Abstract
Cosmetic formulations with natural antioxidants can reduce the oxidative stress caused by solar radiation and pollution. In this context, the aim of this study was to develop and evaluate the clinical efficacy of cosmetic formulations containing olive extract (OE) and Spirulina sp. (SP). For this, rheological behavior, texture, and sensory properties were evaluated. In addition, 31 healthy women with an age of 39 to 60 years, with skin phototypes II and III, and the presence of signs of photoaging on the face were recruited and divided in Group 1 (vehicle formulation) and Group 2 (vehicle with active substances) for this clinical efficacy study. Both groups applied sunscreen daily during the day. The formulations showed non-Newtonian pseudoplastic behaviors and good sensory properties. The clinical evaluation using instrumental measurements showed an increase in skin hydration, an improvement of the skin barrier, and morphological characteristics of the epidermis after 12 weeks of application of the formulations. There was a significant increase in the brightness of the stratum corneum, which suggested a film-forming effect. In addition, both groups had an improvement in the dermis echogenicity, due to the use of sunscreens. Finally, the proposed formulation was effective in protecting the skin and reducing skin changes related to photoaging.
1. Introduction
Consumer preference for natural cosmetic products is increasing over time. The global market for natural skin care products is projected to grow from 2022 to 2030 at a compound annual growth rate (CAGR) of 6.6% [1]. Furthermore, this market had reached a value of USD 6.7 billion by 2021 [1].
Antioxidants of natural origin are commonly used in cosmetic formulations in order to mitigate free radicals and reactive oxygen species (ROS) produced by UV radiation or pollution on the skin [2,3].
Ultraviolet radiation (UV) is one of the main causes of skin aging exposome, followed by pollution [4]. Photoaging is the premature aging of the skin caused by UV radiation. The skin changes caused by extrinsic aging are a rough texture, paleness, a reduction in elasticity, the presence of wrinkles and pigmentary changes, as well as a decreased cell turnover and skin barrier, promoting dryness and desquamation [5,6].
Oxidative skin stress is caused directly by UVA radiation and indirectly by UVB radiation that can be intensified by the combination of ubiquitous pollutants (polycyclic aromatic hydrocarbons such as benzo[a]pyrene) [3]. This oxidative stress leads to the degradation of skin cells [3]. If this oxidative stress is not stopped, DNA damage can occur and even promote cancerous lesions in the skin [3]. So, the use of antioxidants is indicated as a complementary form of photoprotection, in order to prevent or reverse this oxidative stress caused by solar radiation. However, the daily use of sunscreens is also important, as indicated by dermatologists [7].
Sunscreens act in the prevention of photoaging, due to the combination of UV filters in the formulation [7]. Physical and chemical filters protect the skin against UV radiation in a reflective and absorptive way, respectively [8]. In addition, effective sunscreens have a film-forming property, due to polymers or active substances, which help decrease the retention of pollutant particles on the skin [9,10].
The use of antioxidants can improve skin photoprotection, reduce skin oxidative stress, and prevent or attenuate skin changes caused by photoaging [11,12,13]. Furthermore, the association of antioxidants is demonstrated in the literature as a way to improve these characteristics [14,15,16]. Souza and Maia Campos [16] showed the synergistic effect of the association of Spirulina and dimethylmethoxy chromanol loaded in solid lipid nanoparticles in sunscreens, demonstrating an improved barrier function, skin echogenicity, skin mechanical properties, and skin lightening after 84 days.
Among the antioxidants from natural sources, we can highlight olive extract and Spirulina sp. Olive fruit extract (Olea europaea L.) is characterized by containing 20% hydroxytyrosol, tyrosol, and other polyphenols. This combination produces synergistic effects, resulting in high antioxidant properties [17,18]. This ingredient can be used as an emollient, antioxidant, anti-inflammatory, antiviral, antifungal, and antibacterial [19]. One research group has studied the use of olive extract standardized in hydroxytyrosol in reducing the hyperpigmentation caused by photoaging [20].
Spirulina sp. is a blue–green microalgae widely used in cooking and is considered a “superfood” due to its wide range of micronutrients and macronutrients [21]. The composition of Spirulina sp. comprises 55–70% protein, 15–20% carbohydrates, 5–8% lipids, 6–8% minerals (magnesium, manganese), 1.5–2% polyunsaturated fatty acids (PUFAs), linolenic acid, vitamins B1, B2, B3, B6, B9, B12, vitamins C and E, pigments such as zeaxanthin and canthaxanthin, as well as enzymes (C-phicocyanin and alophycocyanin) [22]. The antioxidant activities of Spirulina sp. of scavenging free radicals and preventing oxidative stress are due to the presence of phycocyanins and vitamins in its composition [22]. Its rich composition makes Spirulina sp. a multifunctional ingredient [23].
The topical application of Spirulina sp. in cosmetics has been patented by our research group for its antioxidant effect on the prevention of free radicals and the protective effects in the skin barrier function by clinical studies [24]. Other clinical effects of Spirulina sp. obtained using biotechnological processes have also been investigated by our research group, such as the lightening of solar lentigines and ephelides [20] and its film-forming effect [10].
The development of stable cosmetic formulations with suitable physical–mechanical properties and pleasant sensory properties, alongside a clinical efficacy evaluation, is very important to obtain more effective products. In addition, non-invasive biophysical and skin imaging techniques allow the evaluation of cosmetic products under real usage conditions [25,26]. In this context, the aim of this study was to develop and evaluate the clinical efficacy of cosmetic formulations containing olive extract and Spirulina sp.
2. Materials and Methods
2.1. Materials
The raw materials used in the formulations are described in Table 1.

Table 1.
Raw materials.
The equipment used is described in Table 2.

Table 2.
Equipment.
2.2. Methods
2.2.1. Studied Formulations
The studied formulations were made using a cold process at room temperature (25 °C), due to the presence of an organogel that has the property of cold emulsification. The ingredients of the oil (A) and aqueous (B) phases were weighed separately in a glass beaker. In order to prevent the formation of lumps of xanthan gum, a pre-dispersion was performed with glycerin before the addition of water. After weighing the two phases, A and B, the beaker containing the aqueous phase (B) was poured into the beaker containing the oily phase (A), with constant manual stirring with a glass stirring rod until the emulsification process occurred. After this process, the preservative (phase C) was added to the emulsion.
In formulations F1 to F6, to which the active substances (phase D) were added, a pre-dispersion was made in glass mortar with a pestle. The pre-dispersion of Spirulina sp. was formulated with water (1:10). The pre-dispersion of the olive extract was formulated with vegetable glycerin (1:1). Afterwards, these pre-dispersions were gradually added to the emulsion. When necessary, the pH was adjusted with citric acid solution (1:10)—phase E—to an approximate pH value of 5.5. The vehicle formulation was named F7.
The developed formulations are shown in Table 3.

Table 3.
Qualitative and quantitative composition of formulations 1 to 7.
The stability of formulations F1 to F6 added to olive extract, in combination with or without Spirulina sp., were evaluated in terms of rheological parameters, pH values, and organoleptic characteristics.
Formulations F6 and F7 were evaluated in terms of texture profile, sensory properties, and clinical efficacy.
Rheological Behavior
The rheological behavior of all formulations developed were evaluated. For this, a cone and plate rheometer with the RheocalcT® 1.2.19 software program was used. The conditions for the rheological analyses were as follows: room temperature of 25 °C, 0.5 g samples, CP-52 spindle, rotation speed increased progressively from 0 to 50 rpm at 6 points of one minute duration each, and the reverse occurred from 50 to 0 rpm. The parameters obtained from the rheograms were as follows: minimum apparent viscosity, thixotropy, consistency index, and flow rate. The minimum apparent viscosity was calculated by dividing the shear rate by the shear stress. Thixotropy is the value obtained by integrating the points of the rheological curve. The flow and consistency index were obtained using the Ostwald–de Waele model, represented using the following equation where τ = shear stress (Pa); κ = consistency index (Pa.sn); γ = shear rate (s−1); and n = flow index (dimensionless) (Equation (1)) [27]:
The rheological parameters were analyzed over time in order to verify the rheological stability of the formulations (F1 to F7). The evaluation time points were initial time (T0) and after 7 (T7), 14 (T14), 28 (T28), 56 (T56), and 84 (T84) days.
Accelerated Stability Studies
Before starting the stability tests, the formulations had to pass the centrifugation test that evaluates the presence or absence of phase separation. The centrifugation test was performed with 5 g of formulation in a conical plastic tube and submitted to centrifugation at 3000 rpm for 3 cycles of 30 min each [28]. After verifying that there was no phase separation, all formulations were submitted to accelerated stability studies. The formulations were stored in glass containers with lids (20 g for each formulation), under room temperature conditions (25 °C), protected from light, and at temperatures of 37 °C and 45 °C [28] for a duration of 12 weeks. The parameters evaluated were pH values, organoleptic characteristics (color, odor, and appearance), and rheological properties. The determination of the pH values was performed with the formulations diluted in distilled water in a 1:10 ratio. The evaluations were performed at the initial time (T0) and after 7 (T7), 14 (T14), 28 (T28), 56 (T56), and 84 (T84) days.
Texture Profile
The texture profile was evaluated in formulations F6 and F7, which were chosen to be evaluated in the clinical study. The work of shear parameter was evaluated using a Texture Analyzer equipped with the TTC Spreadability rig HDP/SR and Exponent Connect® software. The cone-shaped probe was inserted at 90° into a cone-shaped receptacle containing the formulation at a defined speed and depth. The flow force of the formulation forced it to flow at 45°, indicating the degree of spreadability. The work of shear is calculated from the area under the positive curve of a graph with axes of force (N) and time (t) [29]. The experimental conditions were as follows: a return distance of 25 mm, a return speed of 20 mm s−1, a contact force of 30 g, a trigger force of 5 g, and a probe penetration distance of 8 mm. The test was performed in triplicate for each formulation.
2.3. Casuistic and Methods
2.3.1. Clinical Study Design
The double-blind, randomized, placebo-controlled clinical trial was conducted after approval by the Ethics Committee of the Faculty of Pharmaceutical Sciences of Ribeirão Preto—University of São Paulo (n° CAAE: 84 599 418.8.0000.5403). In addition, the clinical trial was conducted in accordance with ICH Guidelines on Good Clinical Practice and the Declaration of Helsinki [30,31].
Inclusion criteria were healthy women aged 39 to 60 years (mean ± standard deviation—51.2 ± 5.4), skin phototype II and III, and the presence of signs of photoaging on the face. The exclusion/non-inclusion criteria were pregnant or lactating women, smokers, history of adverse reactions to cosmetics, skin diseases that may impair the analysis region, availability to visit the laboratory during the study. Thirty-one women who met the inclusion criteria were included in the clinical study after giving written informed consent. However, twenty-seven women completed the study, as four research participants were excluded during the study. One of the exclusions was for personal reasons of illness and the other three exclusions were because they were no longer available to return to the laboratory.
In order to ensure a double-blind study, the labeling of the study formulations was placed on the containers by a person unrelated to this study. The identification of the group’s formulations was placed in a sealed envelope that was not opened until the end of the study.
The research participants were randomized into two groups. Group 1 (n = 14) received the vehicle formulation (F7) and Group 2 (n = 13) received the formulation containing the combination of olive extract and Spirulina sp. (F6). Participants were instructed to apply the SPF 30 sunscreen on the face during the day and the study formulations at night every day for the 12 week period of the study. In addition, the research participants were instructed to use the test products in sufficient quantities to apply to the entire face, with the exception of the eye area. This study was conducted in the spring from October to December with initial (T0), after 6 (T6w), and 12 (T12w) week time points. The site where the study was conducted was at the Cosmetic Technology Laboratory located at the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Brazil (21°100 S, 47°480 W).
Instrumental measurements were collected after the participants’ acclimatization period of 20 min in an environment with controlled temperature between 20 °C and 22 °C and a 45% to 55% relative humidity at each time point. The region of analysis was the frontal region of the face.
2.3.2. Sensory Analysis
The sensory analysis was performed with the formulations that were chosen to be evaluated in the clinical study (F6 and F7). Ten previously trained research participants evaluated the formulations with intensity scales in terms of the parameters of spreadability, stickiness, moisture feeling, skin feel after 5 min, and absorption feeling [32]. For this purpose, a standardized amount of formulation (50 μL) was applied with a positive-displacement pipette to a 6 cm2 area on the anterior forearm region with ten circular and orderly movements.
2.3.3. Instrumental Measurements
Stratum Corneum Water Content
The Corneometer® equipment measures skin hydration. Evaluation of the aqueous content of the stratum corneum is performed using the capacitance method of the dielectric constant of water [16]. The results are expressed in arbitrary units (AUs) and were performed in quintuplicate.
Transepidermal Water Loss
The Tewameter® equipment measures the transepidermal water loss (TEWL). A skin barrier assessment is performed using the skin water evaporation method, based on the diffusion principle described by Adolf Fick in 1885 [16]. Data collection occurred after 20 s of the probe remaining in the analysis region under constant pressure by the same operator.
Dermis Thickness and Echogenicity
Dermal thickness and echogenicity were performed using a 20 MHz ultrasound. Real-time images of the skin are produced using ultrasound. The echoes of different amplitudes are the result of the reflection of high-frequency ultrasonic waves on the skin structures. The intensity of the reflected echoes (echogenicity) is visualized in a two-dimensional color image. The echogenicity follows a color scale of white > yellow > red > green > blue > black, where white is high echogenicity and black is low echogenicity [16]. The parameters evaluated using the B-scan module were the thickness and echogenicity of the dermis. The measured echogenicity is the ratio between the number of low echogenicity pixels and the total number of pixels.
Morphological and Structural Skin Characteristics
Morphological and structural skin characteristics of the epidermis were evaluated using Reflectance Confocal Microscopy. Multiple high-resolution images (1000 × 1000 pixels) on a scale of 500 µm × 500 µm are captured from the stratum corneum to the papillary dermis. The Vivastack® system allowed us to obtain the “z” value that was used to calculate the quantitative parameters of epidermal thickness, through the difference of “z” values. The parameters were thickness of the stratum corneum, granular layer, dermal papillae depth, minimum epidermis thickness, maximum epidermis thickness, mean epidermis thickness, and total epidermis thickness [33]. Qualitative parameters were evaluated using standardized scores by Maia Campos and D’Angelo Costa [33], including skin surface homogeneity, furrows morphology, interkeratinocyte brightness, and skin hyperpigmentation. These scores are rated from 0 to 3. A score of 0 indicates a regular or low condition and a score of 3 indicates an irregular or high condition.
Another score standardized by Infante and Maia Campos [34] was also evaluated, in terms of the quality of stratum corneum, stratum granulosum, dermal–epidermal junction, and papillary collagen. These scores are rated from 1 to 5. A score of 5 indicates good skin quality and the opposite is indicated by a score of 1.
In addition, a new score was developed for this study to qualify the reflectance/brightness of the stratum corneum, through a score rated from 0 to 3. Score 0 means low brightness and score 3 means high brightness, as shown in Figure 1.
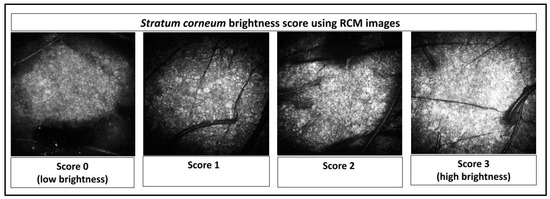
Figure 1.
Stratum corneum brightness score using Reflectance Confocal Microscopic (RCM) images.
Furthermore, the time points analyzed using RCM were the initial time (T0) and that after 12 weeks of the study (T12w).
2.4. Statistical Analysis
Statistical analyses were performed using GraphPad® Prism 9 software. The Shapiro–Wilk test was performed to evaluate the normal distribution of the data. An unpaired Student’s t-test was used for analysis between two independent groups and a paired Student’s t-test was used for analysis between two dependent groups with parametric data. Their counterparts for non-parametric data were Mann–Whitney and Wilcoxon, respectively. Parametric data with three or more groups used one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. Their counterparts for nonparametric data were Kruskal–Wallis, followed by Dunn’s multiple comparison test, respectively. The confidence level adopted was 95%. Analyses were performed in triplicate.
3. Results
3.1. Formulation Development
The evaluation of formulations with or without the sodium metabisulfite—SMBS—was carried out in different concentrations of the olive extract (0.5 and 0.2). In addition, the stability of the olive extract with the association of Spirulina sp. was evaluated. There was no phase separation in any formulation evaluated. Therefore, the accelerated stability was evaluated for 84 days. The rheological results of the formulation development on the evaluation of olive extract stability in formulations alone or in combination with Spirulina sp. (F1–F6) are shown in Figure 2.
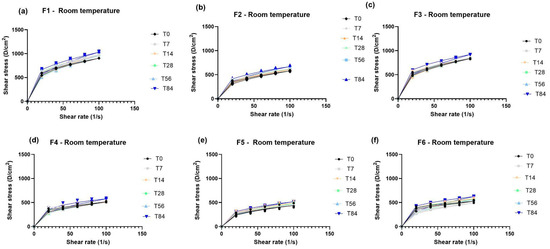
Figure 2.
Rheograms of formulations with or without Sodium Metabisulfite (SMBS) in the presence of olive extract alone or in association with Spirulina sp. when stored at room temperature for (T0), 7 (T7), 14 (T14), 28 (T28), 56 (T56), and 84 (T84) days. F1 = formulation without SMBS with 0.5% w/w olive extract at room temperature (a); F2 = formulation with SMBS with 0.5% w/w olive extract at room temperature (b); F3 = formulation without SMBS with 0.2% w/w of olive extract at room temperature (c); F4 = formulation with SMBS with 0.2% w/w of olive extract at room temperature (d); F5 = formulation with SMBS with 0.5% w/w olive extract and 0.1% w/w Spirulina sp. at room temperature (e); and F6 = SMBS formulation with 0.2% w/w olive extract and 0.1% w/w Spirulina sp. at room temperature (f).
According to the results obtained in the initial time (T0) analysis of the organoleptic characteristics and pH values, we can observe that the presence of SMBS makes the formulation more fluid, light beige in color, and gives it a characteristic odor of SMBS. In the absence of SMBS, the formulation is less fluid, brown in color, and has a characteristic odor of olive extract. Regarding the colors of the formulations, the olive extract promotes a brown color in the formulation and the Spirulina sp. promotes a green color. In the association of olive extract with Spirulina sp., green was the predominant color with a lower concentration of olive extract (0.2% w/w) and for concentration 0.5% w/w, the brown mixed with the green, becoming a darker green, was the predominant color. After 84 days of stability, the color was darker for all the formulations, although the formulations without SMBS were darker than those containing SMBS. The appearance remained the same and the pH had no significant difference for room temperature samples after 84 days (5.6 ± 0.2). However, the pH values had a significant difference in stability at 45 °C after 84 days, changing from 5.6 ± 0.2 to 5.3 ± 0.2. Despite this, the pH of the formulations remained within the physiological skin range of 4 to 6 [35] throughout the stability study.
According to the rheological results, all formulations presented a non-Newtonian pseudoplastic behavior with a flow index below 1. Furthermore, it was observed that the addition of SMBS promoted a significant decrease in formulation viscosity compared to the formulations without it. Moreover, the rheograms retained similar characteristics throughout the 84 days of accelerated stability for the formulations containing SMBS. Therefore, the F6 formulation is the ideal one to proceed with to clinical trials, since the lower concentration (0.2% w/w) of the olive extract was better in stability compared to the 0.5% w/w concentration. Therefore, it is the indicated concentration to be used in association with Spirulina sp.
The evaluation of the stability and the sensory and texture properties of the formulations chosen to be evaluated in the clinical study is presented in Figure 3 and Figure 4. The formulations were considered stable after 84 days of evaluation at all temperatures. The pH value of the formulations remained within the physiological range of the skin. The organoleptic characteristics of the formulations were maintained after 84 days of the stability study. The organoleptic characteristics were white color for the vehicle formulation (F7), dark green color for the association formulation (olive extract + Spirulina sp.), as well as the characteristic odor and gel–cream appearance.
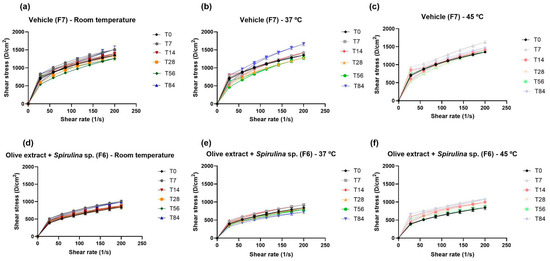
Figure 3.
Rheograms of the vehicle formulation—F7 (a–c) and the formulation containing the association of olive extract with Spirulina sp.—F6 (d–f), when stored at room temperature, 37 °C, and at 45 °C for (T0), 7 (T7), 14 (T14), 28 (T28), 56 (T56), and 84 (T84) days.
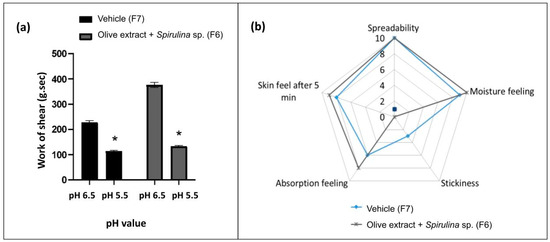
Figure 4.
Work of shear of the vehicle (F7) and formulation containing the association of olive extract with Spirulina sp. (F6) at pH 6.5 and 5.5 (a) and sensory analysis of these formulations at pH 5.5 (b). * Statistically different means when compared to the pH 6.5 value (p < 0.05).
The work of shear of vehicle (F7) and association formulation F6 (olive extract + Spirulina sp.) was evaluated both before and after pH adjustment and showed a significant difference between pH values. A lower work of shear was obtained at lower pH values. The lower work of shear is related to a better spreadability [27]. The lower pH also improves the stability of some actives, such as phenolic compounds [36]. Therefore, a lower pH is interesting to obtain a good spreadability and stability, in addition to keeping the pH close to the physiological pH of the skin [35].
Furthermore, in the sensory analysis of the formulation with the association (olive extract + Spirulina sp.), F6 obtained better results compared to F7, mainly regarding skin feel after 5 min, absorption feeling, and stickiness.
3.2. Clinical Study
The clinical study was conducted after defining the formulations and evaluating the texture and sensory properties, as well as their stability.
The results of the biophysical techniques are presented in Figure 5. There was an increase in stratum corneum water content and a decrease in TEWL after 12 weeks of application of the formulation containing olive extract + Spirulina sp. (F6).
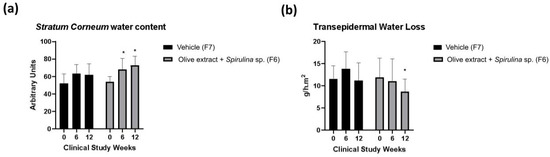
Figure 5.
Stratum corneum water content (a), and transepidermal water loss (b) before (T0), after 6 (T6w), and 12 (T12w) weeks of use of the vehicle (G1–F7) and association with olive extract and Spirulina sp. (G2–F6) formulations. * Significant difference from baseline values (T0) (p < 0.05).
The dermis echogenicity and thickness are shown in Figure 6A and Figure 6B, respectively. There was no variation in dermal thickness for the formulations evaluated. However, the decrease in the echogenicity ratio (hypoechogenic pixels/total number of pixels) was significant for both groups evaluated, which means an improvement of dermis echogenicity, as shown in Figure 6C.
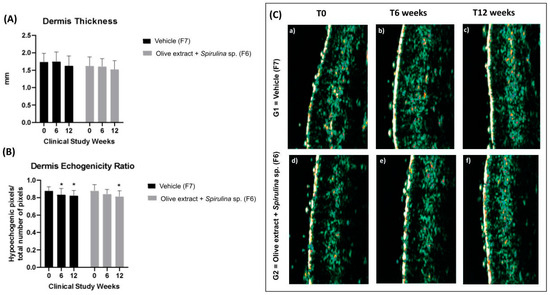
Figure 6.
(A) Dermis thickness before (T0), after 6 (T6w), and 12 (T12w) weeks of use of the vehicle (F7) and association with olive extract and Spirulina sp. (F6) formulations; (B) dermis echogenicity ratio (hypoechogenic pixels/total number of pixels) before (T0), after 6 (T6w), and 12 (T12w) weeks of use of the vehicle and association with olive extract and Spirulina sp. formulations; (C) representative 20 MHz ultrasound images of dermis echogenicity before (T0), after 6 (T6w), and 12 (T12w) weeks of use of the vehicle (G1–F7) and association with olive extract and Spirulina sp. (G2–F6) formulations (echogenicity color scale: white > yellow > red > green > blue > black) * Significant difference from baseline value (T0) (p < 0.05).
The results of the evaluation of the morphological and structural characteristics of the skin assessed using RCM are shown in Table 4, as well as Figure 7 and Figure 8.

Table 4.
Qualitative parameters of epidermal layers using Reflectance Confocal Microscopy.
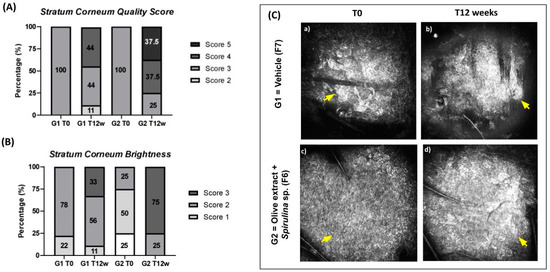
Figure 7.
(A) Stratum corneum quality score at initial time (T0) and after 12 weeks (T12w) of the use of vehicle formulation (G1–F7) and the use of association with olive extract and Spirulina sp. formulation (G2–F6); (B) Stratum corneum brightness at initial time (T0) and after 12 weeks (T12w) of the use of vehicle formulation (G1–F7) and the use of association with olive extract and Spirulina sp. formulation (G2–F6); (C) representative Reflectance Confocal Microscopy images of Stratum corneum. The yellow arrows indicate improved stratum corneum brightness. (Scale: 500 µm × 500 µm).
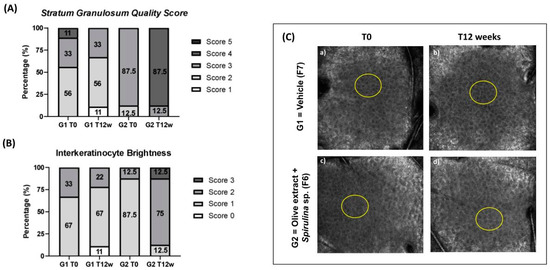
Figure 8.
(A) Stratum granulosum Quality Score at initial time (T0) and after 12 weeks (T12w) of the use of vehicle formulation (G1–F7) and the use of association with olive extract and Spirulina sp. formulation (G2–F6); (B) Interkeratinocyte brightness at initial time (T0) and after 12 weeks (T12w) of the use of vehicle formulation (G1–F7) and the use of association with olive extract and Spirulina sp. formulation (G2–F6); (C) representative Reflectance Confocal Microscopy images of Stratum Granulosum. The yellow circles indicate improved interkeratinocyte brightness and honeycomb pattern. (Scale: 500 µm × 500 µm).
The qualitative parameters that had a significant increase were quality scores of stratum corneum and stratum granulosum for G2. The increase in the stratum corneum thickness was 37.5% and for the stratum granulosum was 34.8%.
Also, the interkeratinocytes’ brightness score had a significant increase of 87.5% for G2.
The stratum corneum brightness scores had a significant increase for both groups. The increase in G1 was 39.5% and for G2, it was 141.5%. We can observe that the increase for G2 was expressive, which shows that there was a positive influence of the association of antioxidants on this result.
However, no significant improvement was observed for the other scores evaluated, such as homogeneity of the skin surface and morphology of the furrows. The scores showed a regular score around 0 from the initial time. This characteristic may be related to the skin morphology in the analysis region, since the frontal region presented few furrows and a homogeneous surface in all timepoints analyzed.
There was no significant variation for the skin hyperpigmentation score, as the score remained at 0, meaning low skin hyperpigmentation during the clinical study. This suggests that the frontal region had a lower incidence of pigmentary changes than the malar region [34,37].
Quality score analysis of the dermal–epidermal junction showed a layer with low density and polycyclic or flattened papillae for both study groups.
Analysis of the papillary collagen quality score showed a layer with low density and quality of collagen fibers for both study groups.
There were no significant differences for the quantitative parameters stratum granulosum thickness, dermal papillae depth, as well as minimum, maximum, and mean epidermal thickness. However, there was a significant increase in stratum corneum thickness of 25.9% and 26.2% for G1 and G2, respectively. There was also a significant increase in total epidermal thickness of 14.9% for G2. Although the increase in stratum corneum thickness occurred for both groups, this increase was only significant in the total epidermis thickness for the group that used the formulation with the association for 12 weeks.
4. Discussion
The development of topical formulations containing antioxidants of natural origin, as well as the evaluation of their stability and clinical efficacy is important for the cosmetic and dermatological area, since it can be used in addition to the daily use of sunscreens.
The use of extracts of natural origin and microalgae in cosmetic formulations is important to increase the benefits of the formulation. However, it is a challenge for formulators to obtain stable formulations. As olive extract is a potent antioxidant, it was necessary to add an antioxidant in the formulation to prevent oxidation of the extract and improve the stability of the active [38].
All formulations developed showed a non-Newtonian pseudoplastic behavior characteristic of cosmetic formulations that facilitates the application of the product on the skin [28]. Formulation F6, with the lowest concentration (0.2% w/w) of olive extract and associated with Spirulina sp., was the ideal association to be used in the clinical trial, since it showed stability and had pleasant sensory results. The association of antioxidants in topical formulations can be used to improve the overall skin condition, as well as to improve skin photoprotection [11,12,13].
The use of the formulation containing the association of antioxidants (F6) in group 2 after 12 weeks of study was shown to increase skin hydration and improve the skin barrier, as there was an increase in the stratum corneum water content and a decrease in TEWL [16]. This effect is likely due to the presence of Spirulina sp. in the formulation, as previous studies have shown that using a formulation containing 0.1% Spirulina sp. for 28 days on mature skin promoted an increase in hydration and an improvement in the skin barrier [39].
There was a significant increase in the echogenicity of the dermis for both groups. This suggests that the daily use of sunscreen promoted an improvement in the echogenicity of the dermis. This finding corroborates with the scientific literature, as Souza and Maia Campos [16] demonstrated the improvement of dermis echogenicity with the use of sunscreens.
An increase in the quality score of the stratum corneum indicates an improvement in the reflectance and shape parameters of this layer [34]. An increase in the quality score of the stratum granulosum indicates an improvement in the parameters related to the honeycomb pattern of the epidermis, an increase in interkeratinocyte reflectance, and of keratinocyte area [34]. The significant results of the scores obtained suggest an improvement in the morphological characteristics of the stratum corneum and stratum granulosum for group 2. These characteristics are related to skin hydration in depth [40].
According to Manfredini et al. [40], an increase in the brightness of interkeratinocytes may be due to the enrichment of lipids and proteins from the formulation applied to the skin and more functional keratinocytes with skin hydration. There was an increase in interkeratinocyte brightness for G2. G1, which used the vehicle formulation (F7), did not show this deep hydration characteristic. Therefore, we can suggest that the use of the formulation containing the association of antioxidants (olive extract + Spirulina sp.—F6) after 12 weeks promotes hydration in the skin’s deeper layers, which can help with cell renewal and can maintain skin physiology and health.
According to the scientific literature [9,10], the increase in the stratum corneum brightness suggests the formation of a film on the skin surface that may be related to an improvement in skin texture. The presence of biocompatible skin emollients in the developed formulations may have favored the improvement of skin texture and, consequently, the formation of the film on the skin surface [10]. Therefore, the increase in the stratum corneum brightness score was significant for both groups. The daily use of sunscreens during the study could also have influenced film formation in both groups, since the sunscreen formulation contains polymers that could have favored this film formation [9].
In addition, the film-forming effect of a formulation on the skin can cause an improvement of the skin barrier, since it prevents the evaporation of transepidermal water, due to the film formed by the formulation and/or active substance with this characteristic [9]. This corroborates with the reduction in TEWL obtained in this study for G2 after 12 weeks of use of the formulation containing the association of antioxidants (olive extract + Spirulina sp.—F6).
The observed film-forming effect may be related to the composition of Spirulina sp. used in the formulation of the association, as it presents carbohydrates in the form of polysaccharides that are considered natural polymers. Spirulina sp. was also investigated by Infante, Leite, and Maia Campos [10] on its effective film-forming effect after 30 min of application on skin and hair. This is the first time that the film-forming effect has been observed long-term (12 weeks).
The presence of polycyclic or flattened papillae at the dermal–epidermal junction, as well as the low density and quality of collagen fibers for both study groups suggests that the skin evaluated during the study has skin photoaging characteristics [35]. These characteristics were not improved with the use of sunscreen and formulations containing the association of antioxidants, which suggests that the association of antioxidants did not reach deeper layers of the skin.
The increase in epidermal thickness may be related to skin hydration, as was observed by Bagcı et al. [41] with the application of a moisturizer to the skin for 2 weeks. An increase in stratum corneum thickness was also observed by Kakuda et al. [9], due to the film formation effects after 1, 2, 4, and 6 h of application of a formulation containing active substances with film-forming properties. In addition, a previous study from our research group showed the film-forming property of Spirulina sp., due to an increase in stratum corneum brightness [10].
In summary, the cosmetic formulation containing the association of proposed natural antioxidants (F6) was stable and effective for the improvement of skin photoaging conditions. In addition, the use of sunscreens is fundamental to prevent skin photoaging damage.
5. Conclusions
The cosmetic formulation containing olive extract and Spirulina sp. in combination (F6) was stable and presented good sensory properties and spreadability.
The proposed formulation (F6) showed a hydrating effect and improved the skin barrier after 12 weeks of daily application.
An improvement in the morphological and structural characteristics of the skin was observed only in the group that applied the formulation containing the association of antioxidants for 12 weeks, by improvement of stratum corneum and stratum granulosum quality scores, as well as an increase in interkeratinocyte reflectance in stratum granulosum and stratum corneum brightness.
Finally, the proposed formulation (F6) was stable and effective in protecting the skin and reducing skin changes related to photoaging.
Author Contributions
G.M.D.C. contributed to study conceptualization, methodology, formal analysis, interpretation of data, writing the original draft, review and editing. P.M.B.G.M.C. contributed to study conceptualization, interpretation of data, writing the original draft, review and editing. All authors provided input on the content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by FAPESP—São Paulo Research Foundation (grant number: 2022/00897-0), CNPQ—Brazilian Council for Scientific and Technological Development (grant number: 119933/2021-7), and CAPES -Coordination of Superior Level Staff Improvement (finantial code 001).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Faculty of Pharmaceutical Sciences of Ribeirão Preto—University of São Paulo (n° CAAE: 84 599 418.8.0000.5403).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank the companies ChemyUnion® (Sorocaba, SP, Brazil), Galena® (Campinas, SP, Brazil), and CP Kelco (Limeira, SP, Brazil) for the raw material samples donated to the School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grand View Research. Natural Skin Care Products Market Size, Share & Trends Analysis Report by Type (Mass, Premium), by Product (Facial Care, Body Care), by End-use (Men, Women), by Distribution Channel, by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/natural-skin-care-products-market (accessed on 21 February 2023).
- Kaur, I.P.; Kapila, M.; Agrawal, R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res. Rev. 2007, 6, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E. Mechanisms of aging and development-A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Chien, A.L.; Kang, S. Photoaging. Dermatol. Clin. 2014, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily photoprotection to prevent photoaging. Photodermatol. Photoimmunol. Photomed. 2021, 37, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Forestier, S. Rationale for sunscreen development. J. Am. Acad. Dermatol. 2008, 58, S133–S138. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, L.; Maia Campos, P.M.B.G.; Bordini Zanin, R.; Noronha Favaro, L. Development of multifunctional sunscreens: Evaluation of Physico-mechanical and film-forming properties. Int. J. Pharm. 2023, 635, 122705. [Google Scholar] [PubMed]
- Infante, V.H.P.; Leite, M.G.A.; Maia Campos, P.M.B.G. Film-Forming Properties of Topical Formulations for Skin and Hair: In Vivo and In Vitro Studies Using Biophysical and Imaging Techniques. AAPS PharmSciTech. 2022, 24, 29. [Google Scholar] [CrossRef]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Gayathri, S.; Bhaskar, J.P.; Krishnan, V.; Balamurugan, K. Analyzing the Synergistic Effects of Antioxidants in Combating Photoaging Using Model Nematode, Caenorhabditis elegans. Photochem. Photobiol. 2020, 96, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jagdeo, J.; Kurtti, A.; Hernandez, S.; Akers, N.; Peterson, S. Novel Vitamin C and E and Green Tea Polyphenols Combination Serum Improves Photoaged Facial Skin. J. Drugs Dermatol. 2021, 20, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Maia Campos, P.M.B.G. Development and photoprotective effect of a sunscreen containing the antioxidants Spirulina and dimethylmethoxy chromanol on sun-induced skin damage. Eur. J. Pharm. Sci. 2017, 104, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, D.; Shahidi, F. Antioxidant properties of tyrosol and hydroxytyrosol saturated fatty acid esters. Food Chem. 2018, 245, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-Y.; Sun, Y.-X.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Foods 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Dauber, C.; Parente, E.; Zucca, M.P.; Gámbaro, A.; Vieitez, I. Olea europea and By-Products: Extraction Methods and Cosmetic Applications. Cosmetics 2023, 10, 112. [Google Scholar] [CrossRef]
- D’Angelo Costa, G.M.; Maia Campos, P.M.B.G. Efficacy of topical antioxidants in the skin hyperpigmentation control: A clinical study by reflectance confocal microscopy. J. Cosmet. Dermatol. 2021, 20, 538–545. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Maddiboyina, B.; Vanamamalai, H.K.; Roy, H.; Ramaiah Gandhi, S.; Kavisri, M.; Moovendhan, M. Food and drug industry applications of microalgae Spirulina platensis: A review. J. Basic Microbiol. 2023, 63, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Corauce Neto, D.; Camargo, F.B., Jr.; Maia Campos, P.M.B.G. Spirulina Containing Cosmetic Composition and Cosmetic Treatment Method. U.S. Patent 20140023676A1, 23 January 2014. [Google Scholar]
- John, A.J.U.K.; Galdo, F.D.; Gush, R.; Worsley, P.R. An evaluation of mechanical and biophysical skin parameters at different body locations. Skin Res. Technol. 2023, 29, e13292. [Google Scholar] [CrossRef] [PubMed]
- Dasgeb, B.; Kainerstorfer, J.; Mehregan, D.; Van Vreede, A.; Gandjbakhche, A. An introduction to primary skin imaging. Int. J. Dermatol. 2013, 52, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Huang, N. Rheological Characterization of Pharmaceutical and Cosmetic Formulations for Cutaneous Applications. Curr. Pharm. Des. 2019, 25, 2349–2363. [Google Scholar] [CrossRef] [PubMed]
- Cosmetics Europe: Guidelines on Stability Testing of Cosmetic Products. 2004. Available online: http://www.cosmeticseurope.eu/files/5914/6407/8121/Guidelines_on_Stability_Testing_of_Cosmetics_CE-CTFA_-_2004.pdf (accessed on 23 April 2020).
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef] [PubMed]
- The International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH). Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). 2016. Available online: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed on 23 June 2020).
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Calixto, L.S.; Infante, V.H.P.; Maia Campos, P.M.B.G. Design and Characterization of Topical Formulations: Correlations Between Instrumental and Sensorial Measurements. AAPS PharmSciTech. 2018, 19, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Maia Campos, P.M.B.G.; D’Angelo Costa, G.M. Evaluation of the influence of the application of a cosmetic formulation on the skin morphological characteristics by Reflectance Confocal Microscopy. Biomed. Biopharm. Res. 2022, 19, 410–423. [Google Scholar]
- Infante, V.H.; Maia Campos, P.M.B.G. Application of a Reflectance Confocal Microscopy Imaging Analysis Score for the Evaluation of Non-Melanogenic Changes in Male Photoaged Skin. Photochem. Photobiol. 2023, 99, 993–1002. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nishad, J. Chapter 5—Stability of plant extracts. In Plant Extracts: Applications in the Food Industry; Mir, S.A., Manickavasagan, A., Shah, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 89–126. [Google Scholar] [CrossRef]
- Haytoglu, N.S.; Gurel, M.S.; Erdemir, A.; Falay, T.; Dolgun, A.; Haytoglu, T.G. Assessment of skin photoaging with reflectance confocal microscopy. Skin Res. Technol. 2014, 20, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ezz El-Din Ibrahim, M.; Alqurashi, R.M.; Alfaraj, F.Y. Antioxidant Activity of Moringa oleifera and Olive Olea europaea L. Leaf Powders and Extracts on Quality and Oxidation Stability of Chicken Burgers. Antioxidants 2022, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Delsin, S.; Mercurio, D.; Fossa, M.M.; Maia Campos, P.M.B.G. Clinical Efficacy of Dermocosmetic Formulations Containing Spirulina Extract on Young and Mature Skin: Effects on the Skin Hydrolipidic Barrier and Structural Properties. Clin. Pharmacol. Biopharm. 2015, 4, 144. [Google Scholar] [CrossRef]
- Manfredini, M.; Mazzaglia, G.; Ciardo, S.; Simonazzi, S.; Farnetani, F.; Longo, C.; Pellacani, G. Does skin hydration influence keratinocyte biology? In vivo evaluation of microscopic skin changes induced by moisturizers by means of reflectance confocal microscopy. Skin Res. Technol. 2013, 19, 299–307. [Google Scholar] [CrossRef]
- Bağcı, I.S.; Ruini, C.; Niesert, A.C.; Horváth, O.N.; Berking, C.; Ruzicka, T.; von Braunmühl, T. Effects of Short-Term Moisturizer Application in Different Ethnic Skin Types: Noninvasive Assessment with Optical Coherence Tomography and Reflectance Confocal Microscopy. Skin Pharmacol. Physiol. 2018, 31, 125–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).