Abstract
Energy input in emulsion manufacturing comprises thermal and mechanical energy, with thermal energy being predominant. In terms of raw material selection, there is a widely accepted belief that natural formulations are more “eco-friendly” than their standard (not natural) counterparts. The aim of this study was to compare the energy consumption and subsequent carbon footprint resulting from the production of two main emulsion types, each represented by its standard and natural variant and made by using different manufacturing processes (hot, hot-cold and cold). This resulted in six samples of oil-in-water (O/W) and water-in-oil (W/O) emulsion types, respectively. Scale-down calculations were used to establish the required homogenisation time and speed of the laboratory homogeniser, necessary to achieve the same shear rates as the chosen industrial vessel. The resulting emulsions were characterised using rheological and textural analysis. The six emulsions within each emulsion type have exhibited sufficiently similar characteristics for the purpose of carbon footprint comparisons. Calculations were conducted to quantify the energy input of hot and hot-cold procedures, followed by cradle-to-gate life cycle analysis (LCA). Energy calculations demonstrated that the hot-cold manufacturing process saved approximately 82% (for O/W) and 86% (for W/O) of thermal energy in comparison to the hot process. LCA has shown that the effects of using natural instead of standard ingredients were negative, i.e., it led to a higher carbon footprint. However, it was dwarfed by the effect of the energy used, specifically thermal energy during manufacturing. This strongly indicates that the most efficient way for companies to reduce their carbon footprint is to use the hot-cold emulsification process.
1. Introduction
The cosmetic industry is under pressure to reduce its negative environmental impact, like all other sectors of society. Being a manufacturing industry, its environmental impact is complex. Scope 1 (direct) and 2 (indirect electricity) greenhouse gas emissions occur mainly during the manufacturing of a cosmetic product, while scope 3 (other indirect) emissions occur during the acquisition of raw materials, distribution, consumer end-use and disposal of the used cosmetic product [1,2]. The main metric of success is the reduction in the carbon footprint of a product or a company, which represents the amount of greenhouse gases released as carbon dioxide equivalent units (CO2e) [1]. This metric mainly reflects energy consumption, as the burning of fossil fuels is the primary source of greenhouse gas emissions [3].
Particularly, the extraction, processing and production of raw materials, as well as the heat consumed during product manufacturing, contribute to the energy consumption issues of the cosmetic industry [4]. Bom, Ribeiro and Marto [5] have developed a sustainability calculator based on the opinions of cosmetic industry experts. According to it, raw material selection and product manufacture contribute 22.7% and 19.6%, respectively, to the overall product sustainability score. Therefore, companies can reduce their carbon footprint by reducing the energy required to manufacture cosmetic raw materials and final products. Energy that cannot be reduced could be obtained from renewable energy sources to further reduce carbon emissions [4].
With an increasing supply of sustainable raw material options, such as natural and cold-processable ingredients, the industry endeavours to substitute current raw materials and manufacturing processes with more sustainable ones without noticeably changing the resulting formulation [6]. Replacing synthetic raw materials with natural or naturally derived ones has the obvious benefit of using renewable feedstock. However, while natural ingredients are often viewed as sustainable and synthetic ingredients as unsustainable, this is not always the case [5]. Aspects beyond the origin of the raw material need to be considered, including the energy consumption of the industrial synthesis vs. plant growth and extraction, as well as the processing of ingredients [4,5]. There appears to be a lack of research comparing the energy consumption between natural and synthetic ingredients and determining whether a switch to natural ingredients benefits the overall carbon footprint of the product.
Emulsions are the most common form of cosmetic product format. Since its constituent phases (usually water and oil) are immiscible, emulsion formation is non-spontaneous and requires the presence of an emulsifier and considerable energy input. Conventional hot-process emulsification uses thermal energy required to heat the phases, mechanical energy necessary for the dispersion of droplets and their homogenisation, and thermal energy for cooling. However, with the development of cold-processable emulsifiers, cold-process emulsification is becoming more widely used [7]. There are also other forms of emulsification in between these two extremes, where the oil phase is heated as normal while the water phase is not heated or only partially heated, which is referred to as hot-cold and hot-warm emulsification, respectively. Lin [8] has suggested the use of lower energy emulsification (LEE) by heating the entire oil phase and parts of the water phase to form a concentrated O/W emulsion before adding the rest of the water phase at room temperature. This allows the use of solid wax thickeners in the oil phase, which could not be used in the cold process [7].
The two main types of emulsions, oil-in-water (O/W) and water-in-oil (W/O), often contain 60–80% water, with the chosen emulsifier determining the type of emulsion [9]. Water has a high heat capacity, meaning it requires considerably more heat to raise the temperature of 1 g of substance by 1 °C than any oil [10]. Hence, cold-process emulsification would be relevant for both emulsion types in order to save energy during manufacturing. In fact, in his seminal paper, Lin [8] suggested that the heating and cooling processes constitute about 95% of the energy consumption during emulsion manufacturing, while mechanical energy only makes up 5%. With the elimination of the heating and cooling step, there is also a significant reduction in manufacturing time, which in turn increases production capacity [7,8,11]. However, very little research has been carried out to quantify these benefits. In a recent paper, Raposo et al. [11] found that O/W emulsions developed by cold process emulsification saved 67% in electrical cost, 36.7% in water cost and 17.1% in total production cost compared to hot process emulsions.
Additionally, there has been little comparison between the properties of emulsions resulting from hot emulsification in comparison to hot-cold and cold emulsification. Lin [12] found that LEE resulted in smaller droplets and a more uniform droplet size distribution for O/W, but not for W/O emulsions. It has also been suggested that the elimination of heating and cooling makes it easier to control the structure of the resulting emulsion [11]. This is in line with the results of Tamburic et al. [13], in which hot process emulsification resulted in less viscous emulsions than cold process emulsification, as heating affected the structure of petrolatum in the investigated W/O creams.
Clearly, further research is required to provide a better understanding of the differences in energy consumption and carbon footprint of products using different variations of the emulsification process. It is also important to quantify the environmental effects of replacing standard (non-natural) ingredients with certified natural ones.
The aim of this study was to compare the energy consumption and cradle-to-gate carbon footprint of physico-chemically comparable emulsions of both W/O and O/W types, made with standard (mainly synthetic) and COSMOS-approved natural ingredients, respectively. The comparison was extended to the use of different manufacturing processes (hot, hot-cold and cold emulsification) for the above emulsion variants.
2. Materials and Methods
2.1. Materials
Table 1 and Table 2 present all the emulsion formulations used in this study. The two standard formulations (one for each emulsion type) were taken from the supplier’s portfolio as examples of commonly used formulations. They contain synthetic ingredients, but not exclusively, which was the reason to avoid the term “synthetic”. They were prepared by the hot and hot-cold emulsification process, respectively, producing standard hot and standard hot-cold samples.

Table 1.
The formulations of O/W standard and natural emulsions used in the study.

Table 2.
The formulations of W/O standard and natural emulsions used in the study.
Standard formulations were then modified, as much as necessary, to produce structurally and sensorily very similar emulsions that could be produced using the cold emulsification process. This has resulted in standard cold samples.
The same preparation and re-formulation processes were applied to the variation that contained COSMOS-approved natural ingredients, which resulted in natural hot, natural hot-cold and natural cold emulsion samples. This approach was applied to both O/W and W/O emulsion types, producing a series of 12 test samples. The diagram depicting the above experimental design is presented in Figure 1.
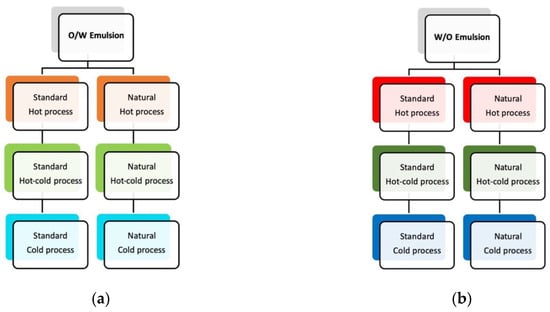
Figure 1.
Experimental design, showing all test samples: (a) standard and natural O/W emulsions; (b) standard and natural W/O emulsions. Both emulsion types were prepared using hot, hot-cold and cold process, respectively.
2.2. Methods
2.2.1. Scale-Down Calculations
Scale-up, the process of increasing batch size from laboratory to manufacturing scale, is challenging, particularly for thermodynamically unstable products such as emulsions. This is due to the differences in geometric, mechanical, thermal and chemical characteristics between the two processes, which can affect the final product quality [14,15]. In this study, scale-down calculations were completed first, aiming to match the manufacturing and laboratory scale conditions to ensure minimal product changes during scaling-up. They have proceeded in the stages shown below.
- Homogenisation Speed Calculations
The specifications of a standard manufacturing vessel, based on the Ekato Unimix S Jet 500 (Ekato, Germany), as well as the laboratory L5M homogeniser (Silverson, Chesham, UK), can be found in Table 3; Table 4, respectively.

Table 3.
Standard manufacturing vessel and process specifications [16].

Table 4.
Laboratory homogeniser standard mixing assembly (Silverson, UK).
The values in Table 3 and Table 4 were used to calculate velocity from Equation (1) [17]:
where V is velocity (m/s), νn is rotational speed (rpm), is the diameter of the rotor (m) and is 3.14. The shear rate was then calculated from Equation (2) [18]:
where is shear rate (s−1), V is velocity (m/s) and h is the gap between stator and rotor (m).
At the given standard manufacturing rotational speed of 3800 rpm, the velocity and shear rate applied to the formulation were calculated using Equations (1) and (2).
The velocity and the rotational speed in rpm of the laboratory homogeniser have to be set to achieve the same shear rate of 23,864 s−1 as the manufacturing vessel, hence:
Therefore, the laboratory homogeniser speed was set at 6600 rpm.
- Effective Power Calculations
The scale-down concept used in this study is based on the premise that two parameters are kept constant: (a) The shear rate, as outlined in the chapter before and (b) the energy input at this specific shear rate. We use water as a model substance because its viscosity is not dependent on shear rate and it is simple to use. We postulate that the energy input for water must be identical at production and lab scale. Using identical shear rates to make emulsions in production and at the lab scale, we then postulate that the viscosity of the produced emulsions would also be identical. This is based on fluid mechanics, which states that the energy input of the rotor-stator system is directly proportional to the viscosity of the fluid. The calculated effective power for water at production and lab scale can be used for the scale-down calculations for emulsions because the values for water and for emulsions obtained under the same conditions are proportional.
Since the effective power determines the duration of homogenisation required to achieve identical energy input at production and lab scale, the first step in the process was to establish the effective power of laboratory homogeniser. To do that, 500 g of water was homogenised using the L5M homogeniser (Silverson, UK) at 6600 rpm for 5 min. The temperature increase was recorded every 30 s and the process was repeated three times. The temperature change over time was plotted and presented in Figure 2. It was noted that the slope of the temperature curve slightly decreased after around 150 s due to heat loss to the surroundings. Therefore, the temperature difference was found using only the change within the first 150 s, resulting in an average temperature increase of 6.13 °C.
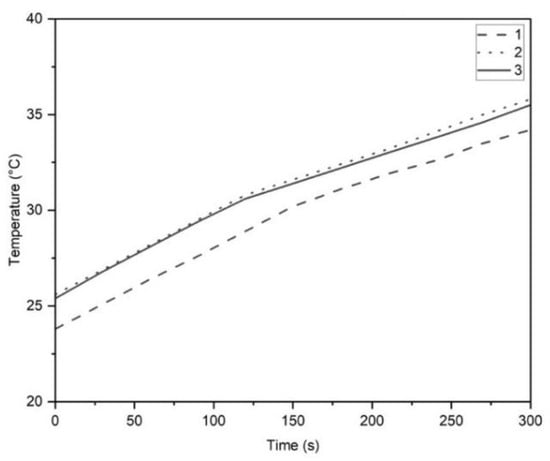
Figure 2.
Assessing effective power of laboratory homogenizer using water as a model material: the temperature increase in water during 5 min of homogenisation at 6600 rpm (n = 3), with an inflection after 150 s.
The effective power of the laboratory homogeniser was calculated using Equation (3) [19]:
where P is power (W), m is mass (kg), Cp is specific heat capacity (J/kg·°C), ΔT is temperature difference (°C) and Δt is time difference (s).
Water-specific heat capacity is known to be 4184 J/kg·°C [10]. When the temperature increase of 6.13 °C during 150 s was included in Equation (3), it showed the power of the laboratory homogenizer to be 85.49 W:
- Homogenisation Time Calculations
The calculated effective power of 85.49 W and the values from Table 4 were used in Equation (4):
where P is specific power (W/kg) and m is mass (kg).
The specific power values of both factory and laboratory homogeniser were calculated using Equation (4), and the ratio between the two values was established.
The resulting ratio was used to convert the manufacturing homogenisation time of 20 min into the required laboratory homogenisation time in order to provide the same power input per unit mass of formulation.
Therefore, the laboratory homogenisation time was set as 2 min and 20 s.
2.2.2. Emulsion Preparation Methods
Having experimentally determined the specific power of the given laboratory homogeniser, calculated the specific power of the intended vessel and calculated the required homogenisation time, the next task was to establish a suitable laboratory preparation method for each of the emulsion variants used in the study. The two diagrams in Figure 3 visualise the laboratory-based emulsification processes used in the production of the 12 test emulsions.
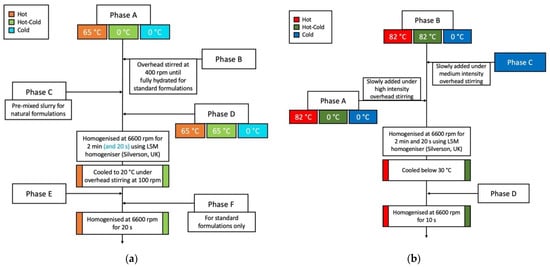
Figure 3.
Laboratory production process for the O/W emulsions (a) and W/O emulsions (b).
To assess initial emulsion stability, centrifuge stress testing was performed on all samples. Each emulsion (5–7 g) was centrifuged in the Heraeus Labofuge 400 (ThermoFisher Scientific, Loughborough, UK) at 4000 rpm for 30 min. A visual assessment of phase separation was completed as a quick indication of emulsion stability [20].
2.2.3. Emulsion Characterisation: Rheology
Laboratory production of the test emulsions was followed by their characterisation using rheological and textural methods approximately 48 h after their preparation.
Rheological tests were carried out at 20.0 °C ± 1.0 °C using the Haake Mars iQ Air Modular Advanced Rheometer with a TMP35 S serrated lower plate and P35/Ti/SE serrated plate rotor (ThermoFisher Scientific, UK), with a 1 mm gap. The accompanying Rheowin software v. 4.91.0021 was used to collect and analyse the data, while OriginPro v. 10 (OriginLab, Northampton, MA, USA) was used to plot the resulting graphs.
Continuous Flow Test
A continuous shear rate sweep from 0.1 to 100 s−1 and back to 0.1 s−1 for 60 s was performed on all samples. Viscosity and flow curves were plotted, and the hysteresis loop area between the up and down flow curves was calculated as a measure of the level of thixotropy [21].
Oscillatory Test
An oscillatory stress (amplitude) sweep was performed at the constant frequency of 1 Hz, from 1 to 500 Pa for O/W and from 1 to 300 Pa for W/O emulsions. Two parameters were identified and plotted against the changes in oscillating shear stress: The complex modulus (G*) and phase angle (δ). Complex modulus G*, also known as rigidity, is a measure of the balance between viscous and elastic components of the material, which are defined as viscous (G″) and elastic (G′) moduli, respectively. Its mathematical expression is . The phase angle δ, also known as the lag phase, shows how much the response (deformation) lags behind the stimulus (shear stress) in this type of test, thereby reflecting the elasticity of the sample. The lower the phase angle, the more elastic the material. The yield stress (a point where internal structure yields under the applied stress and starts flowing) was quantified as the shear stress at which the complex modulus decreased by 10% after departing the linear viscoelastic region [21].
2.2.4. Emulsion Characterisation: Texture Analysis
An immersion/de-immersion test was carried out using the TA.XT Plus Texture Analyser (Stable Micro Systems, Godalming, UK), using a 1-inch cylinder probe P/0.5R. The pre-test speed was 1 mm/s, the test speed was 1.5 mm/s and the post-test speed was 10 mm/s. The immersion distance was 15 mm and the trigger force was 5 g. All experiments were performed in triplicate, at 22.0 °C ± 1.0 °C, using the accompanying Exponent software, v. 6. The highest positive force (firmness) and positive area under the curve (work of penetration) were detected from all tests.
2.2.5. Thermal Analysis by Differential Scanning Calorimetry (DSC)
Small samples (10–20 mg) of the premixed oil phases of standard and natural formulations of both emulsion types (except cold processed) were heated and cooled at 5 °C/min in the DSC 3 STAR System Differential Scanning Calorimeter (Mettler Toledo, Greifensee, Switzerland), alongside a thermally inert reference substance. The accompanying STARe Excellence software, v. 12.1 (Mettler Toledo, Switzerland) was used to provide the thermograms, including melting and crystallisation ranges.
2.2.6. Thermal Energy Calculations
The energy consumed during emulsion manufacturing includes mechanical energy for mixing and homogenisation, and thermal energy for heating and cooling, with the heating energy being predominant [4]. Therefore, the amount of thermal energy that would be consumed to manufacture 500 kg of product in a typical manufacturing vessel was calculated for each of the emulsions used in this study. This was carried out in order to establish the percentage of energy saved when using hot-cold and cold emulsification processes.
Equation (5) [22] was used to calculate thermal energy consumption:
where Q is heat energy (J), m is mass (kg), Cp is specific heat capacity (J/kg·K) and ΔT is the temperature difference (K). The equation used the input values from Table 5 and Table 6. The resulting energy value was doubled because the same amount of energy input is required for both heating and cooling the vessel [22]. The main component of the oil phase was used as a representative of the heat capacity value for the entire oil phase.

Table 5.
Input values for the thermal energy equation for O/W emulsions.

Table 6.
Input values for the thermal energy equation for W/O emulsions.
2.2.7. Life Cycle Analysis
Life cycle analysis (LCA, also known as life cycle assessment) assesses the environmental impact of a product throughout its life cycle [1]. In this study, Benchmark Consulting’s proprietary software was used to calculate the carbon footprint (CO2e) of 500 kg of each emulsion. The cradle-to-gate (from raw material extraction to the product leaving the factory) processes were considered, including raw material extraction, manufacturing and packaging. The best available report for each raw material from the Ecoinvent 3.8 database (Ecoinvent, Switzerland) provided the CO2e figures following the global warming potential of 100 years, as advocated by the Intergovernmental Panel on Climate Change [3]. Raw material reports from Europe were used where available; otherwise, global or rest-of-the-world reports were selected. Only two ingredients had CO2e figures available from their supplier: PEG-7 glyceryl cocoate, used in the standard O/W formulation, and cetyl PEG/PPG-10/1 dimethicone, used in the standard W/O formulation. Both were provided by Evonik [25].
Benchmark Consulting software used its “extruder” machine to model the emulsion manufacturing process. Machine input values included running energy usage (thermal energy consumption converted into kWh), set-up and indirect energy usage (Ecoinvent 3.8) during a 150 min manufacturing time [16]. All the final CO2e figures obtained considered the average European electricity production since different countries use different mixes of electricity sources.
3. Results
3.1. The Results of Rheological Tests
Since the aim of this study was to answer questions regarding the carbon footprint of emulsions, not the development of stable products for commercial use, full long-term stability testing was not carried out. However, a short-term stability test, known as the centrifugal stress test, was performed. All 12 test samples passed it without phase separation, enabling the study to continue.
The formulations of hot and hot-cold processed emulsions were identical (Table 1 and Table 2), which allowed for the comparison of the effect of the manufacturing process on the emulsion’s internal structure. However, the cold-processed emulsions had to undergo a re-formulation process, which proved challenging for both standard and natural variants and for both formulation types. The criteria for successful reformulation were organoleptic and sensorial, aiming to replicate the properties of the original emulsion. The internal structure was then assessed by two rheological methods, continuous flow and oscillatory, which are particularly informative when used in tandem [21].
Figure 4 shows the results of the shear rate sweep and oscillatory stress sweep tests, respectively, for the standard (a and c) and natural formulations (b and d) of the O/W emulsion type. The viscosity curves are shown up to 20 s−1 since they mostly overlap for the rest of the shear rate region. As expected, all emulsions showed shear-thinning behaviour, with yield stress and thixotropy. The numerical results are presented in Table 7, alongside the values of yield stress, obtained from the oscillatory stress sweep. The two emulsions that show different yield values are natural hot and natural hot-cold samples, which require a much lower shear force in order to get the material flowing. In terms of thixotropy, natural hot-cold samples had the lowest and natural cold samples had the highest levels. The latter, which is an adjusted formulation for cold processing, has also displayed the highest viscosity at low shear rates (Figure 4b) and different rigidity (complex modulus G*) and elasticity (phase angle δ) (Figure 4d).
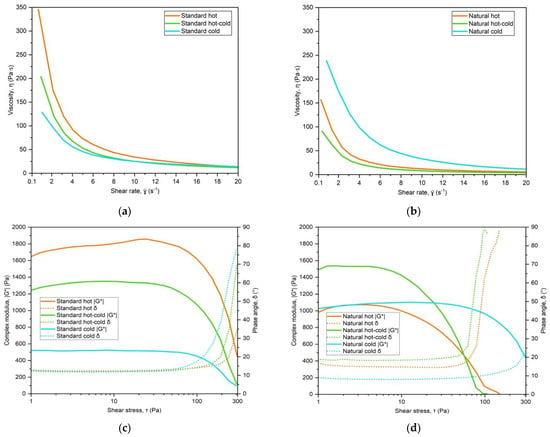
Figure 4.
Viscosity curves (a,b) and the results of oscillatory stress sweep: complex modulus G* and phase angle δ (c,d), for standard and natural O/W emulsions, obtained by hot, hot-cold and cold emulsification process.

Table 7.
The level of thixotropy, expressed as the hysteresis loop area, and the yield stress values of O/W emulsions.
Figure 5 presents the same four sets of results as above, but for the W/O emulsion type. It can be observed that the low shear rate viscosity was the highest for both hot-cold samples, even more pronounced in the case of the natural formulation (Figure 5b). This was reflected in the large hysteresis area and high yield stress for the natural hot-cold sample (Table 8). It also had the highest rigidity and elasticity of all samples (Figure 5d). This leads to the conclusion that the hot-cold process has produced a different internal structure than the hot process for the same formulation.
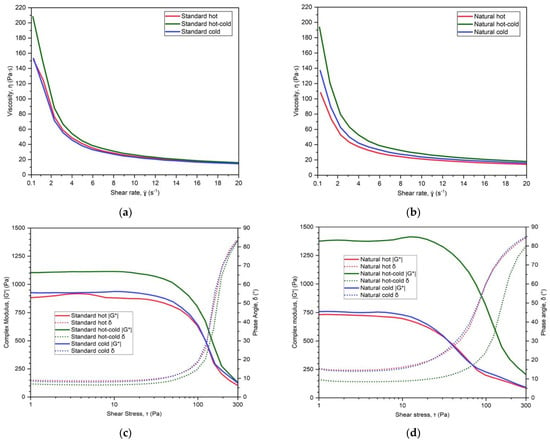
Figure 5.
Viscosity curves (a,b) and the results of oscillatory stress sweep: complex modulus G* and phase angle δ (c,d), for standard and natural W/O emulsions, obtained by hot, hot-cold and cold emulsification process.

Table 8.
The level of thixotropy, expressed as the hysteresis loop area, and the yield stress values of W/O emulsions.
It is important to note that identifying the above small differences was possible due to very sensitive rheological methods, specifically oscillatory stress sweep. It uses very small forces, initially below the yield point of each sample, which are normally not experienced while the product is in use. It could be observed from Figure 4c,d, as well as from Figure 5c,d, that the differences among samples in both rigidity (G*) and elasticity (δ) start to diminish as the force increases above the yield point. Indeed, the viscosity curves show differences only at very low shear rates. This indicated that at the shear rates used during product application of 120 s−1 [26] and higher, the sensory differences would be very small, hence the above two sets of samples were suitable for further study.
3.2. The Results of Texture Analysis
Being sophisticated penetrometry, the texture analysis has complemented the information about the internal structure obtained from rheology. Figure 6 and Figure 7 present the results of the immersion/de-immersion tests for all 12 samples. In the case of O/W emulsions, the natural cold sample has shown the highest firmness and work of penetration (Table 9), mirroring the rheological findings. The three standard samples, including the cold process one, have revealed very similar textural properties (Figure 6a).
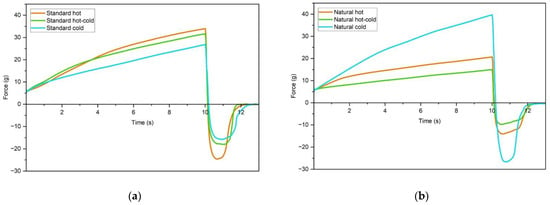
Figure 6.
Immersion/de-immersion curves of O/W emulsions for standard (a) and natural (b) hot, hot-cold and cold process.
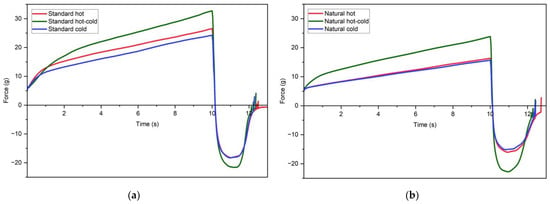
Figure 7.
Immersion/de-immersion curves of W/O emulsions for standard (a) and natural (b) hot, hot-cold and cold process.

Table 9.
Firmness (maximal positive force) and work of penetration (positive area under the curve) for O/W emulsions.
In the case of W/O emulsions, standard formulations had higher firmness and work of penetration values than their natural counterparts (Figure 7, Table 10). For both standard and natural formulations, the hot-cold process formulations had the highest firmness and work of penetration values, which again mirror the results of rheological tests.

Table 10.
Firmness (maximal positive force) and work of penetration (positive area under the curve) for W/O emulsions.
Unlike oscillatory rheology, texture analysis was not sensitive enough to detect the fine differences in the internal structure. For example, the immersion/de-immersion graphs for hot and cold-processed emulsions of both standard and natural formulations look almost identical (Figure 7). This further reinforces the assumption that the sensory differences would be difficult to detect.
3.3. The Results of Thermal Analysis and Thermal Energy Calculations
To check whether the melting point of an oil mixture was the same as the melting point of the highest melting wax, the DSC analysis of all oil phases (except those intended for cold processing) was carried out, and the results are presented in Table 11. Since the melting temperature is not a well-defined point, two temperatures were considered: The onset point, when the material started to melt, and the offset point, where the melting was complete. Moreover, it was important to detect the same for the crystallisation process, specifically its onset point. If the melting temperature used in the hot-cold manufacturing was too close to the onset of crystallisation, this would not allow for the complete melting of the waxes and would have a negative effect on the quality of the emulsion. Normally, a difference of approximately 10 °C between the end of melting and the beginning of crystallisation should be established. For example, in the case of the O/W standard formulation (Table 11), the oil phase should not be melted at 33 °C since the crystallisation starts at 27 °C. The recommended temperature would be around 40 °C, which is still 25 °C less than the temperature that was used.

Table 11.
Results of the differential scanning calorimetry (DSC) analysis of the oil and waxes mixtures used as oil phases in the formulations that required thermal energy input, with the used and recommended melting temperatures.
Table 11 shows that the O/W natural and the W/O standard formulations could not be made by using lower melting temperatures. However, the W/O natural sample could be manufactured by using a 19 °C lower melting temperature than the one commonly used. Since the melting temperature is directly related to the thermal energy used to heat the oil bulk, Equation (5) was used to calculate how much of it could be saved if the heating was reduced. This saving would apply to both hot and hot-cold processes. It is evident from Table 12 that more than 54% and 30% of the thermal energy used for melting could be saved in the case of O/W standard and W/O natural formulations, respectively.

Table 12.
Thermal energy required for the melting and cooling of the oil phase in order to make 500 kg of emulsion: energy normally used, based on the highest melting point wax in the oil phase; minimum energy required, based on the melting point of the whole oil phase obtained by differential scanning calorimetry (DSC); and the percentage of the energy that could be saved.
3.4. The Results of Life Cycle Analysis
Table 13 and Table 14 show the results of the cradle-to-gate LCA for the O/W and W/O emulsions, respectively, calculated for a typical production batch of 500 kg. Original input and output values of the Benchmark Consulting software are provided in the Supplementary Material (Spreadsheet S1 for O/W emulsions and Spreadsheet S2 for W/O emulsions).

Table 13.
Carbon footprint figures (in CO2e for 100 years) for 500 kg of O/W emulsions, produced by Benchmark Consulting and based on Ecoinvent 3.8 sources; Benchmark Consulting is ISO 14067- accredited.

Table 14.
Carbon footprint figures (in CO2e for 100 years) for 500 kg of W/O emulsions, produced by Benchmark Consulting and based on Ecoinvent 3.8 sources; Benchmark Consulting is ISO 14067- accredited.
There are several interesting points concerning the LCA results. Firstly, the CO2e figures for all natural formulations used in this study were higher than those for the standard ones. This is not surprising, knowing how resource-intensive the cultivation of plants normally is, but it goes contrary to the accepted notion that natural formulations are “better for the environment”. Comprehensive evidence for that statement was never presented, while these results reveal the opposite. However, it is important to keep in mind that these are just two examples and that a much wider range of emulsions should be assessed in order to draw a general conclusion. It is possible that in some cases the CO2e figures for natural formulations would be lower than for synthetic or mixed formulations, but it is certainly not a general rule.
Using Equation (5), the savings in thermal energy when switching from a hot to a hot-cold process for the O/W emulsions were calculated to be 83% and 82% for the standard and natural formulations, respectively. Theoretically, the use of a cold process saves 100% of the thermal energy. As can be seen from Table 13, the effect of those savings on the CO2e figures for manufacturing and the total CO2e figures is lower because of the effects of other factors during the product’s journey from cradle to gate. In the case of O/W emulsions, the reduction becomes 27% from hot to hot-cold, 29% from hot to cold for the standard formulations, and 25% and 29% for the natural ones. This makes the overall CO2e savings only 2–4% higher when switching to the cold instead of the hot-cold manufacturing process, while it requires considerable changes in the formulation and possibly equipment.
When applied to W/O emulsions, the energy calculations demonstrated that the hot-cold manufacturing process saves approximately 86% and 85% of thermal energy in comparison to the hot process for the standard and natural formulations, respectively. The LCA results confirmed that the manufacturing process has a major impact on the carbon footprint of the final emulsion, with approximately 25% and 29% CO2e reductions from the hot process to the hot-cold and cold process, respectively, for both standard and natural formulations. This provides an overall CO2e reduction figure of 4% when using a cold instead of a hot-cold emulsification process, similar to the one obtained for the O/W emulsions.
It is important to note from Table 13 and Table 14 that the impact of ingredients on the total product’s CO2e is dwarfed by the impact of manufacturing (i.e., the use of energy). It is considerably smaller than the impact of packaging, which in these theoretical examples is taken to be the same in all cases. Therefore, for an immediate and measurable positive impact on the environment, manufacturers should switch from a hot to a hot-cold emulsification process.
4. Discussion
Scale-up is the most critical parameter in the transfer of a cosmetic formulation from a laboratory prototype to a commercial product. Its purpose is to ensure repeatable large-scale production of a formulation with the same characteristics as laboratory-produced formulations, which is not always straightforward [27]. The scale-up process tends to affect the droplet size distribution in emulsions, which in turn affects their viscosity and sensory properties. Higher specific energy input, often achieved through a higher homogenisation speed and/or longer time, can decrease the droplet size of emulsions [28,29,30]. This means there are more droplets within the same volume and thus a rise in interactions between them, which increases viscosity [14]. This effect is particularly notable at disperse phase concentrations above 60%, which are present within the W/O samples used in this study, since the droplets cannot easily flow past each other and are more likely to be deformed during homogenisation [28,29]. This problem is also seen in cold process emulsification, whereby the scale-up process decreased mean droplet size and increased viscosity [31].
The purpose of scale-down calculations is to minimise the differences in shear rate and mass specific power, and thus the overall energy incorporated into the emulsion, between laboratory and manufacturing scales [30]. This approach should reduce the variability between the laboratory and pilot batches, making the transition faster. Thus, by following the processes of gathering the required information and doing simple calculations described by Equations (1)–(4), manufacturers could reduce both product waste and energy consumption.
Rheological characterisation was used in this study with the main aim of establishing sufficient similarity among the test samples of the same emulsion type in order to take them further into the project. The secondary aim was to assess any differences in the internal structure due to different manufacturing conditions. Based on previous studies [21], a combination of continuous flow and oscillatory tests was used, supported by texture analysis. The shear rate sweep test measured the resistance of materials to flow (viscosity) when exposed to increasing shear rates, from 0.1 to 100 s−1. To assess the level of its structural breakdown and subsequent recovery (the level of thixotropy), a reverse shear rate sweep was performed immediately after. This was accompanied by the oscillatory stress sweep to establish the end of the linear viscoelastic region and the yield stress, as well as the rigidity (complex modulus G*) and elasticity (phase angle δ) of the samples.
In terms of O/W emulsions, hot formulations of both emulsion types have displayed higher low shear rate viscosity than hot-cold, but this has not always translated into higher rigidity (Figure 4). This was the opposite of what was observed in W/O emulsions (Figure 5), whereby the hot-cold process consistently produced higher viscosity and rigidity. This indicates that the mechanisms of semi-solid structuring in the two emulsion types have been affected differently by changes in manufacturing conditions, predominantly the cooling rate during emulsification. In all hot and hot-cold O/W formulations, the main solidification mechanism is the formation of the lamellar gel network, which can withstand elastic deformation and have complex flow properties [32]. Above a certain transition temperature, the cetearyl alcohol/emulsifier mixture in water forms a liquid crystalline Lα phase with a positional disorder of alkyl chains in the plane of lamellae. Upon cooling the positional disorder can transform into hexagonal order, forming a stiffer gel phase known as the Lβ phase [33]. This process is reduced in hot-cold formulations due to the much shorter cooling time, which is something to keep in mind when trying to replicate the rheological properties of the hot-process formulation by using a hot-cold process.
For both standard and natural W/O emulsions, the hot-cold formulations have revealed a stronger internal structure than the hot-processed ones (Figure 5). The oil phase was still heated to the same temperature, but with the addition of the room-temperature water phase, the oil phase would have cooled much quicker during the hot-cold process than the hot process. Cooling rate is an important factor that can impact the crystals formed by a wax in an oil [34]. Multiple studies have demonstrated that the rapid cooling of an oil phase leads to the formation of smaller, non-aggregated wax crystals, which generally produce a weaker network [34,35,36]. However, in this case, the flash cooling of the oil phase occurs simultaneously with the emulsification process, where water droplet formation could have further affected wax crystallisation behaviour [37] and led to the opposite effect.
Because the same formulations have produced higher viscosity using a different manufacturing process, the hot-cold approach provides the possibility of additional savings in materials and energy; hence, it is worth further investigation. However, if structural and therefore rheological and textural changes due to differing cooling rates are not desired, they could be minimised by exploring a hot-warm or low-energy emulsification process in which the oil phase is not cooled down as quickly [8].
In both types of natural formulations, standard synthetic thickeners had to be replaced by COSMOS-approved ones. In O/W emulsions, it was a combination of xanthan gum and bentonite clay (Table 1). The largest hysteresis loop for the O/W natural cold sample is due to the known property of bentonite clay to exert thixotropy because the hydrogen bonds between the swollen clay platelets are easily disrupted by external forces [38].
In contrast, the structuring in the cold W/O emulsions was controlled by the addition of zinc stearate and the volume of the internal (dispersed) water phase. Disperse phase volume is one of two factors found to significantly affect the viscosity of water-in-crude oil emulsions [39]. The increase in viscosity is caused by the higher number of droplets per unit volume and thus more intense interactions among droplets [35]. The increase in disperse phase volume from 74% to 77% has made the standard cold sample a high internal phase emulsion (HIPE), surpassing the close packing limit of 74% [40]. In HIPEs, the droplets are packed tightly and may adopt polygonal shapes [40], but despite that, the same effect of increased viscosity with the increase in the disperse phase volume was observed [29]. This is explained by the decrease in thickness of the continuous layer between droplets, which leads to lower flowability. In conclusion, the addition of zinc stearate and the increase in disperse phase volume in W/O formulations could achieve similar thickening effects as the addition of wax.
The results of texture analysis (Figure 6 and Figure 7) have corroborated the rheological ones, as expected [30]. Particularly, higher firmness was aligned with higher yield stress values, while higher work of penetration correlated with a higher initial viscosity and complex modulus, which matches the findings of Lukic et al. [41]. Texture analysis can also provide insight into the sensorial experience of products. More than 30 years ago, Pompei et al. [42] found that the results of instrumental penetration tests correlate with the sensory panel evaluation of spreadability. More recently, it has been shown that spreadability significantly correlates with firmness [43] and the work of penetration [44], both obtained by texture analysis.
Any potential differences in consumer perception, due to small differences in emulsion structure, could be easily overcome by fine-tuning the formulations until rheologically and texturally identical emulsions are achieved. The same approach would apply to any stability issue that might become apparent with longer stability testing. This would not lead to any notable changes in the use of ingredients or energy. Therefore, the 12 formulations used in this study present a suitable model for the assessment of their thermal energy consumption and total carbon footprint during manufacturing.
The specific heat capacity of water is known to be 4.184 J/kg·K [10], approximately double that for oils. With the high amount of water in commercial emulsions of both types, it is evident that this has the highest impact on energy consumption. When the specific heat capacity value for a particular oil was not available, an average value for the mixed oil phase of 2100 J/kg·K was used [45], which is something that could be improved with further research. Despite the differences in emulsion type and the origin of ingredients among the 12 test samples, the results have shown that a reduction of 82–87% of thermal energy is possible when switching from a hot to a hot-cold emulsification. The fact is that the switch from hot to cold process saves 100% thermal energy, but at the expense of the same differences in the emulsion’s internal structure and its stability profile due to the change in its thickening/stabilising mechanism.
Beyond the above energy savings, the effect of lowering the manufacturing temperature on thermal energy consumption was explored. Approximately 54% and 30% reduction in thermal energy, with a 25 °C and 19 °C decrease in manufacturing temperature, respectively, was achievable (Table 11 and Table 12). This highlights the importance of using the lowest possible manufacturing temperature for a hot or hot-cold process, either by the preliminary DSC analysis or by selecting lower melting point waxes.
The results of LCA revealed an expected reduction in the manufacturing CO2e figure for the hot-cold and cold processes, followed by a reduction in the total carbon footprint CO2e figures (Table 13 and Table 14). The main decrease in overall carbon footprint was observed from the hot to the cold manufacturing process (27–29%), but it was only 2–4% better than from the hot to the hot-cold process. This reduction was much lower than the one obtained from thermal energy calculations because the manufacturing CO2e figure also includes indirect energy usage (such as lights, computers, etc.) and equipment set-up, which can also be very carbon intensive.
Another limitation of the manufacturing CO2e figure used here was that the same manufacturing time was used for the different processes, while the use of colder processes should reduce the overall manufacturing time [7,8,11]. This is not only due to the reduction in heating time but also a big reduction in cooling time, which is necessary even in cold process emulsification because the emulsion heats during homogenisation [11].
The LCA software was adopted from the packaging industry, so the machines used were called “extruders”. The packaging itself was represented by the process of wrapping a finished, manufactured plastic film around a core. In principle, this acts as a placeholder for the process of filling creams into their jars and closing the lids, ready to leave the factory [46]. Although this aspect could be more precise, it does not affect the comparison between the test formulations since the CO2e was identical for all of them.
In the case of the studied formulations, the total CO2e figure of natural raw materials was much higher than that of the standard (Table 13 and Table 14). However, as the overall carbon footprint between standard and natural formulations remained similar, this suggests that the total carbon footprint was dominated by that of the manufacturing process. This opposes Bom, Ribeiro and Marto [5], who assigned raw material selection a higher importance than product manufacture when comparing product life cycle phases based on the opinions of cosmetic industry experts. This also indicates that the small changes necessary to fine-tune the formulations would not have any noticeable effect on the total product’s CO2e.
While these findings are highly dependent on the individual ingredients, they reiterate the fact that natural raw materials are not automatically more sustainable than synthetic raw materials [5,6]. Beyond the natural/synthetic origin of the ingredient, the way the ingredient is synthesised or extracted largely contributes to its sustainability [5]. While extensive energy is consumed in the production of synthetic and semi-synthetic raw materials, the farming, extraction and processing of natural plant-based raw materials can also be very energy intensive [4]. Farming requires energy for machinery, irrigation and fertiliser production [4]. Post-harvest processing and extraction of natural ingredients may require energy for heating, which Glew and Lovett [47] have found to contribute over 75% of the total emissions and carbon footprint of the common natural cosmetic ingredient, shea butter.
One limitation of the current LCA is that the Ecoinvent database does not have reports on every raw material, meaning that the best available reports on similar or constituent ingredients had to be used. The LCA used in this study only considered the climate change impact category, which is the global warming potential of greenhouse gas emissions to the air (in kg CO2e) from fossil and biogenic sources and land use change known as deforestation [48]. There are other aspects of environmental ingredient sustainability, such as emissions to soil and water, biodegradability pattern, bioaccumulation potential, aquatic toxicity, water consumption during production, waste formation, feedstock renewability and risks to animal preservation [6], that are not being considered.
5. Conclusions
This project aimed to compare the thermal energy consumption and cradle-to-gate carbon footprint of physico-chemically comparable standard and natural emulsions manufactured using hot, hot-cold and cold-process emulsification.
Results revealed that rheologically and texturally similar emulsions of both O/W and W/O types could be achieved using different manufacturing processes, although the cold process required considerable re-formulation work. In the case of the W/O emulsions, the hot-cold process resulted in a more viscous, more elastic and firmer cream than its hot-process counterpart. This is an observation that should be explored further as a possible way to save ingredients. However, a wider variety of W/O emulsions would need to be studied in order to draw a more general conclusion in this respect.
The introduced scale-down calculations may help reduce energy consumption, as they can ensure that no more mechanical energy input than needed is used during manufacturing, alongside reducing the need for intermediate pilot-scale batches.
Energy calculations have shown that the bulk (82–87%) of thermal energy, the major type of energy used during emulsion manufacture, would be saved when using a hot-cold emulsification process. While cold process emulsification theoretically saves 100% of thermal energy, it brings about limitations regarding suitable ingredients and challenges in terms of emulsion stability. Overall, cold emulsification presents a less efficient way of achieving sustainability through energy reduction than a hot-cold approach.
The LCA results confirmed that the reduction in thermal energy had a major impact on the cradle-to-gate carbon footprint of 500 kg of final emulsions, which was in the region of 25–29%. For O/W emulsions, the differences in CO2e between the hot-cold and cold process were only 2% for standard formulations and 4% for natural formulations, while for W/O emulsions, the difference was 2% in both cases.
Contrary to expectations, the natural formulations used in this study had a higher carbon footprint than standard formulations. Although dependent on individual raw materials, this finding suggests that cosmetic brands should be cautious when stating the sustainability benefits of natural products without concrete evidence. The use of LCA, with its granular approach, accurate calculations and transparent reporting, should provide the necessary evidence and enable companies to make informed choices.
Overall, this study has shown that the cosmetic industry could address its high energy consumption and consequent contribution to global warming more effectively by changing the manufacturing process than by changing its formulations to those with natural ingredients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics10050132/s1, Spreadsheet S1: LCA Output Tables for O/W Emulsions; Spreadsheet S2: LCA Output Tables for W/O Emulsions.
Author Contributions
Conceptualization, S.T. and E.D.; methodology, S.T. and L.J.F.; software, T.B.; data analysis, S.B.; investigation, J.F. and S.M.; resources, S.T., L.J.F., T.B. and E.D.; writing—original draft preparation, S.T., J.F. and S.M.; writing—review and editing, S.T., J.F and S.M.; visualization, J.F. and S.M.; supervision, S.T.; project management, S.T.; J.F. and S.M. have contributed equally to this project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
3rd Party Data-Restrictions apply to the availability of these data. Data were obtained from Benchmark Global Consulting Ltd. and are available from the authors with the permission of Benchmark Global Consulting Ltd. The Spreadsheets S1 and S2 are submitted with the permission of Benchmark Global Consulting Ltd.
Acknowledgments
The authors would like to acknowledge Surfachem (UK) for the donations of raw materials and Neil’s Yard Remedies (UK) for the sharing of technical information regarding emulsion manufacturing. They are also thankful to Natasha Csonge for technical laboratory support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vital, X. Environmental Impacts of Cosmetic Products, Part 1: The Growing Importance of Metrics. In Sustainability: How the Cosmetics Industry Is Greening Up; Sahota, A., Ed.; John Wiley & Sons: New York, NY, USA, 2014; pp. 17–31. [Google Scholar]
- Van Geem, K.M.; Weckhuysen, B.M. Toward an e-chemistree: Materials for electrification of the chemical industry. MRS Bull. 2021, 46, 1187–1196. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Bennett, C.J.; Brown, M.S. Energy and Waste Management. In Sustainability: How the Cosmetics Industry Is Greening Up; Sahota, A., Ed.; John Wiley & Sons: New York, NY, USA, 2014; pp. 155–174. [Google Scholar]
- Bom, S.; Ribeiro, H.M.; Marto, J. Sustainability Calculator: A Tool to Assess Sustainability in Cosmetic Products. Sustainability 2020, 12, 1437. [Google Scholar] [CrossRef]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A Step Forward on Sustainability in the Cosmetics Industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Cold Processing of Emulsions. Available online: https://www.cosmeticsandtoiletries.com/research/methods-tools/article/21835518/cold-processing-of-emulsions (accessed on 26 April 2023).
- Lin, T.J. Low energy emulsification I: Principles and applications. J. Soc. Cosmet. Chem. 1978, 29, 117–125. [Google Scholar]
- Formulating Water-in-Oil Emulsions: A Scary Endeavor. Available online: https://www.cosmeticsandtoiletries.com/research/methods-tools/article/21833970/formulating-water-in-oil-emulsions-a-scary-endeavor (accessed on 26 April 2023).
- Specific Heat Capacity and Water. Available online: https://www.usgs.gov/special-topics/water-science-school/science/specific-heat-capacity-and-water (accessed on 10 April 2023).
- Raposo, S.; Salgado, A.; Eccleston, G.; Urbano, M.; Ribeiro, H.M. Cold processed oil-in-water emulsions for dermatological purpose: Formulation design and structure analysis. Pharm. Dev. Technol. 2014, 19, 417–429. [Google Scholar] [CrossRef]
- Lin, T.J. Low energy emulsification II: Evaluation of emulsion quality. J. Soc. Cosmet. Chem. 1978, 29, 745–756. [Google Scholar]
- Tamburic, S.; Craig, D.Q.M.; Vuleta, G.; Milic, J. An investigation into the use of thermorheology and texture analysis in the evaluation of W/O creams stabilized with a silicone emulsifier. Pharm. Dev. Technol. 1996, 1, 299–306. [Google Scholar] [CrossRef]
- Block, L.H. Non-Parenteral Liquids and Semisolids. In Pharmaceutical Process Scale-Up; Levin, M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 89–128. [Google Scholar]
- Bom, S.; Fitas, M.; Martins, A.M.; Pinto, P.; Ribeiro, H.M.; Marto, J. Replacing synthetic ingredients by sustainable natural alternatives: A case study using topical O/W emulsions. Molecules 2020, 25, 4887. [Google Scholar] [CrossRef]
- Neal’s Yard Remedies. Manufacturing Method for Face and Body Lotion. Intern. Commun. 2022. [Google Scholar]
- How to Convert RPM to Linear Speed. Available online: https://sciencing.com/convert-rpm-linear-speed-8232280.html (accessed on 29 April 2023).
- Malvern Instruments. A Basic Introduction to Rheology. Available online: https://cdn.technologynetworks.com/TN/Resources/PDF/WP160620BasicIntroRheology.pdf (accessed on 29 April 2023).
- How to Calculate Time to Heat Water. Available online: https://sciencing.com/calculate-temperature-btu-6402970.html (accessed on 29 April 2023).
- IFSCC. IFSCC Monograph Number 2 The Fundamentals of Stability Testing; Micelle Press: Dorset, UK, 1992; pp. 1–23. [Google Scholar]
- Tamburic, S.; Sisson, H.; Cunningham, N.; Stevic, M. Rheological and texture analysis methods for quantifying yield value and level of thixotropy. SOFW J. 2017, 143, 24–30. [Google Scholar]
- Al-Shemmeri, T. Engineering Thermodynamics; Ventus Publishing ApS: Copenhagen, Denmark, 2010; pp. 1–96. [Google Scholar]
- Rojas, E.E.G.; Coimbra, J.S.; Telis-Romero, J. Thermophysical properties of cotton, canola, sunflower and soybean oils as a function of temperature. Int. J. Food Prop. 2013, 16, 1620–1629. [Google Scholar] [CrossRef]
- Nadolny, Z.; Dombek, G. Thermal properties of mixtures of mineral oil and natural ester in terms of their application in the transformer. E3S Web Conf. 2017, 19, 01040. [Google Scholar] [CrossRef]
- Evonik. Carbon Footprint & Feedstock. Intern. Commun. 2023. [Google Scholar]
- Henderson, N.L.; Meer, P.M.; Kostenbauder, H.B. Approximate Rates of Shear Encountered in Some Pharmaceutical Processes. J. Pharm. Sci. 1961, 50, 788–791. [Google Scholar] [CrossRef]
- Durgun, M.E.; Yapar, E.A. A critical step for the cosmetic industry: Scale-up. Univers. J. Pharm. Res. 2021, 6, 77–82. [Google Scholar] [CrossRef]
- Pal, R. Shear Viscosity Behavior of Emulsions of Two Immiscible Liquids. J. Colloid Interface Sci. 2000, 225, 359–366. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhattacharya, A.; Singh, R.; Tabor, R.F. Rheological behavior of high internal phase water-in-oil emulsions: Effects of droplet size, phase mass fractions, salt concentration and aging. Chem. Eng. Sci. 2017, 174, 290–301. [Google Scholar] [CrossRef]
- Calvo, F.; Gomez, J.M.; Alvarez, O.; Ricardez-Sandoval, L. Effect of emulsification parameters on the rheology, texture, and physical stability of cosmetic emulsions: A multiscale approach. Chem. Eng. Res. Des. 2022, 186, 407–415. [Google Scholar] [CrossRef]
- Raposo, S.; Urbano, M.; Ribeiro, H.M. Scale up of a Low Energy Process for the Production of Oil in Water Emulsions. Athens J. Health 2015, 2, 21–30. [Google Scholar] [CrossRef]
- Wang, F.; Larson, R.G. Thixotropic constitutive modelling of shear banding by boundary-induced modulus gradient in lamellar gel networks. J. Rheol. 2023, 67, 35–51. [Google Scholar] [CrossRef]
- Datta, A.; Tanmay, V.S.; Tan, G.X.; Reynolds, G.W.; Jamadagni, S.N.; Larson, R.G. Characterizing the rheology, slip, and velocity profiles of lamellar gel networks. J. Rheol. 2020, 64, 851–862. [Google Scholar] [CrossRef]
- Jana, S.; Martini, S. Effect of high-intensity ultrasound and cooling rate on the crystallization behavior of beeswax in edible oils. J. Agric. Food Chem. 2014, 62, 10192–10202. [Google Scholar] [CrossRef]
- Thompson, D.G.; Taylor, A.S.; Graham, D.E. Emulsification and demulsification related to crude oil production. Colloids Surf. 1985, 15, 175–189. [Google Scholar] [CrossRef]
- Chen, S.; Øye, G.; Sjöblom, J. Rheological Properties of Model and Crude Oil Systems when Wax Precipitate under Quiescent and Flowing Conditions. J. Dispers. Sci. Technol. 2007, 28, 1020–1029. [Google Scholar] [CrossRef]
- Freitas, G.B.; Duncke, A.C.; Barbato, C.N.; de Oliveira, M.C.; Pinto, J.C.; Nele, M. Influence of wax chemical structure on W/O emulsion rheology and stability. Colloids Surf. A Physicochem. Eng. 2018, 558, 45–56. [Google Scholar] [CrossRef]
- IFSCC. IFSCC Monograph Number 3 An Introduction to Rheology; Micelle Press: Dorset, UK, 1997; pp. 1–35. [Google Scholar]
- Kralova, I.; Sjöblom, J.; Øye, G.; Simon, S.; Grimes, B.A.; Paso, K. Heavy Crude Oils/Particle Stabilized Emulsions. Adv. Colloid Interface Sci. 2011, 169, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of recent advances in the preparation, properties, and applications of high internal phase emulsions. Trends Food Sci. Technol. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Cekic, N.; Savic, S. A combined approach in characterization of an effective w/o hand cream: The influence of emollient on textural, sensorial and in vivo skin performance. Int. J. Cosmet. Sci. 2012, 34, 140–149. [Google Scholar] [CrossRef]
- Pompei, C.; Lucisano, M.; Zanoni, B.; Casiraghi, E. Evaluation of spreadability of food products by penetration tests and panel scores. J. Food Sci. 1988, 53, 592–596. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Sousa Lobo, J.M. Comparison between sensory and instrumental characterization of topical formulations: Impact of thickening agents. Int. J. Cosmet. Sci. 2016, 38, 389–398. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B Biointerfaces 2013, 102, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Huber, P. Energy Efficient production of Cosmetics. In Proceedings of the SCS Annual Conference, Cambridge, UK, 4–5 July 2023. [Google Scholar]
- Filling and Closing Line for Cream, Lotion, Makeup and Much More. Available online: https://www.groninger-group.com/en/cosmetics/cosmetic-filling-machines/creme-60/ (accessed on 28 April 2023).
- Glew, D.; Lovett, P.N. Life cycle analysis of shea butter use in cosmetics: From parklands to product, low carbon opportunities. J. Clean. Prod. 2014, 68, 73–80. [Google Scholar] [CrossRef]
- Impact Categories (LCA)—Overview. Available online: https://ecochain.com/knowledge/impact-categories-lca/ (accessed on 28 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).