Abstract
This paper aims to establish a current relationship between pharmaceutical sciences andthe development of perfumes and fragrances, which bring significant economic benefits. For this purpose, historical data are used as a starting point, and galenic, chemical and botanical aspects are discussed in a transversal way. Sources such as Web of Science (WOS) and databases such as Scopus, monographs and various web pages (where scientific–technical documents appear) were used. The results and discussion are based on the selection of the 50 plant species most commonly used in high-quality fragrances. Therefore, this publication should be considered an approach to this subject based on an analysis of a representative sample of data. Some characteristics of perfumes (classification according to the concentration of essential oils and combination of plant extracts) are presented beforehand. The main focus of this work is the botanical and chemical analysis of these described plants, pointing out their common name, correct botanical name, geographical place of origin, used part of the plant and main molecules. The most significant families are aromatic: Rutaceae, Lamiaceae (16.7%) and Apiaceae. The most represented genus is Citrus (with seven species or hybrids). However, it should be noted that natural extracts of good quality natural fragrances may be supplemented with chemically synthesized molecules. Of the 50 botanical species selected, 84% of the extracts have their origin in Tropical Asia, the Middle East and the Mediterranean region. This figure generally coincides with the percentage of medicinal plants (or their extracts) admitted by the European Pharmacopoeia. All parts of a plant (depending on which one it is) can be a source of molecules for the elaboration of these products. The most commonly used parts to obtain extracts are flowers and leaves, a biological circumstance that is explained in this work. In this work, 110 molecules have been found that are part of the essences of perfumes and fragrances; the most frequent are linalool, limonene, 1,8-cineole, eugenol and derivatives, geraniol, vanillin and derivatives, β-caryophyllene, p-cymene, and farnesene and derivatives. However, in order to elaborate a quality perfume, many other molecules must be taken into account, according to the creative experience of the perfumer, which is subject to confidentiality, and chemical analysis according to current legislation, which would avoid fraud, allergy and dermatitis problems.
1. Introduction
Human beings have considered it necessary to improve the appearance and visible properties of the body in order to increase their self-esteem and to be successful in love, war or social life. Hence, they have put their interest in the elaboration of cosmetics and fragrances. Different civilizations have used and continue to use plants for this purpose [1].
Smell is an essential tool for animals, as it is used to link chemical signals or substances of different origins with emotions and memories. It is understandable to think of perfumery as the art of composing fragrances using odorous substances from nature [2]. Following the Helicon considerations [3], the term perfume comes from the alliance of two Latin words: per, “by”, and fumare, “through smoke”, since it was originally considered an odoriferous vapor. Further to this argument, the (IFRA) defines the concept of fragrance as a combination of chemical substances that possess aroma or odor, but it is much more; it encompasses cultural, historical, social, economic and emotional values. We must thus understand that fragrance is the very “soul” of perfume [4].
Economic reports from different countries show that the economic impact generated by the personal external care of the organism with the use of cosmetics and fragrances translates into millions of euros [5]. In addition, people are interested in the characteristics of the products they buy [1].
It is essential to highlight in the following pages the evolution of the use of perfume in different periods and civilizations. Perfume formulas have changed according to their uses and composition, requiring a high level of knowledge of botany, chemistry and pharmaceutical technology, as these sciences have been developed with new analytical and technical instruments.
If we go back in history, at the dawn of mankind, there was a certain interest in the use of aromatic substances for hunting, to repel insects and even in religious rites in the form of incense (Boswellia sacra Floeck.) [6].
The Sumerians began to manufacture the first perfumes for personal, medicinal and ceremonial use. The first chemical record of the history manufactured fragrances with flowers, oils, myrrh and balsams by boiling, enfloration, sublimation, filtration and pseudo-distillation can be attributed to Tapputi-Belatekallim (4th century BC) [7].
The importance of perfume reached high levels during Ancient Egypt, and it was considered a significant status symbol. At this time, techniques such as maceration in animal fats and the pressing of aromatic plants were developed. Thus, fragrances based on rose (Rosa L.), violet (Viola odorata L.) and saffron (Crocus sativus L.) were elaborated.
Greece and Rome borrowed from Egypt the numerous uses of perfumes, which had a social stigma according to the quality and quantity used. It was common to use fragrances for personal hygiene, insect repellent, worship ceremonies and festivities. They did not skimp on containers, which were made of glass, alabaster or ivory. Authors such as Theophrastus, Pliny and Dioscorides wrote protocols for the manufacture of perfumes and descriptions of romatis scents.
Theophrastus refers to ways of coloring perfumes and ointments. The dye used for red perfumes was alkannet from the plant Anchusa officinalis L. (borage), while the sweet perfume of marjoram (Origanum majorana L.) was painted with a dye called “chroma (=colour)”, a root imported from Syria. According to Theophrastus, the quantity of raw materials combined was directly relevant to the quality, complexity and distinctiveness of the produced flavor. In mixed perfumes, the targeted scent came as a result of all the ingredients together rather than having a dominant one [8].
In Christianity, the production of fragrances fell to apothecaries and monasteries for therapeutic use [9]. In Islamic times, there are records of the use of perfume by Arabs since the 6th century, and its use was considered a religious duty according to the words of the prophet Mohammed. Its use was also popular for its medicinal properties, according to Hippocrates’ theory of humor. Physicians such as Avicenna used them for therapeutic purposes as a precursor of present aromatherapy. It also included techniques such as filtration and distillation and even highlighted the side effects of essential oils. Fragrances, made up at that time of aromatic waters, oils and ointments, were manufactured on a small scale in small laboratories and apothecaries. Plants such as jasmine (Jasminum officinale L.), clove (Syzygium aromaticum (L.) Merr & L. M. Perry)) camphor (Cinnamomum camphora (L.) J. Presl), narcissus (Narcissus L.), wallflower (Erysimum cheiri (L.) Crantz) and roses (Rosa L.) were used [10].
In the 14th century, Queen Elizabeth Piast of Hungary popularized the Queen’s Water or Eau de Hungary, a fragrance with supposed rejuvenating and curative properties based on alcohol, rosemary water (Rosmarinus officinalis L.), rose water, orange blossom, cedar (Cedrala odorata L.) and turpentine [1,11]. Also, as a consequence of plague epidemics and the idea that aromas avoid diseases, the custom of perfuming clothes and accessories appeared, causing a furor among the wealthy classes [12,13].
During the 17th and 18th centuries, the manufacture of perfumes was in the hands of Italy and France. In these years, the oldest “perfume” in the world reappeared, the aforementioned eau de Cologne or Eau de Toilette. At the court of Versailles (France), eau de cologne was a great success, originally prepared by Florentine nuns as Aqua de Regina—bergamot extracts—its formula was acquired by an Italian apothecary who manufactured the fragrance in Cologne in 1729. A breakthrough at the time was Aqua Mirabilis—by Gian Paolo Feminis—a citrus perfume with alcohol, bergamot (Citrus × bergamia Risso & Point.), lemon (Citrus x limon (L.) Osbeck) and orange (Citrus aurantium L.). His grandson, Giovanni Maria Farina, created the popular perfume Aqua de Cologne from essential oils and alcohol. At this time, laws appeared that regulated fragrances, differentiating them from medicines and making eau de cologne undrinkable [14].
The modern age coincided with the worldwide expansion of the perfume industry, making Grasse, in southeastern France, the perfume capital from the Renaissance to the mid-19th century. Advances in organic chemistry found their way into the world of perfumery, including synthetic molecules in perfumes at the hands of the Guerlain and Houbigant houses. The latter developed Fougère Royale in 1884, giving rise to the Fougère family of perfumes, which simulates the supposed scent of a fern [15]. In these years, pharmacists were the true experts in the elaboration of fragrances; since they were the experts in plant extracts for medicinal use, they possessed the necessary instruments, mainly distillers. It should be noted at this point that the current giant cosmetic companies were founded at the beginning of the 20th century by chemists and pharmacists in the United States of America and France. A well-known and current cosmetics company had its origin in the house-to-house sale of books, and along with the books, it also sold colognes manufactured by a pharmacist friend [16]. In the Spanish Pharmacopoeia of 1954, there are still 22 monographs for obtaining essences “Essentiae” and 6 monographs for obtaining waters, such as Aqua destillata floris auriantii (with freshly harvested petals of Citrus auriantium). All of them use steam distillation [17].
In 1921, Chanel nº 5 was born, a perfume that included five types of aldehydes: jasmine, orange blossom, rose, sandalwood (Santalum alum L.) and vetiver (Crysopogon zizanioides (L.) Roberty) that bring a unique freshness that has survived to this day. The second half of the 20th century would be marked by the use of patchouli (Pogostemon cablin Benth), fresh waters and masculine perfumes in men’s daily routine [11]. In recent years, the production of fragrances prioritized quantity and marketing over quality, investing especially in advertising by famous faces who founded their own lines [18].
With these considerations in mind, it seems interesting to recall the World Health Organization’s definition of health, which may in part be related to perfumes and fragrances: health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity. Proper care of the external appearance of the body contributes to this complete state of well-being. Therefore, there is a growing demand for information on this subject [1]. One of the main objectives of this publication is to provide the chemist–pharmacist, and other professionals working in the field of the manufacture of perfumes, fragrances and cosmetics in general, with a global vision that takes into account various aspects of the issue. This information is traditionally known, but it is scattered and very fragmented in publications from different areas of knowledge. For these reasons, we have carried out this transversal, integrative and synthetic work in which we describe some aspects of these odorous products, such as the classification of different types of fragrances according to the time that their effects last in the olfactory perception; mixing of different plant extracts to obtain different types of fragrances; traditional and alternative methods of obtaining essential oils; botanical considerations on the plant extracts used; and other phytochemical, allergenic and toxicological observations described in the literature or in the European Pharmacopoeia.
2. Methodology
A search for printed bibliographic material was carried out at the CRAI (Resource Center for Learning and Research) of the University of Seville, where books were found (with difficult access) on the history of perfume, as well as its cultural and social relevance in different civilizations around the world. The Web of Science and the Scopus database were consulted on the Internet. When the keyword “Frargance” was entered into the search engine, the number of documents found was very high, so the search had to be narrowed down with the term review. The initial results were 12,114 documents vs. 88 (WOS) and 33,984 vs. 3548 (Scopus). Given this number of documents, the most recent articles were analyzed. When this prospection was carried out, it could be seen that most of the documents were monographs of specific molecules on perfumes and fragrances dealing with chemical synthesis, chemical analysis, toxicity and allergenic character; hence, only the documents necessary to meet our objective were taken into account.
In this case, the criterion chosen was to gather a representative sample of 50 plants relevant to the production of perfumes and fragrances, following the information provided on their websites by different commercial companies that produce these products, such as Calvin Klein, Gucci, Chanel, Christian Dior and Guerlain [19,20]. However, these websites may change and may not last over time.
The scientific names of the species and plant families were reviewed in the WFO Theplantlist [21], eFloras [22] and Mobot [23]. Each species also details its geographical origin, the part used in perfumery and the main composition of its essential oil supported by a bibliography, such as Fitoterapia.net [24] (a professional page where complete monographs of plants with therapeutic action appear, related to the Spanish Pharmacopoeia and Theplantlist).
3. Results and Discussion
3.1. Some Considerations on the Classification of Perfumes and Fragrances
The classification of perfume and fragrances is not homogeneous, and there are no globally agreed categories for these products. To separate the characteristics of these products, it is necessary to resort to the concentration of essential oils in each formulation [25] and to the type of olfactory notes they offer [26,27]. Fragrances consist of a mixture of essential oils or other volatile aromatic compounds (often synthetic), solvents and “fixatives” (substances used to improve stability and reduce slow evaporation). Typical solvents are ethanol or a mixture of ethanol and water [28]. The denomination of the type of perfume or derivatives can be established according to the amount of pure fragrance concentration (although there are no exact amounts) and the time in which the formulation is perpetuated in the body odor. According to the above, this type of product would be divided into four classes: fresh water (approximate essence concentration of 7%), toilet water (approximate essence concentration of 12–15%), perfume water (approximate essence concentration of 17–20%), fragrance (approximate essence concentration of 30–40%) (Figure 1). It is for this simple reason that it is not the same to apply a parfum or a perfume water, in which evolution and fixation over time are usually very long-lasting (one application would be enough for the whole day), as it is to apply other types of lighter compositions such as toilet water or fresh water (Figure 1).
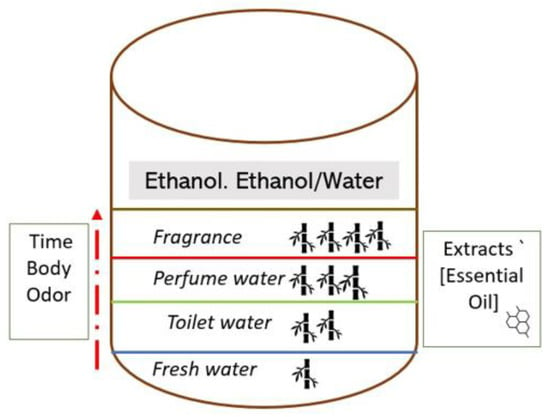
Figure 1.
Types of fragrances according to their concentration of essence and duration of effect. Own elaboration based on reference [25].
On the other hand, some perfumes offer different versions of the same scent. The difference between them lies not only in the percentages of pure essence but, in some cases, also in a variation in the notes that enhance, for example, a fresh character or their intensity and duration. It is therefore necessary to separate their composition into layers or hierarchical notes in the well-known olfactory pyramid (Figure 2), in which we distinguish high, medium and low notes, which are described below:
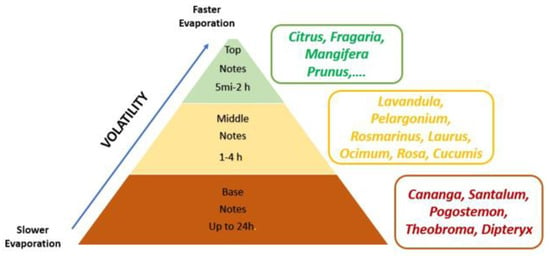
Figure 2.
Olfactory pyramid. Own elaboration from citations [25,26].
Top notes. These are the most volatile notes with the lowest molecular weight. As a consequence, they are the first to be detected. They are usually made up of stimulating and energizing aromas, giving freshness to the formula. However, since they are so volatile, they disappear within 5 to 20 min after the application of the perfume (they can last up to two hours). Citrus fruits (lemon, grapefruit and lime essential oils) generally fall into this category as they have light and lively aromas. Fruit notes such as strawberry (Fragaria L.), mango (Mangifera indica L.), cherry (Prunus avium L.) or peach ((Prunus persica (L.) Stokes), etc., are also included.
Middle or heart notes. They form the body of the perfume. They are detected after the evaporation of the top notes, changing the initially perceived scent of the product. Flowers and herbs make up this category because of their strong and powerful but comforting scent. They are more muted compared to the top notes but last for 15 to 60 min on the skin (up to 4 h). Among other plants used for these notes are lavender (Lavandula angustifolia Mill.), geranium (Pelargonium graveolens L’Her), rosemary (Rosmarinus officinalis L.), laurel (Laurus nobilis L.), basil (Ocimum basilicum L.), rose (Rosa sp.) and cucumber (Cucumis sativus L.).
Base notes. They are rich, soporific and aphrodisiac. When they emerge from the perfume, they emerge strongly, and it is difficult not to notice their presence. They usually take an hour to appear and last up to a day due to their higher molecular weight. The components are usually extracted from sap, roots, fruits or wood. Some notes in this category are: cananga or ylang-ylang (Cananga odorata (Lam.) Hook. f. & Thomson), sandalwood (Santalum album), patchouli (Pogostemon cablin Benth.), vanilla (Vanilla planifolia Andrews), chocolate (Theobroma cacao L.), tonka bean (Dipteryx odorata (Aubl.) Orsyth f.), etc.
3.2. Methods of Obtaining Essences
As shown below, the list of raw materials of plant origin is very broad, and it is complex to establish a classification of perfumes according to their plant composition since extracts can often be combined with each other to obtain different characteristics and notes [29]. This complexity is illustrated in Figure 3 (inspired by Euler diagrams), in which an entire architecture of floral notes and fragrances is visualized from circles.
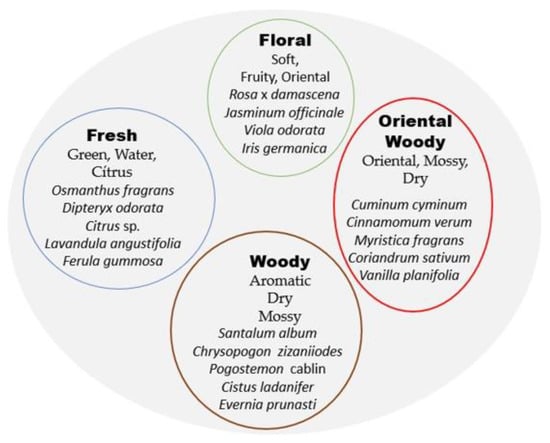
Figure 3.
Denomination of perfumes and fragrances according to the combination of notes provided by the different plant extracts. Own elaboration based on citation [29].
Figure 3 is a simplification that is taken into account to produce a whole catalog of plant-based fragrances, starting from four basic floral notes (floral, fresh, woody and oriental), which in turn are subdivided into smaller categories. This figure, as an example, shows some of the plant associations that are scientifically responsible for the properties of the fragrance wheel used to demonstrate the complexity of perfume and derivatives and perhaps for marketing purposes.
As mentioned in the introduction, the use of fragrances is increasing demand, and this has forced synthetic organic chemistry to obtain molecules similar to those found in nature without resorting to plants. This is the case of the best-known synthetic fragrance in history: Iso E Super® [30]. However, in the field of cosmetics in general (including fragrances), people prefer products of natural origin because they are considered safer and more effective [1]. For the industries that produce quality perfumes, natural extracts, namely essential oils, cretins, resinoids and absolutes, are produced from mainly plant-derived raw materials. The most commonly used methods are summarised below [26,30,31] (Figure 4):
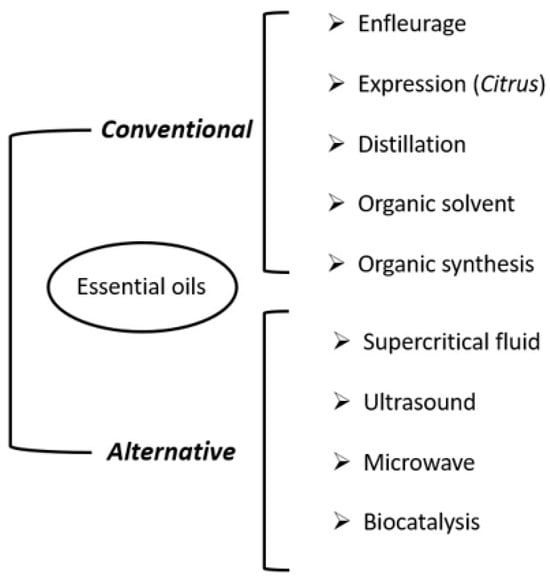
Figure 4.
Summary of methods for obtaining essences. Own elaboration.
Enfleurage. The flowers are put in contact with refined and odorless animal fats for several days to extract the essences until an ointment is obtained from which the absolute is extracted (the most concentrated fragrance and most appreciated in perfumery). This method has been used to extract essential oils from jasmine (Jasminum officinale L.) and rose (Rosa L.) flowers [32].
Expression. A mechanical method is usually used on citrus fruits, the oils of which are found in the pericarp (mesocarp) of the fruit. Water and oil are separated by centrifugation [33].
Steam distillation. Distillation is the most frequent method used in the industry to extract essences, whether simple, fractionated or by steam distillation, depending on the boiling points and volatility of the products to be separated. In the collecting flask, aqueous and oily faces are easy to separate. The “perfumed” waters can be used to make rose water or orange water. Through steam distillation, basil oils (Ocimum basilicum L.) are obtained, an ancient plant cultivated in India for its medicinal and aromatic properties [34].
Solvent extraction. Large quantities of plants macerated in hexane or acetates are used. It is based on the different solubility of the mixture in the solvent, and the soluble part is obtained in the organic solvent (concrete); it is not very soluble in alcohol. In this sense, absolutes are much nobler, richer and easier to use in perfumery. An example of the use of this method is the extraction of essential oils from the tobacco (Nicotiana tabacum L.) leaf [35].
Tinctures and resinoids. These are the result of macerating raw materials with alcohol. They are then heated, and tinctures are obtained, which are similar to absolutes. This method can be applied to the extraction of essential oils from numerous plants: (Labdanum—Cistus ladanifer L.; Tolu Balsam—Myroxylon toluiferum Harms; Tonka—Dipteryx odorata (Aubl.) Forsyth f.; Myrrh—Commiphora myrrha T. (Nees) Engl, etc. [36].
On the other hand, if we analyze alternative methods for obtaining these products, we must take into account the concept of so-called “green chemistry”. New techniques are currently being developed for the extraction of essential oils. These new alternative methods aim at a more efficient extraction. We can mention the use of supercritical fluids, microwave-assisted extraction and ultrasound. They are recognized as efficient extraction methods and can significantly reduce the amount of solvents and extraction times and improve yields and the quality of the essential oil. However, these methods are predominantly exploited at the laboratory scale [37,38]. Another alternative to produce some fragrance base molecules is the use of biocatalysis in which, from plant cell cultures, immobilized enzymes or metabolic products of some microorganisms (bacteria, yeasts and other fungi) [39]. For example, several strains of Pseudomonas putida, Aspergillus niger, Corynebacterium sp., Arthrobacter globiformis and Serratia sp. produce vanillin as an intermediate compound in the microbial degradation of various substrates such as ferulic acid, stilbenes, phenolic acids, lignin, eugenol and isoeugenol [40].
3.3. Botanical Considerations on Perfumes and Fragrances
As can be deduced from what has been developed up to this point, “Botanical Science” is an inexcusable factor to elaborate or at least fully understand the significance of products with a good quality odor, whether of the body or of the rest of the things to suppress unpleasant aromas. This desideratum is within the reach of a pharmaceutical botanist. For this reason, we dedicate this epigraph to make botanical considerations on this subject, in which we take into account, as already mentioned, a reasonable and representative quantity of plants (50), which are presented in alphabetical order (Table 1) by their common name in English, followed by their correct and complete scientific name, the first geographical origin, botanical family according to the current systematics and part or organ used. This table also shows the main components of essential oils found in plant extracts, taking into account that the number of phytomolecules may be higher depending on the extract. This is a consequence of the application of techniques such as chromatography and spectrometry (high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry (GC-MS), Fourier-transform infrared spectroscopy (FTIR)) and the use of plant extracts in in vitro and in vivo tests [1].

Table 1.
Botanical characteristics of plants [21,22,23,24] and their essential oils as a source of extracts and fragrances.
This table provides a wealth of information on botany in perfume and other phytochemical considerations (which is discussed in the following Section 3.3). From a botanical point of view, we believe that a professional or specialist in the elaboration of perfumes and fragrances should know in depth all the possible aspects of the plants that are the main raw material for the elaboration of these products. It should be noted first of all that the data presented here come, as already indicated, from the choice of the 50 most frequent plants, although this list could be more extensive. For example, minority species that are also used in the formulation of these products have not been considered according to the search criteria: jojoba seed oil (Simmondsia chinensis, (Link) C. K. Schneid) sunflower seed oil (Helianthus annuus L.), sampaguita leaf extract (Jasminum sambac (L.) Aiton), red camellia flower extract (Camellia japonica L.), tobacco leaf extract (Nicotiana tabacum L.), etc. On the other hand, 27 families of Angiosperms (less than 10% of the total number of families) [23], one species of lichen (Evernia prunasti L.) and no species of “gymnosperms” were found. As mentioned above, the Latin scientific name does not appear correctly on the composition label (this also happens in many cases of medicines whose composition is based on medicinal plants, according to our own experience). This could be a minor detail, but the buyer, who has more and more access to the Internet, can research the product they are buying (especially expensive perfumes) and become suspicious simply because of this fact. We are, therefore, very scrupulous in Table 1 to indicate the correct and modern names of the botanical species.
Figure 5 shows that this sample of plants (which at first can be considered “reduced”) is present in almost all the current large groups in which plants are classified from the point of view of molecular genetics in the form of a phylogenetic tree. In the first place, the group of primitive angiosperms appears, with families of Asian origin, rich in spices and whose interest led to the discovery of the American continent and later the first round-the-world voyage by sea [68]. Figure 5 also shows (in green) that only three botanical families are the source of almost 40% of plant extracts in the manufacture of perfumes: Rutaceae and Lamiaceae (16.7% each) and Apiaceae (6%). These figures are not correlated with the quantitative importance according to the number of species that these families have in the angiosperms as a whole (Asteraceae, Orchidaceae, Fabaceae, etc.) [69]. It is also important to note that 58% of the plants named here were studied by Linnaeus. As is well known in the scientific world, Linnaeus was a physician and naturalist who was dedicated to naming and describing many of the plants known since classical-Graeco-Roman antiquity (and other new ones) to which he had access, starting in many cases from descriptions that previous botanists had made with Arabic translations of plants already described by Dioscorides (1st century BC) [68]. It must be taken into account that for hundreds of years, the plants that were studied were for their economic, medicinal and agricultural importance. This fact leads us to think that, in some way, the different beneficial properties of different plants that appear in this work from different points of view are supported by the empirical weight of history.
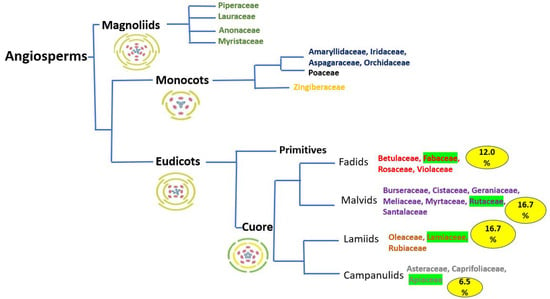
Figure 5.
Botanical systematics in the form of a phylogenetic tree of the families that form part of the fragrances, according to this work. Floral diagrams. Own elaboration from (APGIV) [23].
Figure 5 below shows that the plants most commonly used in perfumes and fragrances are at the lower end of the phylogenetic tree (core angiosperms), characterized by smaller flowers that tend to have calyx and corolla with sepals and petals welded together, which trap insects once they are pollinating the flower. This consideration is not set in stone and may change in the coming years as a consequence of the growing scientific interest in many countries in the study of the various properties of plants (many of them based on traditional and local uses).
If we analyze the initial geographic origins of these plants (although many of these plants can be cultivated far from their initial cradle) (Figure 6), we observe an asymmetry in their origin since a large majority are native to central and tropical Asia and the region where the Mediterranean Sea is located (84%). This figure almost coincides with the origin of the plants or their medicinal derivatives that appear in the Spanish Pharmacopoeia (the same as the European one) (82%) [70]. It could be deduced, then, that the human being, while searching for medicinal plants, has also found plants producing essences to perfume himself, not in vain; many of the molecules responsible for the aromas also have therapeutic action (1,8-cineole, hesperidin, zingiberene, etc.). This is related to the concept of cosmeceuticals. Although perfumes and fragrances are not intended for the treatment of skin problems, some of them can indirectly have some beneficial effects on the skin. However, it should not be forgotten that the mere fact of offering a good body odor can improve some people’s self-esteem and improve their emotional state [1]. As mentioned above, the data shown in Figure 6 reflect the current situation according to the plants collected in this study and may, therefore, change over time.
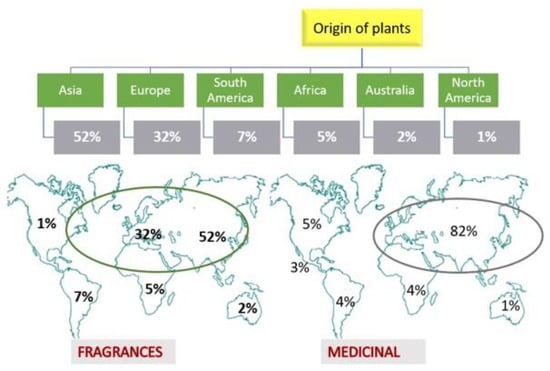
Figure 6.
Regional origin of the plants used in perfumes and fragrances according to this work and origin of the medicinal plants that appear in the Spanish Pharmacopoeia V edition [70]. Own elaboration. The circles indicate the approximate area of distribution of the plants.
Continuing with the analysis of Table 1, so far, we have discussed the botanical taxonomy, nomenclature and geographical origin of the plants. To conclude this section, we comment on other aspects related to the quality control of the extracts, such as the part of the plant used and the microscopic visualization of the pulverized extracts. Figure 7 shows the number of times the plant parts considered in this study appear. It can be observed that all parts (depending on the plant) can be sources of essences, although the use of the flower (34 times) and the leaf (30 times) predominates. On the opposite side are resins (e.g., Tolu balsam) (10 times), stems (e.g., Santalum album), rhizomes (e.g., Zingiber officinale) and roots (e.g., Chrysopogon zizanioides) (8 times). These facts may have multiple biological meanings; plants are immobile organisms and are subject to aggression by herbivores and phytophagous or climatic variations throughout their vegetative development. Hence, they produce secondary metabolites as a form of protection, but in the case of pollination, the odor of flowers (pleasant or unpleasant, day or night) is related to the attraction of the type of pollinating agent—usually insects [71].
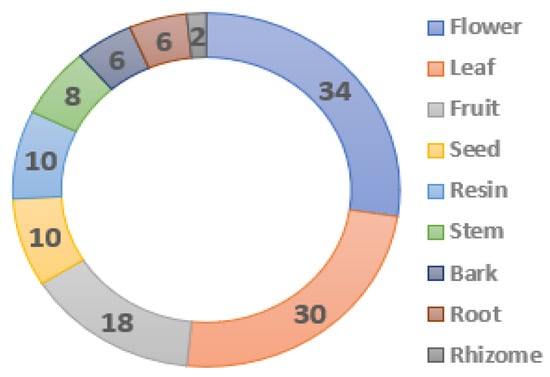
Figure 7.
Part of the plant used to obtain extracts. Own elaboration.
A high percentage of the plants considered here come from warm climates with high seasonal temperatures. The Lamiaceae (Rosmarinus, Lavandula, Thymus, Mentha, etc.) are mainly Mediterranean plants, very aromatic and rich in essences. These essences evaporate at high temperatures and help to cool the plant [60]. The same consideration can be made for the Apiaceae, which have resiniferous canals throughout the plant, especially in the fruit [72]. But this explanation is different in the case of pollination, the odor of flowers (pleasant or unpleasant, day or night). The case of the Rutaceae family may be somewhat different. While the flowers of the genus Citrus are very fragrant, essential oils are very abundant in the mesocarp of the fruit, especially rich lysigenous cavities. The citrus family is native to China and has spread to temperate areas of southern Africa, Australia, the Mediterranean region and other warm regions of the world, forming what is called the “Citrus Belt” [73]. It is possible that its preponderance in the composition of fragrances is the easy obtaining of its components (limonene, myrcene, geraniol, linalool, citronellol) by the simple method of expression of the fruit. Figure 8 shows, as an example, some macroscopic and microscopic images of three plant genera or species that form an essential part in the form of natural extracts for the preparation of these products: Rosmarinus officinalis (Lamiaceae), Pimpinella anisum (Apiaceae) and Citrus (Rutaceae). This figure is complementary to the optical microscope photographs available in the following bibliographical references: Rosmarinus [74], Pimpinella [75] and Citrus [76].
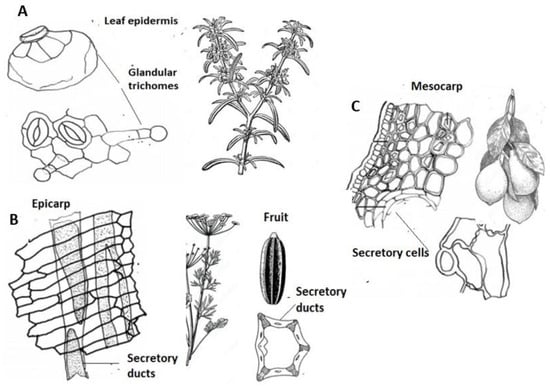
Figure 8.
Microscopic images of secretory cells: (A) Glandular trichomes of the leaf of Rosmarinus officinalis (Lamiaceae). (B) Secretory ducts in the pericarp (epicarp) of Pimpinella anisum (Apiaceae). (C) Secretory cells of the pericarp (mesocarp) of the genus Citrus (Rutaceae). Own elaboration based on Spanish Pharmacopoeia (European] [70].
3.4. Chemical Considerations on Extracts in Perfumes and Fragrances
Currently, more than 3000 chemicals, either natural fragrances or synthetic chemicals, are responsible for the odorous properties of scented products, while a perfume can be composed of a mixture of 20 to more than 200 of them [27].
In this work, 110 molecules were found to be part of perfume essences and fragrances. Table 1 shows the most frequent ones: linalool (13.8%), (α and β pinene—12.74%), limonene (12.74%), 1,8-cineole (10.92%), eugenol and derivatives (9.1%), geraniol (7.28%), vanilla and derivatives (7.28%), β-caryophyllene (4.55%), p-cymene (4.55%), and farnesene and derivatives (3.64%). In general, terpenoids predominate, with an overall presence of more than 80% in the molecules shown in Table 1. The concept of terpenoid refers to a very numerous set of molecules of plant origin whose precursor is isopentenyl diphosphate and are assembled and modified in many different ways, always based on the isopentane skeleton. Most terpenoids have multicyclic structures, which differ from each other not only in functional groups but also in their basic carbon skeleton [77]. These functional groups of volatile terpenoids (alcohols, ketones, aldehydes, esters) are responsible for the different odors of extracts to elaborate fragrances. In these extracts in Table 1, we can also find other non-terpenoid compounds such as eugenol, hesperidin, vanillin, etc. (Figure 9).
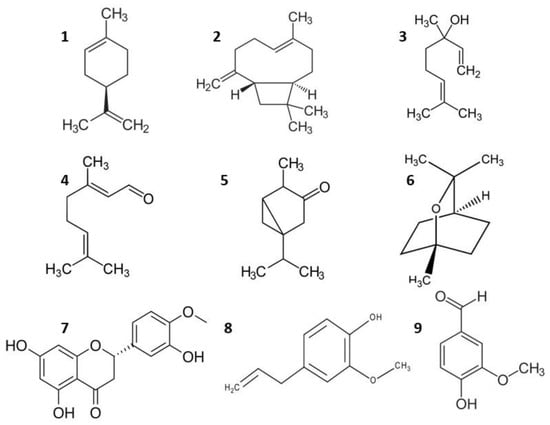
Figure 9.
Some molecular structures found in essential oils: 1: Limonene. 2: β-caryophyllene. 3: Linalool. 4: Citral (Geranial). 5: Thujone. 6: 1,8-cineole. 7: Hesperidin. 8: Eugenol. 9: Vanillin.
Table 1 does not indicate the concentrations of these molecules; since the percentages of essence vary within the same species, there are varieties that require optimal conditions of climate, soil, adequate cultivation and collection at the time of the year in which the essential oils are of the highest quality. In the case of citrus fruits, it is worth mentioning the results of Bourgou’s research [42], in which the proportion of essential oil molecules in the pericarp of four Citrus species (Citrus auriantium, C. limon, C. sinensis and C. reticulata) ranges from 0.46 to 2.70% depending on the ripening stage of the fruit. In this regard, we can add the following example for the analytical control, according to the European Pharmacopoeia [70] of the flower of Citrus auriantium. It must contain at least 8.0% total flavonoids, expressed as naringin (in the dried drug and in the unopened flower). This text sets out the botanical characteristics of the plant and the elements of the powder for microscopic analysis. It provides data for performing thin-layer chromatography and the steps for spectroscopic titration, where absorbance at 530 nm is measured to calculate the percentage of flavonoids.
In recent years, both official institutions and the industry have become aware of the problem of fragrance allergy, and there have been important changes in legislation. Until a few years ago, manufacturers were not obliged to specify the chemical compounds considered as fragrances contained in the product, and now they are committed to declaring them in some cases under a confidentiality contract since their composition is subject to analysis by chromatography, spectroscopy, mass spectrometry and electronic nose methods [77,78]. Previously, people allergic to fragrances had to avoid any hygiene and household products containing fragrances in their composition, which was a significant limitation considering the wide distribution of these substances. The current European regulation requires the mandatory declaration of 26 fragrances recognized as contact allergens on cosmetic product labels since 2005 (geraniol, Evernia prunasti L., eugenol, cumarine, farnesol, linalool, limonene, etc.). These fragrances should be indicated on the label if present in a concentration ≥10 ppm [78]. Perfume or similar is regulated by European legislation within the cosmetics section and is controlled by the Spanish Agency of Medicines and Health Products, in which incidence is made on labeling the toxic or allergenic nature of some of the components [79]. Like the rest of cosmetics, perfumes and their components require rigorous quality controls to ensure their safety since their natural origin is not synonymous with safety. An example is bergamot essential oil, which, in the presence of sunlight, can cause Berloque dermatitis, characterized by the presence of erythema and brown pigmentation in the exposed area. In addition to phototoxicity, as in the previous case, essential oils can produce photosensitization, irritation, or short- and long-term toxicity by accumulation, either by systemic or inhaled routes [78]. Finally, we would like to emphasize that in the elaboration of quality fragrances, it would not be correct to mix certain molecules (which can be obtained by chemical synthesis to obtain a fragrant product). The most advisable is to use combinations of extracts, in which the majority of molecules are accompanied by others in lower concentration but can give the fragrance a special characteristic, which is why the experience of the perfumer is very important, who must apply the Latin phrase Fiat Secundum artem or let it be performed according to art (empirical and personal knowledge, often secret).
4. Conclusions
The current society increasingly uses cosmetics and fragrances to contribute to a better opinion of itself. This has been a constant in history and in different civilizations. People have more information about what is happening around them, in this case, about personal hygiene issues. The quality, origin and production of fragrances are no exception, since the individual in particular likes to know as much as possible about the process that the product they buy undergoes, especially because it can be expensive.
The composition of perfumes is very complex, using multiple plant extracts combined with each other, sometimes joined with molecules produced by chemical synthesis. This study highlights the traditional and current importance of plants (bio-renewable raw material) together with the most innovative chemical and modern technological transformation, without forgetting that some plant derivatives can be toxic.
These general considerations (traditional and current) must be taken into account by the pharmacist, as a specialist who must meet the needs of the public. All this is closely related to Pharmaceutical Botany as a “Green Science” and source of many resources in the future”.
In short, this work offers general, synthetic and, at the same time, representative information, and interdisciplinary researchers are encouraged to continue to delve into all aspects of this subject.
Author Contributions
All the contributions of this work have been carried out by the 3 authors, F.J.G.-M., L.B.-D. and E.M.-T. In the following way: “Conceptualization, F.J.G.-M., L.B.-D. and E.M.-T.; Methodology, F.J.G.-M., L.B.-D. and E.M.-T.; Validation, F.J.G.-M., L.B.-D. and E.M.-T.; Formal Analysis, F.J.G.-M., L.B.-D. and E.M.-T.; Investigation, F.J.G.-M., L.B.-D. and E.M.-T.; Resources, F.J.G.-M., L.B.-D. and E.M.-T.; Data Curation F.J.G.-M., L.B.-D. and E.M.-T.; Writing—Original Draft, F.J.G.-M., L.B.-D. and E.M.-T.; Preparation, F.J.G.-M., L.B.-D. and E.M.-T.; Writing—Review & Editing, F.J.G.-M., L.B.-D. and E.M.-T.; Visualization, F.J.G.-M., L.B.-D. and E.M.-T.; Supervision, Project Administration, F.J.G.-M., L.B.-D. and E.M.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study not require ethical approval.
Informed Consent Statement
All authors approve the publication of this work.
Data Availability Statement
All data used is available without restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Herz, R.; Eliassen, J.; Beland, S.; Souza, T. Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia 2004, 42, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Helicon. Hutchinson Dictionary of Word Origins; Helicon Publishing: Abingdon, UK, 2006. [Google Scholar]
- International Fragrance Association (IFRA). Available online: https://ifrafragrance.org/home (accessed on 23 April 2023).
- Economy of Cosmetics. Available online: https://www.oxfordeconomics.com/resource/the-economic-impact-of-the-fragrance-industry/ (accessed on 1 July 2023).
- Boy de García, M. Historia del Perfume Origen y Evolución. Available online: https://dossierinteractivo.com/historia-del-perfume-origen-y-evolucion/ (accessed on 1 December 2022).
- Muñoz-Páez, A.; Garritz, A. Mujeres y química Parte I. De la antigüedad al siglo XVII. Edu. Quím. 2013, 24, 2. [Google Scholar] [CrossRef]
- Voudouri, D.; Tesseromatis, C. Perfumery from Myth to Antiquity. Int. J. Med. Pharm. 2015, 3, 41–55. [Google Scholar] [CrossRef]
- Davis, P. Aromaterapia de la A a la Z, 10th ed.; EDAF: Madrid, Spain, 1993. [Google Scholar]
- King, A. Medieval islamicate aromatherapy: Medical perspectives on aromatics and perfumes. Senses Soc. 2022, 17, 37–51. [Google Scholar] [CrossRef]
- Academia del Perfume. Available online: https://www.academiadelperfume.com/ (accessed on 20 November 2022).
- Reinarz, J. Past Scents: Historical Perspectives on Smell, 1st ed.; University of Illinois Press: Champaign, IL, USA, 2014. [Google Scholar]
- Pybus, D.; Sell, C. The Chemistry of Fragrances; RSC: Cambridge, UK, 1999. [Google Scholar]
- Burger, P.; Plainfosse, H.; Brochet, X.; Fernandez, X. Extraction of natural fragrance ingredients: History overview and future trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef]
- Guerlain. Available online: https://www.guerlain.com/es/es-es/p/aqua-allegoria-rosa-rossa-harvest---eau-de-toilette-P014700.html (accessed on 30 December 2022).
- Oh, C.H.; Rugman, A.M. The Regional Sales of Multinationals in the Word Cosmetics Industry. Available online: http://kelley.iu.edu/riharbau/repec/iuk/wpaper/bepp2006-20-oh-rugman.pdf (accessed on 30 November 2022).
- Gaviño-González, F.A.; González-Minero, F.J. A propósito del Estudio Botánico de la Farmacopea Española, 9th ed.; Ende: Seville, Spain, 2021. [Google Scholar]
- Gilbert, K. Perfume: The Art and Craft of Fragrance; Ryland Peters & Small: New York, NY, USA, 2013. [Google Scholar]
- Dior. Available online: https://www.dior.com/en_th/beauty/products/Y0996491-jadore-parfum-deau-alcohol-free-eau-de-parfum-floral-notes (accessed on 30 December 2022).
- Chanel. Available online: https://www.chanel.com/es/perfumes/p/120090/n19-extrait-parfum/ (accessed on 20 December 2022).
- WFO. The Plant List. Available online: http://www.theplantlist.org/ (accessed on 23 April 2023).
- eFloras. Available online: http://www.efloras.org/ (accessed on 20 April 2023).
- Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 15 June 2023).
- Fitoterapia. Available online: https://www.fitoterapia.net/vademecum/ (accessed on 1 December 2022).
- Denominación de Fragancias. Available online: https://www.academiadelperfume.com/tipos-de-fragancias/ (accessed on 1 December 2022).
- Hornsey, S. Make Your Own Perfume; Begbroke, Spring Hill: Oxford, UK, 2011. [Google Scholar]
- Dove, R. The Essence of Perfume; Black Dog: London, UK, 2014. [Google Scholar]
- Manay, A.; Saeidnia, P. Cosmetics and personal care products k. In Encyclopedia of Toxicology; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Rueda Cosmética Europea. Available online: https://www.perfumecosmetics-eu.com/es/la-rueda-de-las-fragancias-de-michael-edwards/ (accessed on 1 December 2022).
- Stepanyul, A.; Kirschning, A. Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase. Beilstein J. Org. Chem. 2019, 15, 2590–2602. [Google Scholar] [CrossRef]
- Tascone, O.; Roy, C.; Filippi, J.J.; Meierhenrich, W.J. Use, analysis, and regulation of pesticidesin natural extracts, essential oils, concretes, and absolutes. Anal. Bioanal. Chem. 2014, 406, 971–980. [Google Scholar] [CrossRef]
- Soe’eib, S.; Puspa-Asri, N.; Dwi-Saptati, A.S.; Diah-Agustina, P. Enfleurage essential oil from jasmine and rose used cold fat adsorbent. J. Ilm. Widya Tek. 2016, 15, 58–61. [Google Scholar]
- El-Toumya, A.; Husseinb, A. Cold Pressed Yuzu (Citrus Junos Sieb. ex Tanaka) Oil Sayed; Cold pressed oils; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Pushpangadan, P.; George, C.K. Basil. In Handbook of Herbs and Spices; Woodhead Publishing: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2012. [Google Scholar]
- Zhang, X.; Gao, H.; Zhang, L.; Liu, D.; Ye, X. Extraction of essential oil from discarded tobacco leaves by solvent extraction and steam distillation, and identification of its chemical composition. Ind. Crops Prod. 2012, 39, 162–169. [Google Scholar] [CrossRef]
- Perfume Chemiclas. Available online: https://perfumechemicals.wordpress.com/2014/04/30/on-tinctures-and-infusions/ (accessed on 23 October 2023).
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical Fluid Extraction of Plant Flavors and Fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C.; Koidis, A. Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 31–38. [Google Scholar]
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic preparation of natural flavours and fragrantes. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A.; Mahmoud, K.F.; Amin, A.A.; EL Banna, H.A. Application of Biotechnology to the Production of Natural Flavor and Fragrance Chemicals. Res. J. Pharmaceut. Biol. Chem. Sci. 2016, 7, 2670–2717. [Google Scholar]
- Venkateshwarlu, G.; Selvaraj, Y. Changes in the Peel Oil Composition of Kagzi Lime (Citrus aurantifolia Swingle) during Ripening. J. Essent. Oil Res. 2000, 12, 50–52. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemm, I.; Saïdani, M.; Tounsi, M.S. Changes of peel essential oil composition of four Tunisian citrus during fruit maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef]
- Anonymous. Occurrence of Umbelliferone in the Seeds of Dipteryx odorata (Aubl.). Willd. J. Agríc. Food Chem. 1982, 30, 609–610. [Google Scholar]
- Pujiarti, R.; Kusumadewi, A. Chemical Compounds, Physicochemical Properties, and Antioxidant Activity of A. cardamomum Leaves and Rhizomes Oils on Different Distillation Time. Wood Res. J. 2020, 11, 35–40. [Google Scholar] [CrossRef]
- Nogueira, T.S.; Passos, M.D.; Nascimento, L.P.; Arantes, M.B.; Monteiro, N.O.; Boeno, S.I.; de Carvalho Junior, A.; Azevedo, O.D.; Terra, W.D.; Vieira, M.G.; et al. Molecules Chemical Compounds and Biologic Activities: A Review of Cedrela Genus. Molecules 2020, 25, 5401. [Google Scholar] [CrossRef]
- Ameh, O.E.; Achika, J.I.; Bello, N.M.; Owolaja, A.J. Extraction and Formulation of Perfume from Cymbopogon citratus (Lemongrass). J. Appl. Sci. Environ. Manag. 2021, 25, 1461–1463. [Google Scholar] [CrossRef]
- Silva, F.; Moura, C.; Mendes, M.F.; Pessoa, F.L.P. Extraction of citronella (Cymbopogon nardus) essential oil ussing supercritical CO2: Exprimental data and mathematical modeling. Braz. J. Chem. Eng. 2011, 28, 343–350. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Hamdi, N.; Miladi, R.; Abdelkafi, S. Myrtus communis Essential Oil: Chemical Composition and Antimicrobial Activities against Food Spoilage Pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose Flowers—A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Talebi-Kouyakhi, E.; Naghavi, M.R.; Alayhs, M. Study of the essential oil variation of Ferula gummosa samples from Iran. Chem. Nat. Comp. 2008, 44, 124–126. [Google Scholar] [CrossRef]
- Xiao, W.; Li, S.; Wang, S.; Tang-Ho, C. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2017, 25, 43–61. [Google Scholar] [CrossRef]
- Lo, C.M.; Han, J.; Wong, E.S.W. Chemistry in Aromatherapy–Extraction and Analysis of Essential Oils from Plants of Chamomilla recutita, Cymbopogon nardus, Jasminum officinale and Pelargonium graveolens. Biomed. Pharmacol. J. 2020, 13, 1339–1350. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Corazzaa, M.L.; Ndiayea, P.M.; Dalla-Santa, O.R.; Cardozoc, L.; Scheer, A.P. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluids 2013, 80, 44–49. [Google Scholar] [CrossRef]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.; Díaz, T.S.; Silva, A.M. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Perriot, R.; Breme, K.; Meierhenrich, U.J.; Carenini, E.; Ferrando, G.; Baldovini, N. Chemical Composition of French Mimosa Absolute Oil. J. Agric. Food Chem. 2010, 58, 1844–1849. [Google Scholar] [CrossRef]
- Hanuša, L.O.; Řezankab, T.; Dembitskya, V.M.; Moussaief, A. Myrrha—Commiphora Chemistry. Biomed. Papers 2005, 149, 3–28. [Google Scholar]
- Baranauskienė, R.; Venskutonis, P.R. Supercritical CO2 Extraction of Narcissus poeticus L. Flowers for the Isolation of Volatile Fragrance Compounds. Molecules 2022, 27, 353. [Google Scholar] [CrossRef]
- Joulain, D.; Tabacchi, R. Lichen extracts as raw materials in perfumery. Part 1: Oakmoss. Flavour. Fragr. J. 2009, 24, 49–61. [Google Scholar] [CrossRef]
- Donelian, A.; Carlson, L.H.C.; Lopes, T.J.; Machadoa, R.A.F. Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J. Supercrit. Fluids 2009, 48, 15–20. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Hartman-Petrycka, M.; Lebiedowska, A. The Assessment of Quality of Products Called Sandalwood Oil Based on the Information Provided by Manufacturer of the Oil on Polish, German, and English Websites. Evid.-Based Complement. Altern. Med. 2021, 2021, 9934143. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.S.; Nautiyal, M.C.; Figueredo, G.; Rana, V.S. Effect of Post Harvest Drying Methods on the Essential Oil Composition of Nardostachys jatamansi DC. TEOP 2017, 20, 1090–1096. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.; McFeeters, R.; Setzer, W. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Moon, U.R.; Bera, P.; Samanta, T.; Mitra, A. The in vitro antioxidant capacities of Polianthes tuberosa L. flower extracts. Acta Physiol. Plant 2014, 36, 10. [Google Scholar] [CrossRef]
- Singletary, K.W. Vanilla. Potential Health Benefits. Nutr. Today 2020, 55, 186–196. [Google Scholar] [CrossRef]
- Grover, M.; Behl, T.; Virmani, T.; Bhatia, S.; Al-Harrasi, A.; Aleya, L. Chrysopogon zizanioides—A review on its pharmacognosy, chemical composition and pharmacological activities. Environ. Sci. Pollut. Res. 2021, 28, 44667–44692. [Google Scholar] [CrossRef]
- Chandra, D.; Kohli, G.; Prasad, K.; Bisht, G.; Punetha, V.D.; Khetwal, K.S.; Devrani, M.K.; Pandey, H.K. Phytochemical and Ethnomedicinal Uses of Family Violaceae. Curr. Res. Chem. 2015, 7, 44–52. [Google Scholar] [CrossRef]
- González-Minero, F.J.; González-García, A.; Venegas-Fito, C.J. Botánica Miscelánea. Apuntes Sobre los Herbarios y su Relación con la Farmacia; Ende: Seville, Spain, 2020. [Google Scholar]
- Christenhusz, M.J.M.; Fay, M.F.; Chase, M.W. Plants of the World. An Illustrated Encyclopedia of Vascular Plants; Kew Publishing Royal Botanic Gardens: Kew, UK, 2017. [Google Scholar]
- Real Farmacopea Española, 5th ed. Available online: https://extranet.boe.es/farmacopea/ (accessed on 23 June 2023).
- Olorunshola, Y. Why Do Plants Smell? Available online: https://www.kew.org/read-and-watch/why-do-plants-smell#:~:text=For%20plants%2C%20smell%20is%20a,attract%20pollinators%20and%20repel%20predators (accessed on 23 July 2023).
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). Biochem Mol. Biol. Plants. 2000, 24, 1250–1319. [Google Scholar]
- Devesa-Alcaraz, J.A.; Carrión-García, J.S. Las Plantas Con Flor; Universidad de Córdoba: Córdoba, Spain, 2012. [Google Scholar]
- Olmos, E.; Sánchez-Blanco, M.J.; Ferrández, T.; Alarcón, J.J. Subcellular Effects of Drought Stress in Rosmarinus officinalis. Plant Biol. 2007, 9, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jonoubi, P.; Majd, A.; Marouf, A.; Amini, S. Investigation the structure of vegetative organs and development of reproductive organs of Pimpinella anisum L. Nova Biol. Reper. 2015, 2, 151–165. [Google Scholar] [CrossRef][Green Version]
- Svoboda, K.P.; Svoboda, T.G. Secretory Structures of Aromatic and Medicinal Plants; Microscopix Publications: Powys, UK, 2000. [Google Scholar]
- Saint-Lary, L.; Roy, C.; Paris, J.P.; Martin, J.F.; Thomas, O.T.; Fernandez, X. Metabolomics for the Authentication of Natural Extracts Used in Flavors and Fragrances: The Case Study of Violet Leaf Absolutes from Viola odorata. Chem. Biodivers. 2016, 13, 737–747. [Google Scholar] [CrossRef]
- Arribas, M.P.; Soro, P.; Silvestre, J.F. Dermatitis de contacto alérgica por fragancias. Actas Dermosifiliogr. 2012, 103, 874–879. [Google Scholar] [CrossRef]
- Legislative Framework of Cosmetics of the European Union. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:32017R0542 (accessed on 23 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).