Antioxidative Potentials of Eleutherine bulbosa Bulb and Its Utilization in Topical Cosmetic Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Eleutherine bulbosa Extract
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. Determination of Anthraquinone
2.6. DPPH Radicals Scavenging Assay
2.7. Determination of Reducing Power
2.8. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.9. Anti-Elastase Activity
2.10. Anti-Collagenase Activity
2.11. Cytotoxicity Test
2.12. Qualification of Active Compounds by UHPLC-QTOF-MS
2.13. Utilization of E. bulbosa Bulb Extract in Cosmetic Preparation
2.14. Accelerated Stability Test of Cosmetic Emulsion
3. Results
3.1. Preparation of Eleutherine bulbosa Extract
3.2. Qualification of Active Compounds by UHPLC-QTOF-MS
3.3. Anti-Elastase and Anti-Collagenase Activity
3.4. Cytotoxicity Test
3.5. Utilization of E. bulbosa Extract in Cosmetic Formulation
3.5.1. Preparation of the Extract
3.5.2. Use of E. bulbosa Bulb Concentrated Extract in Emulsion Formulation
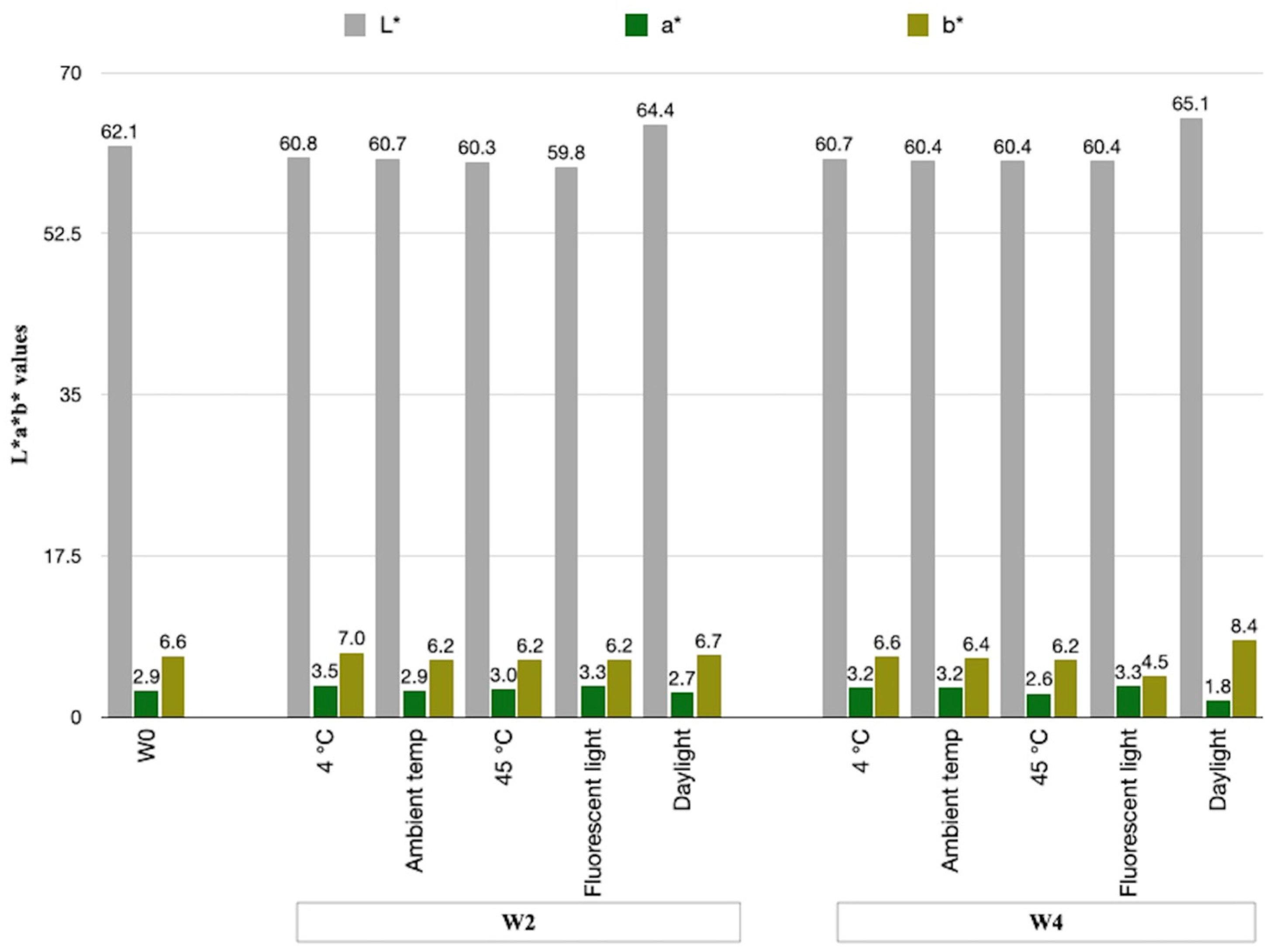
3.5.3. Physical Stability Study of the E. bulbosa Emulsion Cream
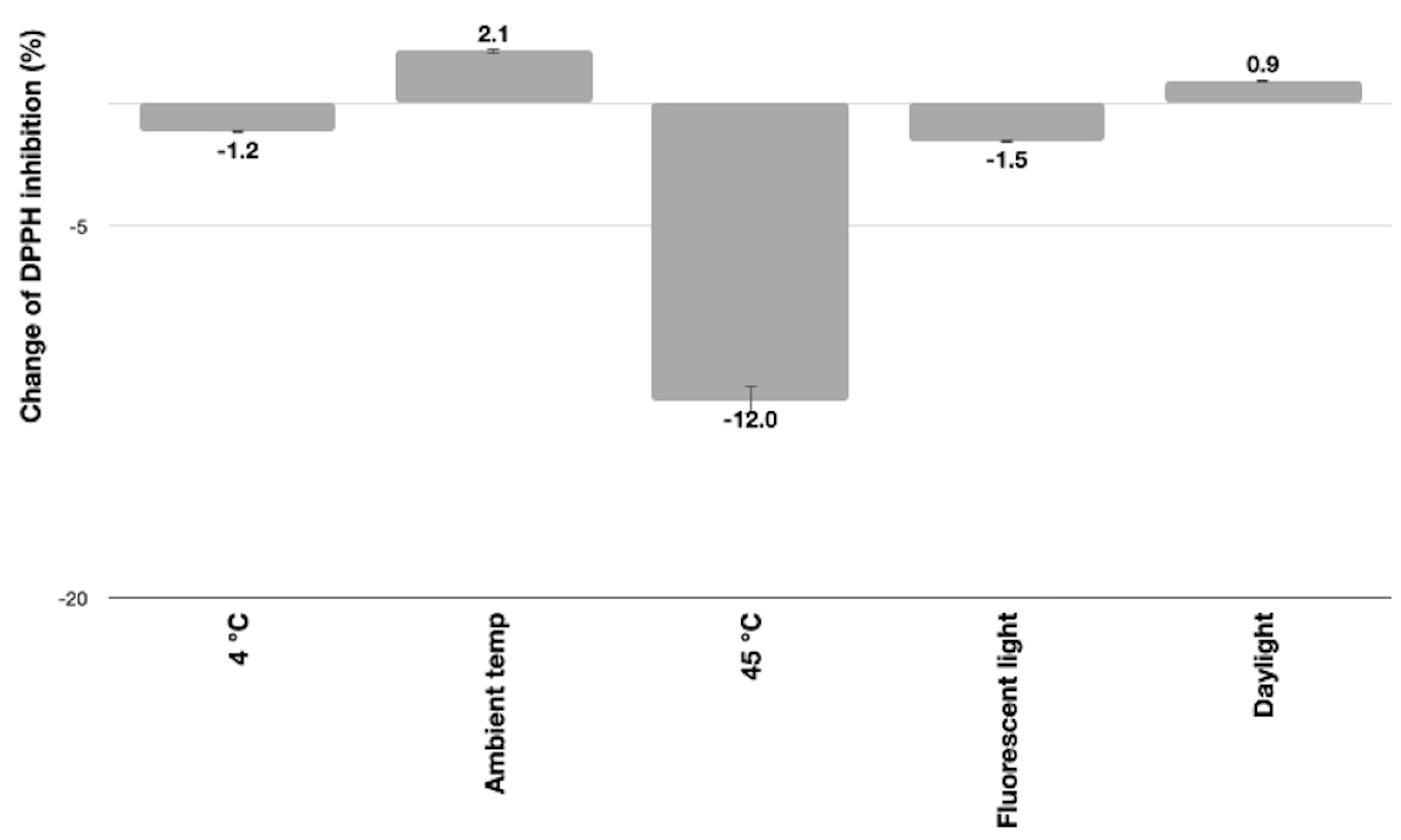
3.5.4. Antioxidant Activity of the E. bulbosa Emulsion Cream
4. Discussion
4.1. Antioxidative and Cytotoxicity Activity of E. bulbosa Bulb
4.2. Utilization of E. bulbosa Extract in Cosmetic Formulation
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamarudin, A.A.; Sayuti, N.H.; Saad, N.; Razak, N.A.A.; Esa, N.M. Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the Pharmacological Activities and Its Prospects for Application. Int. J. Mol. Sci. 2021, 22, 6747. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.L.; Moraes, D.F.; Cartagenes, M.; Do Amaral, F.M.; Guerra, R.N. Eleutherine bulbous (Mill.) Urb.: A Review Study. J. Med. Plants Res. 2016, 10, 286–297. [Google Scholar]
- Insanu, M.; Kusmardiyani, S.; Hartati, R. Recent Studies on Phytochemicals and Pharmacological Effects of Eleutherine americana Merr. Procedia Chem. 2014, 13, 221–228. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, Q.H.; He, Y.; Wang, H. Advances in Studies on Chemical Constituents and Pharmacological Activities of Eleutherine americana. Asia-Pac. Trad. Med. 2015, 11, 39–42. [Google Scholar]
- Ifesan, B.O.; Siripongvutikorn, S.; Hutadilok-Towatana, N.; Voravuthikunchai, S.P. Evaluation of The Ability of Eleutherine americana Crude Extract As Natural Food Additive in Cooked Pork. J. Food Sci. 2009, 74, M352–M357. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.R.; Palazzino, G.; Federici, E.; Iurilli, R.; Galeffi, C.; Chifundera, K.; Nicoletti, M. Polyketides from Eleuthe-rine bulbosa. Nat. Prod. Res. 2010, 24, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, I.W.; Arung, E.T.; Rosamah, E.; Purwatiningsih, S.; Kuspradini, H.; Astuti, J.; Kim, Y.U.; Shimizu, K. Antidermatophyte and Antimelanogenesis Compound from Eleutherine americana Grown in Indonesia. J. Nat. Med. 2010, 64, 223–226. [Google Scholar] [CrossRef]
- Harlita, T.D.; Oedjijono; Asnani, A. The Antibacterial Activity of Dayak Onion (Eleutherine palmifolia (L.) Merr) towards Pathogenic Bacteria. Trop. Life Sci. Res. 2018, 29, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ardhany, S.D.; Putra, C.D.; Novaryatiin, S. Modification of Anti-acne Bawang Dayak (Eleutherine bulbosa (Mill.) Urb.) Cream to Propionibacterium acnes. J. Adv. Pharm. Technol. Res. 2021, 12, 94–98. [Google Scholar] [CrossRef]
- Novaryatiin, S.; Amalia, N.R.; Ardhany, S.D. Formulation of Anti Acne Loose Powder of Bawang Dayak (Eleutherine bulbosa (Mill.) Urb.) Ethanol Extract. Borneo J. Pharm. 2022, 5, 153–160. [Google Scholar] [CrossRef]
- Kamarudin, A.A.; Esa, N.M.; Saad, N.; Sayuti, N.H.; Razak, N.A.A. Heat Assisted Extraction of Phenolic Compounds from Eleutherine bulbosa (Mill.) bulb and Its Bioactive Profiles Using Response Surface Methodology. Ind. Crop Prod. 2020, 144, 112064. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Latif, A.S. Potential of Phenolic compounds From Pomegranate (Punica granatum L.) By-product with Significant Antioxidant and Therapeutic Effects: A Narrative Review. Saudi J. Biol. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–148. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Krisdaphong, P. Antioxidant Activity of Pandanus amaryllifolius Leaf and Root Extract and its Application in Topical Emulsion. Trop. J. Pharm. Res. 2013, 12, 425–431. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Khoomsab, R.; Khoomsab, K. Extraction and Determination of Anthraquinone from Herbal Plant as Bird Repellent. Sci. Technol. Asia 2019, 24, 14–20. [Google Scholar]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro Antioxidant Activity of Hydro Alcoholic Extract from the Fruit Pulp of Cassia fistula Linn. Ayu 2013, 34, 209–214. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) As a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kubán, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in Vegetables—Evaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Lee, S.H.; Sancheti, S.; Sancheti, S.; Seo, S.-Y. Potent Antielastase and Antltyrosinase Activities of Astilbe chenesis. Am. J. Pharm. Toxicol. 2009, 4, 127–129. [Google Scholar]
- Kim, S.J.; Sancheti, S.A.; Sancheti, S.S.; Um, B.H.; Yu, S.M.; Seo, S.Y. Effect of 1,2,3,4,6-penta-O-Galloyl-beta-D-Glucose on Elastase and Hyaluronidase Activities and Its Type II Collagen Expression. Acta Pol. Pharm. 2010, 67, 145–150. [Google Scholar] [PubMed]
- Park, H.; Sin, B.Y.; Kim, H.P. Inhibition of Collagenase by Anti-inflammatory Synthetic Flavones. J. Appl. Pharmacol. 2006, 14, 36–39. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Whangsomnuek, N.; Mungmai, L.; Mengamphan, K.; Amornlerdpison, D. Efficiency of Skin Whitening Cream Containing Etlingera elatior Flower and Leaf Extracts in Volunteers. Cosmetics 2019, 6, 39. [Google Scholar] [CrossRef]
- Singh, M.; Seth, P.; Poddar, S. Comparative Analysis of Four Facial Foundation Lotions with Reference to Its Antioxidant Richness and Bio-Safety. Cosmetics 2017, 4, 12. [Google Scholar] [CrossRef]
- Saralamp, P.; Chuakul, W.; Temsiririrkkul, R.; Clayton, T. Medicinal Plants in Thailand; Amarin Printing and Publishing: Bangkok, Thailand, 1996; Volume 1. [Google Scholar]
- Mahmudah, S.; Muntaha, A.; Muhlisin, A. Effectiveness of Dayak (Eleutherine palmifollia (L) Merr) Extracts Against Escherichia coli In Vitro. Trop. Health Med. Res. 2019, 1, 44–48. [Google Scholar] [CrossRef]
- Herlina; Asnani, A.; Diastuti, H. The application of red pigments from Streptomyces K-4B and Dayak onions (Eleutherine palmifolia (L.) Merr.) in colouring glycerine soap. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 172, p. 012023. [Google Scholar]
- Tsai, C.E.; Lin, L.H. DPPH Scavenging Capacity of Extracts from Camellia Seed Dregs Using Polyol Compounds as Solvents. Heliyon 2019, 5, e02315. [Google Scholar] [CrossRef]
- Morabandza, C.J.; Okiemy-Akieli, M.G.; Okiemy, E.; Andzi-Barhé, T.; Ongoka, P.R. Total Phenols, Total Flavonoids Content; Antioxidant and Anti-fungal Activities of Ethanolic and Aqueous Extracts of Eleutherine bulbosa (Iridaceae). World J. Pharm. Sci. 2016, 4, 252–255. [Google Scholar]
- Komura, H.; Mizukawa, K.; Minakata, H.; Huan, H.; Qin, G.; Xu, R. New Anthraquinones from Eleutherine americana. Chem. Pharm. Bull. 1983, 31, 4206–4208. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, H.; Wang, C.; Li, Y.; Ding, J.; Ushio, S.; Hiroshi, N.; Yoichi, I. Hongconin, A new Naphthalene Derivative from Hong-Cong the Rhizome of Eleutherine americana Merr and Heyne (Iridaceae). Chem. Pharm. Bull. 1986, 34, 2743–2746. [Google Scholar]
- Hara, H.; Maruyama, N.; Yamashita, S.; Hayashi, Y.; Lee, K.H.; Bastow, F.K.; Chirul; Marumoto, N.; Imakura, Y. Elecanacin, a Novel New Naphthoquinone from the Bulb of Eleutherine americana. Chem. Pharm. Bull. 1997, 45, 1714–1716. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Hemtasin, C.; Chakthong, S.; Voravuthikunchai, S.P.; Olawumi, I.B. Naphthoquinones, Anthraquinones and Naphthalene Derivatives from the Bulbs of Eleutherine americana. Planta Medica 2010, 76, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Calhelha, R.C.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds. Molecules 2020, 25, 2497. [Google Scholar] [CrossRef] [PubMed]
- Myo, H.; Yaowiwat, N.; Pongkorpsakol, P.; Aonbangkhen, C.; Khat-Udomkiri, N. Butylene Glycol Used as a Sustainable Solvent for Extracting Bioactive Compounds from Camellia sinensis Flowers with Ultrasound-Assisted Extraction. ACS Omega 2023, 8, 4976–4987. [Google Scholar] [CrossRef] [PubMed]
- Jimtaisong, A.; Saewan, N. Efficiency Evaluation of Topical Emulsion of Croton thorelii Gagnep. Extract and Its Related Properties. Maejo Int. J. Sci. Technol. 2022, 16, 124–134. [Google Scholar]
- Bianchi, C.; Ceriotti, G. Chemical and Pharmacological Investigations of Constituents of Eleutherine bulbosa(Miller) Urb. (Iridaceae). J. Pharm. Sci. 1975, 64, 1305–1308. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, F.; Qu, G.; Wang, N.; Yao, X. Studies on Antifungal Constituents Isolated from Eleutherine americana. Zhongguo Yaowu Huaxue Zazhi 2005, 15, 157–161. [Google Scholar]
- Hong, J.H.; Yu, E.S.; Han, A.R.; Nam, J.W.; Seo, E.K.; Hwang, E.S. Isoeleutherin and Eleutherinol, Naturally Occurring Selective Modulators of Th Cell-mediated Immune Responses. Biochem. Biophys. Res. Commun. 2008, 371, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Paramapojn, S.; Ganzera, M.; Gritsanapan, W.; Stuppner, H. Analysis of Naphthoquinone Derivatives in the Asian Medicinal Plant Eleutherine americana by RP-HPLC and LC–MS. J. Pharm. Biomed. Anal. 2008, 47, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Kamiloglu, S.; Petroni, K.; Mishra, A.P.; Monserrat-Mesquida, M.; Sureda, A.; Martorell, M.; Aidarbekovna, D.S.; Yessimsiitova, Z.; et al. Recent Advances in the Therapeutic Potential of Emodin for Human Health. Biomed. Pharmacother. 2022, 154, 113555. [Google Scholar] [CrossRef] [PubMed]
- Li, R.R.; Liu, X.F.; Feng, S.X.; Shu, S.N.; Wang, P.Y.; Zhang, N.; Li, J.S.; Qu, L.B. Pharmacodynamics of Five Anthraquinones (Aloe-emodin, Emodin, Rhein, Chysophanol, and Physcion) and Reciprocal Pharmacokinetic Interaction in Rats with Cerebral Ischemia. Molecules 2019, 24, 1898. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef] [PubMed]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative Study of Flavonoids in Experimental Models of Inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Yusha’u, Y.; Adam, U.; Ibrahim, S.; Muhammad, M. Ameliorative Effect of Rutin Supplement on Chronic Unpredictable Mild Stress-Induced Depressive Phenotypes in Mice. Nig. J. Neurosci. 2022, 13, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, Anti-elastase and Anti-oxidant Activities of Extracts from 21 Plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Lee, K.K.; Kim, J.H.; Cho, J.J.; Choi, J.D. Inhibitory Effects of 150 Plant Extracts on Elastase Activity, and Their Anti-inflammatory Effects. Int. J. Cosmet. Sci. 1999, 21, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Pimple, B.P.; Badole, S.L. Chapter 67—Polyphenols: A Remedy for Skin Wrinkles; Watson, R.R., Victor, R., Preedy Zibadi, S., Eds.; Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2014; pp. 861–869. ISBN 9780123984562. Available online: https://www.sciencedirect.com/science/article/pii/B9780123984562000670 (accessed on 1 May 2024). [CrossRef]
- Rani, V.S. In vitro Cytotoxic Activity and Preliminary Phytochemical Analysis of the Crude Extracts of Eleutherine bulbosa (Miller), Urban. World J. Pharm. Res. 2017, 7, 1022–1029. [Google Scholar]
- Lubis, I.A.; Ichwan, M.F.; Mustofa, M.; Satria, D. Anticancer Activity of Eleutherine bulbosa (Mill.) Urb. Extract on WiDr cell line in vitro. In Proceedings of the 2nd Public Health International Conference (PHICo 2017), Medan, Indonesia, 18–19 December 2017; Atlantis Press: Dordrecht, The Netherlands; pp. 123–127. [Google Scholar]
- Mutiah, R.; Choiroh, F.; Annisa, R.; Listiyana, A. Combinational Effect of Eleutherine palmifolia (L.) Merr Extract and Doxorubicin Chemotherapy on HeLa Cervical Cancer Cells. In AIP Conference Proceedings July 2019; AIP Publishing LLC.: New York, NY, USA, 2019; Volume 2120, No. 1; p. 070001. [Google Scholar]
- Ardhany, S.D.; Novaryatiin, S.; Pratomo, G.S. Irritation Test of Bawang Dayak (Eleutherine bulbosa (Mill.) Urb.) Loose Powder for Acne Vulgaris. Biomed. Pharmacol. J. 2022, 15, 2209. [Google Scholar] [CrossRef]
- Ardhany, S.D.; Novaryatiin, S.; Pratama, M.R.F.; Utar, Z. Irritation Test of Bawang Dayak (Eleutherine bulbosa (Mill.) Urb.) Extract Cream with Human Patch Test Method. J. Farm. Sains Praktis. 2021, 7, 74–80. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chattopadhyay, P.; Goyary, D.; Mazumder, P.M.; Veer, V. Eleutherine indica L. Accelerates In vivo Cutaneous Wound Healing by Stimulating Smad-mediated Collagen Production. J. Ethnopharmacol. 2013, 146, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.A.; Badhe, Y.S.; Farde, P.D.; Hegde, M.V.; Zanwar, A.A. Long-term Storage Stability Assessment of Omega-3-Fatty Acid Emulsified Formulation Containing Micronutrients. J. Pharm. Innov. 2022, 17, 1126–1135. [Google Scholar] [CrossRef]
- Smaoui, S.; ben Hlima, H.; ben Chobba, I.; Kadri, A. Development and Stability Studies of Sunscreen Cream Formulations Containing Three Photo-protective Filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour Difference ∆E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Costa, R.; Santos, L. Delivery Systems for Cosmetics—From Manufacturing to the Skin of Natural Antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Babaei Mahani Nezhad, S.; Pozharitskaya, O.N.; Shikov, A.N. Potential of the Ethyl Acetate Fraction of Padina boergesenii as a Natural UV filter in Sunscreen Cream Formulation. Life 2023, 13, 239. [Google Scholar] [CrossRef]




| Ingredients (INCI Name) | % w/w | Function |
|---|---|---|
| Oil phase | ||
| Caprylic/capric triglyceride | 4.0 | Skin conditioning—Occlusive |
| Jojoba oil | 4.0 | Skin conditioning—Emollient |
| Glyceryl stearate | 1.5 | Emulsifier |
| Sorbitan stearate | 1.8 | Emulsifier |
| Theobroma cacao (Cocoa) seed butter | 3.0 | Emollient |
| Beeswax | 6.0 | Viscosity controlling wax |
| Stearic acid | 0.4 | Emulsion stabilizer |
| Water phase | ||
| Deionized water | qs. to 100 | Solvent |
| Polyacrylate crosspolymer-11 | 1.7 | Thickening agent |
| Disodium EDTA | 0.2 | Chelating agent |
| Butylene glycol | 3.0 | Humectant |
| Glycerin | 2.0 | Humectant |
| Sodium PCA | 4.0 | Humectant |
| Polysorbate 60 | 2.2 | Emulsifier |
| Diazolidinyl urea (and) Iodopropynyl butylcarbamate (and) Propylene glycol | 0.3 | Preservative |
| E. bulbosa bulb extract | 1–2 | Natural antioxidant active |
| Bioactive Compounds and Biological Activities | DI Water | EtOH | PG | EtOH/PG | |||
|---|---|---|---|---|---|---|---|
| Fresh | Dry | Fresh | Dry | Fresh | Dry | Fresh | |
| Total phenolic content (mgGAE/mL extract) | 41.58 ± 1.85 a | 32.51 ± 0.23 | 70.91 ± 2.30 b | 24.58 ± 1.71 | 74.05 ± 0.67 b | 22.11 ± 0.75 | 87.60 ± 2.00 c |
| Total Flavonoids (µgQE/mL extract) | 4.25 ± 0.58 a | 1.22 ± 0.44 | 25.97 ± 0.66 b | 12.01 ± 1.22 | 12.20 ± 0.58 c | 5.07 ± 0.77 | 26.22 ± 1.23 d |
| Anthraquinone (mg anthraquinone/mL extract) | 0.55 ± 0.01 a | 0.34 ± 0.01 | 1.50 ± 0.01 b | 0.65 ± 0.00 | 1.34 ± 0.01 c | 0.41 ± 0.00 | 1.531 ± 0.008 d |
| DPPH (mgAAE/mL extract) | 0.178 ± 0.003 a | 0.180 ± 0.005 a | 0.432 ± 0.002 b | 0.157 ± 0.005 | 0.423 ± 0.005 b | 0.114 ± 0.002 | 0.493 ± 0.002 c |
| Reducing power (mgAAE/mL extract) | 0.195 ± 0.009 a | 0.211 ± 0.042 a | 0.442 ± 0.033 b | 0.185 ± 0.019 | 0.410 ± 0.040 c | 0.159 ± 0.015 | 0.509 ± 0.002 d |
| FRAP assay (mgTE/mL extract) | 1.09 ± 0.05 a | 1.03± 0.02 a | 3.73 ± 0.18 b | 1.30± 0.07 | 3.10 ± 0.06 c | 0.89 ± 0.01 | 4.46 ± 0.01 d |
| Type | Proposed Compounds | Molecular Formula | Retention Time (min) | Adduct Ions (ESI−/ESI+) | Molecular Weight | Theoretical m/z | Observed m/z | Error (ppm) |
|---|---|---|---|---|---|---|---|---|
| Naphthalene | Eleutherol | C14H12O4 | 19.866 | [M + H]+ + [-H2O] | 244.0737 | 227.0703 | 227.0702 | 0.24 |
| Hongconin | C16H16O5 | 18.532 | [M + H]+ | 288.1002 | 289.1071 | 289.1076 | −1.89 | |
| Dihydroeleutherinol | C15H14O4 | 19.071 | [M − H]− | 258.0892 | 257.0819 | 257.082 | −0.09 | |
| Eleutherinol | C15H12O4 | 17.821 | [M − H]− | 256.073 | 255.0663 | 255.0663 | 0.01 | |
| Naphthoquinone | Eleutherin | C16H16O4 | 19.594 | [M + H]+ | 272.1051 | 273.1121 | 273.1123 | −0.72 |
| Anthraquinone | Chrysophanol (1,8-dihydroxy-3-methyl-anthraquinone) | C15H10O4 | 20.410 | [M − H]− | 254.0581 | 253.0506 | 253.0507 | −0.33 |
| Emodin | C15H10O5 | 19.295 | [M − H]− | 270.0529 | 269.0455 | 269.0458 | −0.9 | |
| 4,8-dihydroxy-3-methoxy-1-methyl antraquinone-2-carboxylic acid methyl ester | C18H14O7 | 20.336 | [M − H]− | 342.0743 | 341.0667 | 341.0671 | −1.3 | |
| Coumarin | Rutaretin | C14H14O5 | 18.795 | [M + H]+ + [-H2O] | 262.0844 | 245.0808 | 245.0812 | −1.65 |
| Chalcone | 2-Hydroxy-3,4,6 trimethoxydihydro-chalcone | C18H20O5 | 20.190 | [M + H]+ | 316.1315 | 317.1384 | 317.1388 | −1.53 |
| Glycoside | Eleutherinoside A | C21H22O9 | 15.907 | [M − H]− | 418.1269 | 417.1191 | 417.1198 | −1.64 |
| Myricetin derivative | Myricetin 3,7,3′,4′-tetramethyl ether | C19H18O8 | 21.218 | [M + H]+ + [-H2O] | 374.1003 | 357.0969 | 357.0970 | −0.34 |
| Quercetin derivative | Quercetin 3-isobutyrate | C19H16O8 | 21.138 | [M − H]− | 372.085 | 371.0772 | 371.0778 | −1.54 |
| Quercetin 4′-isobutyrate | C19H16O8 | 19.413 | [M + H]+ + [-H2O] | 372.084 | 355.0812 | 355.0804 | 2.39 | |
| Epicatechin and its derivative | Epicatechin | C15H14O6 | 11.936 | [M − H]− | 290.079 | 289.0718 | 289.0717 | 0.29 |
| 4-Methyl-epicatechin | C16H16O6 | 16.629 | [M + Na]+ | 304.946 | 327.0839 | 327.0839 | 0.02 | |
| Epicatechin 5,3′-dimethyl ether | C17H18O6 | 18.091 | [M + Na]+ | 318.1104 | 341.0996 | 341.0997 | −0.28 | |
| 3,4-Methylenedioxy epicatechin 5,7-dimethyl ether | C18H18O6 | 19.758 | [M + H]+ + [-H2O] | 330.1106 | 313.1071 | 313.1078 | −1.38 | |
| Catechin and its derivative | Catechin | C15H14O6 | 9.227 | [M − H]− | 290.0791 | 289.0718 | 289.0719 | |
| Catechin 7-O-apiofuranoside | C20H22O10 | 12.136 | [M − H]− | 422.1215 | 421.1140 | 421.1142 | −0.48 | |
| Catechin-3′-methyl ether | C16H16O6 | 16.931 | [M − H]− | 304.0951 | 303.0874 | 303.0879 | −1.58 | |
| Catechin 3-O-alpha-L-rhamnoside | C21H24O10 | 15.854 | [M + H]+ + [-H2O] | 436.1369 | 419.1337 | 419.1336 | 0.12 | |
| 4′-O-Methylcatechin | C16H16O6 | 17.696 | [M + Na]+ | 304.944 | 327.0839 | 327.0837 | 0.52 | |
| Catechin 3-O-rutinoside | C27H34O15 | 18.114 | [M + H]+ | 598.1819 | 599.1885 | 599.1893 | −1.3 | |
| Catechin 5,7,3′-trimethyl ether | C18H20O6 | 18.511 | [M + Na]+ | 332.1266 | 355.1152 | 355.1159 | −1.88 | |
| Catechin 3′,4′-diglucoside | C27H34O16 | 20.478 | [M + H]+ + [-H2O] | 614.1765 | 597.1728 | 597.1735 | −1.16 | |
| Epigallocatechin and its derivative | Epigallocatechin | C15H14O6 | 18.607 | [M + Na]+ + [-H2O] | 306.0742 | 322.0526 | 322.0526 | −0.06 |
| Epigallocatechin 3-O-(3,5-di-O- methylgallate) | C24H22O11 | 15.955 | [M − H]− | 486.1146 | 485.1089 | 485.1072 | 3.54 |
| Properties | Characteristics |
|---|---|
| Appearance | Deep red liquid with characteristic odor |
| pH | 6.07 ± 0.01 |
| Total phenolic content (mgGAE/mL extract) | 136.65 ± 3.62 |
| Total Flavonoids (µg QE/mL extract) | 28.12 ± 0.87 |
| Anthraquinone (mg anthraquinone/mL extract) | 2.39 ± 0.03 |
| DPPH (mgAAE/mL extract) | 1.034 ± 0.002 |
| IC50 (DPPH, g/mL) | 0.0097 ± 0.0002 |
| Reducing power (mgAAE/mL extract) | 0.649 ± 0.008 |
| FRAP assay (mgTE/mL extract) | 6.765 ± 0.042 |
| Storage Condition | ΔE* (W2) | ΔE* (W4) |
|---|---|---|
| 4 °C | 1.48 ± 0.34 | 1.40 ± 0.16 |
| Ambient temperature (30–35 °C) | 1.41 ± 0.29 | 1.71 ± 0.35 |
| 45 °C | 1.79 ± 0.14 | 1.72 ± 0.10 |
| Fluorescent light (8 h/day) | 2.37 ± 0.31 | 2.78 ± 0.16 |
| Daylight (8 h/day) | 2.34 ± 0.20 | 3.85 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyachariwat, N.; Jimtaisong, A.; Saewan, N. Antioxidative Potentials of Eleutherine bulbosa Bulb and Its Utilization in Topical Cosmetic Emulsion. Cosmetics 2024, 11, 111. https://doi.org/10.3390/cosmetics11040111
Panyachariwat N, Jimtaisong A, Saewan N. Antioxidative Potentials of Eleutherine bulbosa Bulb and Its Utilization in Topical Cosmetic Emulsion. Cosmetics. 2024; 11(4):111. https://doi.org/10.3390/cosmetics11040111
Chicago/Turabian StylePanyachariwat, Nattakan, Ampa Jimtaisong, and Nisakorn Saewan. 2024. "Antioxidative Potentials of Eleutherine bulbosa Bulb and Its Utilization in Topical Cosmetic Emulsion" Cosmetics 11, no. 4: 111. https://doi.org/10.3390/cosmetics11040111
APA StylePanyachariwat, N., Jimtaisong, A., & Saewan, N. (2024). Antioxidative Potentials of Eleutherine bulbosa Bulb and Its Utilization in Topical Cosmetic Emulsion. Cosmetics, 11(4), 111. https://doi.org/10.3390/cosmetics11040111






