Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Analysis
2.2. LS-TSW Microbiome Analysis
2.3. Cell Culture
2.4. Induction of Inflammation Using LPS
2.5. Cytokine Arrays
2.6. Induction of Inflammation Using TNF-α and IFN-γ
2.7. Total RNA Isolation and Real-Time quantitative Polymerase Chain Reaction (RT-qPCR)
3. Results
3.1. La Solia, Thermal Spring Water Chemical Characterization
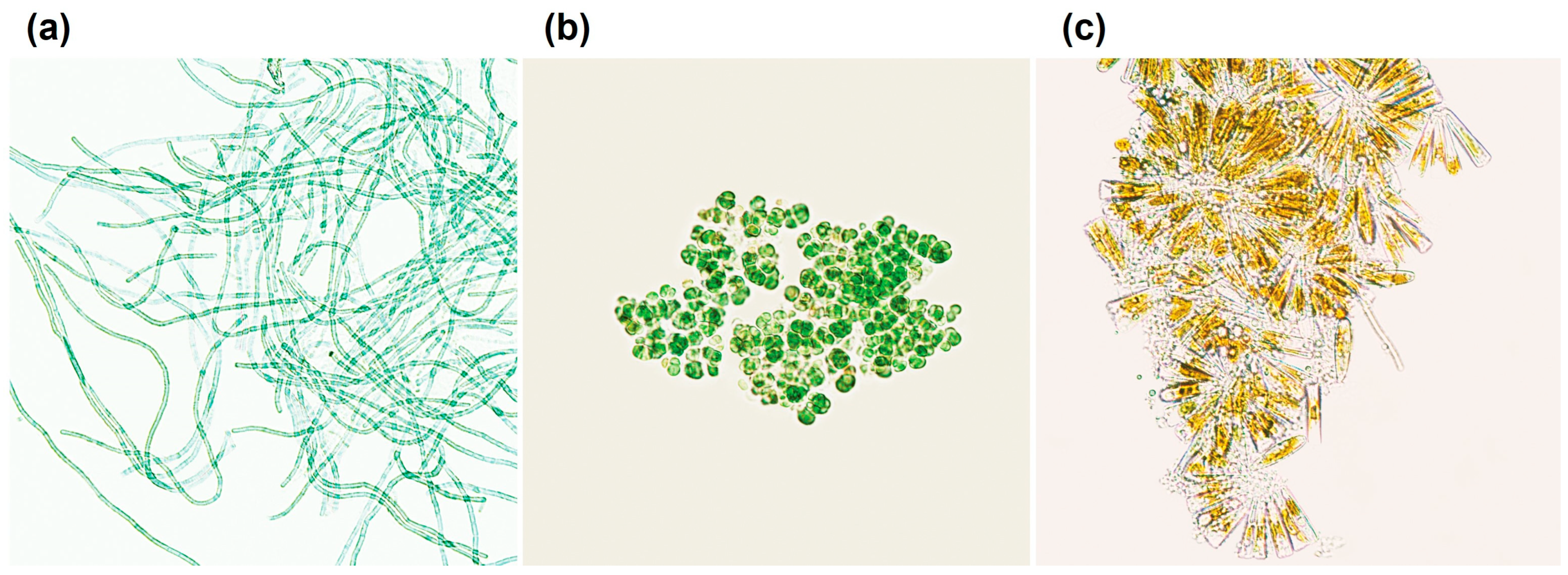
3.2. La Solia Microbiological Characterization
3.2.1. Cyanobacteria
3.2.2. Chlorophyta
3.2.3. Diatoms
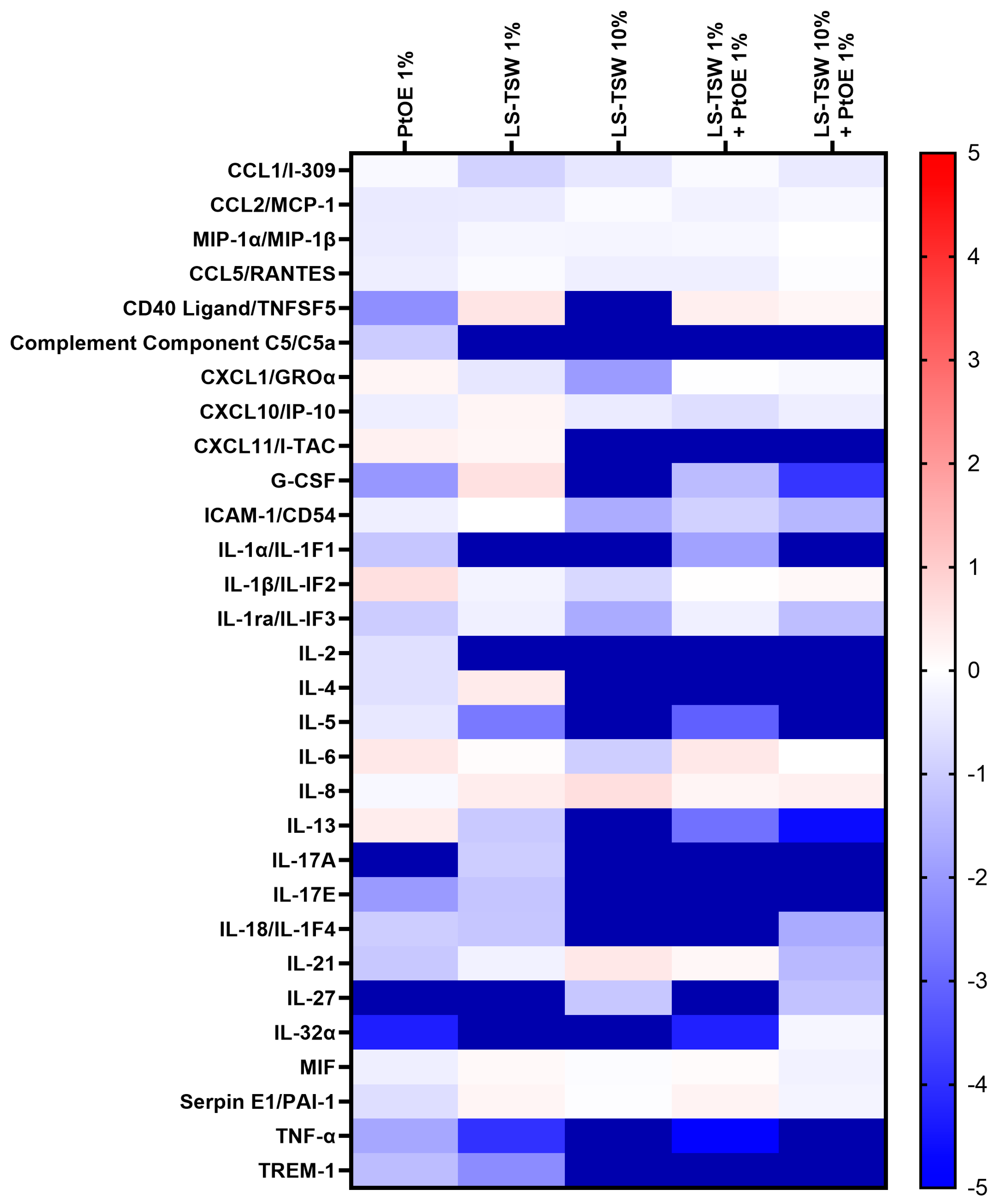
3.3. Immunomodulatory Activity of a Combination of La Solia Thermal Water and Phaeodactylum Tricornutum Oil Extract: Aquammunist
3.3.1. Aquammunist Showed Potential Anti-Inflammatory Properties in In Vitro Conditions
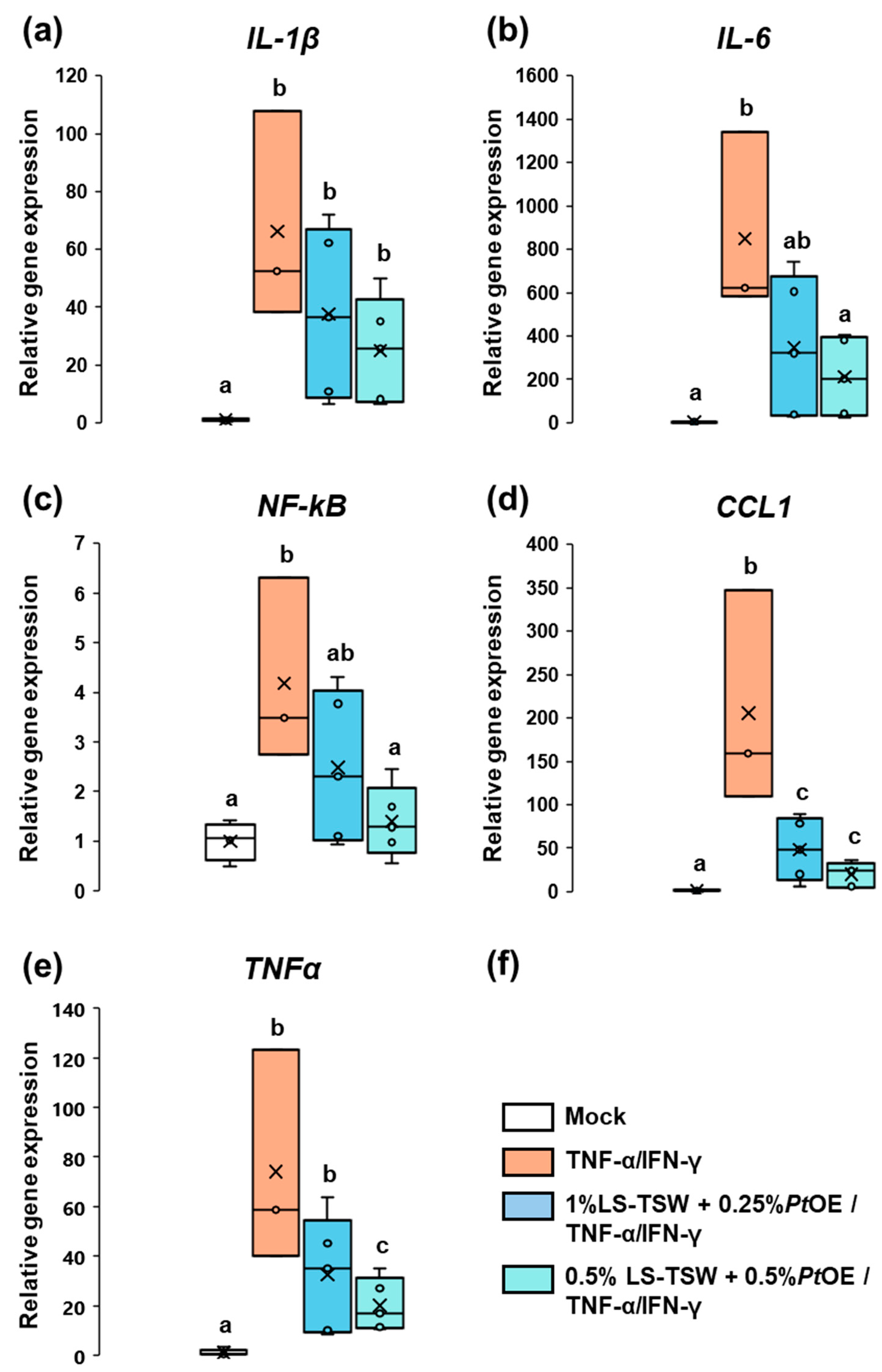
3.3.2. Aquammunist Displays an Effective Control of Skin Inflammation in In Vitro Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.J.; Lin, L.L.; McMeniman, E.; Wu, J.; Kao, Y.-C.; Kumari, S.; Boyle, G.M.; Wells, J.W.; Soyer, H.P.; Gonzalez-Cruz, J.L. Cytokine/Chemokine Assessment as a Complementary Diagnostic Tool for Inflammatory Skin Diseases. Front. Immunol. 2022, 13, 1028435. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in Patients with Inflammatory Skin Diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef] [PubMed]
- Antelo, D.A.P.; Leta da Costa Rocha, A. Cosmetic Approach in Patients with Acne and Rosacea. In Daily Routine in Cosmetic Dermatology; Issa, M.C.A., Tamura, B., Eds.; Clinical Approaches and Procedures in Cosmetic Dermatology; Springer: Cham, Switzerland, 2016; pp. 1–28. ISBN 978-3-319-20250-1. [Google Scholar]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Ständer, S.; Szepietowski, J.; Reich, A.; Wallengren, J.; Evers, A.; Takamori, K.; Brenaut, E.; Gall-Ianotto, C.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dai, R.; Li, L. The Prevalence of Self-Declared Sensitive Skin: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 1779–1788. [Google Scholar] [CrossRef]
- Legeas, C.; Misery, L.; Fluhr, J.W.; Roudot, A.-C.; Ficheux, A.-S.; Brenaut, E. Proposal for Cut-off Scores for Sensitive Skin on Sensitive Scale-10 in a Group of Adult Women. Acta Derm. Venereol. 2021, 101, 1498. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.-L.; Wu, Y.; Welsh, B.; Abad-Casintahan, M.F.; Tseng, C.-J.; Sharad, J.; Jung, S.; Rojanamatin, J.; Sitohang, I.B.S.; Chan, H.N.K. Expert Consensus on Holistic Skin Care Routine: Focus on Acne, Rosacea, Atopic Dermatitis, and Sensitive Skin Syndrome. J. Cosmet. Dermatol. 2023, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Tapia, A.; Serra-Baldrich, E.; Prieto Cabezas, L.; González-Guerra, E.; López-Estebaranz, J.L. Diagnóstico y tratamiento del síndrome de piel sensible: Un algoritmo para la práctica clínica habitual. Actas Dermo-Sifiliográficas 2019, 110, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wen, J.; Wu, W.; Peng, Q.; Cui, X.; He, L. A Review of Factors Influencing Sensitive Skin: An Emphasis on Built Environment Characteristics. Front. Public Health 2023, 11, 1269314. [Google Scholar] [CrossRef]
- Sinha, S.; Meena, N. The Sensitive Skin: Do’s and Don’ts. In Skin Diseases in Females; Sarkar, R., Sinha, S., Eds.; Springer: Singapore, 2022; pp. 471–486. ISBN 9789811660658. [Google Scholar]
- Lee, H.-P.; Choi, Y.-J.; Cho, K.-A.; Woo, S.-Y.; Yun, S.-T.; Lee, J.T.; Kim, H.J.; Lee, K.-H.; Kim, J.-W. Effect of Spa Spring Water on Cytokine Expression in Human Keratinocyte HaCaT Cells and on Differentiation of CD4+ T Cells. Ann. Dermatol. 2012, 24, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Dermothermal Cosmetics: Added Value for Thermal Centers. In Proceedings of the 1st InternationalCongress on Water Healing Spa and Quality of Life, Ourense, Spain, 23–24 September 2015; pp. 1–7. [Google Scholar]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Hydrobiome of Thermal Waters: Potential Use in Dermocosmetics. Cosmetics 2023, 10, 94. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Resende, D.I.S.P.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Mosxou, D.; Letsiou, S. Exploring the Protective Effects of Phaeodactylum Tricornutum Extract on LPS-Treated Fibroblasts. Cosmetics 2021, 8, 76. [Google Scholar] [CrossRef]
- Lee, A.-H.; Shin, H.-Y.; Park, J.-H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from Microalgae Phaeodactylum Tricornutum Inhibits Pro-Inflammatory Cytokines by Regulating Both NF-κB and NLRP3 Inflammasome Activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, S.; Rödiger, S.; Kriegner, A.; Vierlinger, K.; Weinhäusel, A. A Survey of Tools for the Analysis of Quantitative PCR (qPCR) Data. Biomol. Detect. Quantif. 2014, 1, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Rodrigues, M.; Mourelle, M.L.; Araujo, A.R.T.S. Thermal Spring Waters as an Active Ingredient in Cosmetic Formulations. Cosmetics 2023, 10, 27. [Google Scholar] [CrossRef]
- McGregor, G.B.; Rasmussen, J.P. Cyanobacterial Composition of Microbial Mats from an Australian Thermal Spring: A Polyphasic Evaluation. FEMS Microbiol. Ecol. 2008, 63, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Hamlaoui, S.; Piquet, B.; Toutirais, G.; Yéprémian, C.; Reinhardt, A.; Duperron, S.; Marie, B.; Demay, J.; Bernard, C. Diversity of Cyanobacteria from Thermal Muds (Balaruc-Les-Bains, France) with the Description of Pseudochroococcus Coutei Gen. Nov., Sp. Nov. FEMS Microbes 2021, 2, xtab006. [Google Scholar] [CrossRef] [PubMed]
- Leira, M.; Meijide-Failde, R.; Torres, E. Diatom Communities in Thermo-Mineral Springs of Galicia (NW Spain). Diatom Res. 2017, 32, 29–42. [Google Scholar] [CrossRef]
- Krajenbrink, H.J.; White, J.C.; Dunbar, M.J.; Wood, P.J. Macroinvertebrate and Diatom Community Responses to Thermal Alterations below Water Supply Reservoirs. River Res. Appl. 2022, 38, 595–612. [Google Scholar] [CrossRef]
- Verma, P.; Thapliyal, K. Diatom Analysis in Thermophilic Water Bodies: A Study on Hot Water Springs of Madhya Pradesh. Indian J. Forensic Med. Pathol. 2023, 16, 133–143. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum Tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Yongmanitchai, W.; Ward, O.P. Screening of Algae for Potential Alternative Sources of Eicosapentaenoic Acid. Phytochemistry 1991, 30, 2963–2967. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Awwad, F.; Diamond, A.; Desgagné-Penix, I. Diatoms’ Breakthroughs in Biotechnology: Phaeodactylum Tricornutum as a Model for Producing High-Added Value Molecules. Am. J. Plant Sci. 2020, 11, 1632–1670. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Growth of and Omega-3 Fatty Acid Production by Phaeodactylum Tricornutum under Different Culture Conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Chang, H.-H.; Chou, S.-C.; Chu, T.W.; Hsu, Y.-J.; Hsiao, C.-Y.; Lo, Y.-H.; Wu, N.-L.; Chang, D.-C.; Hung, C.-F. Evaluation of the Anti-Atopic Dermatitis Effects of α-Boswellic Acid on Tnf-α/Ifn-γ-Stimulated HaCat Cells and DNCB-Induced BALB/c Mice. Int. J. Mol. Sci. 2022, 23, 9863. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.P.; Merial-Kieny, C.; Nocera, T.; Mery, S. Comparative Benefit of Two Thermal Spring Waters after Photodynamic Therapy Procedure. J. Cosmet. Dermatol. 2007, 6, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Ribet, V.; Alvarez-Georges, S.; Sibaud, V.; Guerrero, D.; Schmitt, A.-M.; Redoulès, D. Modulation of Interleukin-8 and Staphylococcal Flora by Avène Hydrotherapy in Patients Suffering from Chronic Inflammatory Dermatoses. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Mias, C.; Maret, A.; Gontier, E.; Carrasco, C.; Satge, C.; Bessou-Touya, S.; Coubetergues, H.; Bennett-Kennett, R.; Dauskardt, R.H.; Duplan, H. Protective Properties of Avène Thermal Spring Water on Biomechanical, Ultrastructural and Clinical Parameters of Human Skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.; Costa, P.C.; Bahia, M.F. Effect of São Pedro Do Sul Thermal Water on Skin Irritation. Int. J. Cosmet. Sci. 2010, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Vaz, C.V.; Silva, A.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R.; Pereira, C.; Cruz, M.T.; et al. In Vitro Evaluation of Potential Benefits of a Silica-Rich Thermal Water (Monfortinho Thermal Water) in Hyperkeratotic Skin Conditions. Int. J. Biometeorol. 2020, 64, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Verdy, C.; Branka, J.-E.; Lefeuvre, L. Modulation of Sodium-Dependent Transporters Expression in Normal Human Keratinocytes by a Sodium Rich Isotonic Thermal Water. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 254–262. [Google Scholar] [CrossRef]
- Eysteinsdóttir, J.H.; Ólafsson, J.H.; Agnarsson, B.A.; Lúðvíksson, B.R.; Sigurgeirsson, B. Psoriasis Treatment: Faster and Long-Standing Results after Bathing in Geothermal Seawater. A Randomized Trial of Three UVB Phototherapy Regimens. Photodermatol. Photoimmunol. Photomed. 2014, 30, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Eysteinsdóttir, J.H.; Sigurgeirsson, B.; Ólafsson, J.H.; Fridriksson, T.; Agnarsson, B.A.; Davíðsson, S.; Valdimarsson, H.; Lúðvíksson, B.R. The Role of Th17/Tc17 Peripheral Blood T Cells in Psoriasis and Their Positive Therapeutic Response. Scand. J. Immunol. 2013, 78, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Moysan, A.; Morlière, P.; Marquis, I.; Richard, A.; Dubertret, L. Effects of Selenium on UVA-Lnduced Lipid Peroxidation in Cultured Human Skin Fibroblasts. Skin Pharmacol. 2009, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Celerier, P.; Litoux, P.; Dreno, B.; Richard, A. Modulatory Effects of Selenium and Strontium Salts on Keratinocyte-Derived Inflammatory Cytokines. Arch. Dermatol. Res. 1995, 287, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Rougier, A.; Richard, A. Preventing Effect of a Selenium-Rich Thermal Water on UV and Chemically Induced Dermatitis. In Proceedings of the Congress of the International Federation Societies of Cosmetic Chemists, Montreux, Switzerland, 18 September 1995. [Google Scholar]
- Rougier, A.; Richard, A. A Selenium-Rich Spring Water Prevents UV and Chemically Induced Inflammation. J. Am. Acad. Dermatol. 2012, 66, AB68. [Google Scholar] [CrossRef]
- Seite, S. Thermal Waters as Cosmeceuticals: La Roche-Posay Thermal Spring Water Example. Clin. Cosmet. Investig. Dermatol. 2013, 6, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Oron, M.; Merrik, E.; Ma’or, Z.; Ben-Amitai, D.; Yogev, H.; Zvulunov, A. A Dead Sea Water-Enriched Body Cream Improves Skin Severity Scores in Children with Atopic Dermatitis. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 71–78. [Google Scholar] [CrossRef]
- Portugal-Cohen, M.; Oron, M.; Cohen, D.; Ma’or, Z. Antipollution Skin Protection—A New Paradigm and Its Demonstration on Two Active Compounds. Clin. Cosmet. Investig. Dermatol. 2017, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tsoureli-Nikita, E.; Menchini, G.; Ghersetich, I.; Hercogova, J. Alternative Treatment of Psoriasis with Balneotherapy Using Leopoldine Spa Water. J. Eur. Acad. Dermatol. Venereol. JEADV 2002, 16, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Hercogova, J.; Stanghellini, E.; Tsoureli-Nikita, E.; Menchini, G. Inhibitory Effects of Leopoldine Spa Water on Inflammation Caused by Sodium Lauryl Sulphate. J. Eur. Acad. Dermatol. Venereol. JEADV 2002, 16, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Madeira, A.; Marto, J.; Graça, A.; Pinto, P.; Ribeiro, H. Monfortinho Thermal Water-Based Creams: Effects on Skin Hydration, Psoriasis, and Eczema in Adults. Cosmetics 2019, 6, 56. [Google Scholar] [CrossRef]
- Joly, F.; Galoppin, L.; Bordat, P.; Cousse, H.; Neuzil, E. Calcium and Bicarbonate Ions Mediate the Inhibition of Mast Cell Histamine Release by Avène Spa Water. Fundam. Clin. Pharmacol. 2000, 14, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, F.; Garcia-Mace, J.L.; Joly, F. In Vitro Effects of Uriage Spring Water on the Apoptosis of Human Eosinophils. Fundam. Clin. Pharmacol. 1998, 12, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Zöller, N.; Valesky, E.; Hofmann, M.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Impact of Different Spa Waters on Inflammation Parameters in Human Keratinocyte HaCaT Cells. Ann. Dermatol. 2015, 27, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Deleuran, M.; Georgescu, V.; Jean-Decoster, C. An Emollient Containing Aquaphilus Dolomiae Extract Is Effective in the Management of Xerosis and Pruritus: An International, Real-World Study. Dermatol. Ther. 2020, 10, 1013–1029. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, A.B.; Brynjolfsdottir, A.; Olafsdottir, E.S.; Hardardottir, I.; Freysdottir, J. Exopolysaccharides from Cyanobacterium aponinum Induce a Regulatory Dendritic Cell Phenotype and Inhibit SYK and CLEC7A Expression in Dendritic Cells, T Cells and Keratinocytes. Int. Immunopharmacol. 2019, 69, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, A.B.; Omarsdottir, S.; Brynjolfsdottir, A.; Paulsen, B.S.; Olafsdottir, E.S.; Freysdottir, J. Exopolysaccharides from Cyanobacterium Aponinum from the Blue Lagoon in Iceland Increase IL-10 Secretion by Human Dendritic Cells and Their Ability to Reduce the IL-17+RORγt+/IL-10+FoxP3+ Ratio in CD4+ T Cells. Immunol. Lett. 2015, 163, 157–162. [Google Scholar] [CrossRef]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass Nutrient Profiles of the Microalga Phaeodactylum Tricornutum. J. Food Biochem. 2001, 25, 57–76. [Google Scholar] [CrossRef]
- Geider, R.; La Roche, J. Redfield Revisited: Variability of C:N:P in Marine Microalgae and Its Biochemical Basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Karim, M.M.; Das, R.; Park, M.N.; Chandran, D.; et al. Fucoxanthin: A Promising Phytochemical on Diverse Pharmacological Targets. Front. Pharmacol. 2022, 13, 929442. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, K.T.; Hwang, S.; Choi, K.Y.; Park, Y.J.; Choi, J.-H.; Truong, T.Q.; Kim, S.M. Nanoencapsulation Enhances the Bioavailability of Fucoxanthin in Microalga Phaeodactylum tricornutum Extract. Food Chem. 2023, 403, 134348. [Google Scholar] [CrossRef] [PubMed]
- Uchi, H.; Terao, H.; Koga, T.; Furue, M. Cytokines and Chemokines in the Epidermis. J. Dermatol. Sci. 2000, 24, S29–S38. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, M.S.; Shear, N.H. Biologics in Patients with Skin Diseases. J. Allergy Clin. Immunol. 2017, 139, 1423–1430. [Google Scholar] [CrossRef]
- Yao, Y.; Ravn Jørgensen, A.-H.; Thomsen, S.F. Biologics for Chronic Inflammatory Skin Diseases: An Update for the Clinician. J. Dermatol. Treat. 2020, 31, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Stieger, M.; Yawalkar, N.; Kakeda, M. Cytokines and Chemokines in Irritant Contact Dermatitis. Mediat. Inflamm. 2013, 2013, 916497. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, G.; Campanati, A.; Molinelli, E.; Offidani, A. Biologic Therapy in Inflammatory and Immunomediated Skin Diseases: Safety Profile. Curr. Drug Saf. 2016, 11, 12–21. [Google Scholar] [CrossRef] [PubMed]
| Anions/Cations/Trace Elements and Other Compounds | |
|---|---|
| Chloride (mg/L) | 3200 |
| Sulphate (mg/L) | 300 |
| Bicarbonate (mg/L) | 210 |
| Nitrate (mg/L) | 2 |
| Fluoride (mg/L) | <0.5 |
| Phosphate (mg/L) | <0.04 |
| Silica SiO2 (mg/L) | 27.1 |
| Calcium (mg/L) | 184 |
| Magnesium (mg/L) | 36 |
| Potassium (mg/L) | 19 |
| Sodium (mg/L) | 1956 |
| Iron (mg/L) | <0.1 |
| Manganese (μg/L) | 67 |
| Boron (μg/L) | 214 |
| Cadmium (μg/L) | 6.57 |
| Zinc (μg/L) | 174.0 |
| Copper (μg/L) | 427.0 |
| Selenium (μg/L) | 11.97 |
| Barium (μg/L) | 96 |
| A/C/T/Other | La Solia (Cantabria Labs) | Uriage | Avène | La Roche-Posay | Vichy | Saint-Gervais |
|---|---|---|---|---|---|---|
| Chloride (mg/L) | 3200 | 3500 | 5.4 | 22.6 | -- | 530 |
| Sulphate (mg/L) | 300 | 2860 | 13.1 | 56.1 | 182.39 | 1812 |
| Bicarbonate (mg/L) | 210 | 390 | 226.7 | 387 | 4818.63 | 247 |
| Nitrate (mg/L) | 2 | -- | 1.4 | 1.6 | -- | -- |
| Fluoride (mg/L) | <0.5 | -- | 0.1 | 0.2 | 7.67 | -- |
| Phosphate (mg/L) | <0.04 | -- | 0.3 | <0.1 | 0.210 | -- |
| Silica SiO2 (mg/L) | 27.1 | 42 | 14 | 31.6 | 11.78 * | -- |
| Calcium (mg/L) | 184 | 600 | 42.7 | 149.0 | 165.61 | 234 |
| Magnesium (mg/L) | 36 | 125 | 21.2 | 4.4 | 12.08 | 26.8 |
| Potassium (mg/L) | 19 | 45.5 | 0.8 | 1.9 | 103.56 | 29 |
| Sodium (mg/L) | 1956 | 2360 | 4.8 | 1.3 | 1862.88 | 944 |
| Iron (mg/L) | <0.1 | -- | <0.1 | <0.005 | 0.810 | <30 |
| Manganese (μg/L) | 67 | 0.154 | <0.1 | <0.003 | 0.208 | 0.327 |
| Boron (μg/L) | 214 | -- | 220 | -- | 970 | 5030 |
| Cadmium (μg/L) | 6.57 | -- | 2 | -- | -- | -- |
| Zinc (μg/L) | 174.0 | 160 | 20 | <5 | -- | 57 |
| Copper (μg/L) | 427.0 | 75 | <5 | <5 | -- | <2 |
| Selenium (μg/L) | 11.97 | -- | <5 | 53 | -- | -- |
| Barium (μg/L) | 96 | -- | 220 | -- | -- | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourelle, M.L.; Segura de Yebra, J.; Ayats, J.; Vitale, M.; López Sánchez, A. Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract. Cosmetics 2024, 11, 62. https://doi.org/10.3390/cosmetics11020062
Mourelle ML, Segura de Yebra J, Ayats J, Vitale M, López Sánchez A. Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract. Cosmetics. 2024; 11(2):62. https://doi.org/10.3390/cosmetics11020062
Chicago/Turabian StyleMourelle, Maria Lourdes, Jordi Segura de Yebra, Jordi Ayats, Maria Vitale, and Ana López Sánchez. 2024. "Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract" Cosmetics 11, no. 2: 62. https://doi.org/10.3390/cosmetics11020062
APA StyleMourelle, M. L., Segura de Yebra, J., Ayats, J., Vitale, M., & López Sánchez, A. (2024). Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract. Cosmetics, 11(2), 62. https://doi.org/10.3390/cosmetics11020062







