Microtopography and Barrier Function in Healthy Skin: Differences between Forearm, Cheek and Palm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Skin Microtopography and Epidermal Barrier Function Parameters
2.3. Other Variables
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Sample Characteristics
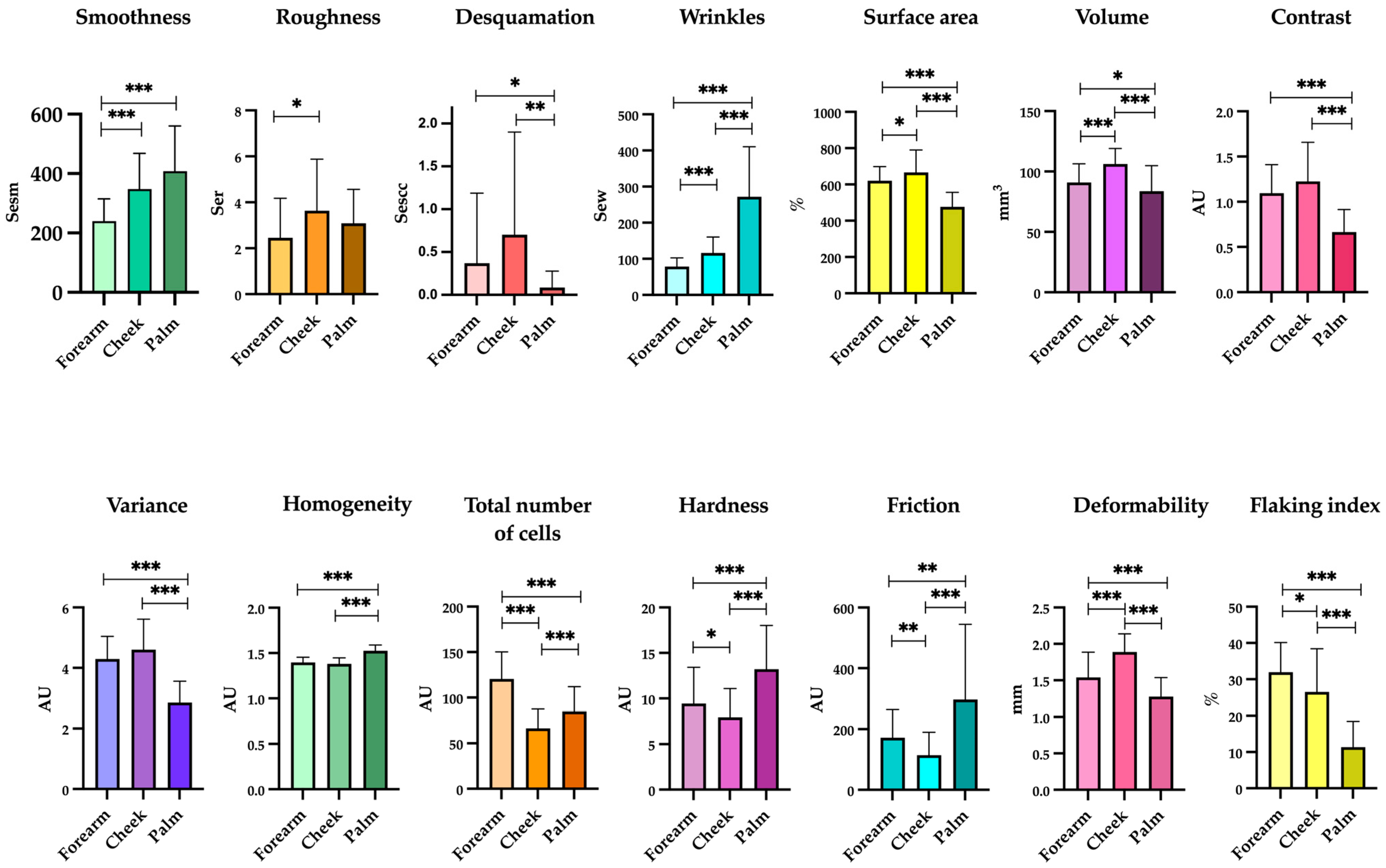
3.2. Skin Homeostasis and Epidermal Barrier Function Changed between Body Sites
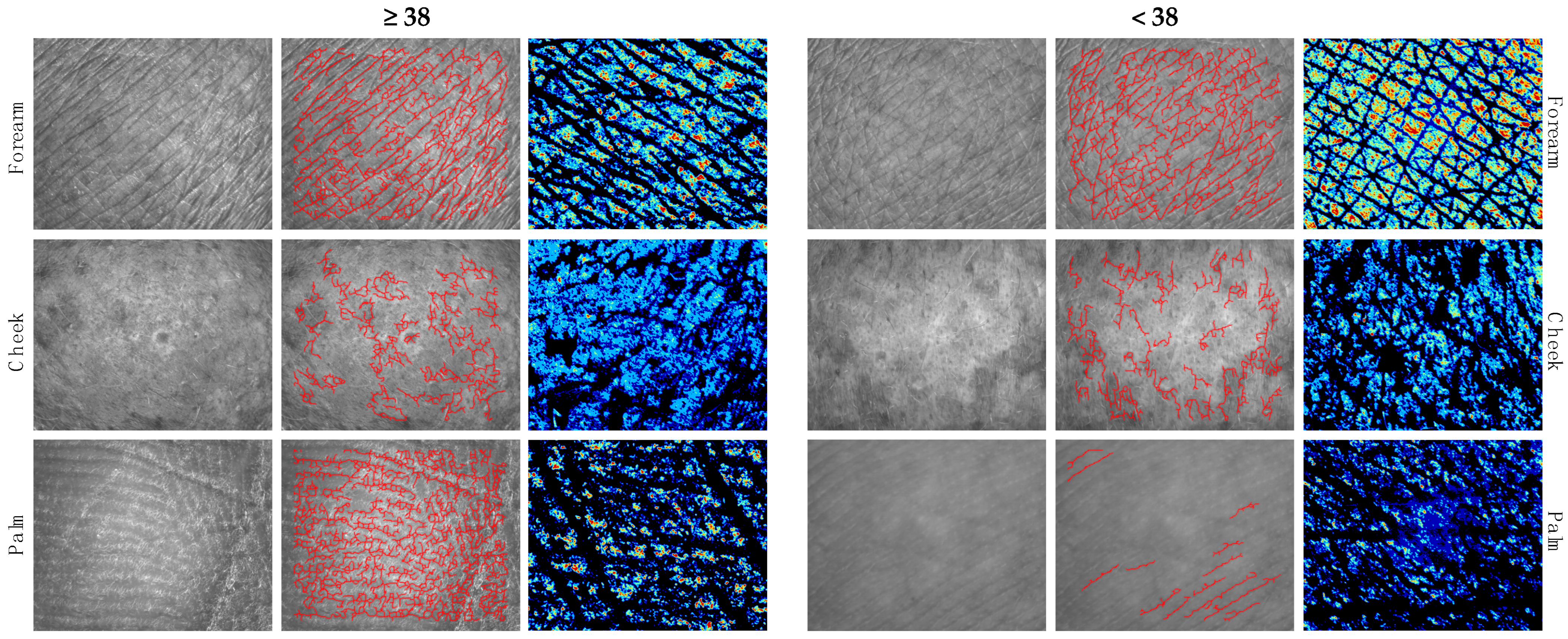
3.3. Differences in Skin Microtopography and Epidermal Barrier Function in Subjects ≥38 and <38 Years
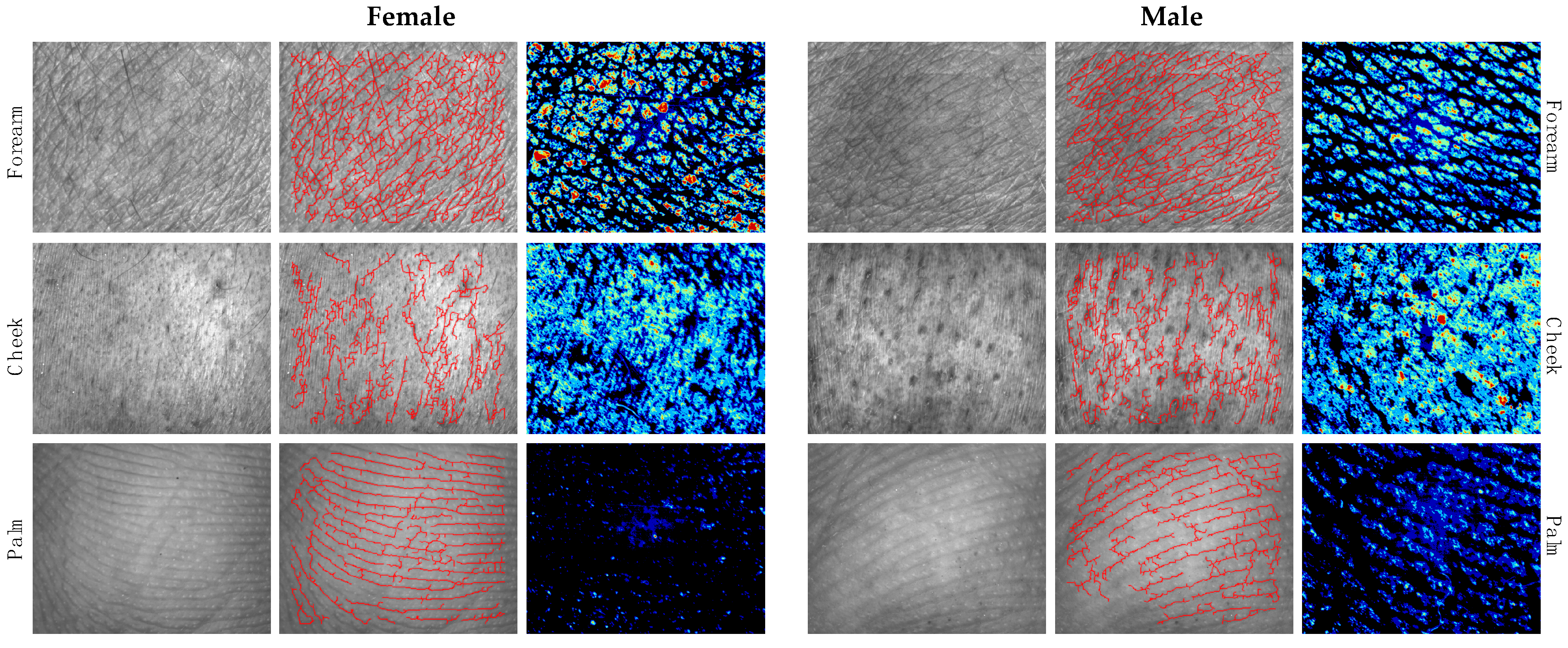
3.4. Differences in Skin Microtopography and Epidermal Barrier Function between Genders
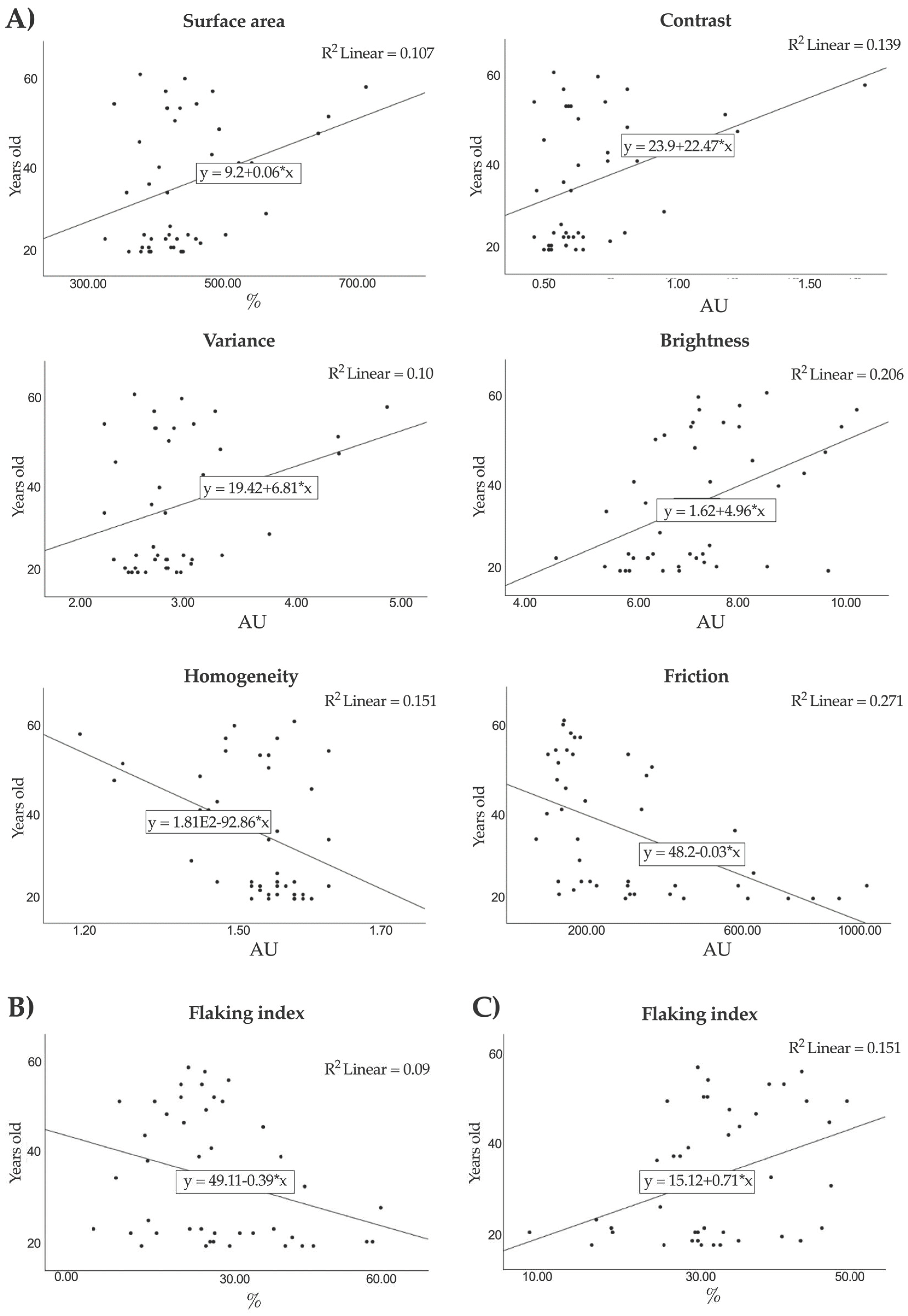
3.5. Correlation between Skin Biophysical Parameters and Age
3.6. Influences of Toxic Habits (Tobacco and Alcohol Use) on Skin Microtopography and Epidermal Barrier Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorado, J.G.; Fraile, P.A. Anatomía y Fisiología de La Piel. Pediatr. Integral 2021, 24, 156.e1–156.e13. [Google Scholar]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and Function of the Epidermis Related to Barrier Properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Espinosa-Rueda, M.I.; Montero-Vilchez, T.; Martinez-Lopez, A.; Molina-Leyva, A.; Sierra-Sánchez, A.; Arias-Santiago, S.; Buendia-Eisman, A. Cutaneous Homeostasis and Epidermal Barrier Function in a Young Healthy Caucasian Population. Eur. J. Dermatol. 2021, 31, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Morales, D.; Montero-Vilchez, T.; Arias-Santiago, S. Study of Skin Barrier Function in Psoriasis: The Impact of Emollients. Life 2021, 11, 651. [Google Scholar] [CrossRef]
- Trojahn, C.; Schario, M.; Dobos, G.; Blume-Peytavi, U.; Kottner, J. Reliability and Validity of Two in Vivo Measurements for Skin Surface Topography in Aged Adults. Skin Res. Technol. 2015, 21, 54–60. [Google Scholar] [CrossRef]
- Theek, C.; Tronnier, H.; Heinrich, U.; Braun, N. Surface Evaluation of Living Skin (SELS) Parameter Correlation Analysis Using Data Taken from Astronauts Working under Extreme Conditions of Microgravity. Ski. Res. Technol. 2020, 26, 105–111. [Google Scholar] [CrossRef]
- Kuklinski, L.F.; Zens, M.S.; Perry, A.E.; Green, A.C.; Karagas, M.R. Skin Microtopography as a Measure of Photoaging and Risk of Squamous Cell Carcinoma of the Skin in a US Population. Photodermatol. Photoimmunol. Photomed. 2017, 33, 41. [Google Scholar] [CrossRef]
- Holman, C.D.J.; Armstrong, B.K.; Evans, P.R.; Lumsden, G.J.; Dallimore, K.J.; Meehan, C.J.; Beagley, J.; Gibson, I.M. Relationship of Solar Keratosis and History of Skin Cancer to Objective Measures of Actinic Skin Damage. Br. J. Dermatol. 1984, 110, 129–138. [Google Scholar] [CrossRef]
- Nanzadsuren, T.; Myatav, T.; Dorjkhuu, A.; Ganbat, M.; Batbold, C.; Batsuuri, B.; Byamba, K. Skin Aging Risk Factors: A Nationwide Population Study in Mongolia Risk Factors of Skin Aging. PLoS ONE 2022, 17, e249506. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Martinez-Lopez, A.; Sierra-Sanchez, A.; Soler-Gongora, M.; Jimenez-Mejias, E.; Molina-Leyva, A.; Buendia-Eisman, A.; Arias-Santiago, S. Erythema Increase Predicts Psoriasis Improvement after Phototherapy. J. Clin. Med. 2021, 10, 3897. [Google Scholar] [CrossRef] [PubMed]
- Epidermal, S.; Monteiro Rodrigues, L.; Querleux, B.; Gyulai, R.; Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; et al. Epidermal Barrier Function and Skin Homeostasis in Atopic Dermatitis: The Impact of Age. Life 2022, 12, 132. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Martinez-Lopez, A.; Cuenca-Barrales, C.; Rodriguez-Tejero, A.; Molina-Leyva, A.; Arias-Santiago, S. Impact of Gloves and Mask Use on Epidermal Barrier Function in Health Care Workers. Dermatitis 2021, 32, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Chen, D. The Effect of Sun Tan Lotion on Skin by Using Skin TEWL and Skin Water Content Measurements. Sensors 2022, 22, 3595. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Toro, A.; Montero-Vilchez, T.; Muñoz-Baeza, J.; Sanabria-De-la-Torre, R.; Buendia-Eisman, A.; Arias-Santiago, S. The Assessment of Skin Homeostasis Changes after Using Different Types of Excipients in Healthy Individuals. Int. J. Environ. Res. Public Health 2022, 19, 16678. [Google Scholar] [CrossRef] [PubMed]
- John, A.J.U.K.; Galdo, F.D.; Gush, R.; Worsley, P.R. An Evaluation of Mechanical and Biophysical Skin Parameters at Different Body Locations. Skin Res. Technol. 2023, 29, e13292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Gan, Y.; Yang, M.; He, C.; Jia, Y. Lipidomics Analysis of Facial Skin Surface Lipids between Forehead and Cheek: Association between Lipidome, TEWL, and PH. J. Cosmet. Dermatol. 2020, 19, 2752–2758. [Google Scholar] [CrossRef]
- Seo, J.I.; Ham, H.I.; Baek, J.H.; Shin, M.K. An Objective Skin-Type Classification Based on Non-Invasive Biophysical Parameters. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 444–452. [Google Scholar] [CrossRef]
- Tagami, H. Location-Related Differences in Structure and Function of the Stratum Corneum with Special Emphasis on Those of the Facial Skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef]
- Prost-Squarcioni, C. Histology of Skin and Hair Follicle. Med. Sci. 2006, 22, 131–137. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The Relationship between Skin Function, Barrier Properties, and Body-Dependent Factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.W.; Baek, J.H.; Koh, J.S.; Hwang, J.K. The Seasonal Variation in Skin Hydration, Sebum, Scaliness, Brightness and Elasticity in Korean Females. Skin Res. Technol. 2015, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial Barrier Hypothesis: Effect of the External Exposome on the Microbiome and Epithelial Barriers in Allergic Disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.d.A.; Hiraishi, C.F.; Macedo, P.I.d.S.; Pinto, C.A.S.d.O.; Gregório, J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. HPLC-TBARS-EVSC (High-Performance Liquid Chromatography–Thiobarbituric Acid Reactive Substances—Ex Vivo Stratum Corneum) Protocol: Selection of the Subjects and Approach to Present the Results. Int. J. Cosmet. Sci. 2023, 45, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International Guidelines for the in Vivo Assessment of Skin Properties in Non-Clinical Settings: Part 2. Transepidermal Water Loss and Skin Hydration. Skin Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Firooz, A.; Sadr, B.; Babakoohi, S.; Sarraf-Yazdy, M.; Fanian, F.; Kazerouni-Timsar, A.; Nassiri-Kashani, M.; Naghizadeh, M.M.; Dowlati, Y. Variation of Biophysical Parameters of the Skin with Age, Gender, and Body Region. Sci. World J. 2012, 2012, 386936. [Google Scholar] [CrossRef]
- Wilhelm, K.P.; Cua, A.B.; Maibach, H.I. Skin Aging. Effect on Transepidermal Water Loss, Stratum Corneum Hydration, Skin Surface PH, and Casual Sebum Content. Arch. Dermatol. 1991, 127, 1806–1809. [Google Scholar] [CrossRef]
- Humbert, P.; Maibach, H.I.; Fanian, F.; Agache, P. Agache’s Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants: Second Edition; Springer: Cham, Switzerland, 2017; pp. 1–1651. [Google Scholar] [CrossRef]
- Cua, A.B.; Wilhelm, K.P.; Maibach, H.I. Elastic Properties of Human Skin: Relation to Age, Sex, and Anatomical Region. Arch. Dermatol. Res. 1990, 282, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ya-Xian, Z.; Suetake, T.; Tagami, H. Number of Cell Layers of the Stratum Corneum in Normal Skin—Relationship to the Anatomical Location on the Body, Age, Sex and Physical Parameters. Arch. Dermatol. Res. 1999, 291, 555–559. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Mielcarek, A.; Nowak, I. Evaluation of Sex-Related Changes in Skin Topography and Structure Using Innovative Skin Testing Equipment. Skin Res. Technol. 2018, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D’arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.B.; Kamath, P.P.; Chatterjee, S.; Bhattacherjee, R.; Nayak, U.Y. Recent Updates on Nanocosmeceutical Skin Care and Anti-Aging Products. Curr. Pharm. Des. 2022, 28, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.R.; Kupper, T.S. Immunity at the Surface: Homeostatic Mechanisms of the Skin Immune System. Life Sci. 1996, 58, 1485–1507. [Google Scholar] [CrossRef] [PubMed]
- Sunderkötter, C. Aging and the Skin Immune System. Arch. Dermatol. 1997, 133, 1256. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging Skin Aging and Senescence. Trends Mol. Med. 2021, 28, 97–109. [Google Scholar] [CrossRef]
- Alonso-Bartolomé, R.; Rodríguez, D.P.; Rodríguez-Jiménez, P.; Ruiz-Rodríguez, R. Method R: Efficacy of a Cosmetic Routine Comprising Six Topical Lipid-Encapsulated Products. J. Cosmet. Dermatol. 2022, 21, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.K.; Khan, B.A.; Akhtar, N.; Chowdhry, F.; Khan, H.; Bakhsh, S.; Khan, S.; Rasul, A. Fabrication of Tamarindus Indica Seeds Extract Loaded-Cream for Photo-Aged Skin: Visioscan® Studies. Postepy Dermatol. Alergol. 2017, 34, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-Aging and Brightening Effects of a Topical Treatment Containing Vitamin C, Vitamin E, and Raspberry Leaf Cell Culture Extract: A Split-Face, Randomized Controlled Trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef] [PubMed]
| Sociodemographic Characteristics | Participants (n = 44) |
|---|---|
| Age (years) | 38.8 ± 15.0 |
| Sex | |
| Female | 29 (65.9%) |
| Male | 15 (39.1%) |
| Weight (kg) | 67.5 ± 12.9 |
| Height (cm) | 169.1 ± 10.0 |
| Phototype | |
| II | 10 (22.7%) |
| III | 27 (61.4%) |
| IV | 7 (15.9%) |
| Marital status | |
| Single | 25 (56.8%) |
| Married | 16 (36.4%) |
| Divorced | 2 (4.5%) |
| Widowed | 1 (2.3%) |
| Level of education | |
| Basic | 9 (20.5%) |
| Higher | 35 (79.5%) |
| Occupation | |
| Physician | 10 (22.7) |
| Student | 11 (25.0) |
| Administrative | 6 (13.6) |
| Miscellaneous | 17 (38.7) |
| Smoking habit (yes) | 14 (31.8%) |
| Cigarettes/day | 2.1 ± 4.5 |
| Alcohol use (yes) | 22 (50.0%) |
| Units/week | 0.9 ± 1.2 |
| Moisturizer use (>3 days/week) | |
| Body | 28 (63.6%) |
| Face | 33 (75.0%) |
| Sun exposure (hours/week) | 5 ± 4.6 |
| Sunscreen use | |
| Never | 3 (6.8%) |
| Sometimes | 9 (20.5%) |
| Always | 32 (72.7%) |
| Parameter | Forearm | Cheek | Palm | p * | p ** | p *** |
|---|---|---|---|---|---|---|
| Smoothness (Sesm) | 240.02 ± 74.67 | 348.16 ± 119.99 | 408.19 ± 152.33 | <0.001 | <0.001 | 0.050 |
| Roughness (Ser) | 2.45 ± 1.72 | 3.64 ± 2.24 | 3.08 ± 1.48 | 0.005 | 0.051 | 0.188 |
| Desquamation (Sesc) | 0.37 ± 0.82 | 0.70 ± 1.98 | 0.09 ± 0.19 | 0.128 | 0.022 | 0.002 |
| Wrinkles (Sew) | 78.61 ± 23.33 | 116.32 ± 44.04 | 271.96 ± 138.55 | <0.001 | <0.001 | <0.001 |
| Surface area (%) | 620.62 ± 77.51 | 666.69 ± 123.18 | 477.05 ± 79.13 | 0.025 | <0.001 | <0.001 |
| Volume (mm3) | 90.88 ± 15.49 | 106.16 ± 12.83 | 83.64 ± 21.2 | <0.001 | 0.041 | <0.001 |
| Contrast (AU) | 1.09 ± 0.31 | 1.23 ± 0.43 | 0.67 ± 0.25 | 0.088 | <0.001 | <0.001 |
| Variance (AU) | * 4.29 ± 0.75 | 4.60 ± 1.01 | 2.85 ± 0.70 | 0.064 | <0.001 | <0.001 |
| Homogeneity (AU) | 1.40 ± 0.06 | 1.38 ± 0.07 | 1.52 ± 0.06 | 0.155 | <0.001 | <0.001 |
| Total number of cells (AU) | 120.48 ± 29.75 | 66.36 ± 21.17 | 85.07 ± 26.04 | <0.001 | <0.001 | <0.001 |
| Hardness (AU) | 9.44 ± 3.98 | 7.94 ± 3.14 | 13.22 ± 4.79 | 0.038 | <0.001 | <0.001 |
| Friction (AU) | 172.21 ± 91.80 | 114.23 ± 75.79 | 269.95 ± 248.04 | 0.002 | 0.002 | <0.001 |
| Brightness (AU) | 8.36 ± 8.34 | 7.21 ± 1.3 | 7.50 ± 1.37 | 0.377 | 0.514 | 0.274 |
| Deformability (mm) | 1.54 ± 0.35 | 1.89 ± 0.25 | 1.28 ± 0.26 | <0.001 | <0.001 | <0.001 |
| Flaking index (%) | 31.93 ± 8.2 | 26.54 ± 11.86 | 11.33 ± 7.1 | 0.019 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria-de la Torre, R.; Ceres-Muñoz, M.; Pretel-Lara, C.; Montero-Vílchez, T.; Arias-Santiago, S. Microtopography and Barrier Function in Healthy Skin: Differences between Forearm, Cheek and Palm. Cosmetics 2024, 11, 5. https://doi.org/10.3390/cosmetics11010005
Sanabria-de la Torre R, Ceres-Muñoz M, Pretel-Lara C, Montero-Vílchez T, Arias-Santiago S. Microtopography and Barrier Function in Healthy Skin: Differences between Forearm, Cheek and Palm. Cosmetics. 2024; 11(1):5. https://doi.org/10.3390/cosmetics11010005
Chicago/Turabian StyleSanabria-de la Torre, Raquel, María Ceres-Muñoz, Carlota Pretel-Lara, Trinidad Montero-Vílchez, and Salvador Arias-Santiago. 2024. "Microtopography and Barrier Function in Healthy Skin: Differences between Forearm, Cheek and Palm" Cosmetics 11, no. 1: 5. https://doi.org/10.3390/cosmetics11010005
APA StyleSanabria-de la Torre, R., Ceres-Muñoz, M., Pretel-Lara, C., Montero-Vílchez, T., & Arias-Santiago, S. (2024). Microtopography and Barrier Function in Healthy Skin: Differences between Forearm, Cheek and Palm. Cosmetics, 11(1), 5. https://doi.org/10.3390/cosmetics11010005










