in the submandibular region (
Figure 2) [
11,
12]. In total, 0.07 mL was applied in the ventral tongue and 0.1 mL in the submandibular region [
12,
13]. The substance was injected through a 27 G needle, adapted to a 1.0 mL syringe. The needle was inserted at an angle of approximately 30°, with the bevel facing downwards for the deposition of the material. The region was palpated with the free hand to confirm the insertion of the needle in the layer of interest [
11,
12,
13].
In the control group, the same technique as employed in the PCL group was utilized; however, a saline solution was injected. Eight animals from each group were euthanized 24 h after the application of either PCL or saline solution. The remaining animals were euthanized 30 and 90 days later. Euthanasia was induced via the inhalation of an overdose of isoflurane. The animals were monitored throughout the experiment, and pain and stress were minimized with the application of analgesic—dipyrone (150 mg/kg/day via IP).
2.3. Macroscopic Assessment
Following euthanasia, a macroscopic evaluation of the sites where the material was applied was conducted. Reactions such as erythema, edema, ulceration, suppuration, nodule formation, and hematoma were recorded.
2.4. Specimen Preparation
After macroscopic evaluation, the tongue, skin of the submandibular region, and the submandibular glands were dissected. The specimens were then immersed in a 10% buffered formalin solution where they remained for 24 h. After fixation, the specimens were sectioned, underwent routine histological processing, and were embedded in paraffin. Two sections, each 5 µm thick, were obtained from each specimen of the tongue and skin. These sections were stained with hematoxylin-eosin (H&E) and Masson’s trichrome. Additionally, the submandibular gland specimens were stained with H&E.
2.5. Histological Analysis
Histological analysis was performed by a calibrated and blinded examiner. For calibration, 20 microscopic fields were analyzed in duplicate, at intervals of seven days, in no predetermined order. The intra-examiner agreement was assessed using the intraclass correlation coefficient, and the obtained value was 0.86.
Initially, the slides were qualitatively analyzed across their entire length using an Olympus® light microscope (model BX50, Olympus, Tokyo, Japan). The inflammatory process, the presence of the material, and the thickness of the epidermis/dermis were evaluated in the H&E stained slides The percentage of collagen was evaluated and quantified in the slides stained with Masson’s trichrome.
On the tongue slides, which were stained in H&E, three histological fields adjacent to the sites where the material was injected were captured at a magnification of 100×. The captures were performed using an Olympus
® light microscope (model BX-50, Olympus, Tokyo, Japan) connected to an Olympus DP-73 digital camera. These images were saved in the Tagged Image File Format (TIFF). Inflammatory activity was evaluated by the presence or absence of neutrophils, lymphocytes, plasma cells, eosinophils, macrophages, multinucleated giant cells, edema, and hyperemia. Inflammation was quantified using the
Image-Pro Plus 4.1 software, (Media Cybernetics, Bethesda, MD, EUA). A grid of 540 points was superimposed on each image. The percentages of material present, inflammatory tissue, edema, and normal tissue were calculated using the manual point counting technique [
12].
From the skin samples taken from the submandibular region and stained with H&E, three histological fields were captured at 40× magnification. The images were then saved in TIFF. The total thickness of the epidermis/dermis was determined with the measurements tool, also from the Image-Pro Plus 4.1 software. At three different points within each histological field, a cross-sectional line was drawn from the uppermost point of the epithelial tissue to the beginning of the adipose tissue, maintaining the same angle. For each lamina, the mean total thickness of the epidermis/dermis was established.
On the tongue and skin slides, which were stained with Masson’s trichrome, three histological fields adjacent to the site where the material was applied were captured at 100X magnification and saved in TIFF. The percentage of collagen, represented by the blue color, was determined using the semi-automated segmentation technique in the Image-Pro Plus 4.1 software. The proportion of collagen fibers was determined by calculating the area occupied by them relative to the total area of each field.
The histological sections of the submandibular glands, which were stained in H&E, were analyzed qualitatively for the presence of PCL, inflammatory activity, and morphological changes in the glandular parenchyma and stroma.
2.6. Statistical Analysis
Data were initially analyzed using descriptive statistics. To compare the percentage of material present, inflammatory process, edema, and normal tissue between the experimental times in the PCL group, the ANOVA test was used, followed by Tukey’s post hoc test. To compare epidermis/dermis thickness and collagen percentage between the PCL and control groups, Student’s t-test was used. In the PCL group, Student’s t-test was also used to compare the percentage of collagen (on the tongue and skin) between the periods of 30 and 90 days. The value established to reject the null hypothesis was p ≤ 0.05. SPSS software version 23.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
4. Discussion
The growing demand for esthetic procedures has significantly changed the landscape of the biomedical field. Numerous materials have been used for esthetic purposes; therefore, it is crucial to understand how they function and to be aware of any potential unreported adverse effects. The current in vivo study examined the macroscopic changes and both early and late tissue histology responses induced by the PCL dermal filler. The tongue was one of the sites chosen for the application of the material because, in addition to having been used in previous studies [
11,
12,
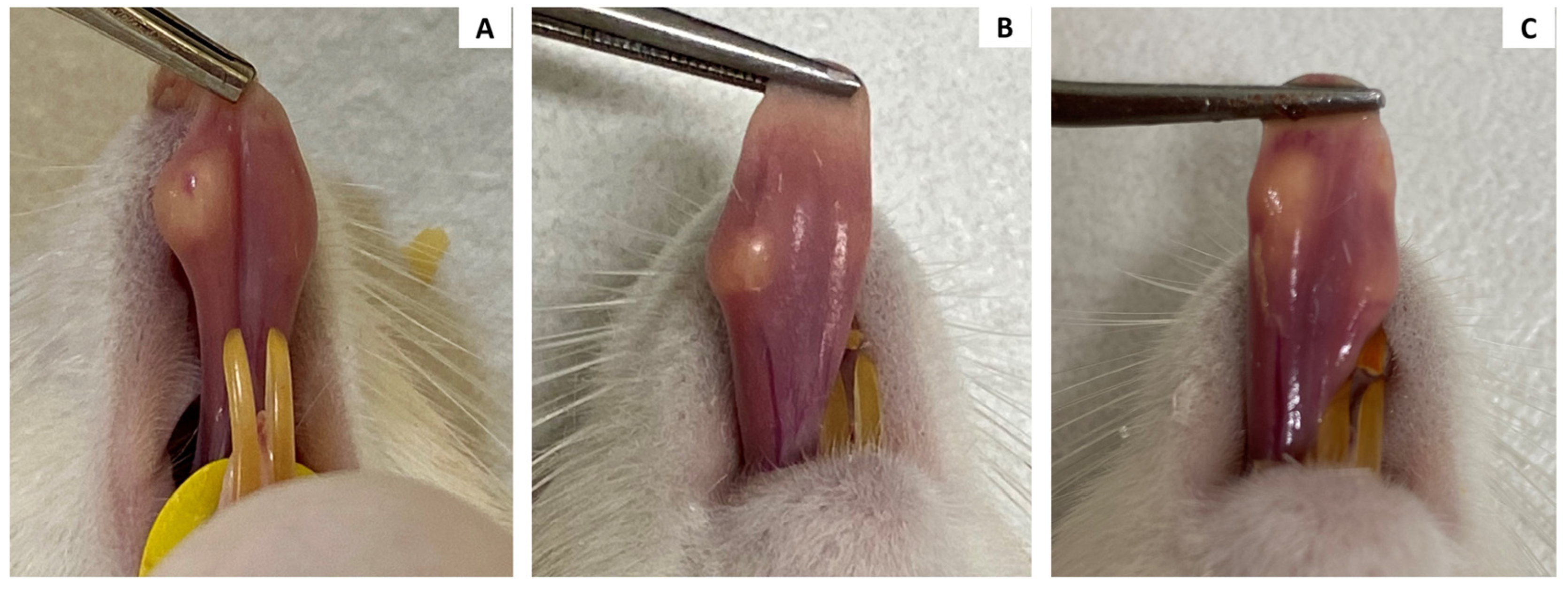
13], it is an anatomically more protected site and less vulnerable to external traumatic factors. In addition, there are few studies evaluating histological changes in orofacial tissues resulting from the application of PCL. Despite the small volume of injected material, the formation of nodular and yellowish lesions was observed on the tongue of most animals. The formation of nodules in the region where PCL is applied may result from errors in the application technique, such as very superficial injection or an excessive quantity [
1,
14,
15], which did not occur in our study. It is noteworthy that these lesions were not transient, as they remained in the animals of the 30 day and 90 day subgroups. In addition, they showed firmer consistency in these periods compared to the 24 h subgroup. The highly mobile characteristic of this anatomical structure may have contributed to the formation of the nodules. Kim [
16] pointed out that nodules formed after PCL injection can take two years or more to disappear spontaneously.
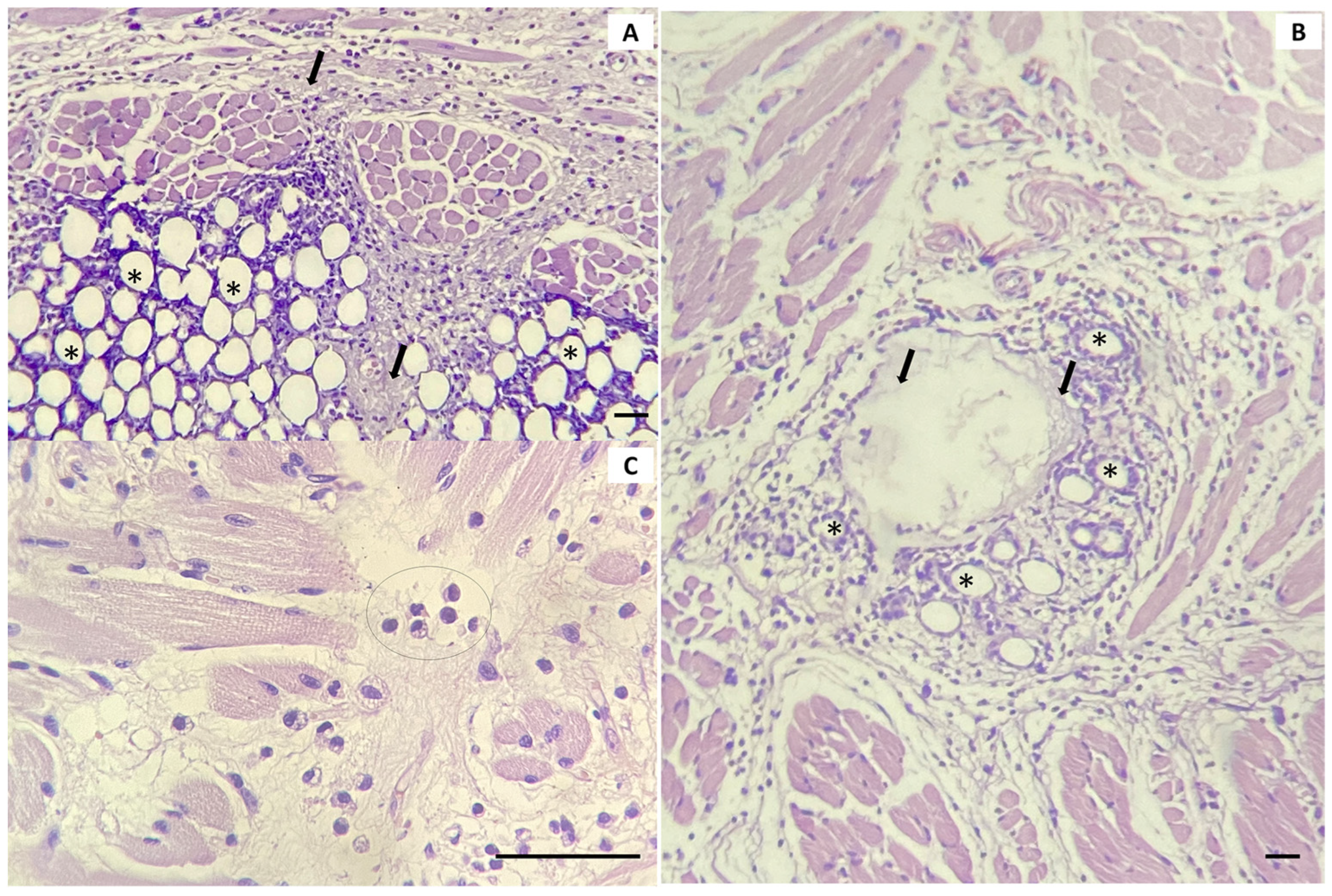
In the histological analysis, we evaluated and quantified the inflammatory process and possible reactions that the interaction of the product with the organism could trigger. Edema was quantified separately from inflammatory infiltrate, as it was more expressive at 24 h, while at 30 days and 90 days, it was not present. The results showed an increase in the inflammatory process at 30 days and 90 days, formed predominantly by macrophages and multinucleated giant cells, which were not present in the 24 h subgroup. On the other hand, the quantity of the material decreased at 30 and 90 days, which can be attributed to the reabsorption of the CMC gel. In the initial weeks, the CMC gel is responsible for the immediate volumizing effect, and, over time, it is replaced by the tissue reaction induced by PCL [
1,
2,
3,
4,
5,
6,
7,
17].
Still, on the tongue, the histological findings revealed the presence of macrophages and multinucleated giant cells around the PCL microspheres, characterizing a reaction to a foreign body. Although the histological description suggests the appearance of foreign body granulomas, according to Lemperle et al. [
18], the presence of giant cells is not a typical sign of such granulomas. Instead, it indicates the existence of a material that is too large to be phagocytosed by macrophages. These giant cells fuse to combat the foreign substance. In other words, the microparticles are intentionally implanted to stimulate a foreign body reaction. Their encapsulation by fibrous tissue, associated with the presence of cells such as macrophages, ensures a more flexible implant. In another study, the author pointed out that giant cells can produce several interleukins, which stimulate fibroblasts to produce collagen during the degradation of PCL particles [
16]. These histological findings help us to better understand the nodular lesions present on the tongue. After 24 h, the nodules were softer because of the large amount of CMC gel. However, at 30 and 90 days, the consistency was firmer as a result of a cluster of PCL microspheres surrounded by multinucleated giant cells and collagen fibers upon microscopic examination.
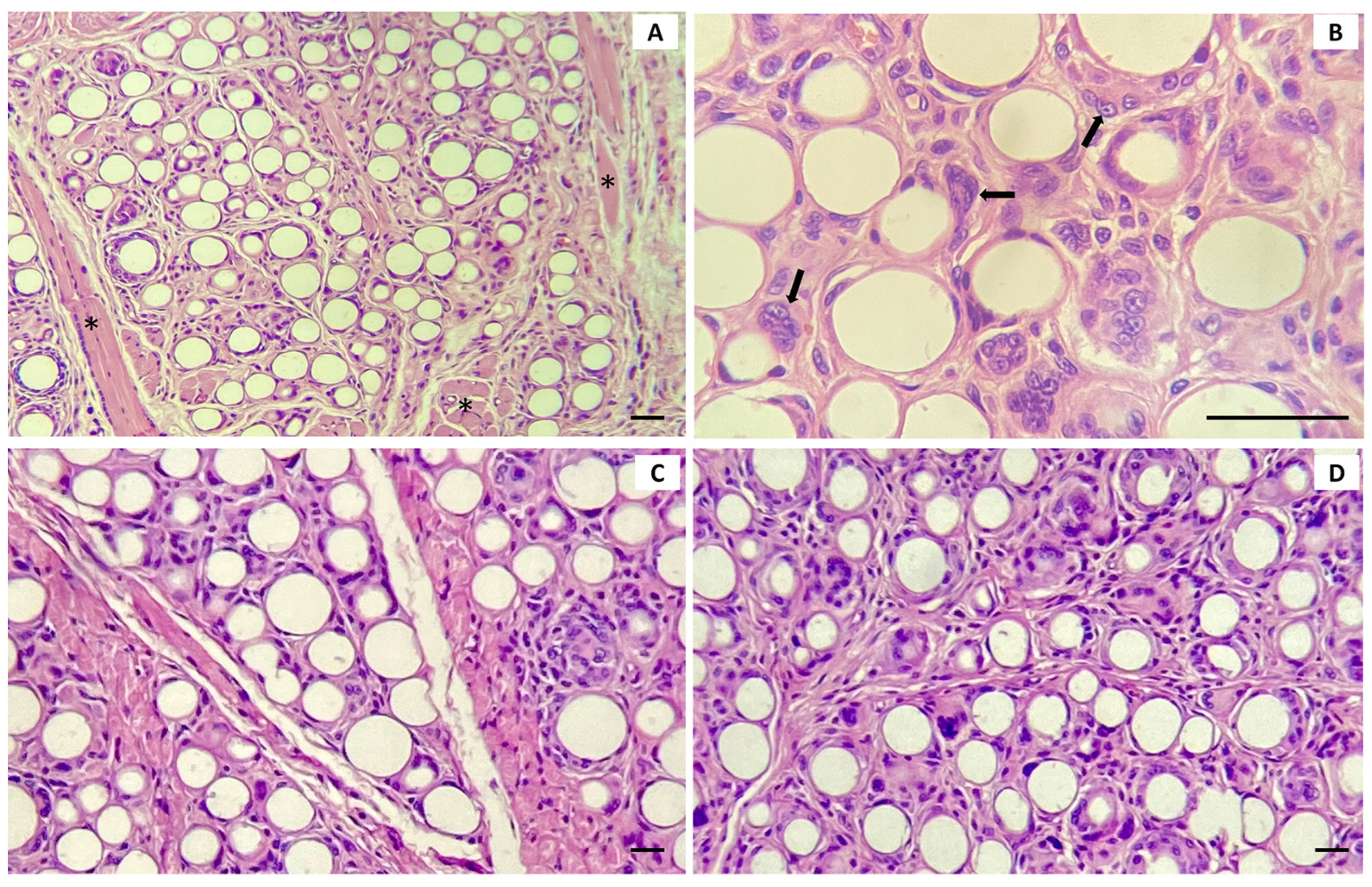
In this study, it was possible to observe the neoformation of collagen around the PCL spheres on the tongue, in the subgroups of 30 and 90 days. Although the 90 day subgroup presented values higher than the 30 day subgroup, the difference between both was not significant. This result may be related to time, as one of the limitations of this study was the 90 day period, and the variables analyzed may change over a longer period. We chose not to compare the collagen present in the tongue of the PCL group and the control group, because in the control group, the PCL spheres were not present, while in the experimental group, it was possible to observe them in all tongue samples, which may contribute to measurement biases.
In the dermis, it was possible to compare the percentage of collagen between the groups. The percentage of collagen was significantly higher in the PCL group compared to the control, both at 30 and 90 days, confirming the biostimulatory capacity of collagen that is related to the material. Yanatma et al. [
19] and Sezer et al. [
20] conducted studies comparing collagen formation in an animal model. The animals that received PCL showed a higher collagen density, which confirmed our findings. Collagen formation in the dermis after PCL filler injection involves cellular and tissue responses. Initially, the material is coated with protein, and macrophages migrate to the site, encapsulating the filler. The healing cascade then leads to collagen formation. In the early stages, there is the formation of granulation tissue and the appearance of type III collagen. This is followed by the long-term production of type I collagen [
1].
Another histological variable analyzed in our study was the thickness of the epidermis/dermis in the skin samples from the submandibular region. This variable was analyzed only in the periods of 30 and 90 days. The choice to exclude the 24 h timeframe was due to the potential bias introduced by edema. Thirty days after PCL injection, the experimental group showed significantly higher values than the control group; however, this difference was not significant after ninety days. Yanatman et al. [
19], Sezer et al. [
20], and Hong et al. [
21] also evaluated skin thickness after PCL filler injection in rats. Yanatman et al. [
19] observed that at two months, there was no significant difference between the experimental and control groups. At four months, the thickness of the dermis was significantly higher in the experimental group. In the study by Sezer et al. [
20], the thickness of the dermis in the group that received PCL was significantly greater both at two and four months. Kim [
16] highlighted that the increase in the thickness of the dermis is directly associated with the formation of collagen, which PCL induces.
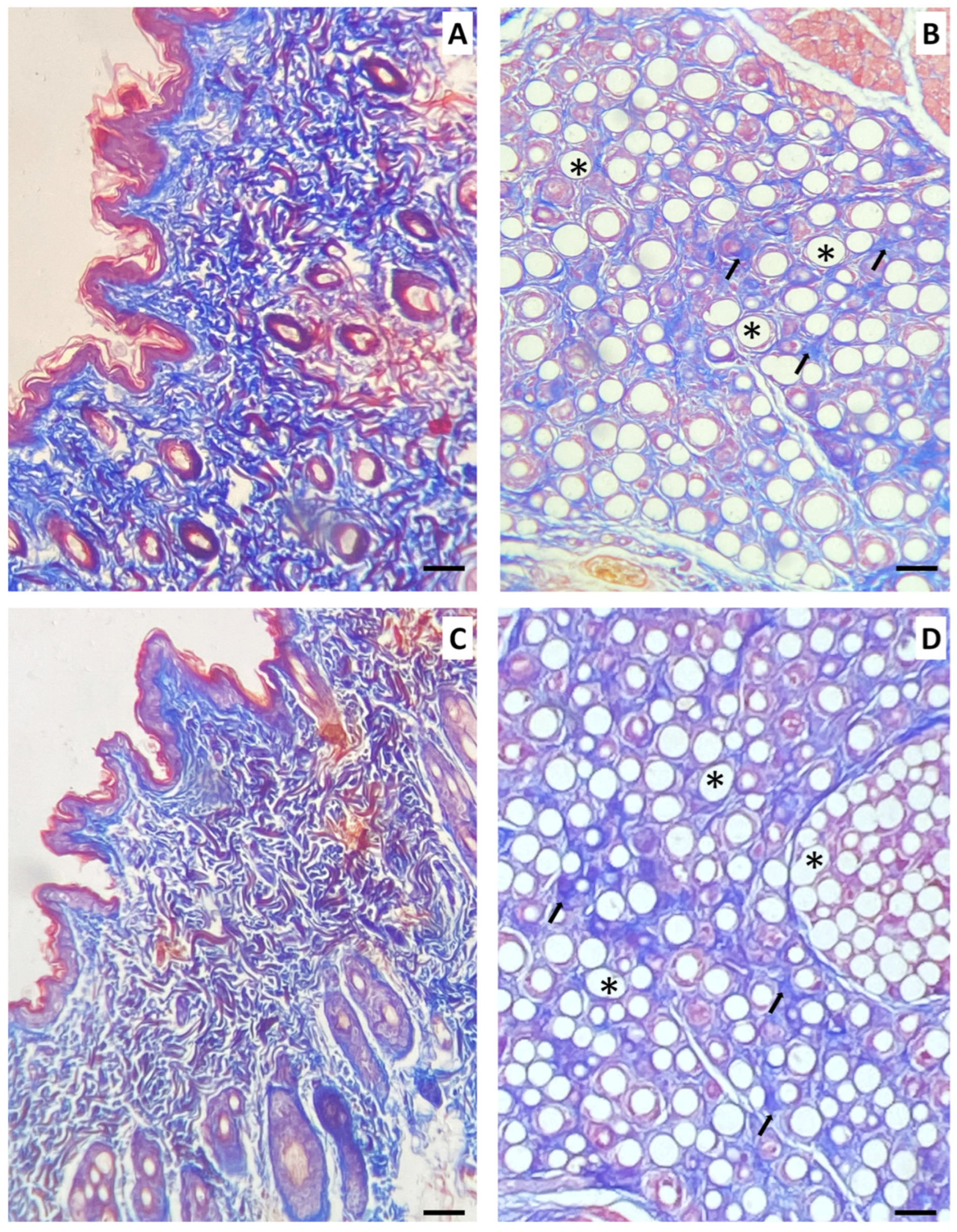
In our study, we also examined potential histological alterations in the submandibular glands. This investigation was initiated because the material was injected into a region near these anatomical structures. As described, the material was observed mainly in the glandular periphery, and despite the intense inflammatory process, no significant morphological changes were observed in the glandular parenchyma. In some of the samples, PCL spheres were observed within the gland. These spheres were surrounded by macrophages and multinucleated giant cells, yet the glandular parenchyma was preserved.
However, as already pointed out, one of the limitations of our study is the short period of analysis. It is estimated that the durability of this product within the tissues is approximately one year. As such, it has the potential to induce histological and macroscopic changes that extend beyond those observed at the 90 day mark. Another limitation of this study was the use of a saline solution in the control group, rather than the injection of CMC gel, which is the vehicle for the product used. This could have provided evidence about the influence of CMC in the outcomes studied.