High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products
Abstract
1. Introduction
2. Sustainability in Cosmetics
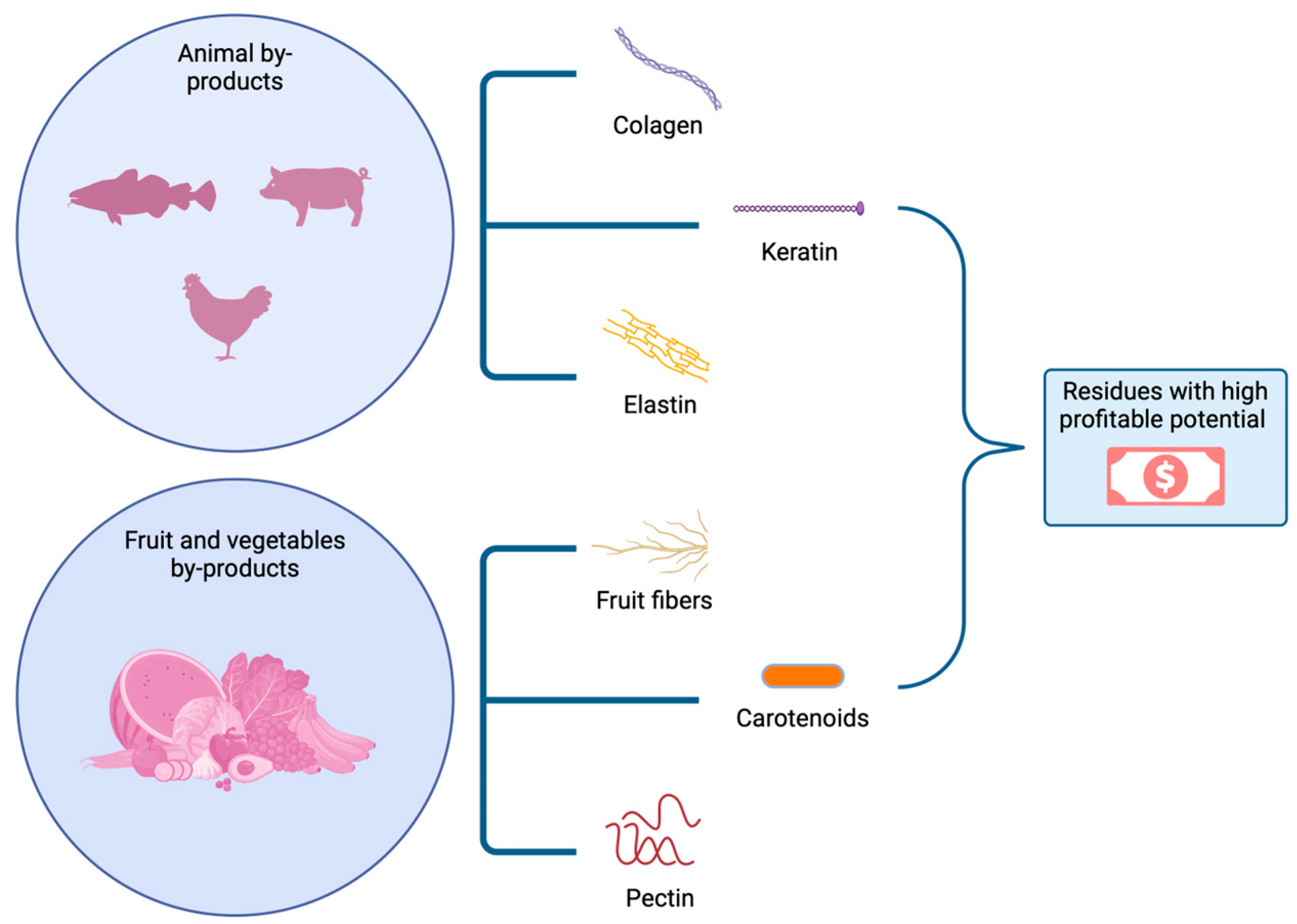
3. Sources of Plant and Animal Waste
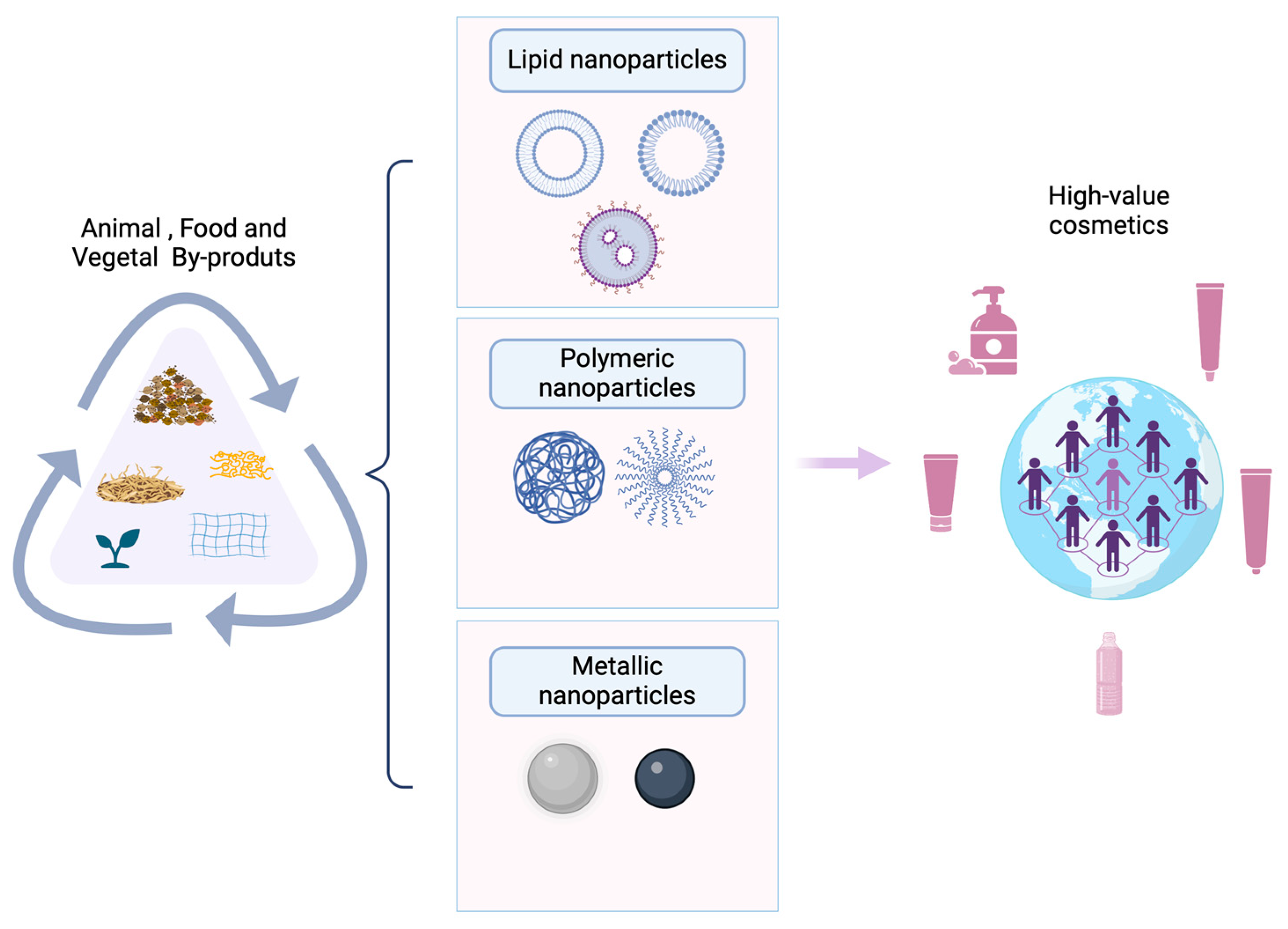
4. Nanotechnology in Cosmetics
4.1. Lipid NPs
| Type of Nanoparticle | Composition | Active Ingredient | Characterization | Physical-Chemical Properties | Formulation | Biological Performance | Reference |
|---|---|---|---|---|---|---|---|
| SLN | Glyceryl monostearate, polysorbate 80 | KDPAG | DLS, in vitro release and cytocompatibility, antioxidant and anti-aging properties, in vivo anti-wrinkling activity, histological analysis and SOD activity | 170–176 nm, PDI 0.23–0.45, ZP −22 mV | Cream | Controlled release, cytocompatibility in human keratinocytes, improved antioxidant, anti-aging, and anti-wrinkling activities | [52] |
| NLC | Bee wax, Myverol® RX GMS 95P, Chemal OA-20 and different fruit seed oils (blackcurrant, blackberry, raspberry, strawberry and plum) | PUFAs | Differential scanning calorimetry, proton nuclear magnetic resonance, DLS, oxidative stability | 274–355 nm, PDI 0.15–0.44, ZP −28–−38.5 mV, pH 3.9–4.2 | - | Increased protection against oxidative degradation | [104] |
| NLC | Precifac® ATO, Miglyol® 812 and polysorbate 60 | Caffeine | DLS, in vitro permeation and cytocompatibility, colloidal stability, morphology | 178–183 nm, PDI > 0.25, ZP −23–−30 mV | - | Spherical morphology, increased skin permeation, dose-dependent cytotoxicity in human keratinocytes, desirable colloidal stability | [105] |
| LPS | Soy lecithin, 50 mM Tris-HCl buffer (pH 8.0) | Lysolecithin | DLS, morphology | 84–144 nm | - | Spherical vesicles, successful modification of soy lecithin into lysolecithin, and production of oleic and linolenic acids. | [106] |
| NEs | Catfish oil, lemon oil, polysorbate 80, anionic co-surfactant | PUFAs + antioxidants | DLS, antioxidant, antibacterial, anti-inflammatory and cytotoxic properties | 44 nm, PDI 0.07, ZP −5 mV | - | Increased antioxidant properties, broad antibacterial spectrum, anti-inflammatory properties, and cytocompatibility in human fibroblasts | [109] |
| NEs | Raspberry seed oil, polysorbate 80 or polyglycerol ester mixture as surfactant + association with different (natural or synthetic) antioxidants | PUFAs | DLS, oxidative stability | 41–255 nm, PDI 0.059–0.125 | - | Increased protection against temperature-induced oxidative degradation | [110] |
4.2. Polymeric Nanoparticles
| Type of Nanoparticle | Composition | Active Ingredient | Characterization | Physical-Chemical Properties | Formulation | Biological Performance | Reference |
|---|---|---|---|---|---|---|---|
| Polymeric nanoparticle | Chitosan, flaxseed gum, hyaluronic acid | Ferulic acid | Size, PDI, zeta potential, TEM, FT-IR, interfacial tension and wettability. | 262.4 nm, PDI 0.25, zeta +36.2 mV | Pickering emulsion | Improved skin permeation and retention, increased stability in the emulsion | [127] |
| Polymeric nanoparticle | Poly(lactic acid), poly(vinyl alcohol) | Olive leaves extracts | Size, PDI, zeta potential, SEM, EE%, DSC, FT-IR. | 246.3 nm, PDI 0.21, zeta −27.5 mV, EE 49.2% | Cream | Increased stability in the emulsion | [129] |
4.3. Inorganic Nanoparticles
| Type of Nanoparticle | Composition | Active Ingredient | Characterization | Physical-Chemical Properties | Formulation | Biological Performance | Reference |
|---|---|---|---|---|---|---|---|
| Metallic nanoparticle | Leaf extract; hexahydrated zinc nitrate 0.1 | TiO2 | X-ray diffraction | >50 nm | Thermally stable pure crystals | Antioxidant agents | [134] |
| Metallic nanoparticle | OPE-AgNPs | Ag | UV | 15–20 nm | Aqueous solution | Bio-nanosynthesis, antitumoral, and microbial control | [135] |
| Inorganic nanoparticle | Corn aqueous extract- silver nanoparticle (ACCS-Ag NP) | ACCS | TEM | 34.7 ± 8.6 nm ACCS; 5–10 nm Ag | Hydrogel | Bacterial inactivation | [136] |
| Metallic nanoparticle | Titanium oxo solution | TiO2 | FE-SEM | 30–40 nm | Nanocrystals | Antimicrobial; anticancer; and photocatalytic activity | [137] |
| Metallic nanoparticle | Aqueous solution | ZnO | SEM | 9–14 NM | Nanocrystals | Photocatalytic activity | [138] |
| Metallic nanoparticle | Aqueous solution | ZnONPs | DLS, Zeta | 170 nm; Zeta −21.0 mV | Crystals | Antibacterial activities; antioxidant | [139] |
| Metallic nanoparticle | Aqueous solution | DtbP-AgNPs | DLS, Zeta | 78.02 nm; Zeta −33.3 mV | Biological activities showed remarkable cytotoxicity and anti-diabetic effects, along with reasonable antioxidant activity | [140] |
5. Patents of Beauty Products Derived from Plant and Animal By-Products and Their Nanotechnological Aspects
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. Towards a Circular Economy: Business Rationale for an Accelerated Transition; Ellen MacArthur Foundation: Isle of Wight, UK, 2015. [Google Scholar]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Cagno, R. Di High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S. Antioxidants in Dermatology*. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Nasir, H.; Mohd-Setapar, S.H. Natural Ingredients in Cosmetics from Malaysian Plants: A Review. Sains Malays. 2018, 47, 951–959. [Google Scholar] [CrossRef]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant Extracts and Natural Compounds Used against UVB-Induced Photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of Nanoparticles through Plant as Nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043002. [Google Scholar] [CrossRef]
- Geng, X. The Application of Nanotechnology in Natural Cosmeceuticals. Theor. Nat. Sci. 2023, 3, 110–116. [Google Scholar] [CrossRef]
- Sharma, A.; Kuhad, A.; Bhandari, R. Novel Nanotechnological Approaches for Treatment of Skin-Aging. J. Tissue Viability 2022, 31, 374–386. [Google Scholar] [CrossRef]
- Siler-Marinkovic, S. Liposomes as Drug Delivery Systems in Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 15–38. [Google Scholar]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol 2021, 14, 867–887. [Google Scholar] [CrossRef]
- Yentekakis, I.V. The 10th Anniversary of Nanomaterials—Recent Advances in Environmental Nanoscience and Nanotechnology. Nanomaterials 2022, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Report on the Implementation of the EMA-EUnetHTA Work Plan 2017–2021; European Medicines Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gran View Research Beauty And Personal Care Products Market Growth & Trends. Available online: https://www.grandviewresearch.com/press-release/global-beauty-personal-care-products-market (accessed on 24 April 2024).
- Fonseca-Santos, B.; Antonio Corrêa, M.; Chorilli, M. Sustainability, Natural and Organic Cosmetics: Consumer, Products, Efficacy, Toxicological and Regulatory Considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- Shum, K. How Cosmetics Retailer Lush Is Making Purposeful Profit through Circular Processes|GreenBiz. Available online: https://www.greenbiz.com/article/how-cosmetics-retailer-lush-making-purposeful-profit-through-circular-processes (accessed on 24 April 2024).
- Almeida, M.; Santos, D.; Soares, B.B.; Farias De Sousa, L.; Alves, E.C. Cleaner Production Alternatives for a Cosmetics Industry in Southern Bahia. Indep. J. Manag. Prod. 2021, 12, 1068–1086. [Google Scholar] [CrossRef]
- Fröhlich, E.; Steinbiß, K. Circular Economy Applies to Beauty Industry. In Responsible Consumption and Production; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Purwanto, P.; Permana-Citra, A.D. Recycling and Processing of Solid Waste into Products of the Cosmetic Packaging Industry. J. Phys. Conf. Ser. 2019, 1295, 012042. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Confetto, M.G.; Palazzo, M.; Ferri, M.A.; Normando, M. Brand Activism for Sustainable Development Goals: A Comparative Analysis in the Beauty and Personal Care Industry. Sustainability 2023, 15, 6245. [Google Scholar] [CrossRef]
- Nikolova, A. Sustainability in the Beauty and Personal Care Industry: Trends, Best Practices, and Opportunities. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Hameed, A.; Fatima, G.R.; Malik, K.; Muqadas, A.; Fazal-ur-Rehman, M. Scope of Nanotechnology in Cosmetics: Dermatology and Skin Care Products. J. Med. Chem. Sci. 2019, 2, 9–16. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “Sisters” α-Helices of Collagen, Elastin and Keratin Recovered from Animal by-Products: Functionality, Bioactivity and Trends of Application. Trends Food Sci. Technol. 2016, 51, 65–75. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.H.; Kim, S.K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M. Seeds Recovered from Industry By-Products of Nine Fruit Species with a High Potential Utility as a Source of Unconventional Oil for Biodiesel and Cosmetic and Pharmaceutical Sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Ko, H.C.; Jang, M.G.; Oh, J.M.; Park, J.Y.; Kim, J.E.; Kim, J.-W.; Baek, S.; Han, S.H.; Kim, S.-J. Changes in Chemical Composition and Antioxidant Activity of Dried Citrus Unshiu Peel after Roasting. LWT 2020, 131, 109612. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Van Tran, V.; Moon, J.Y.; Chae, M.; Park, D.; Lee, Y.C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Castro-Silva, S.; Martins, R.C. Olive Oil Extraction Industry Wastewater Treatment by Coagulation and Fenton’s Process. J. Water Process Eng. 2021, 39, 101818. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Allegro, M.; Marto, J.; Pedras, B.; Oliveira, N.G.; Paiva, A.; Barreiros, S.; Gonçalves, L.M.; Simões, P. Converting Spent Coffee Grounds into Bioactive Extracts with Potential Skin Antiaging and Lightening Effects. ACS Sustain. Chem. Eng. 2018, 6, 6289–6295. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Yi, R.; Bai, K.; Wang, G.; Tan, R.; Sun, S.; Xu, N. Electrodialysis Extraction of Pufferfish Skin (Takifugu Flavidus): A Promising Source of Collagen. Mar. Drugs 2019, 17, 25. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Ronza, P.; Garcia-Oliveira, P.; Pereira, A.G.; Losada, A.P.; Prieto, M.A.; Quiroga, M.I.; Simal-Gandara, J. Aquaculture as a Circular Bio-Economy Model with Galicia as a Study Case: How to Transform Waste into Revalorized by-Products. Trends Food Sci. Technol. 2022, 119, 23–35. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry-an Update. Cosmetics 2018, 5, 2. [Google Scholar] [CrossRef]
- Rocca, R.; Acerbi, F.; Fumagalli, L.; Taisch, M. Sustainability Paradigm in the Cosmetics Industry: State of the Art. Clean. Waste Syst. 2022, 3, 100057. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M. A Sustainable Life Cycle for Cosmetics: From Design and Development to Post-Use Phase. Sustain. Chem. Pharm. 2023, 35, 101178. [Google Scholar] [CrossRef]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A Review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Beal, T.; Gardner, C.D.; Herrero, M.; Iannotti, L.L.; Merbold, L.; Nordhagen, S.; Mottet, A. Friend or Foe? The Role of Animal-Source Foods in Healthy and Environmentally Sustainable Diets. J. Nutr. 2023, 153, 409–425. [Google Scholar] [CrossRef]
- Anastasiadis, F.; Manikas, I.; Apostolidou, I.; Wahbeh, S. The Role of Traceability in End-to-End Circular Agri-Food Supply Chains. Ind. Mark. Manag. 2022, 104, 196–211. [Google Scholar] [CrossRef]
- Rizos, V.; Behrens, A.; van der Gaast, W.; Hofman, E.; Ioannou, A.; Kafyeke, T.; Flamos, A.; Rinaldi, R.; Papadelis, S.; Hirschnitz-Garbers, M.; et al. Implementation of Circular Economy Business Models by Small and Medium-Sized Enterprises (SMEs): Barriers and Enablers. Sustainability 2016, 8, 1212. [Google Scholar] [CrossRef]
- Chang, J.; Yu, B.; Saltzman, W.M.; Girardi, M. Nanoparticles as a Therapeutic Delivery System for Skin Cancer Prevention and Treatment. JID Innov. 2023, 3, 100197. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Yu, C.; Huang, Q.; Du, Y.-Z. Nano-Drug Delivery Systems in Wound Treatment and Skin Regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef]
- Hatem, S.; Elkheshen, S.A.; Kamel, A.O.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Elezaby, R.S.; El Hoffy, N.M. Functionalized Chitosan Nanoparticles for Cutaneous Delivery of a Skin Whitening Agent: An Approach to Clinically Augment the Therapeutic Efficacy for Melasma Treatment. Drug Deliv. 2022, 29, 1212–1231. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) Var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.M.; Barbinta-Patrascu, M.E. Organic and Biogenic Nanocarriers as Bio-Friendly Systems for Bioactive Compounds’ Delivery: State-of-the Art and Challenges. Materials 2023, 16, 7550. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals 2024, 17, 329. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Setapar, S.H.M.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Borchert, H.-H. Penetration and Release Studies of Positively and Negatively Charged Nanoemulsions—Is There a Benefit of the Positive Charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The Impact of Nanoparticle Shape on Cellular Internalisation and Transport: What Do the Different Analysis Methods Tell Us? Mater. Horiz. 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.-B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical Application, Patent Repository, Clinical Trial and Regulatory Updates on Hydrogel: An Extensive Review. Gels 2021, 7, 207. [Google Scholar] [CrossRef]
- Santos, J.S.; Barradas, T.N.; Tavares, G.D. Advances in Nanotechnology-based Hair Care Products Applied to Hair Shaft and Hair Scalp Disorders. Int. J. Cosmet. Sci. 2022, 44, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Rajendran, S. Current Commercial Nanocosmetic Products. In Nanocosmetics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 445–453. [Google Scholar]
- Santos, A.C.; Rodrigues, D.; Sequeira, J.A.D.; Pereira, I.; Simões, A.; Costa, D.; Peixoto, D.; Costa, G.; Veiga, F. Nanotechnological Breakthroughs in the Development of Topical Phytocompounds-Based Formulations. Int. J. Pharm. 2019, 572, 118787. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Babu, R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-Based Dermatological Formulations: Dermopharmaceutical and Cosmetic Applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S.; Rohilla, A.; Narwal, S.; Dureja, H.; Bhagwat, D.P. Global Trends of Cosmeceutical in Nanotechnology: A Review. Pharm. Nanotechnol. 2023, 11, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Pires, P.C.; Fonseca, M.; Costa, G.; Giram, P.S.; Mazzola, P.G.; Bell, V.; Mascarenhas-Melo, F.; Veiga, F.; Paiva-Santos, A.C. Nanomaterials in Cosmetics: An Outlook for European Regulatory Requirements and a Step Forward in Sustainability. Cosmetics 2023, 10, 53. [Google Scholar] [CrossRef]
- Melo, A.; Seragiotto Amadeu, M.; Lancellotti, M.; Maria De Hollanda, L.; Machado, D. The Role of Nanomaterials in Cosmetics: National and International Legislative Aspects. Quim. Nova 2015, 38, 599–603. [Google Scholar] [CrossRef]
- Allan, J.; Belz, S.; Hoeveler, A.; Hugas, M.; Okuda, H.; Patri, A.; Rauscher, H.; Silva, P.; Slikker, W.; Sokull-Kluettgen, B.; et al. Regulatory Landscape of Nanotechnology and Nanoplastics from a Global Perspective. Regul. Toxicol. Pharmacol. 2021, 122, 104885. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Martel-Estrada, S.-A.; Morales-Cardona, A.-I.; Vargas-Requena, C.-L.; Rubio-Lara, J.-A.; Martínez-Pérez, C.-A.; Jimenez-Vega, F. Delivery Systems in Nanocosmeceuticals. Rev. Adv. Mater. Sci. 2022, 61, 901–930. [Google Scholar] [CrossRef]
- Eloy, J.O.; Claro de Souza, M.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as Carriers of Hydrophilic Small Molecule Drugs: Strategies to Enhance Encapsulation and Delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Ahmadi Ashtiani, H.R.; Bishe, P.; Lashgari, N.-A.; Nilforoushzadeh, M.A.; Zare, S. Liposomes in Cosmetics. J. Ski. Stem Cell 2016, 3, e65815. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and Innovation in the Manufacturing Process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- de Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and Physical Properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Calixto, G.; Graminha, M.; Cerecetto, H.; González, M.; Chorilli, M. Development, Characterization, and in Vitro Biological Performance of Fluconazole-Loaded Microemulsions for the Topical Treatment of Cutaneous Leishmaniasis. Biomed. Res. Int. 2015, 2015, 396894. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, Protection, and Release of Hydrophilic Active Components: Potential and Limitations of Colloidal Delivery Systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.; Aldawsari, M.; Alalaiwe, A.; Mirza, M.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.; Rana, R.; Sambhakar, S.; Chourasia, M.K. Nanocosmeceuticals: Trends and Recent Advancements in Self Care. AAPS PharmSciTech 2024, 25, 51. [Google Scholar] [CrossRef]
- Oliveira, C.; Coelho, C.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Nanocarriers as Active Ingredients Enhancers in the Cosmetic Industry—The European and North America Regulation Challenges. Molecules 2022, 27, 1669. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Madkhali, O.A. Perspectives and Prospective on Solid Lipid Nanoparticles as Drug Delivery Systems. Molecules 2022, 27, 1543. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Goncalez, M.L.; Rigon, R.B.; Pereira-Da-Silva, M.A.; Chorilli, M. Curcumin-Loaded Cationic Solid Lipid Nanoparticles as a Potential Platform for the Treatment of Skin Disorders. Pharmazie 2017, 72, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani, Y.; Patravale, V.B.; S, B. Solid Lipid Nanoparticles for Hydrophilic Drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid Lipid Nanoparticles as Promising Tool for Intraocular Tobramycin Delivery: Pharmacokinetic Studies on Rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for Preparation of Nanostructured Lipid Carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Zaid, A.-N. Features, Applications, and Sustainability of Lipid Nanoparticles in Cosmeceuticals. Saudi Pharm. J. 2022, 30, 53–65. [Google Scholar] [CrossRef]
- Ahmad, J. Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders. Cosmetics 2021, 8, 84. [Google Scholar] [CrossRef]
- Lahkar, S.; Das, M.K. Smart Lipid Nanoparticles for Cosmetic Use. In Nanocosmeceuticals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 307–317. [Google Scholar]
- Krasodomska, O.; Paolicelli, P.; Cesa, S.; Casadei, M.A.; Jungnickel, C. Protection and Viability of Fruit Seeds Oils by Nanostructured Lipid Carrier (NLC) Nanosuspensions. J. Colloid Interface Sci. 2016, 479, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.H.; Reis, S.; Oliveira, M.B.P.P. Permeation of Topically Applied Caffeine from a Food by—Product in Cosmetic Formulations: Is Nanoscale in Vitro Approach an Option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hung, C.-F.; Chen, B.-H. Preparation of Coffee Oil-Algae Oil-Based Nanoemulsions and the Study of Their Inhibition Effect on UVA-Induced Skin Damage in Mice and Melanoma Cell Growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef]
- Szczepańska, P.; Rychlicka, M.; Moroz, P.; Janek, T.; Gliszczyńska, A.; Lazar, Z. Elevating Phospholipids Production Yarrowia Lipolytica from Crude Glycerol. Int. J. Mol. Sci. 2022, 23, 10737. [Google Scholar] [CrossRef]
- Lee, H.-R.; Kwon, S.-Y.; Choi, S.-A.; Lee, J.-H.; Lee, H.-S.; Park, J.-B. Valorization of Soy Lecithin by Enzyme Cascade Reactions Including a Phospholipase A2, a Fatty Acid Double-Bond Hydratase, and/or a Photoactivated Decarboxylase. J. Agric. Food Chem. 2022, 70, 10818–10825. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Salleh, H.M.; Moniruzzaman, M. Preparation, Characterization and Biological Activities of an Oil-in-Water Nanoemulsion from Fish By-Products and Lemon Oil by Ultrasonication Method. Molecules 2022, 27, 6725. [Google Scholar] [CrossRef] [PubMed]
- Gledovic, A.; Janosevic-Lezaic, A.; Tamburic, S.; Savic, S. Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability. Antioxidants 2022, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Sánchez-López, E.; Ettcheto, M.; López-Machado, A.; Espina, M.; Souto, E.B.; Galindo, R.; Camins, A.; García, M.L.; Turowski, P. Current Advances in the Development of Novel Polymeric Nanoparticles for the Treatment of Neurodegenerative Diseases. Nanomedicine 2020, 15, 1239–1261. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Gajbhiye, S. Dendrimers for Skin Delivery of Cosmeceuticals. In Nanocosmeceuticals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 389–429. [Google Scholar]
- Sorroza-Martínez, K.; González-Méndez, I.; Martínez-Serrano, R.D.; Solano, J.D.; Ruiu, A.; Illescas, J.; Zhu, X.X.; Rivera, E. Efficient Modification of PAMAM G1 Dendrimer Surface with β-Cyclodextrin Units by CuAAC: Impact on the Water Solubility and Cytotoxicity. RSC Adv. 2020, 10, 25557–25566. [Google Scholar] [CrossRef]
- Li, X.; Cao, C.; Wei, P.; Xu, M.; Liu, Z.; Liu, L.; Zhong, Y.; Li, R.; Zhou, Y.; Yi, T. Self-Assembly of Amphiphilic Peptides for Recognizing High Furin-Expressing Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 12327–12334. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Hanafy, N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Papavasileiou, K.D.; Papadopoulos, M.G.; Xenakis, A. Reverse Micelles As Antioxidant Carriers: An Experimental and Molecular Dynamics Study. Langmuir 2017, 33, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Dhapte-Pawar, V.; Kadam, S.; Saptarsi, S.; Kenjale, P.P. Nanocosmeceuticals: Facets and Aspects. Future Sci. OA 2020, 6, FSO613. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Dristant, U.; Mukherjee, K.; Saha, S.; Maity, D. An Overview of Polymeric Nanoparticles-Based Drug Delivery System in Cancer Treatment. Technol. Cancer Res. Treat. 2023, 22, 153303382311520. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Erdman, W.; Yuan, Y.; Mohamed, M.A.; Xie, R.; Wang, Y.; Gong, S.; Cheng, C. Crosslinked Polymer Nanocapsules for Therapeutic, Diagnostic, and Theranostic Applications. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1653. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed Gum Solution Functional Properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Lee, Y.-Y.; Qiu, C.; Zeng, X.; Wang, Y. Pickering Emulsions Stabilized by Chitosan-Flaxseed Gum-Hyaluronic Acid Nanoparticles for Controlled Topical Release of Ferulic Acid. Int. J. Biol. Macromol. 2024, 255, 128086. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Olive Tree (Olea europaea L.) Leaf as a Waste by-Product of Table Olive and Olive Oil Industry: A Review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Support for the Safe Use of Zinc Oxide Nanoparticle Sunscreens: Lack of Skin Penetration or Cellular Toxicity after Repeated Application in Volunteers. J. Investig. Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered Silica: Properties, Applications and Toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee of Consumer Safety—SCCS; Bernauer, U. Opinion of the Scientific Committee on Consumer Safety (SCCS)—Revision of the Opinion on Hydroxyapatite (Nano) in Cosmetic Products. Regul. Toxicol. Pharmacol. 2018, 98, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Somaiah, S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Thryallis glauca (Cav.) Kuntze and Their Role as Antioxidant and Antibacterial. Microsc. Res. Tech. 2022, 85, 2835–2847. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Ghodake, G.S.; Jiang, Y.Y.; Kim, D.S.; Saratale, G.D. Exploiting Fruit Byproducts for Eco-Friendly Nanosynthesis: Citrus × Clementina Peel Extract Mediated Fabrication of Silver Nanoparticles with High Efficacy against Microbial Pathogens and Rat Glial Tumor C6 Cells. Environ. Sci. Pollut. Res. 2018, 25, 10250–10263. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Mazumder, P.; Kumar, M. Corn Cob Silica as an Antibacterial Support for Silver Nanoparticles: Efficacy on Escherichia coli and Listeria monocytogenes. Environ. Monit. Assess 2018, 190, 583. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Vijayakumar, S.; Vidhya, E.; Bukhari, N.A.; Hatamleh, A.A.; Nilavukkarasi, M.; Pham, T.H. TiO2 Nanoparticles Derived from Egg Shell Waste: Eco Synthesis, Characterization, Biological and Photocatalytic Applications. Environ. Res. 2022, 214, 113829. [Google Scholar] [CrossRef] [PubMed]
- Wary, R.R.; Baglari, S.; Brahma, D.; Gautam, U.K.; Kalita, P.; Baruah, M.B. Synthesis, Characterization, and Photocatalytic Activity of ZnO Nanoparticles Using Water Extract of Waste Coconut Husk. Environ. Sci. Pollut. Res. 2022, 29, 42837–42848. [Google Scholar] [CrossRef]
- Easmin, S.; Bhattacharyya, M.; Pal, K.; Das, P.; Sahu, R.; Nandi, G.; Dewanjee, S.; Paul, P.; Haydar, M.S.; Roy, S.; et al. Papaya Peel Extract-Mediated Green Synthesis of Zinc Oxide Nanoparticles and Determination of Their Antioxidant, Antibacterial, and Photocatalytic Properties. Bioprocess Biosyst. Eng. 2024, 47, 65–74. [Google Scholar] [CrossRef]
- Patra, J.K.; Shin, H.S.; Das, G. Characterization and Evaluation of Multiple Biological Activities of Silver Nanoparticles Fabricated from Dragon Tongue Bean Outer Peel Extract. Int. J. Nanomed. 2021, 16, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B. Evaluation of Environmental Exposure Models for Engineered Nanomaterials in a Regulatory Context. NanoImpact 2017, 8, 38–47. [Google Scholar] [CrossRef]
- Levine, A. Sunscreen Use and Awareness of Chemical Toxicity among Beach Goers in Hawaii Prior to a Ban on the Sale of Sunscreens Containing Ingredients Found to Be Toxic to Coral Reef Ecosystems. Mar. Policy 2020, 117, 103875. [Google Scholar] [CrossRef]
- Weon, S.Y.; Rang, H.Y.; Soo, K.J.; Jeong, Y.E.; Min, K.H.; Sang, L.H.; Jun, P.S.; Tai, L.C. PDRN Preparation Method of Nanoparticles Containing Anemarrhena Asphodeloides Extract Polydeoxyribonucleotide and Physiologically Active Materials and Cosmetic Composition Comprising the Same. Patent KR102534797B1, 30 May 2023. [Google Scholar]
- Hyun, K.H.; Ho, N.U. PLGA Nanoparticles for Cosmetic Comprising Natural Plant Extracts and a Method for Producing the Same. Patent KR102304048B1, 27 September 2021. [Google Scholar]
- Yang, F.; Zhou, Z.; Li, M.; Guo, M.; Zhou, Z.; Guo, Y. Essence Emulsion Composition Containing Lipid Nanoparticles with Wrinkle-Removing and Tightening Effects and Preparation Method. Patent CN113730295A, 3 December 2021. [Google Scholar]
- Ruch, W.G.C.; Ravanelli, P.L.; Dragani, R.R.; Vidal, S.M. Composition of Lipid Nanoparticle Containing Vitis Vinifera Extract, Cosmetic Uses of a Composition of Lipid Nanoparticle Containing Vitis Vinifera Extract, Antioxidant Dermocosmetic Product and for Preventing Skin Aging and Skin Care Method. Patent EP4380538A1, 12 June 2024. [Google Scholar]
- Andrzej, W.; Przemyslaw, D.; Izabela, N.; Marta, M. A Method of Obtaining of Lipid Nanoparticles Synthesised on the Basis of Marine Microalgae (Schizochytrium) and Lipids Obtained from Diatoms (Halamphora). Patent PL244834B1, 19 August 2021. [Google Scholar]
- Kim, Y.M.; Chang, K.H. Rigida Pine Bark Extract with Improved Stability Encapsulated Nanoparticles and Manufacturing Method Cosmetic Composition Comprising the Same. Patent KR102617427B1, 27 December 2023. [Google Scholar]
- Yun, Z.; Qiuna, Z.; Xuanyan, J.; Rongxi, J.; Zhonghe, Y.; Yanjun, J. Dendrobium-Derived Exosome-like Vesicles and Preparation and Application Thereof in Skin-Care Cosmetics. Patent CN114159373B, 22 October 2021. [Google Scholar]
- Chunqiang, L.; Xue, Z.; Hongyu, L.; Hongfei, Z.; Di, Y.; Junhua, S.; Anan, D.; Dongnan, L.; Lu, Y.; Xinlei, W. Preparation and Application of Modified Pea Protein-Chitosan Nanoparticles. Patent CN1149477107B, 20 October 2021. [Google Scholar]
- Wen, L.; Qingqiu, F.; Chunmei, W.; Jiawen, W. Composite Whitening Active Matter Nanoparticles as Well as Preparation Method and Application. Patent CN113440453A, 28 September 2021. [Google Scholar]
- Wen, C. Orbit Express Liposome Nanoparticles with Low Energy and Thermal Stability, and Preparation Method and Application. Patent CN113616603, 9 November 2021. [Google Scholar]
| Document | Composition | Active Ingredient | Characterization | Physical-Chemical Properties | Formulation | Biological Performance |
|---|---|---|---|---|---|---|
| KR10-2534797 | Anemarrhena asphodeloides extract polydeoxyribonucleotide, hyaluronic acid, collagen, glutathione, vitamin B2, menadione, thioctic acid, and vitamin C | PDRN | DLS, in vitro release and biocompatibility, moisturizing and anti-aging properties, in vivo anti-wrinkle activity, histological analysis and SOD activity. | 143 nm. 40.9–5.3 mV | Skin lotions, skin toners, packs, nutritional creams | Moisturization, antioxidant, skin inflammation alleviation, skin barrier reinforcement, wrinkle improvement, and elasticity |
| KR10-2304048 B1 | Neem oil, thyme oil, and geraniol oil, chitosan, PLGA, PLA | Matrine, phosphatidylcholine | LS13 320, absorption measurement, time absorption, in vitro permeation | 145.8–227.7 n, 10.252 ppm | - | Antibacterial action, skin soothing effect, skin-whitening effect, anti-inflammatory |
| CN113730295 A | Sucrose stearate, sorbitan olivetoleate, polysorbate 20, laureth-23, polysorbate-80, isocetyl steareth-20, a mixture of glyceryl stearate and PEG-75 stearate, poloxamer 407, glyceryl stearate, ceteareth-25 | Acetyl tetrapeptide-5, palmitoyl tripeptide-1 and palmitoyl tetrapeptide-7, and Ascophyllum nodosum extract | Safety test, test samples | - | - | Wrinkle-removing and tightening effects |
| WO2023/010188 A1 | Water, Capric/Caprylic Acid, Triglyceride, Oleic acid, Linoleic acid, PPG-15 stearyl ether, Poloxamer, Steareth, Steareth-, Vitis vinifera extract1, Phenoxyethanol, Caprylyl glycol0, BHT, disodium EDTA, Sodium Metabisulfite | Anthocyanins, procyanidins, catechins, gallic acid, quercetin, kaempferol, myricetin and isoramnetin, caftaric acids, resveratrol | encapsulation efficiency, pH, skin permeation and antioxidant effect, nanozetasizer, Gene modulation assay | 99.97%, 4.043–4.173, −30.9 mV | - | Antioxidant, anti-aging, anti-inflammatory, whitening effect, photoprotection, gene modulation activities |
| CN113616603 A | PEG, ethanol, DMSO | Naringenin, hyaluronic acid | DLS, thermal stability | 140 nm | Facial masks, lotions or creams and the drugs are creams | Thermodynamic stability, nano-carrier, remove wrinkle, increase elasticity, prevent aging |
| EP4137125 A1 | Glycerol stearate, cetyltrimethylammonium bromide, Tween 80 | Algae lipids, oil from marine microalgae, lipids obtained from diatoms | DLS, PDI, zeta potential | 100–300 nm, PDI ≤ 0.26, +40 mV | - | Nanocarrier, treatment of eczema, regenerative processes during skin |
| KR10-2617427 B1 | Pine bark extract, phospholipids, ethanol, cholesterol, yolk lecithin (phosphatidylcholine), hydrogenatethyrestin, soy lecithin, lysolecithin, sphingomyelin, phosphatidylinositol, phosphatidic acid, phosphatidylethanolamine, phosphatidylserine, phosphatidylglycerol, phosphatidylinositol-4,5-diphosphoric acid, cardiolipin, and plasmarogen | Catechin, quercitrin | encapsulation rate, DLS cell 5oxicity 5est in vitro, skin moisturization increase rate, percutaneous moisture loss reduction rate | 90%, 100 nm, 12,5 μg/mL, 7.05%, 7.48 | - | Treatment of topical dermatitis, prevent itching and skin damage caused by skin inflammation, skin soothing, regenerating a skin barrier, skin itching, alleviating or preventing skin erythema |
| CN114159373 B | PBS, Dendrobium extract, tyrosine solution | Dendrobium nobile exosome vesicle | beta-galactosidase inhibition test of fibroblasts, type I collagen promotion assay for human fibroblasts, tyrosinase inhibition assay, test in vivo | 0.5%, 11.2%, 18.3% and 35.2% | - | Anti-aging, whitening effects |
| CN114947107 B | Protein-chitosan, distilled water, acetic acid solution, corn oil | Globulin | deacetylation degree, optimum ultrasonic power, rheology, ionic strength stability, Zeta potential |
≥90%, 100 s,
375 W | Pickering emulsion | Hydrophobicity, thermal stability and the ionic strength stability |
| CN113440453 A | Chinaberry extract, maltodextrin, coconut oil, corn oil, caprylic/capric triglyceride, grape seed oil, evening primrose oil, jojoba oil and olive oil, caprylic/capric triglyceride, squalane, glicerol, butanodiol, propilenoglicol, dipropilenoglicol, gliceril poliéter-7, gliceril poliéter-26, polietilenoglicol-400 e gliceril poliéter de glicose-20 | Alpha-arbutin, hydrolyzed conchiolin | DLS | 50–120 nm | Emulsion, cream and facial mask | Anti-inflammatory, repairing and whitening effect |
| CN116139062 A | Lotus flower, epsilon-polylysine, gamma-cyclodextrin and deionized water, epsilon-polylysine | Total flavonoids, proteins, vitamin C, fat, carbohydrate, carotene, thiamine, nicotinic acid | inhibition rate melanin synthesis | 65% | - | Antioxidation, wrinkle resistance, moisture preservation, whitening, color spot fading, skin brightening, the like on skin, inhibition of melanin synthesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhani, G.B.B.; Di Filippo, L.D.; de Paula, G.A.; Mantovanelli, V.R.; da Fonseca, P.P.; Tashiro, F.M.; Monteiro, D.C.; Fonseca-Santos, B.; Duarte, J.L.; Chorilli, M. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics 2024, 11, 112. https://doi.org/10.3390/cosmetics11040112
Nhani GBB, Di Filippo LD, de Paula GA, Mantovanelli VR, da Fonseca PP, Tashiro FM, Monteiro DC, Fonseca-Santos B, Duarte JL, Chorilli M. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics. 2024; 11(4):112. https://doi.org/10.3390/cosmetics11040112
Chicago/Turabian StyleNhani, Gabriela Braga Barros, Leonardo Delello Di Filippo, Geanne Aparecida de Paula, Vitoria Ribeiro Mantovanelli, Patricia Pereira da Fonseca, Felipe Mota Tashiro, Diana Coêlho Monteiro, Bruno Fonseca-Santos, Jonatas L. Duarte, and Marlus Chorilli. 2024. "High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products" Cosmetics 11, no. 4: 112. https://doi.org/10.3390/cosmetics11040112
APA StyleNhani, G. B. B., Di Filippo, L. D., de Paula, G. A., Mantovanelli, V. R., da Fonseca, P. P., Tashiro, F. M., Monteiro, D. C., Fonseca-Santos, B., Duarte, J. L., & Chorilli, M. (2024). High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics, 11(4), 112. https://doi.org/10.3390/cosmetics11040112








