Abstract
This review was written with the aim of examining the effects that cause an insult, such as a wound, to an organ, such as the skin. Before examining the cellular mechanisms relating to wound healing, the reader is invited to read about the structure of the skin as a necessary basis for understanding the final aim of this review. The structure of the skin as a basis for understanding the phenomena relating to wound healing is addressed, taking into account the updated literature that addresses the numerous problems of the skin microenvironment. Starting from this awareness, the paragraphs dedicated to wound healing become complicated when this phenomenon is not implemented and therefore while the problems of chronic wounds, keloids, and hypertrophic scars are addressed, these are pathologies that are still difficult to understand and treat today.
1. Introduction
This review describes the skin as a well-balanced system, in which an injury, such as a wound, alters the organ’s homeostasis and leads to intricate complications. Understanding the skin system is essential for comprehending the mechanisms involved in wound healing. The other part of the review focuses on the mechanisms implicated in wound healing, with particular emphasis to the cellular mechanisms involved. Therefore, there are many bibliographical entries dedicated to this topic that are notable for their relevance, indicating that the topic is still subject to ongoing debate. When the skin fails to adequately respond to an injury, problems associated with impaired wound healing occur. In this instance, the authors specifically examine chronic wounds, hypertrophic scars, and keloids, which are still not well understood. As a result, numerous laboratories are currently investigating effector cells related to these issues. Undoubtedly, focusing on effector cells as a subject of study is a good starting point. However, it is crucial for the ongoing research to include the skin microenvironment and its interactions with the external environment. In addition, it is important to recognize that reactions to cellular infiltration are influenced by the cutaneous nervous system. The authors argue that the nervous system interacts with and regulates the responses of the microenvironment. The reflections included in the review are also focused on wound dressing. This topic is highly significant and continuously developing.
2. General Morphological Characteristics of the Skin
The integument of the body is made up of the skin, or cutis, and the subcutaneous connective tissue, or hypodermis. The skin is a flattened anatomical structure with a very large surface area that covers the entire external surface of the body and continues with the mucous membranes at the orifices of the cavities that open to the outside. The skin is made up of an epithelial tissue on the surface, called the epidermis, and at depth, of a connective tissue, called the dermis. This is followed by the subcutaneous connective tissue, rich in fat, which reaches the bands that cover the muscles or bones, depending on body location. The skin contains a series of appendages that complete its structure and function, including hair, nails, and glands of various kinds, which develop from the epidermis and with the participation of the dermis [1,2,3,4] (Figure 1).
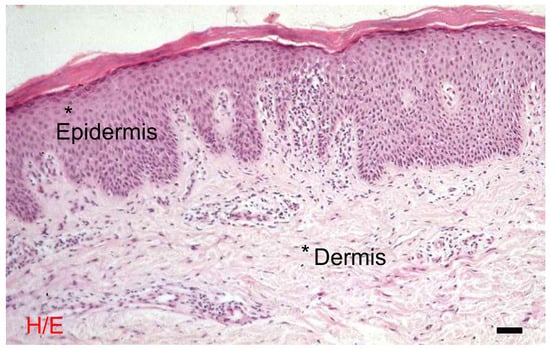
Figure 1.
The skin: morphological differences between the epidermis and the dermis. Hematoxylin eosin, bar = 10 microns. The photograph is a property of one of the authors (SB).
The skin, without considering the subcutaneous layer, represents a conspicuous fraction of total body weight, around 5–6%; its surface is in relation to height and—less strictly—to body weight: in an adult 1.70 cm tall and weighing 70 kg, the skin surface is approximately 1.8 m2. Special tables are available for estimating body surface area, which is closely related to metabolism and consequently is the basis for the dosage of many drugs, better than body weight. The skin surface is distributed among the various body areas, which is taken into account when estimating the relative extension of injuries such as burns; specific tables are followed for the estimate. A good approximation, easy to remember, is provided by the division of the body surface into 11 districts, each equal to 9% of the total surface: (1) the head and neck, (2) the anterior surface of the thorax, (3) the posterior surface of the thorax, (4) the anterior surface of the abdomen, (5) the posterior surface of the abdomen, (6 and 7) the anterior surface of each lower limb, (8 and 9) the posterior surface of each lower limb, and (10 and 11) each upper limb; the remaining 1% is attributed to the perineum [5,6,7,8].
The thickness of the skin varies from a minimum of around 0.5 mm (for example, in the eyelids) to a maximum of around 4 mm (in the nape of the neck); the epidermis represents a small part of the total and its thickness is also variable, between 50 and 150 µm in the greatest part of the body (so-called thin skin, a commonly used term even if strictly speaking it is inappropriate because it does not take into account the thickness of the dermis), and reaches 1.5 mm in the palm of the hand and the sole of the foot (so-called thick skin—see the considerations regarding thin skin), and even more in the case of calluses. The thickness of the subcutaneous tissue varies greatly based on body location (maximum in the abdomen and buttocks, smaller in the trunk, decreasing towards the periphery of the limbs, minimum in the head and neck) and based on the state of nutrition: the subcutaneous tissue is in fact the seat of the adipose panniculus, which represents the body’s largest reserve of fat [5,6,7,8].
On the surface of the skin, in areas with thick skin, there are skin ridges (skin papillae), elongated reliefs alternating with skin furrows and forming characteristic designs, the dermatoglyphics; on the rest of the skin surface, skin furrows delimit flat diamond-shaped surfaces. Temporary or permanent skin folds form in relation to muscles (muscle folds) and joints (joint folds). Wrinkles form on the skin surface in relation to aging; they are more marked on surfaces exposed to solar radiation. On the skin surface, you can see the orifices of the hair follicles, sweat glands, some sebaceous glands, and, where present, apocrine glands and mammary glands, the latter opening on the apex of the nipple [9,10,11,12].
The skin is distensible and elastic; the tension of the skin at rest is not the same in all directions but depends on the organization of the collagen and elastic fibers in the dermis and is greater in one direction and less in the direction perpendicular to it; this must be taken into account when making surgical incisions, which must be oriented as far as possible along the axis of greatest tension to prevent diastasis of scars [1,2,3].
3. Functions of the Skin
The skin provides protection against external agents such as mechanical, chemical, and thermal insults; infections; water and aqueous solutions; electromagnetic radiation; and electric currents. This function is carried out mainly by the epidermis, which exposes to the outside a rather hard and at the same time flexible surface and which has, in its thickness, a layer impermeable to water and ions; furthermore, it contains cells that produce a pigment, melanin, which—together with the epidermal thickness as a whole—protects against visible radiation. The dermis provides valid mechanical, compact support and elastic, due to the rich content of connective fibers. The subcutaneous layer guarantees the cushioning of traumas, pressure exerted by the weight of the body, and objects placed on the body or actively grasped [1,4,6,7,8,12].
The skin provides essential protection against the uncontrolled dispersion of water from the body surface, thanks to the impermeability of the epidermis. Instead, it provides a regulated dispersion of the heat produced within the body through skin circulation and the secretion of sweat, which allows heat to be dispersed even when the external temperature is higher than the internal temperature of the body. The subcutaneous layer guarantees thermal insulation between the inside of the body and the skin, so as to prevent heat loss by radiation and conduction [1,4,6,7,8,12].
The skin represents a powerful means of communication between individuals and the outside world. It allows personal recognition, interpersonal interactions, and interactions with domestic and wild animals thanks to its color, observable and palpable surface profile, odor, the presence of personal identifying marks, and the possible presence of signs of diseases of both the integument and internal organs. Furthermore, the skin allows it to receive numerous and fundamental stimuli from the external environment, both tactile, vibratory, and thermal [1,4,6,7,8,12].
The subcutaneous tissue, in addition to its mechanical and thermal insulating role, acts as the body’s main nutritional reserve and, thanks to the metabolic activity of adipose cells, is a source of hormones involved in the regulation of nutrition and various metabolic cycles [1,4,6,7,8,12].
4. The Epidermis
The epidermis is a keratinized compound pavement lining the epithelium. It consists of various layers, each made up of one or more planes of cells, which proceeding from the dermis towards the surface are as follows: the basal layer, the spinous layer, the granular layer, and the corneous layers (Figure 2). In areas with thick skin and around the outlet of the hair follicles, the deepest part of the corneum has characteristics and forms an identifiable layer, the stratum lucidum. Each layer has structural and functional peculiarities. The main cellular contingent of the epidermis is made up of keratinocytes, i.e., the cells, generated in the basal layer, which can differentiate and largely do differentiate into cells of the stratum corneum. Minor populations, overall around 10–15% of epidermal cells, are represented by other cell types, distinguished by origin, function, and fate, including the following: melanocytes, Langerhans cells, Merkel cells, and lymphocytes [1,5,6,8,9,10].
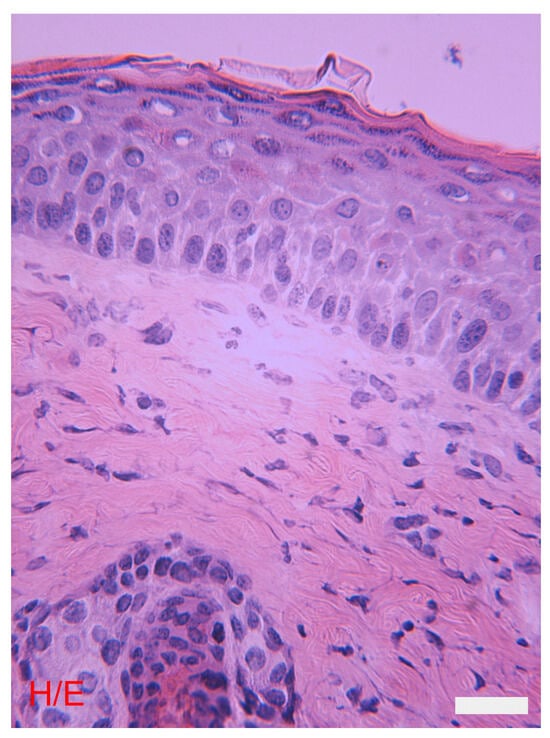
Figure 2.
The skin: morphological differences between the epidermis and the dermis. The epidermis and the various layers that compose it. The underlying dermis has various cell types, a sebaceous gland, and a hair follicle. Hematoxylin eosin, bar = 10 microns. The photograph is a property of one of the authors (SB).
The term keratin was initially used to indicate the stratum corneum; it subsequently indicated the material inside the cells of this layer, consisting largely of filamentous molecules, with a complex and peculiar molecular structure. It has therefore been found that complexes of these same molecules are also found in the deeper layers of the epidermis, where they appear as thin cytoplasmic fibrils, called tonofibrils, in turn made up of tonofilaments visible under an electron microscope. Tonofilaments represent the intermediate filaments of keratinocytes, and tonofibrils are also sometimes designated as keratin. The characteristic proteins of keratin and tonofilaments are called cytokeratins, sometimes briefly just “keratins”; there are different types, which constitute a family of molecules whose members form the intermediate filaments not only of keratinocytes, but also of the epithelial cells of the mucous surfaces and of the exocrine and endocrine glands. In each tonofilament, there are two types of cytokeratins, one acidic and one basic, joined together [1,5,6,8,9,10].
The basal layer consists of cubic or elongated cells perpendicular to the implant surface. The cell surface towards the dermis is more serrated the greater the development of the dermal papillae. This surface is responsible for adhesion to the dermis via the basement membrane [see below], to which the cells are joined along their entire surface; adhesion is reinforced by numerous hemidesmosomes [see below]. The basal layer is also called the germinative layer, because the cells of this layer can reproduce and give rise both to new basal cells and to cells that abandon the basal layer and move on to the subsequent layers of the epidermis, up to the superficial layer. The reproductive capacity is not the same for all the cells of the basal layer: some of them are true stem cells, that is, they reproduce slowly but are able to maintain proliferative capacity for the entire life of the organism and, if stimulated, to completely regenerate the tissue; others are expanding cells, which are generated from stem cells and reproduce for a limited number of cell cycles, so that many elements destined to differentiate are generated from them. Basal cells synthesize type 5 and type 14 cytokeratins, which are organized into tonofilaments and, in turn, into tonofibrils which are inserted into the plasma membrane through desmosomes [see below], which stabilize intercellular adhesion. Among the basal cells, melanocytes are found and melanin granules, or melanosomes, are transferred from the latter cells to the basal keratinocytes, where they tend to arrange themselves between the nucleus and the cell side facing the epidermal surface [1,5,6,8,9,10].
Melanocytes have a dendritic shape, that is, they consist of a spherical or oval body from which extensions of decreasing calibers branch off and bifurcate several times. These cells derive from melanoblasts, which in turn originate from neural crest cells, migrate to various body sites, and then penetrate the epidermis during prenatal life; they are cells capable of dividing, whose average density is around 1000 per mm2 of the epidermal surface. The numerical density of melanocytes is independent of the color of the skin, which depends—as melanin does—on the activity of the melanocytes themselves and on the persistence of melanin in the keratinocytes. Melanin is a molecule derived from the oxidation, cyclization, and polymerization of the amino acid tyrosine; the first stages are catalyzed by a special enzyme, tyrosinase, and the subsequent stages seem to occur spontaneously. Tyrosinase is synthesized by melanocytes in the cell body and is stored in cytoplasmic granules, called melanosomes, which progressively fill with melanin. Mature melanosomes are also called melanin granules; they migrate into the dendrites (thanks to the intervention of microtubules) and are transferred to the keratinocytes, probably through the detachment of portions of the dendrites and their phagocytosis by the keratinocytes. In White and Asian subjects, melanin is limited to the basal layer, while in Black subjects it is found up to the superficial layer. The melanin granules, once phagocytosed, can remain in lysosomes, where they are digested (particularly in subjects with poorly pigmented skin), or they can be released into the cytoplasm and persist for a long time (particularly in subjects with black skin). There are different types of melanin: that typical of well-pigmented subjects (i.e., brown and black skin), called eumelanin and in which there is an extensive polymerization of the oxidized tyrosine monomers, and those of blond and rutile subjects, called pheomelanin, in which the polymerization occurs and stops prematurely, and the resulting compound binds to proteins [1,5,6,8,9,10].
The normal color of the skin depends not only on melanin, but also on the hemoglobin of the blood flowing in the dermal vessels, on the stratum corneum, which is yellowish and is particularly thick in Asian people, and on the reflective power of the epidermis, and is therefore conditioned by ambient light. In pathological conditions, pigments of various origins can come into play (biliary, blood, etc.) [1,5,6,8,9,10].
The basal layer also includes Merkel cells; they are rounded, with short and rigid offshoots, are joined by desmosomes to adjacent keratinocytes, and contain small cytoplasmic granules, visible under an electron microscope. Approximately half of Merkel cells are in contact with expansions of nerve fibers that have the characteristics of receptor endings. It is believed that Merkel cells respond to tactile stimulation of the epidermis by secreting the contents of their granules, which is capable of stimulating adjacent nerve endings, promoting the dilation of dermal vessels to allow the correct functioning of the endings themselves and stimulating the growth of nerve fibers towards the epidermis. These last two functions can also be carried out when the nerve fibers do not reach the Merkel cells, but stop at the most superficial portion of the dermis [1,5,6,8,9,10].
The spinous layer is made up of one or, more frequently, multiple planes of large cells in the shape of a many-sided polyhedron, from whose surface short, thin protuberances radiate, and from which they take their name. Tonofibrils are abundant in the cytoplasm and are made up of type 1 and type 10 cytokeratins, which are added to those of type 5 and type 14 already produced in the basal layer. The cells are joined together at the tips of the spines, where there are intercellular junctions called desmosomes. Here the membranes are joined by specific molecules and on the cytoplasmic face there is a plate of protein material, to which tonofibrils are inserted. Solidarity is thus created between the tonofibril scaffold of a cell and that of the contiguous cells which allows the complex of the epidermis to withstand mechanical stresses to which it is subjected without tearing. Small granules also form in the cells of the stratum spinosum, containing lipids arranged in alternate layers, acid hydrolases, and variously named granules (Odland granules, “membrane coating granules”, keratinosomes, lamellated granules); their contents will be secreted into the space between the cells in the stratum granulosum [see below]. Among the lipids of Odland’s granules, acyl-glucosyl-ceramides are abundant, in which a fatty acid (di-unsaturated: linoleic acid) esterifies the terminal hydroxyl group of an omega-fatty acid, which in turn is joined with an amide bond to sphingosine; the latter, in turn, is joined by a glycosidic bond to a glucose residue. Due to the length of the omega-fatty acid linked to sphingosine and the presence of a hydrophilic zone in correspondence with the ester bond between the terminal alcohol group of the omega-fatty acid and linoleic acid, these lipids continue from a lamella, guaranteeing its orderly stratification [1,5,6,8,9,10].
The stratum granulosum is made up of flattened cells with a regular surface (there are no longer spines); in the cytoplasm there are basophilic granules of so-called keratohyalin, made up of a mixture of proteins with a small amount of ribonucleic acid; these are the proteins that will contribute to the formation of the stratum corneum. The thicker the stratum corneum is, the thicker the stratum granulosum is; where the latter is thin the granular layer appears discontinuous. Odland’s granules are secreted on the surface of the cells facing the stratum corneum: the lipid component seals the intercellular spaces, simultaneously guaranteeing the impermeability of the epidermis both against the loss of water from within the organism and against water and water-soluble substances coming from outside; the enzymatic component contributes to modifying the lipid molecules of the Odland granules so that they are arranged correctly in relation to the cells, and to degrading molecules of the desmosomes, preparing the conditions for the detachment of the cells from the surface of the stratum corneum. It has recently been demonstrated that the cells of the granulosa state are joined together by occluding bands, which contribute significantly to ensuring the impermeability of the tissue; this mechanism contributes with intercellular lipids to waterproofing and neither one nor the other alone seems sufficient to guarantee this result [1,5,6,8,9,10].
The stratum corneum is composed of the corneocytes, flattened cells with exceptional characteristics. Corneocytes have no nucleus and organelles, are significantly dehydrated, and contain only intermediate filaments (tonofilaments) arranged in various directions parallel to the cell surface, thickly and regularly packed and held together by a homogeneous and dense matrix. The cell membrane is also particular, as it appears thicker than usual, particularly in the internal layer of the membrane itself, and has a lipid envelope on the external side. This is formed by omega-hydroxy-ceramides (derived from acyl-glucosyl-ceramides) which insert themselves into the plasma membrane, binding with the end of the sphingosine to the involucrin (see below) and crossing the entire thickness of the membrane, and they interact with intercellular lipids, helping to regulate their stratification and solidarity with the plasma membrane. The tonofilaments no longer attach to the membrane in dense plaques as in the underlying layers, and where there are desmosomes, an electron-opaque material remains in the space between the cells, homogeneous and separated from each of the adjacent cell membranes by a layer transparent to the electrons. Remnants of the lipid material of Odland’s granules are found among the cells of the deeper layers of the stratum corneum. Of the lipids of the stratum corneum, approximately half (by weight) is made up of ceramides (derived from glucosyl-ceramides), a quarter of cholesterol, 10–15% of free fatty acids, and the rest of other lipids, the most important of which it is cholesterol sulfate. Proteins (loricrin, involucrin), synthesized in the underlying layers and are arranged on the cytoplasmic side of the membrane itself, contribute to making the cell membrane thick and rigid. Proteins, which are also synthesized in the underlying layers and had contributed to forming the keratohyalin granules, contribute to cementing the tonofilaments together; the various protein components are joined together by the action of transglutaminase in the transition between the stratum granulosum and the stratum corneum. This transition is sudden, so it is exceptional to see cells undergoing transformation [1,5,6,8,9,10].
In locations with thick skin and near the outlet of the hair follicles, the deeper part of the corneum presents cells with the appearance of corneocytes, but containing small lipid drops; this material was given the name eleidin, a descriptive term devoid of any chemical meaning. This portion of the epidermis is called the stratum lucidum because where it is continuous, as in the sole of the foot and the palm of the hand, if the stratum corneum is gradually removed with a sharp edge, the plane appears transparent compared to deeper planes. and which corresponds precisely to the planes containing the eleidin granules [1,5,6,8,9,10].
5. The Dermis
Below the epidermis is the dermis, a dense fibrous connective tissue (Figure 3). The most superficial portion of this tissue is called the papillary dermis, the deeper and more abundant portion is called the reticular dermis. In the papillary dermis, the fiber bundles are fine and have narrow meshes; in the reticular dermis, the bundles are coarse and have relatively loose meshes. For a better localization of structures and possible alterations in the dermis, the terms superficial, medium, and deep dermis are often used, the first corresponding approximately to the papillary dermis, the other two at subsequent levels of the reticular dermis [1,2,5,6,10,11].
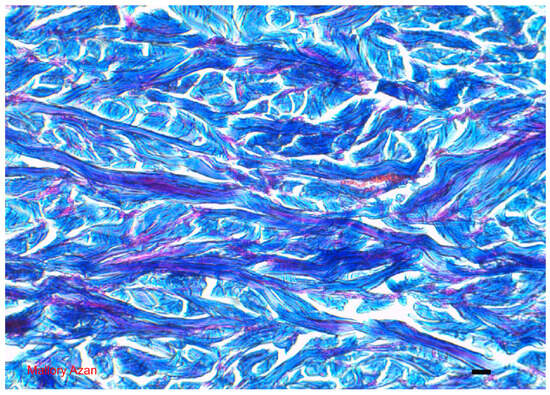
Figure 3.
Dermis: different features of the collagen fibers which histologically define the type of tissue considered Mallory Azan = 10 microns. The photograph is a property of one of the authors (SB).
The dermis contains an abundant extracellular matrix, consisting of both fibers and an amorphous component (anist ground substance). There are multiple types of fibers. The predominant ones are collagen fibers, with a slightly sinuous course arranged in various directions. Collagen fibers are polymers of a protein, tropocollagen or collagen (when this term is used at a molecular level it is used as a noun; to define the corresponding fibers it is instead used as an adjective). There are different types of collagens, the one that makes up the collagen fibers of the dermis is type I collagen, and at most there may be traces of other types. The collagen molecules, once secreted outside the cells, organize themselves into fibrils visible under an electron microscope, a few tens of nanometers thick and with a characteristic transversal striation. Several fibrils together form a fiber visible under an optical microscope, remaining cemented together by a so-called “intrafiber” amorphous matrix. The newly formed collagen fibrils are stabilized within several weeks by covalent bonds between the various constituent molecules, becoming definitively insoluble. Collagen fibers are inextensible and very resistant to tension; the deformability of the dermis depends on the possibility of the individual fibers straightening and the fiber mesh extending due to a change in the angle between the individual fibers until some of them become straight and aligned with the force exerted, after which the dermis can no longer extend [1,2,5,6,10,11].
The second most abundant are the elastic fibers. These are made up of a scaffold of tubular microfibrils, so called because they have a small cylindrical cavity in the center and an amorphous matrix, which is the predominant part. The tubular microfibrils are made up of a glycoprotein, called fibrillin, and are inextensible; the amorphous matrix is made up of another protein, called elastin, and is responsible for the elastic behavior that gives the name to these fibers: they can be easily relaxed by the application of even a slight tension and when the applied force ceases, they recover their original shape and size. In the dermis, the elastic fibers are not perfectly released, but instead remain in a certain basic tension; this tension is not identical in various directions and in each body location there is an axis along which the tension is greater and perpendicular to the previous direction, in which the tension is less. Consequently, a rounded wound tends to deform into an oval, with the major axis positioned in the direction in which the basic tension of the tissue is greatest. This is considered when making surgical cuts on the skin, so as to reduce the tension applied to the wound when it is stitched [1,2,5,6,10,11].
The amorphous fundamental substance consists largely of water, in which large molecules of glycoproteins and proteoglycans are suspended. Glycoproteins have a protein axis to which short, sometimes branched, carbohydrate chains are joined; proteoglycans also have a protein axis, to which glycosaminoglycans are joined, i.e., long, straight glycidic chains, made up of the succession repeated many times of a different dimer depending on the glycosaminoglycan, containing both carboxylic and sulfonic acid groups (derived from sulfate). The proteoglycans in turn bind with one end of the protein chain to another, very long non-sulphated glycosaminoglycan, hyaluronic acid. Part of the fundamental substance anista is found, as already mentioned, inside the collagen fibers, cementing together the collagen fibrils visible under an electron microscope. Part of it, however, is found between the fibers (the so-called amorphous “interfiber” matrix), where it represents an ideal medium for the life of cells, for the diffusion of water and small solutes which also move within the glycoproteins and proteoglycans between a chain and the other molecules of this type, for the diffusion of macromolecules that move in the interstices between glycoproteins and proteoglycans, and for the maintenance of a water reserve which is coordinated by the glycoproteins and proteoglycans thanks to their ability to form hydrogen bridges. Therefore, the fundamental substance anista is also responsible for the turgor of the dermis and its resistance and elasticity in the face of compression [1,2,5,6,10,11].
The dermis contains numerous cells, in particular fibroblasts and macrophages. Fibroblasts are responsible for the synthesis of the extracellular matrix; the individual constituent molecules are synthesized and secreted by fibroblasts and then organized into microscopic structures in the intercellular space. The synthesis, intracellular maturation, and secretion of collagen molecules and the definitive stabilization of collagen fibers require the intervention of numerous enzymes; defects (mostly congenital) of these enzymes are responsible for defects in the constitution of collagen, resulting in abnormal laxity and sometimes fragility of the dermis, as well as of other structures rich in collagen fibers, such as the capsules and joint ligaments. Tubular microfibrils are first formed from elastic fibers, then the elastic component is deposited around and in the middle. Fibroblasts are also responsible for the renewal of the extracellular matrix, which is reabsorbed thanks to the secretion of hydrolytic enzymes, active at a physiological pH and capable of degrading the various molecules of the matrix itself, after which new molecules are synthesized. Some fibroblasts are specialized to adhere to collagen fibers and contract, exerting traction on the fibers themselves and causing a retraction of the dermis; these cells are called myofibroblasts and are at work in the healing processes and in some pathological conditions. The question is still open as to whether myofibroblasts are continuously present in the tissues or differentiate from time to time and, in this case, whether they do so starting from pluripotent stem elements or from already determined precursors. Macrophages help to reabsorb foreign material and portions of tissue damaged by various types of insults, a typical task of these cells at all body sites [1,2,5,6,10,11].
Close to the epidermis and the skin appendages, there is a specialized layer of the extracellular matrix, called the basement membrane. Under a light microscope, the basement membrane is well demonstrated with silver impregnations. A combined examination with an optical microscope and an electron microscope demonstrates that three layers can be recognized in the basement membrane; starting from the surface of the epithelial cell, a thin layer transparent to electrons called the lamina rare or lamina lucidum (20–30 nm thick), another layer opaque to electrons called the lamina dense (20–50 nm thick), and finally a layer faded towards the depth of the dermis called the reticular lamina, a few microns thick. The lamina rare and lamina dense together constitute the basal lamina. The lamina rare is the glycocalyx of the epithelial cell. The lamina dense is a mixture of type IV collagen molecules and non-collagenous glycoproteins, among which laminin prevails; collagen IV does not form fibrils but remains as isolated molecules. The lamina dense is the most characteristic portion of the basement membrane and is sometimes identified with the latter, par excellence. The reticular lamina contains a series of fibrous structures, anchored on one side to the lamina dense and which continue into the dermis on the other side. Reticular fibers, anchoring fibrils, and so-called oxytalan fibers are found here. Reticular fibers are similar to collagen ones, from which they are distinguished by their thin dimensions, their argyrophilicity, and their tendency to form delicate networks. They are made up of collagen, mainly type III. In other tissues, reticular fibers can also be found in the connective tissue network, not limited to basement membranes. Anchoring fibrils are exclusive to the basement membrane; they are made up of type VII collagen. Oxythalanic fibers are bundles of tubular microfibrils without an elastic component; they connect the lamina dense to elastic fibers located deeper in the dermis, to transmit mechanical forces that must be elastically absorbed. The basement membrane is a connection area between the various tissues, and the epithelial cells adhere to it with specific molecules. Adhesion is reinforced by special junctions, called hemidesmosomes; their appearance under an electron microscope is that of a half desmosome, with tonofilaments inserted into a dense plaque on the internal face of the plasma membrane of the epithelial cell and anchoring filaments subtending through the lamina rare. Chemically, the molecules are different from those of desmosomes, but the functional meaning is similar. The fibrils of the reticular lamina adhere to the deep face of the lamina dense, which in turn connects to the fibers located more deeply and guarantees solidarity between the basal lamina and the rest of the dermis. The basement membrane, thanks to the felt-like structure of the lamina dense, also represents a barrier to the diffusion of macromolecular complexes. For this reason, antigen–antibody complexes, which form in the dermis due to various diseases, localize in the basement membrane. The basement membrane also represents a source of signals for the cells that are in contact with it, thanks also to specific interactions between membrane molecules of these cells and molecules from the lamina dense. Among the consequences of these signals is the abandonment of the cell cycle and instead the beginning of an irreversible differentiation process by the cells in which they abandon the basal layer of the epithelium [1,2,5,6,10,11].
6. Subcutaneous
In the hypodermis, the adipose tissue is arranged in lobules separated by fiber-rich shoots called retinacula, which connect on one side to the deep face of the dermis and on the other to the muscular fascia or periosteum, depending on the location. In most of the body, the subcutaneous tissue is organized into a superficial, areolar, layer, and a deep, lamellar layer. In the first, the lobules are almost rounded, and in the second they are flattened parallel to the body surface and are less rich in cells, so the retinacula that run slightly less than parallel to the surface prevail. The deep subcutaneous layer represents a mobile area, which allows the sliding of the skin and the areolar layer with respect to the deep planes. The deep layer is missing in the palms of the hands and the soles of the feet, which explains the firm anchoring of the dermis to the muscle fascia in these locations. In the head, neck, and proximal part of the thigh, the two layers of the subcutaneous layer are separated by a superficial fascia; in this band, the fur muscles develop in the head and neck. Subcutaneous bags, congenital or acquired, develop in the thickness of the deep layer [1,3,5,6,9,10].
7. Vascularization and Innervation of the Skin
Arteries reach the skin through the subcutaneous retinacula and form a deep plexus on the deep surface of the dermis, from which branches rise to form a subpapillary plexus which gives rise to other vessels that push close to the epidermis. The veins follow a reverse path, forming four plexuses in the dermis, one subpapillary, two subsequent ones further away from the epidermis, and one on the deep border of the dermis. The lymphatic vessels follow the path of the veins but form only two plexuses, one subpapillary and one deep. The papillary capillary bed is very developed, but no more than a fifth of this bed serves the metabolic needs of the skin, the rest serves to circulate blood for cooling the body in a strictly regulated manner. When heat must not be dispersed, a large part of the blood is short-circuited directly from the arteries to the veins through tortuous arteriovenous anastomoses with a thick muscular wall, called the glomas [1,5,6,10].
The skin is home to a rich nervous network. Part of the fibers are efferent, destined for the vessels and glands. A large part is instead afferent and refers to sensory endings that vary in location and microscopic organization (Figure 4). Receptor endings are found in the epidermis, many as free fibers and some as disk-shaped expansions abutting Merkel cells. In the dermis, there are both nerve endings, represented by free fibers between the fiber bundles, and encapsulated endings, made up of various types of corpuscles which are characterized by their shape, size, location, and structural organization. Among the latter we can mention the Meissner corpuscles, located in the dermal papillae of which they repeat their shape, with a capsule that closes them only from the side towards the base of the papillae; the Pacinian corpuscles, located in the dermis and subcutaneous tissue, oval in shape, with a capsule that completely surrounds them; the Ruffini corpuscles, also located in the dermis and subcutaneous tissue, tapered with a capsule open at the two poles and crossed by collagen fibers; and the Krause clubs and the Golgi–Mazzoni corpuscles, relatively smaller and simpler than the previous ones, also used for the reception of tactile stimuli. Hair is also important for skin sensitivity: around hair, there is a sort of palisade of nerve endings, which are stimulated every time the hair is flexed. The sensitivity, served by the cutaneous innervation, is tactile and thermal; furthermore, each termination, if stimulated too intensely, can generate painful sensations. The encapsulated corpuscles are selectively sensitive to different types of tactile stimuli, due to their structure; it can be remembered that Meissner’s corpuscles sense surface pressure, Pacini’s corpuscles sense vibrations (so-called pallesthetic sensitivity) and probably deep pressure, and Ruffini’s corpuscles detect the distension of the tissue [13,14].
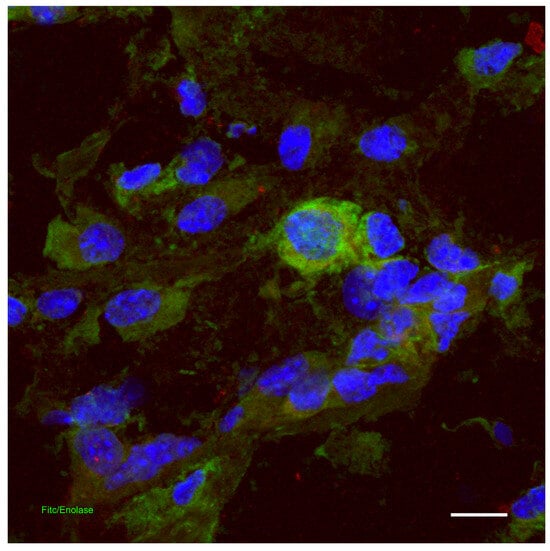
Figure 4.
Dermis: indirect immunofluorescence for the detection of neuronal enolase in the skin. Confocal microscopy, scale bar = 100 microns. The photograph is a property of one of the authors (SB).
8. Skin Immune System
The skin is a barrier between our organism and the outside world and is equipped with an active defense system both against infectious agents and against tumors whose formation is favored by exposure to environmental carcinogens, including ultraviolet radiation. These defenses also come into action against soluble molecules, regardless of their danger, which can generate inflammatory reactions of no benefit to the organism, and are indeed harmful to the skin itself. The entire epidermis participates in this defense, with its complex structure and the desquamation of the corneocytes, as well as the skin appendages, in particular the glands which contribute to determining unfavorable conditions on the epidermal surface for pathogenic microorganisms. Innervation also contributes to defense mechanisms, both by perceiving potentially harmful stimuli and by secreting neurotransmitters capable of influencing the microcirculation as well as the differentiation and perhaps the function of specific defense cells. Specifically, however, the skin’s immune defenses are the task of a large set of cells specialized for this purpose which constitute the so-called cutaneous immune system. This system includes cells specialized for the presentation of antigens, macrophages, mast cells (MCs), and lymphocytes [15,16,17].
Cells specialized for the presentation of antigens are found in both the epidermis and the dermis; these are dendritic cells (DCs), so called due to their shape (they have an oval cell body from which extensions branch out, similar to tree branches). The dendritic cells of the immune system possess a set of membrane and intracytoplasmic molecules and can carry out complex metabolic activities that allow them to absorb antigenic molecules, extract fragments through controlled hydrolysis and expose these fragments, called epitopes, together on their membrane to accessory molecules, in order to stimulate T lymphocytes specific for these epitopes and thus trigger immune responses (so-called antigen presentation). DCs are able to present antigens both to helper T lymphocytes, thus starting a chain of events that leads to the secretion of antibodies, and to cytotoxic T lymphocytes, directly responsible for defense against viruses and tumor cells; the membrane molecules responsible for the presentation of antigens to helper T lymphocytes are those of class II histocompatibility, and the molecules responsible for this presentation to cytotoxic T lymphocytes are those of class I histocompatibility. During the presentation of epitopes to lymphocytes, dendritic cells are capable of exposing so-called costimulatory molecules on their surface, which are essential for initiating the immune response of “virgin” or “naïve” T lymphocytes, i.e., those that have never yet encountered the epitopes for which they are specific, and are the only lymphocytes at play in primary immune responses. DCs appear to be essential for the stimulation of primary immune responses; they are also able to stimulate secondary responses which involve memory T lymphocytes, but during secondary responses, other cells such as macrophages can also present antigens to the T lymphocytes. To present antigens during primary responses, dendritic cells move from the site where they absorbed the antigens to the lymph nodes or spleen, the only sites from which the virgin lymphocytes transit; in secondary responses, antigen presentation can also occur in peripheral sites, therefore even in the skin itself, where memory T lymphocytes transit. The dendritic cells that present antigens to the T lymphocytes derive from circulating precursors of the monocyte family and proceed with their differentiation in the tissues, becoming so-called immature dendritic cells. Once the antigens have been absorbed and as they move towards the lymphoid organs, they undergo a further differentiation stage, becoming so-called mature dendritic cells, no longer capable of absorbing antigens but capable of presenting the previously absorbed antigens to lymphocytes [18,19].
The dendritic cells of the epidermis are called Langerhans cells (LCs) (Figure 5). They are located at various heights in the basal layer and, more often, in the spinous layer. Their dendrites insinuate themselves between the keratinocytes, to which they are joined thanks to an adhesion molecule, E-cadherin. They contain characteristic inclusions in the cytoplasm such as Birbeck granules, involved in the processes of absorption and processing of antigens. They also express characteristic membrane antigens, such as CD1a, which appear to participate in the presentation of bacterial molecules to lymphocytes. Langerhans cells, under normal conditions, renew themselves very slowly, with a renewal time of more than 18 months; this time can be reduced noticeably, even after a few days, following stimuli that cause a drastic numerical reduction in LCs, such as exposure to type B ultraviolet radiation and antiblastic drugs [19,20].
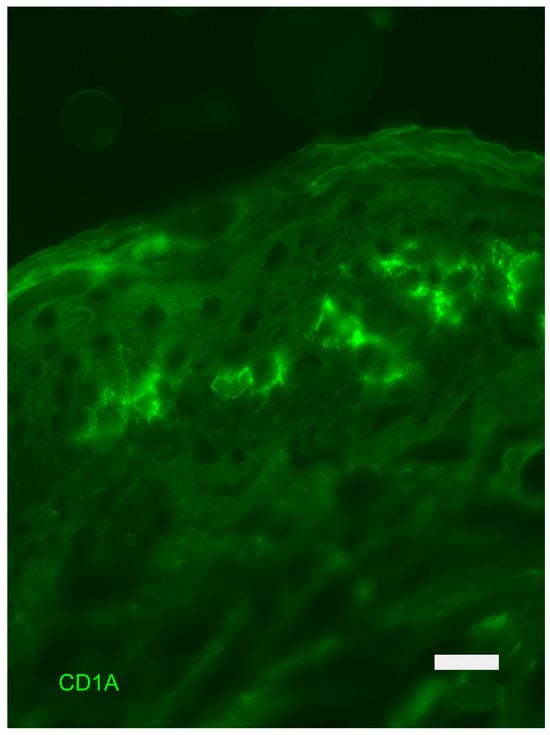
Figure 5.
Epidermis: indirect immunofluorescence for the detection of CD1a-positive Langerhans cells. Fluorescence Microscopy, scale bar = 10 microns. The photograph is a property of one of the authors (SB).
In the dermis, there are dendritic cells typical of connective tissues, which express neither E-cadherin, CD1a, nor other molecules characteristic of LCs and which have some traits in common with macrophages, such as a rich lysosomal complement; their renewal time is short, on the order of a few weeks. Both LCs and dermal dendritic cells are effective stimulants of immune responses; LCs appear particularly effective for stimulating responses to low concentrations of antigens (as might be found in the early stages of viral infections and carcinogenesis). Unfortunately, dendritic cells can also represent a route of entry and transfer to the lymph nodes of the human immunodeficiency virus (HIV), which can enter these cells using both CD4 and other membrane molecules expressed by dendritic cells as a receptor [20].
The dermis also hosts macrophages responsible for engulfing foreign substances of various kinds, such as living agents, inert powders (such as tattoo pigments), and cells, if the extracellular matrix is damaged by trauma, burns, and other insults. Macrophages are derived from monocytes, like dendritic cells, and can participate in the presentation of antigens during secondary immune responses [20].
MCs are equipped with characteristic secretory granules (Figure 6), which contain histamine, heparin, and TNFalpha. These cells respond to numerous stimuli, both directly irritating (such as trauma, burns, and vegetal and animal stinging substances), and immune-mediated; in particular, MCs possess receptors for a particular type of antibodies, immunoglobulin E, which thereby attach themselves to the cell membrane. When an antigen binds to immunoglobulin E attached to the membrane of MCs, the latter rapidly secrete their granules. Local reactions thus occur in the vessels and innervation, which contribute to the organism’s alarm and defense reaction. In addition to the molecules stored in the cytoplasmic granules, once stimulated, MCs also rapidly secrete active derivatives of arachidonic acid (prostaglandins and leukotrienes) and derivatives of other membrane lipids (PAF). Over a period of time ranging from about twenty minutes to a few days they synthesize and secrete numerous cytokines, capable of influencing the recruitment, differentiation, and function of both different types of leukocytes and fibroblasts, thus stimulating defensive and repair processes of tissue damage. Excessive stimulation of MCs leads to a massive secretion of histamine, which can itself cause damage to the tissue or to the entire organism, as occurs in allergic reactions [21].
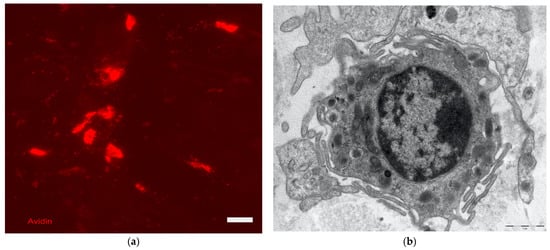
Figure 6.
Dermis: on the left (a) affinity cytochemistry for mast cell localization (stained with avidin in red), Fluorescence Microscopy, scale bar = 10 microns; on the right (b) ultrastructure of a mast cell, electron microscopy, scale bar 1 micron. The photographs are a property of one of the authors (SB).
Memory lymphocytes recirculate through the skin and small numbers are found in the dermis and epidermis; in the latter location, we also find a type of T lymphocyte (so-called gamma-delta) which seems to be involved in responses against some types of bacterial antigens. Normally, the skin does not host B lymphocytes, which can accumulate in this area in pathological conditions [20].
The description of skin appendages is beyond the scope of this review. However, for the interested reader, they are reported below: hair [22,23], nail [1,24], sebaceous glands [25,26], sweat glands [27,28], apocrine sweat glands [29,30], and mammary glands [31,32].
9. Skin pH
The surface of the epidermis is covered by a thin hydrolipidic emulsion; the aqueous part is provided by sweat, and the lipid part by sebum and the secretion of the apocrine glands, where these are present. This emulsion helps to keep the epidermal surface soft and flexible, preventing the formation of cracks and irritation of the tissues due to external agents. Lipids include a portion of fatty acids which, together with the acids originally present in the material that flakes from the stratum corneum and carbonic acid, formed from carbon dioxide—both produced in the tissues and present in the atmosphere—ensure that the surface of the epidermis is acidic, on average around a pH of 5.5 [33,34].
This acidity makes the epidermal surface inhospitable to many bacteria and fungi that could be a source of disease. Although it can sometimes be overcome by infectious agents, this protection is also very valid and helps to make infections rare. The superficial hydrolipidic layer, however, is not important for the waterproofing of the epidermis, which instead depends on the lipid material between the keratinocytes, coming from the Odland granules, and from the occluding junctions between the keratinocytes of the granulosa layer. Absorption through the epidermis is possible with relative ease for fat-soluble molecules, even if these find a certain obstacle in the basement membrane—capable of slowing down but not preventing their passage. It is possible to stimulate percutaneous absorption by massaging the skin and using occlusive dressings: the closed and humid environment thus formed is capable of altering the functional characteristics of the epidermis and allowing the passage of molecules of various kinds. The skin can thus be exploited for the administration of medicines, in particular, substances that dissolve in fats and water-soluble substances but of which must be absorbed slowly and continuously over a long period of time [33,34].
10. Skin Development
The skin develops on the body’s surface from two components, one epithelial and one connective. The surface ectoderm forms the epidermis; at the beginning, this is made up of a single layer of basal cells and is covered by a layer of flattened cells, called periderm, which guarantees impermeability thanks to occluding junctions, and which peels off every four months. In the meantime, all the layers of the epidermis are formed, and this takes on the waterproofing function when the periderm peels off. The dermis is formed partly from common mesenchyme derived from the mesoderm of the lateral plate (and in the cephalic region also from the neural crest), partly from cells migrated from the dermatomes, i.e., from each of the somites, which are located on the sides of the dorsal cord and of the neural tube. The orderly migration of dermatome cells provides spatial clues (probably molecules immobilized in the extracellular matrix) for the orderly growth of sensitive nerve fibers: the cutaneous innervation is therefore segmental and each strip of skin, which at the beginning of development is parallel to the body axis, derives its innervation from the cells of the specific ganglion of that level. In the fetus and in postnatal life, the shape of these territories of nervous distribution, called dermatomes, becomes oblique due to greater body growth at a distance from the neuraxis than in its proximity; the dermatomes extend into the limbs and can be recognized by imagining the limbs themselves in abduction, at 90° with respect to the axis of the trunk, with the palms of the hands and the soles of the feet facing forward [35,36].
In advanced age, there is an atrophy of all the tissues of the skin, with consequent modification of the appearance and mechanical characteristics. In areas exposed to sunlight (so-called photoexposed areas) there is instead a degeneration of the skin tissues, with atrophy or hyperkeratosis of the epidermis, the appearance of hyperpigmented patches, and the formation of altered and functionally ineffective elastic fibers; the loss of elasticity of the skin is the main cause of the formation of wrinkles. Senile skin has a high risk of the onset of epithelial and melanotic tumors, especially in photoexposed areas [37,38].
11. Disturbances in the Skin Homeostasis: The History of Wound Healing
11.1. Historical Aspect of a Wound
The Smith Papyrus, written in 1700 BC, provided the first known description of wounds. Historically, medics in ancient Egypt, Greece, India, and Europe developed delicate techniques for treating wounds. These treatments included removing foreign objects, suturing, using clean materials to cover wounds, and preserving damaged tissues from corrosive substances. In the fourteenth century, there was a growing number of bullet wounds which led to the emergence of a new age focused on aiding the healing of these wounds. The use of boiling oil, hot cautery, and scalding water has been replaced by the practice of gentle washing with warmed heated water and the administration of light salves. Ambroise Pare, a renowned French army surgeon in the mid-sixteenth century, reestablished the use of gentle techniques for wound healing. John Hunter, William Stewart, Halsted, and Alexis are notable clinical biologists who have shown that reducing tissue damage leads to fast and efficient healing [39].
11.2. Issues Related to the Study and Management of Wound Healing
In the field of public health, impaired wound healing (WH) appears to be a big concern due to the fact that the therapy of this condition requires treatments that are both expensive and difficult. The United States of America is home to millions of people who require treatment for chronic wounds (CWs), and it is estimated that a total of USD one billion is spent annually on this matter. The increased prevalence of aging, diabetes, and other risk factors in the population is primarily responsible for the escalation of this burden [40,41,42]. In Europe, a situation quite similar to this one is observed, and in the United Kingdom, over 200,000 people have a chronic wound. Some estimates place the number of people who potentially be affected by CWs at more than 1.5 million [43,44,45,46]. Because of this, WH has developed into a subspecialty, and fellowship programs are now available at a number of academic facilities for a wide range of medical professions, such as general practitioners, vascular surgeons, nurses, and dermatologists [43,44,45,46]. There has been a desire for the implementation of new technology in WH. Additionally, this discomfort is compounded by the fact that the understanding of the cellular mechanisms associated with wound healing has not yet reached the point where it is relevant to the general public. Instead, it is more focused on niche problems, in which, in contrast to certain fields of study, only a small number of researchers engage in conversation with one another about the issues that are being considered [40,41,42,43,44,45,46].
11.3. Vertebrates and Wound Healing
WH is a universally preserved evolutionary process observed in all species. Nevertheless, the results of the healing process in the skin vary between different species. Certain lesser vertebrates, such as zebrafish, axolotls, and xenopus, possess the remarkable capacity to fully regenerate their skin without any scarring. However, in higher vertebrates like humans, the process of skin regeneration requires the creation of scar tissue. While the latter method fulfills the skin’s essential role in preventing infections and dehydration, it can have severe aesthetic and psychological effects, significantly diminishing the affected person’s quality of life. It is noteworthy that the scientific literature has documented instances of scar-free healing in fetal skin. Additionally, the regeneration of skin appendages has been observed in adult skin after the occurrence of significant wounds [47,48,49,50,51].
11.4. Wound Healing Modalities
Wounds can heal in three different ways:
By first intention: this is the case for stab wounds, of which surgical wounds represent an example. These may be linear or have great curvature; they have clear edges and are sutured. This procedure, by minimizing the loss of substance by bringing the flaps together, favors their filling with granulation tissue, and leads to fast healing times and good aesthetic results.
By secondary intention: this concerns non-sutured wounds which are therefore left open, by choice or by necessity. In these cases, the granulation tissue, which forms on the bottom of the lesion, must proceed from below to the surface to fill it, through a process that takes longer and can cause serious blemishes.
By third intention: this type of healing concerns surgical wounds that have undergone partial or total dehiscence in the post-operative course. The treatment of this complication normally involves the complete reopening of the wound, its thorough cleansing, the removal of the mortified areas, and adequate plastering. Secondarily, following an evaluation of the local situation and after excluding the presence of foci of infection, the flaps can be sutured again. This will favor the healing process which, in this case, will be said to take place by third intention [52,53,54].
11.5. Macroscopic Evidence of Wound Healing
- (a)
- One to three days following the injury: this phase is characterized by the formation of a primary clot (blood clot), activation of the epidermal boundaries, and an early inflammatory response.
- (b)
- Four to seven days following the injury: this stage is characterized morphologically by the development of crusts. Histological examination shows epidermal edge migration, selective proliferation of early granulation tissue, and an inflammatory response with a high concentration of macrophages and lymphocytes.
- (c)
- Eight–twelve days after the injury: morphologic investigations show that the crust has detached. Histological results reveal the development of a new epidermis, which by day 12 begins to differentiate. Furthermore, the initiation of cutaneous closure occurs simultaneously with the creation of granulation tissue. The attenuation of the inflammatory response coincides with this phase.
- (d)
- Twelve to 30 days post-injury: matrix remodeling, terminal differentiation of the newly produced epidermis, elevated elastic fiber content, and enhanced wound strength all occur during this stage [55,56,57].
11.6. Acute Inflammatory Reaction
Living organisms exhibit the characteristic of responding to external stimulation. When the stimulation is perceived as an affront to the tissue’s integrity, the tissue responds by initiating an inflammatory reaction. This phenomenon, first described by Hunter in 1793, encompasses various distinct mechanisms, including biochemical induction, angiogenesis, and cellular response, among others. These procedures have the dual purpose of eliminating the harmful stimulus and restoring the affected tissue. The phenomenon, known as the vital reaction as described by Strassman in 1954 based on Plenk’s concept from 1786, is integrated within the stated occurrences but is not directly related. This encompasses additional events that are not exclusively classified as part of the inflammatory response, such as the gathering of platelets, the activation of complements by coagulation factors, and the metabolism of prostaglandins [58,59,60,61].
11.7. Summary of Principal Events in Wound Healing
Wound healing involves a complex interaction between different cell types, which classically culminates in four overlapping phases: hemostasis, inflammation, proliferation, and remodeling. This process ends with the formation of a scar (see Table 1).

Table 1.
Cellular and molecular events in wound healing.
During hemostasis, endothelial cells secrete the von Willebrand factor, promoting platelet adhesion. Platelets not only contribute to clot formation but also release several chemicals that can stimulate or regulate inflammation. The release of these mediators culminates in the creation of a fibrin clot, which blocks the lesion and halts bleeding. Arteries that are injured quickly constrict due to the contraction of smooth muscle, which is caused by elevated levels of calcium in the cytoplasm. Tissue hypoxia and acidity occur rapidly as a result of diminished blood flow caused by constriction of the arterioles. This stimulates the generation of vasoactive metabolites, which leads to the reflexive widening of blood arteries and the relaxing of arterial vessels. The duration of this phase is a short span of time, typically lasting only a few minutes [4,21,42,62,63,64,65,66,67,68] (see Table 1).
During the inflammatory phase, MCs induce vasodilation by releasing histamine or serotonin, as well as other mediators like histamine and TNF-alpha. Neutrophils are recruited from injured blood arteries and drawn by interleukin 1, tumor necrosis factor alpha, and bacterial toxins (Figure 7). Activated neutrophils eradicate germs and cell debris, thus promoting wound healing by producing reactive oxygen species, antimicrobial peptides, and proteolytic enzymes. Leukocytes also secrete cytokines and growth factors to commence the proliferative phase. During the later phase of inflammation, there is a transition in the types of macrophages present. They go from being pro-inflammatory (M1 phagocytic) to anti-inflammatory (M2 pro-regenerative). Additionally, neutrophil apoptosis, or programmed cell death, takes place. In addition, keratinocytes, which are other types of cells, contribute to this process by generating inflammatory cytokines. Research has demonstrated that LCs, a specific type of dendritic cells, significantly multiply during the first hour following an injury. These cells play a crucial role in the initial stage of wound healing by releasing substances that stimulate the growth of other cells engaged in this process. The typical duration of this phase ranges from 0 to 3 days. Additional substances, such as cytokines, matrix proteins, and enzymes, also participate in the inflammatory phase. Chemokines are of special significance in the process of wound healing since they have a vital function in attracting neutrophils and lymphocytes to coordinate the initial phases of this process [4,21,42,62,63,64,65,66,67,68]. (see Table 1).
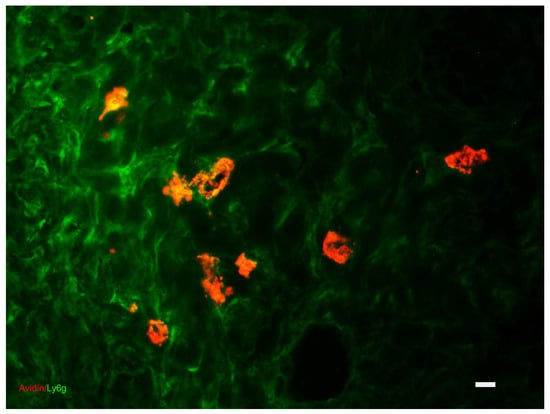
Figure 7.
Dermis: indirect immunofluorescence and affinity cytochemistry for the colocalization of granulocytes (stained with Ly6g antibody in green) and MCs (stained with avidin in red). Fluorescence Microscopy, scale bar = 10 microns. The photograph is a property of one of the authors (SB).
During the proliferative phase, fibroblasts contribute to the synthesis of granulation tissue and regulate the migration and proliferation of keratinocytes as well as the formation of new blood vessels (angiogenesis). In this stage, the initial event involves the migration of keratinocytes across the injured dermis. Fibroblasts and macrophages then replace the fibrin matrix with granulation tissue composed of hyaluronic acid, proteoglycans, glycoproteins, and collagen type III. This freshly generated tissue acts as a new base for the movement of keratinocytes in the later stages of the healing process. Endothelial cells undertake many mitotic cycles to construct numerous vessels, hence fueling neo-angiogenesis (Figure 8). These blood arteries have a crucial function in promoting the development of new tissue at the location of the lesion. Macrophages and MCs play a vital role in supplying a continuous amount of growth factors necessary to facilitate this process. In the epidermis, the keratinocytes located at the outside edge of the injured area divide and migrate toward the center until the two edges are connected, thus restoring the protective function of the epithelial barrier. The duration of this phase spans from 3 to 12 days [4,21,42,62,63,64,65,66,67,68] (see Table 1).
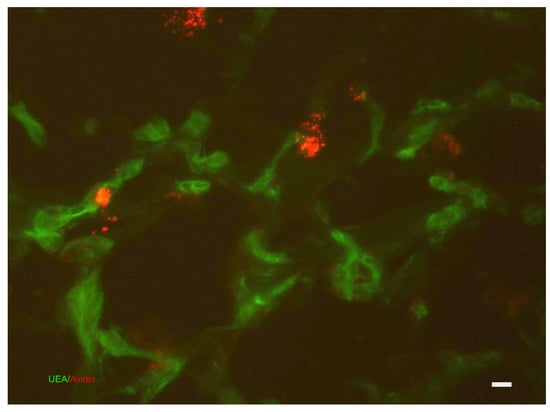
Figure 8.
Dermis: affinity cytochemistry for the colocalization of vessels (stained with Griffonia (Bainderaea) simplicifolia) and MCs (stained with avidin in red). The photograph is a property of one of the authors (SB).
During the maturation phase, the main processes involve the restoration of collagen and the contraction of wounds. The contraction of wounds is caused by the transformation of wound fibroblasts (Figure 9) into myofibroblasts, which express alpha-smooth muscle actin. The remodeling phase is also controlled by several growth factors that regulate the transitions between the mesenchymal–mesenchymal and endothelial–mesenchymal phenotypes. Furthermore, these changes take place via the transforming growth factor beta or the Notch signaling pathways, which suppress the production of cadherin in endothelial cells. Beta2AR has also been identified as a crucial molecule that facilitates the epithelial–mesenchymal transition pathway. At this stage, MCs stimulate fibroblasts to produce collagen; secrete growth factors and cytokines that control the transformation from fibroblasts to myofibroblasts; and release matrix metalloproteinases, which start breaking down the extracellular matrix. As a result, there is a decrease in the production of the extracellular matrix and alterations to its constituents. More precisely, type III collagen takes the place of type I collagen, while elastin, which is not present in the granulation tissue, reemerges. The apoptosis of specific cell types in the granulation tissue is a crucial occurrence in the healing process of wounds [4,21,42,62,63,64,65,66,67,68] (see Table 1).
Figure 9.
Dermis: indirect immunofluorescence for the localization of fibroblasts (stained with HSP47g antibody in green). The photograph is a property of one of the authors (SB).
11.8. The Scar
These processes lead to the development of a scar in both children and adults. During this stage, the tensile strength of the skin increases and becomes similar to that of undamaged skin. This is achieved through the process of collagen cross-linking, facilitated by the enzyme lysyl oxidase. The duration of this phase ranges from 3 days to 6 months. Scar development entails the restructuring of the granulation tissue, with the involvement of matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases TIMPs), which have a crucial function. Consequently, the production of the extra-cellular matrix is diminished, and its constituents undergo alterations. Type III collagen is substituted with type I collagen, and the elastin, which is not present in the granulation tissue, reemerges. It is important to note that the death of different types of cells, which results in a large decrease in the number of cells in the granulation tissue, is a crucial factor in the healing of wounds. The origin of the phenotype of fibrocytes in the dermis is a subject of disagreement. Some argue that it arises from myofibroblastic forms that gradually lose their normal morphological characteristics, while others believe it comes from forms that differentiate later in the described process [4,21,42,62,63,64,65,66,67,68] (see Table 1).
11.9. Differences with Fetal Wound Healing
Experimental animal and human research has established that scarless healing is limited to the early phase of gestation. The capacity for skin regeneration in human fetuses diminishes at 22 weeks of gestation. The primary factors contributing to scarless healing in fetal skin, as opposed to adult skin, include a diminished inflammatory response, variances in the structure and composition of the extracellular matrix, mechanical loading in fetal skin, disparities in the secretion and sensitivity of transforming growth factor (TGF) beta, reduced angiogenesis, and morphological alterations in keratinocytes during re-epithelialization. Furthermore, fetal fibroblasts exhibit an “overactive morphology” resulting in elevated levels of extracellular matrix constituents. Ultimately, the initial restructuring of fetal skin exhibits reduced levels of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) compared to adult skin [47,69,70].
12. Alterations in the Normal Wound Healing Process
12.1. Chronic Skin Lesions
According to the international literature, a skin lesion is considered chronic if it does not heal in six to eight weeks. The inflammatory response in these lesions persists over time, maintaining a balance between degenerative and productive phenomena, without following the regular, systematic, and timely sequence of the repair process, or proceeds through these phases without regaining the tissue’s anatomical and functional integrity.
There are numerous reasons for the process’s delay, which result in its blocking and, in fact, its chronicity. In probabilistic terms, it is possible to have a number of clinical pictures equal to 1406 [45,71,72,73,74,75,76], given that approximately 140 pathologies can act in this way and that the average comorbidity over the age of 65 is approximately 6 (85% of the population is affected by at least one chronic disease; 30% have three or more chronic diseases).
The causes of skin ulcers include a variety of clinical manifestations and syndromes that are well-known, documented in the literature, and whose management is outside the purview of this study. Protease activity increases when the inflammatory phase of ulcers persists, leading to the breakdown of growth factors and other molecular cues that support the reparative phase. The excessive production of hydrolytic enzymes and proinflammatory cytokines inhibits the predominance of reparative activities over destructive ones in chronic ulcers [77,78,79,80,81]. Protease activity reduction through intervention has also been suggested as a way to preserve endogenous growth factors and maintain the regular healing process. The production of new tissue and the breakdown of old tissue must coexist in balance for the body to heal. To do this, in acute wounds, the fibrinolytic systems and matrix metalloproteinases (MMPs) work together to help get rid of fibrin and the damaged extracellular matrix (ECM), favoring its remodeling by making the macromolecules blocked in the matrix available and favoring cell migration. High levels of MMPs and a decrease in TIMPs have been highlighted in chronic skin lesions. This leads to a subsequent change in the matrix’s reorganization and an increase in its degradation [77,78,79,80,81].
Furthermore, a decrease in the tissue concentration of nitric oxide (NO) (especially during malnutrition, diabetes mellitus, corticosteroid therapy, ischemia, and smoking exposure), tissue ischemia, and a high number of neutrophils (with consequent increased degradation of the ECM) have been highlighted in the formation of pericapillary fibrin sheaths (especially in diabetic lesions) [77,78,79,80,81].
Generally, the processes involved in chronic wound healing are similar to those in acute wound healing. However, the dysregulation of MMP secretion is significantly linked to chronic wounds, which prolongs the inflammatory stage. The primary cause of long-lasting inflammation in chronic wounds (CWs) is mostly attributed to the presence of different cell types inside the cell infiltrate [77,78,79,80,81]. Neutrophils are excessively present in the wound and release a substantial amount of MMPs. These enzymes not only damage the connective tissue matrix and elastase, but also deactivate essential proteins involved in the healing process, such as PDGF and TGFbeta [77,78,79,80,81].
Nevertheless, it is important to consider how immune cells interact with keratinocytes. This process occurs through the release of several signaling molecules, although the exact role of these cells in the development of a cell wall is not completely comprehended. In chronic wounds, keratinocytes exhibit the expression of genes associated with partial proliferative activation, which could perhaps account for the observed epidermal hyperproliferation near the borders of the ulcer. In addition, the fibroblasts have no response to the migratory stimulant TGFbeta. This is evident in significantly decreased levels of TGFbetaR, as well as reduced levels of the subsequent components of the TGFbetaR signaling cascade [77,78,79,80,81].
The significance of the immune system’s interactions with the neurological system in regulating wound healing processes should be noted [82]. Recent research has shown that mast cell contact with nerve cells that carry neurotransmitters important in the healing of wounds, including the calcitonin gene-related peptide (CGRP), nerve growth factor (NGF), neurokinin A (NKA), neuropeptide Y (NPY), substance P (SP), Protein Gene Product 9.5 (PGP 9.5), vasoactive intestinal peptide (VIP), and NO, are frequently observed in chronic wounds [83,84,85,86]. This phenomenon can be attributed to the release of the ECM by fibroblasts, along with the elevation of TGFbeta levels and the reaction of cellular infiltrates [83,84,85,86].
Furthermore, infections are an important and frequent cause of blockage of the reparative process: the increase in the bacterial load leads to the persistence of the inflammatory phase, with the production of high levels of MMPs and consequent exacerbation of the destructive processes in the ECM. It is now known and demonstrated that certain bacterial species, once attached to a substrate, produce a polysaccharide matrix rich in proteins and other metabolic products; within this structure (biofilm), the bacteria aggregate to form micro-colonies, protected by the action of antimicrobial agents, both due to reduced penetration of these through the biofilm matrix and due to phenomena of genetic mutation of the bacteria, which alter their sensitivity to antibiotics [41,87].
Biofilms intermittently release individual bacterial cells that can colonize new surfaces or weaken the collagen matrix in healing ulcers, causing “re-ulceration.” In the biofilm, the bacteria settle selectively, limit the colonization of new bacteria, store energy in the polysaccharide matrix, and interact with each other with the transfer of genetic material (Quorum Sensing); when conditions in the biofilm change, these interactions can determine which cells live, which die, and which abandon the colony. Furthermore, during infection, the skin lesions often appear to be more secretive: the link between infection and an increase in exudate is represented by the release of proteases and endotoxins by the microorganisms themselves which demolish the extracellular matrix, favoring a further and massive divestment of mediators who amplify the local phlogistic picture. The exudate contains, in fact, elevated levels of pro-inflammatory cytokines and MMPs, and macromolecules, such as fibrin and albumin, which inhibit growth factors [41,87].
12.2. Keloids
Keloids are fibrous proliferative, prominent skin scar-like lesions of the skin, characterized by a persistent, gradual growth beyond the wound margins into the surrounding healthy skin. Due to the characteristics of keloids, they can cause severe pain, chronic pruritus, psychosocial impairment, and motor striction to the patients, which leaves a heavy burden [88,89,90,91,92].
Since keloids are mainly characterized by increased proliferation of fibroblasts, anextensive overproduction of ECM components, and the consequent decrease in MMPs, research on keloids has primarily focused on the role of fibroblasts in keloid developments [88,89,90,91,92]. However, recently, researchers have noticed that the surrounding immune microenvironment can play a vital role in the development of keloids. The increased number of T cells, LCs, MCs, and macrophages in keloids as opposed to normal tissues is associated with skin fibrosis [88,89,90,91,92]. The keloid fibroblasts and immune cells can interact with each other and develop a synergistic and complex regulation of molecules and pathways, which contribute to the development of keloids. Among these immune cells, macrophages play a key role in cutaneous wound healing by modulating the microenvironment during the different healing phases. In particular, M2 macrophages are associated with fibrosis and scarring and persist in keloids [88,89,90,91,92].
12.3. Hypertrophic Scars
Scarring is a normal consequence of the healing process. The wound healing processes of burn victims may result in the formation of a fibrotic hypertrophic scar. This type of scar is characterized by its elevated, red, rigid appearance, and is associated with significant functional and cosmetic complications [93,94]. Hypertrophic scar formation appears to be associated with a diverse range of subsequent processes, including aberrant extracellular matrix production, increased neovascularization, abnormal extracellular matrix remodeling, prolonged re-epithelialization, and prolonged inflammation. Directly and indirectly, platelets, macrophages, T-lymphocytes, MCs, Langerhans cells, and keratinocytes contribute to the stimulation of fibroblasts, which generate an excess of the extracellular matrix. The causal relationship between these processes and the formation of atypical scar tissue is still unknown; however, there is evidence suggesting that immunological responses in the immediate aftermath of injury may have a significant impact [93,94].
12.4. Wound Dressings
The choice of dressing is determined by the characteristics of the wound, including its kind, depth, location, and size, as well as the amount of discharge, presence of infection, and level of wound adhesion. Conventional wound dressings, such as cotton bandages and gauze, absorb a significant amount of moisture from the wound. This excessive absorption causes the wound surface to become excessively dry, resulting in a decrease in the rate of healing and increased pain while removing the dressing. On the other hand, a wide variety of polymers in the form of films, foams, and gels have been created, which can create an ideal environment for the wound healing process [95,96,97,98].
An ideal wound dressing should possess the following characteristics: (a) effectively regulate moisture levels around the wound, (b) facilitate efficient transmission of gases, (c) remove excess exudates, (d) safeguard the wound against infections and microorganisms, (e) minimize surface necrosis of the wound, (6) provide mechanical protection, (f) be easily changed and removed, (g) be biocompatible, biodegradable, elastic, and nontoxic, (h) alleviate wound pain, and (i) be cost-effective. Dressings are categorized into antibacterial, absorbent, occlusive, adherence, and debridement based on their clinical performance. Dressings are available in several physical forms, including ointments, films, foams, and gels [95,96,97,98].
Based on their source, these materials are classified into three categories: animal-derived, plant-derived, and synthetic.
Animal origin collagen dressings have demonstrated the capacity to facilitate the wound healing process. During acute wound healing, the growth of tissue is regulated by chemical mediators, which result in the production of the extracellular matrix, formation of granulation tissue, maturation of the tissue, and ultimately closure of the wound. Consequently, stubborn wounds might be expedited in their healing process by utilizing collagen dressings [95,96,97,98].
Herbal dressings are typically arranged in layers, with the secondary layer being either another herbal dressing or a different material. These dressings may use thermoplastic material in their structure to enhance flexibility or are secured via mechanical means. Herbal dressings have the disadvantage of strongly sticking to the surface of the wound [95,96,97,98].
Synthetic origin polyurethane is extensively utilized in wound dressing applications due to its excellent biocompatibility, satisfactory mechanical properties, minimal cytotoxicity, appropriate flexibility, and gas permeability [95,96,97,98].
Nonmedicated dressings often utilize hydrocolloids and alginates, which are mostly available in the form of gel, film, and laminar structures. Alginate dressings are mostly manufactured as foams or conforming materials for packing cavity wounds. Alginate dressings have the ability to undergo gelation upon contact with wound exudates. The creation of a robust hydrophilic gel leads to increased absorption, which limits the flow of wound fluids and reduces the likelihood of bacterial infection [95,96,97,98].
Medicated dressings utilize active chemicals that have advanced, similar to pharmaceutical medications and the materials used in drug delivery. The included pharmacological substances, e.g., antibiotics, enhance the process of cleansing the wound bed by removing necrotic tissue, eradicating infection, and ultimately promoting tissue regeneration [95,96,97,98].
In summary, it appears that manufacturing composite dressings comprising the diverse constituents implicated in the process of wound healing is the most viable option for attaining a comprehensive therapeutic outcome and expediting the recovery phase. However, it is essential to identify specialized therapy procedures for individual wounds. Novel methodologies can effectively assist in this kind of treatment; nevertheless, it is imperative to evaluate these sophisticated dressings through clinical studies in order to expedite their implementation in routine clinical settings [95,96,97,98].
13. Conclusions
In this review, we discuss and explore how the skin reacts to an insult such as a wound. The understanding of this mechanism is facilitated if one has knowledge of the skin microenvironment with its main protagonists where MCs play a crucial role. In acute wounds, the activation of MCs, either by different cell types or by the stimuli present in the microenvironment, results in the release of TNFalpha, which promotes the differentiation of dendritic cells. Additionally, MC activation leads to the secretion of various substances that can induce angiogenesis, as well as the release of extracellular matrix by fibroblasts. Consequently, this triggers a cellular response in the infiltrate in order to repair the injury. These events are also associated with the production of TGFbeta by various cells in the microenvironment, leading to the differentiation of macrophages into the M1 and M2 phenotypes as the primary outcome. The latter category of cells, most likely in conjunction with keratinocytes, stimulate fibroblasts to undergo differentiation into myofibroblasts. The action of TGFbeta is also associated with the development of plasmacytoid dendritic cells, which can interact with Treg cells to promote tolerance by expressing CD45 in these cell types during wound healing processes [21,46,62].
When this delicate mechanism is altered in some way, we are faced with major problems such as chronic wounds, keloids, or hypertrophic scars. In chronic wounds, there is further evidence for the important role of these cells. In various articles, it has been reported that MCs show an increased degranulation index in chronic wounds. This event leads to modifications of the inflammatory infiltrate, with various responses from the various cell types involved [21,62,78,83,84,85,86]. Among the cytokines produced here is TGFbeta, which plays a crucial role in the various stages leading to the healing of chronic wounds [86]. In this type of wound, as already noted, an interaction between MCs and neuronal cells is described [83,84]. The release of the nerve mediators involved in wound healing is, in fact, linked to the interaction between these types of cells [21,62,78,83,84,85,86]. Even today, the mechanisms relating to the development of hypertrophic scars or keloids are almost unknown [88,89,90,91,92,93,94]. From the information reported in this review, there is little data, but we are beginning to glimpse the awareness among the authors that the skin microenvironment is of fundamental importance and it follows that the hope of a cure is now perhaps closer [21,46,62,76,78,99,100,101,102,103]. Certainly, the road to knowledge is still long but the integration between doctor and biologist is, to say the least, crucial in the development of such events.
Author Contributions
Conceptualization, M.F.-G., J.N.-R. and S.B.; resources, M.F.-G., J.N.-R. and S.B., data curation, M.F.-G., J.N.-R. and S.B. writing—original draft preparation, M.F.-G., J.N.-R. and S.B.; writing—review and editing, M.F.-G., J.N.-R. and S.B.; visualization, M.F.-G., J.N.-R. and S.B.; supervision, M.F.-G., J.N.-R. and S.B.; project administration, M.F.-G., J.N.-R. and S.B.; funding acquisition, M.F.-G. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are indebted to P. Romagnoli, P. Nardini, G., Paroli, C. Peroni, and R. Sansoni for their kindness, courtesy, and skillful assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Denomination | Acronym |
| Mast Cell (s) | MC |
| Dendritic cell (s) | DC |
| Langerhans cell (s) | LC |
| Wound Healing | WH |
| Chronic Wounds | CW |
| Matrix Metalloproteinases | MMPs |
| Tissue Inhibitors of Metalloproteinases | TIMP |
| Platelet Derived Growth Factor | PDGF |
| Interleukin | IL |
| Tumor Necrosis Factor | TNF |
| Fibroblast Growth Factor | FGF |
| Granulocyte-Macrophage Colony Stimulating Factor | GM-CSF |
| Nitric Oxide | NO |
| Transforming Growth Factor | TGF |
| Glycosaminoglycans | GAGs |
| Extracellular Matrix | ECM |
| Nerve Growth Factor | NGF |
| Hepatocyte Growth Factor | HGF |
| Heparin-Binding Epidermal Growth Factor | HB-EGF |
| Vascular Endothelial Growth Factor | VEGF |
| Calcitonin Gene Related Peptide | CGRP |
| Neurokinin A | NKA |
| Neuropeptide Y | NPY |
| Substance P | SP |
| Protein Gene Product 9.5 | PGP 9.5 |
| Vaso Active Intestinal Peptide | VIP |
References
- Joey, E.; Cheong, L.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy/Wound Manag. 2006, 52, 24–37. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Sorg, H.; Sorg, C.G.G. Skin wound healing: Of players, patterns, and processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier function of the skin: “la raison d’être” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Johansson, J.A.; Headon, D.J. Regionalisation of the skin. Semin. Cell Dev. Biol. 2014, 26, 3–10. [Google Scholar] [CrossRef]
- Mc Grath, J.A.; Llitto, J. Anatomy and organization of human skin. In Rook’s Textbook of Dermatology, 8th ed.; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Wiley Blachwell: Hoboken, NY, USA, 2010; Chapter 3. [Google Scholar]
- Fernandez-Flores, A. Regional variations in the histology of the skin. Am. J. Dermatopathol. 2015, 37, 737–754. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A. Aging skin: Histology, physiology, and pathology. Facial Plast. Surg. Clin. N. Am. 2015, 19, 229–234. [Google Scholar] [CrossRef]
- Laverdet, B.; Danigo, A.; Girard, D.; Magy, L.; Demiot, C.; Desmoulière, A. Skin innervation: Important roles during normal and pathological cutaneous repair. Histol. Histopathol. 2015, 30, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Baguneid, M.; Bayat, A. The role of neuromediators and innervation in cutaneous wound healing. Acta Derm.-Venereol. 2016, 96, 587–594. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.S. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin. Microbiol. Rev. 2019, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Merana, G.R.; Harris-Tryon, T.; Scharschmidt, T.C. Skin immunity: Dissecting the complex biology of our body’s outer barrier. Mucosal Immunol. 2022, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef] [PubMed]
- Focardi, M.; Puliti, E.; Grifoni, R.; Palandri, M.; Bugelli, V.; Pinchi, V.; Norelli, G.A.; Bacci, S. Immunohistochemical localization of Langerhans cells as a tool for vitality in hanging mark wounds: A pilot study. Aust. J. Forensic Sci. 2020, 52, 393–405. [Google Scholar] [CrossRef]
- Bacci, S.; Defraia, B.; Cinci, L.; Calosi, L.; Guasti, D.; Pieri, L.; Lotti, V.; Bonelli, A.; Romagnoli, P. Immunohistochemical analysis of dendritic cells in skin lesions: Correlations with survival time. Forensic Sci. Int. 2014, 244, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S. Fine regulation during wound healing by mast cells, a physiological role not yet clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef]
- Martel, J.L.; Miao, J.H.; Badri, T. Anatomy, Hair Follicle. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470321/ (accessed on 29 May 2024).
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Di Nardo, A.; Bosch, T.C.G.; Paus, R. Exploring the human hair follicle microbiome. Br. J. Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef] [PubMed]
- De Berker, D. Nail anatomy. Clin. Dermatol. 2013, 31, 509–515. [Google Scholar] [CrossRef]
- Okoro, O.E.; Camera, E.; Flori, E.; Ottaviani, M. Insulin and the sebaceous gland function. Front. Physiol. 2023, 14, 1252972. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato-Endocrinol. 2011, 3, 41–49. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 17, 211–259. [Google Scholar] [CrossRef] [PubMed]
- Kurata, R.; Futaki, S.; Nakano, I.; Fujita, F.; Tanemura, A.; Murota, H.; Katayama, I.; Okada, F.; Sekiguchi, K. Three-dimensional cell shapes and arrangements in human sweat glands as revealed by whole-mount immunostaining. PLoS ONE 2017, 21, e0178709. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhang, L.; Chen, J.; Wang, C.; Zhao, J.; Liu, X.; Yan, Y.; Tang, Y.; Chen, Z.; Li, H. Differential antigen expression between human apocrine sweat glands and eccrine sweat glands. Eur. J. Histochem. 2023, 67, 3559. [Google Scholar] [CrossRef]
- Saga, K. Histochemical and immunohistochemical markers for human eccrine and apocrine sweat glands: An aid for histopathologic differentiation of sweat gland tumors. J. Investig. Dermatol. Symp. Proc. 2001, 6, 49–53. [Google Scholar] [CrossRef]
- Ferreira, T.; Gama, A.; Seixas, F.; Faustino-Rocha, A.I.; Lopes, C.; Gaspar, V.M.; Mano, J.F.; Medeiros, R.; Oliveira, P.A. Mammary glands of women, female dogs and female rats: Similarities and differences to be considered in breast cancer research. Vet. Sci. 2023, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Szychlinska, M.A.; Aiello, F.C.; Vecchio, G.M.; Salvatorelli, L.; Magro, G.; Imbesi, R. Mammary gland: From embryogenesis to adult life. Acta Histochem. 2015, 117, 379–385. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Blaak, J.; Staib, P. The relation of pH and skin cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Visscher, M.O.; Adam, R.; Brink, S.; Odio, M. Newborn infant skin: Physiology, development, and care. Clin. Dermatol. 2015, 33, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Lang, C.; Lien, W.H. Concise review: Wnt signaling pathways in skin development and epidermal stem cells. Stem Cells 2018, 36, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 113, 203. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J.; Veysey, E.C.; Finlay, A.Y. Aging and the Skin. In Brocklehurst’s Textbook of Geriatric Medicine and Gerontology, 8th ed.; Fillit, H.M., Rockwood, K., Young, J., Eds.; Elsevier: Philadelphia, PA, USA, 2017; Chapter 25. [Google Scholar]
- Chhabra, S.; Chhabra, N.; Kaur, A.; Gupta, N. Wound healing concepts in clinical practice of OMFS. J. Maxillofac. Oral Surg. 2017, 16, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Andersen, C.; Black, J.; de Leon, J.; Fife, C.; Lantis Ii, J.C.; Niezgoda, J.; Snyder, R.; Sumpio, B.; Tettelbach, W.; et al. Management of chronic wounds: Diagnosis, preparation, treatment, and follow-up. Wounds 2017, 29, S19–S36. [Google Scholar] [PubMed]
- Babalska, Z.L.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound antiseptics and european guidelines for antiseptic application in wound treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Katayama, S.; Vuola, J.; Kankuri, E. Human wound-healing research: Issues and perspectives for studies using wide-scale analytic platforms. Adv. Wound Care New Rochelle 2014, 3, 264–271. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Chidambara Murthy, K.N. Challenges in healing wound: Role of complementary and alternative medicine. Front. Nutr. 2022, 8, 791899. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gould, L.J. Opportunities and challenges of the management of chronic wounds: A multidisciplinary viewpoint. Chronic Wound Care Manag. Res. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S. Cellular mechanisms and therapies in wound healing: Looking toward the future. Biomedicines 2021, 9, 1611. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Arenas Gómez, C.M.; Sabin, K.Z.; Echeverri, K. Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Dev. Dyn. 2020, 249, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.J. Parallels between vertebrate cardiac and cutaneous wound healing and regeneration. npj Regen. Med. 2018, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Belacortu, Y.; Paricio, N. Drosophila as a model of wound healing and tissue regeneration in vertebrates. Dev. Dyn. 2011, 240, 2379–2404. [Google Scholar] [CrossRef]
- Seifert, A.W.; Maden, M. New insights into vertebrate skin regeneration. Int. Rev. Cell Mol. Biol. 2014, 310, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Ozgok Kangal, M.K.; Regan, J.P. Wound Healing 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535406/ (accessed on 29 May 2024).
- Healing by intention. Adv. Ski. Wound Care 2017, 30, 246–247. [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Braiman-Wiksman, L.; Solomonik, I.; Spira, R.; Tennenbaum, T. Novel insights into wound healing sequence of events. Toxicol. Pathol. 2007, 35, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.E.; Enoch, S.; Harding, K.G. Wound assessment. BMJ 2006, 332, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Madkour, F.F.; Hassan, M.M.; Abdo, W.; Khalil, W.F. Wound healing activity of brown algae plus polyherbal extract in normal and alloxan-induced diabetic rats. J. Adv. Vet. Res. 2013, 3, 102–108. [Google Scholar]
- Annoodee, S.; Nasuruddin, D.N. Acute Inflammatory Response. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556083/ (accessed on 29 May 2024).
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Palmblad, J. The acute inflammatory reaction: New concepts for old cells. J. Intern. Med. 2010, 268, 1. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of inflammatory reaction in health and disease. Int. J. Mol. Sci. 2021, 332, 5277. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and molecular processes in wound healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Canedo-Dorantes, L.; Canedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Tyavambiza, C.; Meyer, M.; Meyer, S. Cellular and molecular events of wound healing and the potential of silver based nanoformulations as wound healing agents. Bioengineering 2022, 9, 712. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.A.; Sharun, M.K.; Mamachan, L.; Abualigah, R.; Kumar, A.M.; Pawde, K.; Dhama, S.K.; Maiti, A. Wound healing and skin regeneration: Present status and future directions. J. Exp. Biol. Agric. Sci. 2023, 11, 871–883. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Fetal wound healing. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 27; pp. 579–591. [Google Scholar]
- Yagi, L.H.; Watanuki, L.M.; Isaac, C.; Gemperli, R.; Nakamura, M.Y.; Ladeira, P.R.S. Human fetal wound healing a review of molecular and cellular aspects. Eur. J. Plast. Surg. 2016, 39, 239–246. [Google Scholar] [CrossRef]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, medicine and the future, Healing chronic wounds. Br. Med. J. 2002, 324, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Toporcer, T.; Lakyová, L.; Radonak, J. Venous ulcer-present view on aetiology, diagnostics and therapy. Cas. Lek. Cesk. 2008, 147, 199–205. [Google Scholar] [PubMed]
- Günter, C.I.; Machens, H.G. New strategies in clinical care of skin wound healing. Eur. Surg. Res. 2012, 49, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, B.M.; Järbrink, K.; Martinengo, L.; Car, J.; Harding, K.; Schmidtchen, A. Need for improved definition of chronic wounds in clinical studies. Acta Derm. Venereol. 2018, 12, 157–158. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Bacci, S.; Pérez González, L.A.; Bermejo-Martínez, M.; Cecilia-Matilla, A.; Hernández-Bule, M.L. The role of physical therapies in wound healing and assisted scarring. Int. J. Mol. Sci. 2023, 19, 7487. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Grandi, V.; Corsi, A.; Pimpinelli, N.; Bacci, S. Cellular mechanisms in acute and chronic wounds after PDT Therapy: An Update. Biomedicines 2022, 10, 1624. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Prim. 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 2004, 5, 575–581. [Google Scholar] [CrossRef]
- Grandi, V.; Paroli, G.; Puliti, E.; Bacci, S.; Pimpinelli, N. Single ALA-PDT irradiation induces increase in MC degranulation and neuropeptide acute response in chronic venous ulcers: A pilot study. Photodiagn. Photodyn. Ther. 2021, 34, 102222. [Google Scholar] [CrossRef] [PubMed]
- Nardini, P.; Notari, L.; Magazzini, M.; Mariani, B.; Rossi, F.; Rossi, S.; Van Aardt, E.; Marszalek, K.; Grandi, V.; Corsi, A.; et al. Neuroimmunomodulatory Effect of NO on chronic wound healing after photodynamic therapy. Photodiagnosis Photodyn. Ther. 2024, 47, 104078. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Lecci, P.P.; Bacci, S.; Cappugi, P.; Pimpinelli, N. Early activation of fibroblasts during PDT treatment in leg ulcers. G. Ital. Dermatol. Venereol. 2016, 151, 223–229. [Google Scholar]
- Grandi, V.; Bacci, S.; Corsi, A.; Sessa, M.; Puliti, E.; Murciano, N.; Scavone, F.; Cappugi, P.; Pimpinelli, N. ALA-PDT exerts beneficial effects on chronic venous ulcers by inducing changes in inflammatory microenvironment, especially through increased TGF-beta release: A pilot clinical and translational study. Photodiagnosis Photodyn. Ther. 2018, 21, 252–256. [Google Scholar] [CrossRef]
- Kadam, S.; Nadkarni, S.; Lele, J.; Sakhalkar, S.; Mokashi, P.; Kaushik, K.S. Bioengineered platforms for chronic wound infection studies: How can we make them more human-relevant? Front. Bioeng. Biotechnol. 2019, 7, 418. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J. Gibbs S: The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front. Cell Dev. Biol. 2020, 8, 360. [Google Scholar] [CrossRef]
- Huang, C.; Ogawa, R. Keloidal pathophysiology: Current notions. Scars Burn. Health 2021, 7, 2059513120980320. [Google Scholar] [CrossRef]
- Magni, G.; Banchelli, M.; Cherchi, F.; Coppi, E.; Fraccalvieri, M.; Rossi, M.; Tatini, F.; Pugliese, A.M.; Rossi Degl’Innocenti, D.; Alfieri, D.; et al. Experimental study on blue light interaction with human keloid-derived fibroblasts. Biomedicines 2020, 8, 573. [Google Scholar] [CrossRef]
- Boyce, D.E.; Ciampolini, J.; Ruge, F.; Murison, M.S.; Harding, K.G. Inflammatory-cell subpopulations in keloid scars. Br. J. Plast. Surg. 2001, 54, 511–516. [Google Scholar] [CrossRef]
- Bagabir, R.; Byers, R.J.; Chaudhry, I.H.; Muller, W.; Paus, R.; Bayat, A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br. J. Dermatol. 2012, 167, 1053–1066. [Google Scholar] [CrossRef]
- Van der Veer, W.M.; Bloemen, M.C.; Ulrich, M.M.; Molema, G.; van Zuijlen, P.P.; Middelkoop, E.; Niessen, F.B. Potential cellular and molecular causes of hypertrophic scar formation. Burns 2009, 35, 15–29. [Google Scholar] [CrossRef]
- Mony, M.P.; Harmon, K.A.; Hess, R.; Dorafshar, A.H.; Shafikhani, S.H. An updated review of hypertrophic scarring. Cells 2023, 12, 678. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int. 2019, 35, 50–57. [Google Scholar]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nanotoday 2021, 41, 101290. [Google Scholar] [CrossRef]
- Rossi, F.; Tatini, F.; Pini, R.; Bacci, S.; De Siena, G.; Cicchi, R.; Pavone, S.; Alfieri, D. Improved wound healing in blue LED treated superficial abrasions. In Proceedings of the European Conferences on Biomedical Optics, Munich, Germany, 12–16 May 2013; p. 88030S. [Google Scholar] [CrossRef]
- Cicchi, R.; Rossi, F.; Alfieri, D.; Bacci, S.; Tatini, F.; De Siena, G.; Paroli, G.; Pini, R.; Pavone, F.S. Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J. Biophotonics 2016, 9, 645–655. [Google Scholar] [CrossRef]
- Magni, G.; Tatini, F.; Siena, G.; Pavone, F.S.; Alfieri, D.; Cicchi, R.; Rossi, M.; Murciano, N.; Paroli, G.; Vannucci, C.; et al. Blue-LED-light photobiomodulation of inflammatory responses and new tissue formation in mouse-skin wounds. Life 2022, 12, 1564. [Google Scholar] [CrossRef]
- Magni, G.; Tatini, F.; Bacci, S.; Paroli, G.; De Siena, G.; Cicchi, R.; Pavone, F.S.; Pini, R.; Rossi, F. Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol. Photoimmunol. Photomed. 2020, 36, 166–168. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Naharro-Rodríguez, J.; Bacci, S.; Fernández-Guarino, M. Unlocking the Power of Light on the Skin: A Comprehensive Review on Photobiomodulation. Int. J. Mol. Sci. 2024, 25, 4483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).