Abstract
The goal of this research was to develop a reliable oil-controlling toner for facial skin with a natural product, Houttuynia cordata Thunb extract (HCE). The developed HCE facial toner showed high stability and had a high satisfaction level. Clinical studies revealed that the sebum value of the skin after using the developed HCE toner for eight weeks decreased (4.6-times lower), which was statistically significant (p-value < 0.05) when compared to the HCE-free toner. The sebum amount on the facial skin after using the HCE toner decreased by approximately two-times compared to the HCE-free toner. In addition, the skin moisture content increased statistically significantly (p-value < 0.05) from the eighth week of treatment compared to the HCE-free toner and was 1.5- and 1.4-times higher for the left and right cheeks, respectively. The average moisture content of the facial skin treated with the HCE toner increased by 2- and 1.4-times compared to the treatment with the HCE-free toner for the left and right cheeks, respectively. Consequently, the HCE toner had anti-sebum and moisturizing efficacy, and the increased reliability of the natural product meant that it could soon be a premium commercial product.
1. Introduction
Facial toners are utilized as cosmeceutical products and have several functions, for example rehydrating the skin, balancing the skin’s pH, tightening skin pores, relieving irritation, and antisepsis [1]. Cleansing faces with a high-pH cleanser might upset the skin. Therefore, toners have been developed to balance the skin’s pH [2]. The main ingredients found in toners are beta hydroxy acid (BHA) and alpha hydroxy acid (AHA). Currently, toners are supplemented with ingredients with functions other than cleaning the face, such as adding moisturizers to reduce the occurrence of pimples and control oiliness on the skin. Oily skin usually refers to a facial condition where the skin has a shiny appearance and greasiness due to excessive sebum production and secretion. This aesthetic problem leads to skin disorders such as acne [3,4]. Therefore, utilizing cosmetics to reduce oiliness is challenging. Important components in toners include essential oils, antioxidants, vitamins, and herbal extracts.
Houttuynia cordata Thunb (H. cordata; Saururaceae) is found in Asian regions. Studies of H. cordata or heartleaf (leaves are heart-shaped) show its benefits for oily, dehydrated, and acne-prone skin. H. cordata extract (HCE) contains humectants and is excellent for skin hydration and maintaining the skin’s moisture [4,5]. It also has powerful anti-allergic properties and is beneficial for dermatitis-/eczema-prone skin. Phenolic compounds from HCE, such as alkaloids and flavonoids (hyperoside, afzelin, and quercetin) are beneficial as active ingredients for cosmetics [4,6,7]. Moreover, many studies have found that HCE contains compounds with antioxidant properties [8], antibacterial properties (strong anti-microbial effects against the bacteria most commonly contributing to acne: Propionibacterium acnes and Staphylococcus epidermidis) [9], antiviral activity [10,11,12], anti-inflammatory properties [13,14], anti-skin-aging properties [15,16] and anti-allergic effects [15,17]. Some compounds have also been found to be severe acute respiratory syndrome coronavirus 2 inhibitors (SARS-CoV-2 inhibitors) [18]. In addition, H. cordata is a suitable ingredient in cosmetic creams for dry and rough skin and preventing chapped skin [19]. H. cordata is also suitable for the development of a toner to control oiliness and add moisture to the face. Nevertheless, there are few research reports on HCE facial toners, and there is a lack of information on safety and quality-control standards. Therefore, further study should be encouraged to test the efficacy of products developed in human clinical trials. Clinical studies require modern equipment and ethics committees in order to meet ethical and safety standards (Declaration of Helsinki). These clinical studies can increase the reliability of and prospects for herbal cosmetics.
Consequently, the ultimate goal of this research was to develop an oil-controlling and moisture-adding facial toner product containing H. cordata and to confirm its anti-sebum efficacy and hydration effects by conducting clinical trials on volunteers.
2. Materials and Methods
2.1. H. cordata Preparation and Extraction
Fresh leaves of H. cordata were purchased from Ton Rak Khon Kaen Herbal Shop, 600 Moo 27, Ban Non-Muang Sila, Muang Khon Kaen District, Khon Kaen Province 40000. They were examined for biological characteristics and their scientific name identified. They were then cleaned and decontaminated. The herbs were set aside and dried at 50 °C to prepare them for extraction.
Then, 50 g of the grinding sample was extracted by the maceration method using 600 g ethanol (95% ethanol). Then, the slurry was sonicated for 30 min, after which the resulting solution was filtered through a filter bag, and the filtrate was concentrated by evaporating with a rotary evaporator under a vacuum (rotary evaporator at a temperature of 50 °C, vacuum of 180 mbar, and rotation of 50 rpm). Finally, the extract was dried in a hot-air oven at 50 °C.
2.2. Facial Toner Formulation
The oil-controlling and moisture-adding facial toners were prepared as two formulations: with and without HCE as F1 and F2, respectively. Deionized water was introduced in two phases (A and B) to facilitate solubility and compatibility. The deionized water contained the highest percentage of any facial toner formula. The facial toners were formulated by mixing the ingredients, as in Table 1. Phase A was mixed well by stirring and heating for 50 °C. It was then cooled to 30 °C, and Phase B was added slowly, while stirring continuously until well combined. Then, Phase C, perfume, and phenoxyethanol, were gradually added to Phase AB. Lastly, the pH values of the facial toners were adjusted to 5.5 using 10% citric acid. In this work, proper facial toners were those that did not leave a greasy feeling on the skin, felt light-weight, and left the skin feeling supple.

Table 1.
The ingredients, percentages, and functions of the facial toner.
2.3. Accelerated Stability Studies
The stability study was conducted using a slightly modified method based on several publications [19,20,21]. An accelerated stability study was conducted under the heating/cooling process. The heating/cooling process was achieved by storing the developed products in a fridge/incubator. The storage temperatures changed between 4 ± 2 °C and 45 ± 2 °C every 24 h for each cycle. The stability study was conducted for six cycles. The stability at ambient temperature was examined for six cycles as well. Then, 10 g of the developed products from each stored cycle was centrifuged at 3000 rpm for 30 min (Centrifuge, Benchtop Rotofix 32A, Bangkok, Thailand). The phase separation and the changes were observed. Besides, the pH values of the developed toners were examined and reported using a pH meter (Bench Top pH Meter, ST300 Ohaus, Shanghai, China) to demonstrate their stability.
2.4. Clinical Test of Facial Toner Efficiency
2.4.1. Volunteer Recruitment
- The study was conducted according to the Declaration of Helsinki [22], and the protocol was authorized by The Ethics Committee of Phranakhon Rajabhat University, Bangkok, Thailand (approved study code: AF05-06; study code: 02.022/6). All subjects were required to sign a consent form to participate in this study. The volunteers had the right to withdraw from the test without consequences or penalties, at any time.
- Inclusion criteria: Twenty-one healthy male and female volunteers, aged 18 years and over, did not have skin diseases and abnormalities such as rashes, erythema, or eczema pregnant, or still had to breastfeed. All volunteers were informed of the test objectives and procedures; for example, during the test, volunteers must use the product every day, once a day, avoid the risk of using other facial products, and avoid working outdoors for a long time, and the volunteers must be aware of the potential side effects. The volunteers must pass allergy and irritation tests as mentioned in Section 2.4.2 before participating in the treatment with the developed products for two months. All subjects were required to give informed consent before participating in the study. The investigators adhered to all criteria and procedures for submitting research applications on human subjects.
- Exclusion criteria: Subjects had a history of corticosteroid or antihistamine use two weeks before the trial.
- Withdrawal criteria of the study: Subjects experienced an allergic reaction to the product during the test, became pregnant, or did not have time for the test.
- Termination of study criteria: subjects had an allergic reaction to the product.
The study flowchart of the participants is shown in Figure 1, describing the trial process.
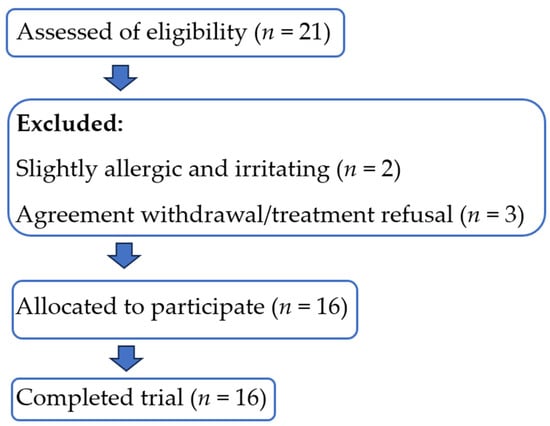
Figure 1.
The study flowchart of the participants.
For clinical testing, the sixteen volunteers who passed through irritation and allergy testing were divided into two groups of 8 volunteers. Each group was treated separately daily with the facial toner with each formulation (F1 and F2 with and without HCE) and observed for eight weeks of follow-up. The study was randomized and placebo-controlled. The volunteers were requested to use ten drops of facial toner, which were absorbed on a cotton pad, once in the morning over eight weeks. All volunteers were allergy-free for one week and had not used steroids and topical products for oily or greasy face treatment for four weeks before the study. The volunteers were requested not to apply any products to the face the night before starting the measurement. The evaluation was planned and conducted at Weeks 0, 5, 6, 7, and 8 using the Sebumeter® SM 815 (Courage+Khazaka electronic GmbH, Ossendorf, Germany) to measure sebum content and the Corneometer® CM 825, Ossendorf, Germany) to measure skin hydration.
2.4.2. Patch Test
The irritation and allergic reactions were evaluated using patch testing [23,24,25]. The researchers recruited the volunteers and conducted the following. All volunteers were informed of the objectives, possible adverse effects, and test procedures and were rewarded for their participation. The approved study code from The Ethics Committee of Phranakhon Rajabhat University, Bangkok, Thailand, AF05-06, was 02.022/6. All volunteers gave consent before entering the study. Volunteers had the right to withdraw from the test at any time without consequence or penalty.
During the patch test method, ten drops of the developed toner were kept on the inside of the arm and in contact with the skin under a 5.5 × 6.5 cm2 occlusive patch. After skin contact for a specified period of time (30 min), the patch was opened and any symptoms of skin irritation were observed. The skin’s irritation was indicated by different ratings, such as without irritation, redness, edema, or redness.
2.4.3. Efficacy Evaluation through Modern Non-Invasive Measurements of the Skin
All volunteers were evaluated for all facial greasiness with the Sebumeter® SM 815 at the baseline or before treatment with the facial toners. The subjects were acclimatized and their faces were cleaned with the cleanser provided in the waiting room (T° = 20–22 °C, humidity 40–60%) for 30 min [26] before the measurement was carried out under the same condition for every evaluation.
It takes 28 days for basal cells to migrate through the epidermis and remove the epidermal layer in humans [27]. Therefore, the evaluation was planned and conducted at Weeks 0, 5, 6, 7, and 8. The sebum content of the three different spots on the T-zone: forehead, cheek, and chin, was analyzed. The undesirable effects were observed, if any, including erythema, edema, scaling, itching, stinging, burning, tightness, or prickling of the skin, and rated as none, mild, severe, or very severe. The evaluation was conducted at the location and with the same lighting at each evaluation by the Sebumeter® SM 815 under the same conditions as the baseline. Anti-sebum efficacy was calculated [28] as follows:
where St = Sebum content (g/cm2) at time interval and S0 = Sebum content (g/cm2) at baseline.
Additionally, an evaluation of skin hydration was conducted as well. The right and left face were measured separately to determine the skin hydration. The skin’s electrical properties depend on the water content of the stratum corneum of the epidermis. Epidermal hydration was analyzed for both groups by measuring the electrical capacitance with the Corneometer®CM 825. The principle of the method relies on the difference between the dielectric constant of water (81) and other substances by measuring the capacitance of a dielectric medium [25]. The Corneometer®CM 825 contains two electrodes with different electrical charges that form an electromagnetic field, which determines the dielectricity of the stratum corneum. The depth of measurement was low (the first 10–20 µm of the stratum corneum) due to the construction of the measuring head of the Corneometer®CM 825. The range of variation of the values of skin hydration degree was between 0 and 130 arbitrary units (a.u.) at the standard working conditions (T° = 20–22 °C, humidity 40–60%). The variations of the values of the skin hydration degree were the following: under 30 a.u.—very dry, between 30 and 45 a.u.—dry, 45 a.u.—sufficiently hydrated, or normal skin higher than 40 a.u. [26,29].
Three repeated measurements were performed at each test, and the means and standard deviations are reported. The paired samples t-test (SPSS Version 26.0) was used to indicate a statistically significant difference.
2.5. Preference Test
For the satisfaction test, both developed facial toners (F1 and F2) were tested by each volunteer (20 people) without knowing the details of each product, using a five-point rating scale questionnaire. The five-point rating scale questionnaire consisted of two parts: personal data: questions in the personal data section were about sex, age, and skin type, and the questions concerning toner products were about spreadability, skin absorption, greasiness, color, and odor. The five-point rating scale ranged from 1 (strongly dissatisfied) to 5 (strongly satisfied). The developed products’ preference was also compared to the commercial product in the blinded experiment [30]. Product preference test: The volunteers tested products that contained HCE (F1) and did not contain HCE (F2), and the third was a commercial product (F3).
3. Results
3.1. H. cordata Preparation and Extraction
The percentage yield of the H. cordata extraction was calculated by the following Equation (1). The percentage yield of the extraction was 3.85%.
The appearance of the extracted powder is shown in Figure 2. The appearance of the extracted H. cordata was dark yellow.

Figure 2.
The appearance of the extracted H. cordata.
3.2. Facial Toner Formulation
Proper facial toners are absorbed quickly, do not have a greasy feeling, and feel soft and supple when touched. The developed facial toners were successfully formulated by mixing the ingredients following Table 1 (Section 2.2). The successfully developed facial toners are presented in Figure 3 as two formulations, with and without HCE, respectively. Furthermore, F1 and F2 were tinted close to the same color using cosmetic-grade pigment.
Figure 3.
The appearance of HCE toner (F1) and HCE-Free toner (F2).
3.3. Accelerated Stability Studies
Samples subjected to changes in temperature can reveal instability quicker than samples stored continuously in one condition. Therefore, the accelerated stability test of this study was performed by heating/cooling for six cycles, as mentioned in Section 2.3. The stability of the developed facial toner was observed by the pH values, color, odor, lamination, turbidity, and precipitation. After the stability test, the pH values of the formulations were within the range of the skin’s pH (5.0–6.0). The pH of the skin surface, or stratum corneum (SC), is typically in the range of 5.0–6.0, as the acid mantle. Maintaining the pH of the SC in the typical range of 5.0–6.0 is very important to provide an effective barrier and maintaining physiological processes, and forming a stabilized double-lamellar structure in these mildly acidic conditions and micellization occurs for pH > 6.0, while a disordered structure occurs for pH < 4.5 [31]. Moreover, the viscosity was suitable for skin application. There was no change in the developed facial toner’s appearance, phase separation, and turbidity. Besides, the results showed no phase separation and precipitation for every cycle, confirmed by centrifugation.
The stability test results of the developed facial toner and placebo facial toner are shown in Table 2. The results revealed the stability of the developed toners with and without HCE (F1 and F2), respectively.

Table 2.
Stability test results of the developed facial toner containing HCE and the placebo facial toner.
3.4. Clinical Test of Facial Toner Efficiency
The volunteers’ reduction in excess sebum after eight weeks of treatment with the HCE toner (F1) was −43.00 g/cm2, while the highest reduction in sebum with the treatment with the placebo toner (F2) was −2.33 g/cm2. The sebum content analysis results showed that the averages for the treatment with the toner containing HCE (F1) decreased the oiliness by −14.17, −7.55, −20.63, and −31.26 in the 5th, 6th, 7th, and 8th weeks, respectively. There was a statistically significant difference in the eighth week (p-value < 0.05) compared to the baseline (before treatment), while for the placebo toner (F2) treatment, the average sebum content decreased by −1.04 and −4.71, −7.96, and −14.79 in the 5th, 6th, 7th, and 8th weeks, respectively. There was a statistically significant difference in the eighth week (p-value < 0.05) compared to the baseline (before treatment), as shown in Table 3. Additionally, the anti-sebum efficacy in the eighth week for the facial toner containing HCE (F1) calculated as Equation (1) was 78%, while for the placebo toner (F2) was 49.3%.

Table 3.
Sebum content in eight weeks.
The overall efficacies for controlling the oil (decreasing the sebum) by the toner containing HCE (F1) were better than the placebo (F2), which could reduce the oiliness about two-times better than F2 at the eighth week of treatment, as shown in Figure 4. The clinical studies revealed that the sebum value of the skin after using the developed toner for eight weeks decreased (4.6-times lower) statistically significantly (p-value < 0.05) compared with before using the toner.
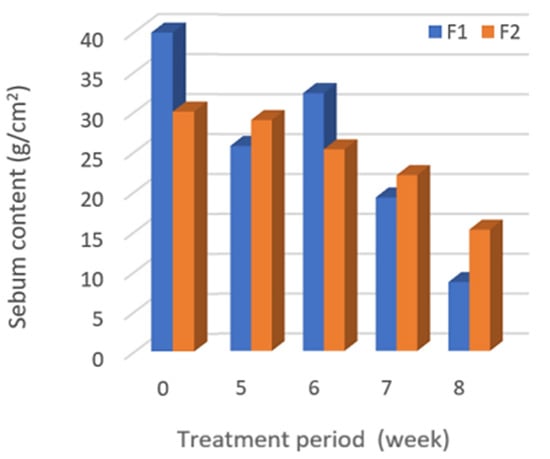
Figure 4.
After treatment with the F1 and F2 toners, the sebum content at different treatment periods (week).
The results of skin moisture analysis on the left side of the face with the Corneometer® CM 825 head showed that the hydration value of the volunteer’s left face after eight weeks, treated with the HCE toner, could increase to a maximum of 57.23 a.u. The maximum increased hydration value, treated with the placebo toner (F2), was 1.27 a.u. The averages for the treatment with the toner containing HCE had increased values in moisture of 7.29, 14.42, 20.33, and 30.44 after treatment for 5, 6, 7, and 8 weeks, respectively. There was a statistically significant difference from the 6-week treatment (p-value < 0.05) compared to the baseline (before treatment). The placebo toner provided a statistically significant difference after the 8-week treatment (p-value < 0.05) compared to the baseline (before treatment), as shown in Table 4.

Table 4.
The left side of the facial skin’s moisture content in eight weeks.
The results of skin moisture analysis on the right side of the face with the Corneometer® CM 825 head showed that the hydration value of the volunteer’s right face after eight weeks of treatment with the HCE (F1) toner increased to a maximum of 41.63 a.u. The maximum increase in the hydration value with the placebo toner (F2) was 5.97 a.u. The averages for the treatment with the toner containing HCE had increased values in moisture as 7.01, 8.39, 17.12, and 24.23 after treatment for 5, 6, 7, and 8 weeks, respectively. Furthermore, there was a statistically significant difference from the fifth week (p-value < 0.05) compared to the baseline (before treatment). Treatment with the placebo toner provided a statistically significant difference after the seventh week of treatment (p-value < 0.05) compared to the baseline (before treatment), as shown in Table 5.

Table 5.
The right side of the facial skin’s moisture content in eight weeks.
The overall efficacies for increasing skin moisture of the toner containing HCE (F1) were better than the placebo (F2). The skin moisture could be increased 2- and 1.4-times more than the HCE-free toner for the left and right cheeks in the eight weeks of treatment, as shown in Figure 5.
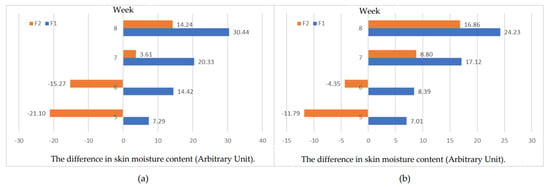
Figure 5.
The difference in skin moisture content after 5–8-week treatment: (a) measurement from the left side of the face; (b) measurement from the right side of the face.
3.5. Preference Test
The satisfaction with the developed facial toner containing HCE as F1 (4.50) was higher than the placebo toner (F2) (4.25). It was less than the commercial toner (F3) (4.55). However, the overall satisfaction with the facial toner containing HCE (F1) and the commercial toner (F3) was not a statistically significant different (p-value > 0.05) using the paired samples t-test, as shown in Table 6.

Table 6.
Average product satisfaction in each aspect.
4. Discussion
Scientific evidence will enhance the reliability of natural products and help them become premium commercial products in the future. This study is the first report to reveal the efficacy of an HCE facial toner. The HCE, widely known as the queen of all spot treatments [32], was developed as an oiliness-controlling and moisture-adding facial toner, and its efficacy was studied regarding its anti-sebum efficacy and hydration effects by conducting clinical trials.
The developed HCE facial toner showed high stability under accelerated heating/cooling testing and revealed a high satisfaction level for spreadability, skin absorption, greasiness, color, and odor. The developed HCE (F1) toner has significantly improved anti-sebum performance. The HCE toner could reduce the amount of sebum by 4.6-times after using the developed toner for eight weeks. There was a statistically significant difference in the eighth week (p-value < 0.05) compared to before the treatment. The sebum content of skin treated with the HCE toner was decreased by approximately two-times compared to the skin treated using the HCE-free toner at Week 8. This oiliness controlling of the developed facial toner may be due to quercetin, the flavonoid in H. cordata extract [4,6]. Quercetin significantly reduces cholesterol levels [33,34], where cholesterol is a component of sebum [35]. The results were consistent with reports that the extract works well for acne-prone skin while gently controlling the sebum [9].
Additionally, the average skin moisture content of the treatment with the HCE facial toner was approximately 2- and 1.4-times higher than that of the HCE-free toner for the left and right cheeks, respectively. This might be the result of polysaccharides from H. cordata extract, i.e., macromolecules from various sugar units such as galacturonic acid, galactose, rhamnose, arabinose, glucuronic acid, glucose, xylose, and mannose in HCE [36,37,38]. Polysaccharides and sugars in skincare are excellent humectants and skin hydrators, meaning they help the skin hold onto water [39]. The results were consistent with several research reports [40,41].
In conclusion, the natural extract (H. cordata extract) was confirmed through the stability and efficacy of its anti-sebum and moisturizing by clinical trials for this study. Therefore, the HCE facial toner in this work demonstrates its readiness and credibility to become a premium commercial product.
Author Contributions
Conceptualization, O.A.; methodology, O.A. and S.N.; software, O.A.; validation, O.A. and S.N.; formal analysis, O.A. and S.N.; investigation, O.A. and S.N.; resources, S.N.; data curation, O.A.; writing—original draft preparation, O.A.; writing—review and editing, O.A. and S.N.; funding acquisition, O.A. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Thailand Science Research and Innovation, Grant Number 178927.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of, PHRANAKHON RAJABHAT UNIVERSITY (protocol code AF05-06 study code: 02.022/6 and date of approval 02.022/6).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The proposed experiments were carried out in the Department of Cosmetic Science, Phranakhon Rajabhat University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chatur, V.M.; Walode, S.G.; Awate, S.A.; Gandhi, M.U.; Thorat, V.S. Formulation and physical characterization of herbal face gel toner. World J. Adv. Res. Rev. 2021, 11, 138–145. [Google Scholar] [CrossRef]
- Science Behind Skin: The Importance of Balancing Your Skin’s pH. Available online: https://yeouth.com/blogs/news/science-behind-skin-the-importance-of-balancing-your-skin-s-ph (accessed on 13 April 2023).
- Wu, Y.; Niu, Y.; Zhong, S.; Liu, H.; Zhen, Y.; Saint-Leger, D.; Verschoore, M. A preliminary investigation of the impact of oily skin on quality of life and concordance of self-perceived skin oiliness and skin surface lipids (sebum). Int. J. Cosmet. Sci. 2013, 35, 442–447. [Google Scholar] [CrossRef]
- Hazarika, N.; Archana, M. The Psychosocial Impact of Acne Vulgaris. Indian J. Dermatol. 2016, 61, 515–520. [Google Scholar] [CrossRef]
- Park, C.I. Study on the Effects of Houttuynia Cordata Extracts on Emulsions. Korea J. Herbol. 2010, 25, 92850731. [Google Scholar]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the aerial parts of Houttuynia cordata attenuate lung inflammation in mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef]
- Panzella, L. Natural phenolic compounds for health, food and cosmetic applications. Antioxidants 2020, 9, 9050427. [Google Scholar] [CrossRef]
- Phosri, S.; Kiattisin, K.; Intharuksa, A.; Janon, R.; Nongkhai, T.A.; Theansungnoen, T. Anti-Aging, Anti-Acne, and Cytotoxic activities of Houttuynia cordata extracts and phytochemicals analysis by LC-MS/MS. Cosmetics 2022, 9, 9060136. [Google Scholar] [CrossRef]
- Wu, L.S.; Si, J.P.; Yuan, X.Q.; Shi, X.R. Quantitive variation of flavonoids in Houttuynia cordata from different geographic origins in china. J. Nat. Med. 2009, 7, 40–46. [Google Scholar]
- Lu, Y.; Wang, X.; Chen, D.; Chen, G. Polystyrene/graphene composite electrode fabricated by in situ polymerization for capillary electrophoretic determination of bioactive constituents in Herba Houttuyniae. Electrophoresis 2011, 32, 1906–1912. [Google Scholar] [CrossRef]
- Ling, L.J.; Lu, Y.; Zhang, Y.Y.; Zhu, H.Y.; Tu, P.; Li, H. Flavonoids from Houttuynia cordata attenuate h1n1-induced acute lung injury in mice via inhibition of influenza virus and toll-like receptor signaling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef]
- Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological effects of Houttuynia cordata Thunb (H. cordata): A comprehensive review. Pharmaceuticals 2022, 15, 1079. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F. Anti-inflammatory functions of Houttuynia cordata Thunb. and its compounds: A perspective on its potential role in rheumatoid arthritis. Exp. Ther. Med. 2015, 10, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A review of phytochemistry and pharmacology and quality control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 29, 10122628. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, I.S.; Kim, J.H.; Kim, J.S.; Kim, D.H.; Yun, C.Y. Suppressive effects of Houttuynia cordata Thunb (Saururaceae) extract on Th2 immune response. J. Ethnopharmacol. 2008, 1, 34–40. [Google Scholar] [CrossRef]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M.; Al-Hemaid, F. Identification of SARS-CoV-2 inhibitors from extracts of Houttuynia cordata Thunb. Saudi J. Biol. Sci. 2021, 28, 7517–7527. [Google Scholar] [CrossRef] [PubMed]
- Timudom, T.; Chaiyasut, C.; Sivamaruthi, B.S.; Tiampasook, P.; Nacapunchai, D. Anti-Sebum efficacy of Phyllanthus Emblica l. (Emblica) toner on facial skin. Appl. Sci. 2020, 10, 8193. [Google Scholar] [CrossRef]
- Chuarienthong, P.; Lourith, N.; Leelapornpisid, P. Clinical efficacy comparison of anti-wrinkle cosmetics containing herbal flavonoids. Int. J. Cosmet Sci. 2010, 32, 99–106. [Google Scholar] [CrossRef]
- Jarupinthusophon, S.; Preechataninrat, P.; Anurukvorakun, O. Development of Liquid Crystal Cream Containing Germinated Brown Rice. Appl. Sci. 2022, 12, 122111113. [Google Scholar] [CrossRef]
- The World Medical Association’s Declaration of Helsinki: Historical and Contemporary Perspectives. Available online: https://www.yumpu.com/en/document/view/34939065/declaration-of-helsinki-historical-contemporary-perspective (accessed on 13 April 2023).
- Hafner, M.F.S.; Rodrigues, A.C.; Lazzarini, R. Allergic contact dermatitis to cosmetics: Retrospective analysis of a population subjected to patch tests between 2004 and 2017. An. Bras. Dermatol. 2020, 95, 696–701. [Google Scholar] [CrossRef]
- Samanta, A.; Agarwal, K.; Naskar, B.N.; De, A. The role of patch testing with Indian cosmetic series in patients with facial pigmented contact dermatitis in India. Indian J. Dermatol. 2021, 66, 81–86. [Google Scholar]
- Li, B.; Cheng, Y.; Tan, Y.; Wang, F.; Hu, W.; Wang, X.; Liu, W.; Krutmann, J.; Wang, S.; Zou, Y. Analysis of factors influencing patch test reactions: Results from a large- population-based study in Chinese. J. Cosmet. Dermatol. 2021, 21, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.M.; Poenaru, E.; Poenaru, C.; Constantin, T. Skin hydration assessment through modern non-invasive bioengineering technologies. Maedica 2014, 9, 33–38. [Google Scholar]
- Milstone, L.M.; Hu, R.H.; Dziura, J.D.; Zhou, J. Impact of epidermal desquamation on tissue stores of iron. J. Dermatol. Sci. 2012, 67, 9–14. [Google Scholar] [CrossRef]
- Meethama, P.; Kanlayavattanakul, M.; Lourith, N. Development and clinical efficacy evaluation of anti-greasy green tea tonner on facial skin. Rev. Bras. Farmacogn. 2018, 28, 214. [Google Scholar] [CrossRef]
- Vasques, L.I.; Leonardi, G.R. User experience in cosmetics: Perception analysis regarding the use of an anti-aging moisturizer. Cosmetics 2023, 10, 10010033. [Google Scholar]
- Rungruangjit, W.; Chankoson, T.; Charoenpornpanichkul, K. Understanding different types of followers’ engagement and the transformation of millennial followers into cosmetic brand evangelists. Behav. Sci. 2023, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.S.; Kong, B.J.; Park, S.M. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Ongredients Houttuynia cordata 95% Mask, Oil Control & Moisture Balancing Face Mask Sheet, Vegan, Cruelty-Free, Organic Clean Beauty, Fragrance-Free, Peta Approved, Eco-Friendly Pouch, 5 Sheets. Available online: https://www.amazon.com/ONGREDIENTS-Houttuynia-Cruelty-Free-Fragrance-Free-Eco-Friendly/dp/B09R7FB3TL (accessed on 13 April 2023).
- Guo, W.; Gong, X.; Li, M. Quercetin Actions on Lipid Profiles in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis. Curr. Pharm Des. 2019, 25, 3087–3095. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Dadgostar, E.; Asemi, Z. The effects of quercetin supplementation on lipid profiles and inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Crit Rev. Food Sci. Nutr. 2020, 60, 1855–1868. [Google Scholar] [CrossRef]
- Pappas, A.; Johnsen, S.; Liu, J.C.; Eisinger, M. Sebum analysis of individuals with and without acne. Dermato-Endocrinology 2009, 3, 157–161. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Pan, Y.; Li, Z.; Zhou, Z.; Pan, Z.; Tai, H.; Xing, Y. Structural characterization and biological activity of polysaccharides from stems of Houttuynia cordata. Foods 2022, 11, 11223622. [Google Scholar] [CrossRef]
- Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R. Antiviral Effects of Houttuynia cordata Polysaccharide Extract on Murine Norovirus-1 (MNV-1)—A Human Norovirus Surrogate. Molecules 2019, 24, 1835. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Biopolysaccharides for Skin Hydrating Cosmetics; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, B. Skin health-promoting effects of natural polysaccharides and their potential application in the cosmetic industry. Polysaccharides 2022, 3, 818–830. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Oliveira, W.F.D.; Silva, P.M.D.S.; Correia, C.T.D.S.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).