Improvement of Human Epidermal Barrier Structure and Lipid Profile in Xerotic- and Atopic-Prone Skin via Application of a Plant-Oil and Urea Containing pH 4.5 Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Ethical Considerations, and Subjects
2.2. Test Product Specification
2.3. Non-Invasive Biophysical Measurements
2.4. Visual and Subjective Skin Evaluation
2.5. SC Lipid Lamellae and Profile Analysis
2.6. Statistical Analysis
3. Results
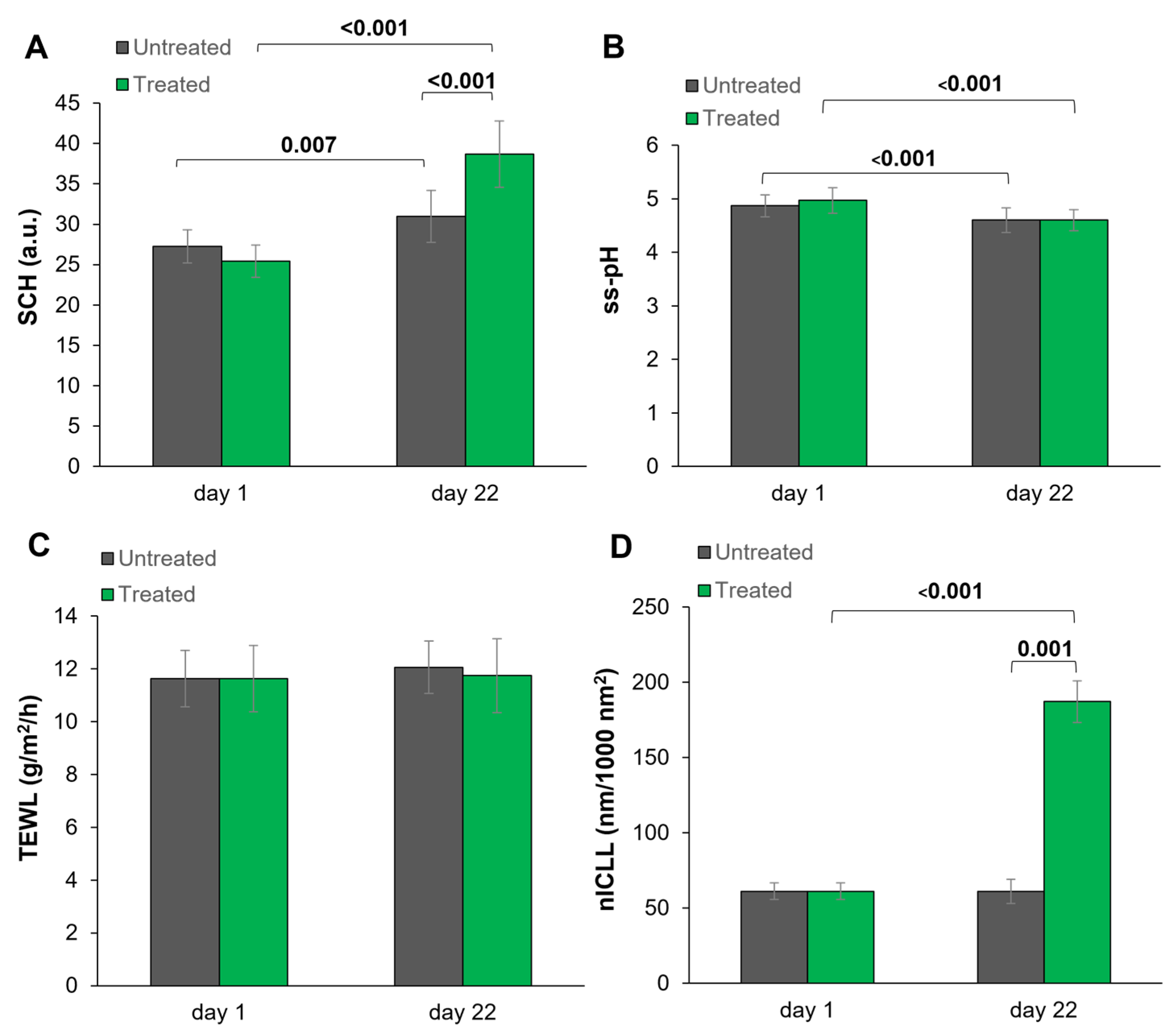
3.1. Non-Invasive Biophysical Measurements
3.2. Visual and Subjective Skin Evaluation
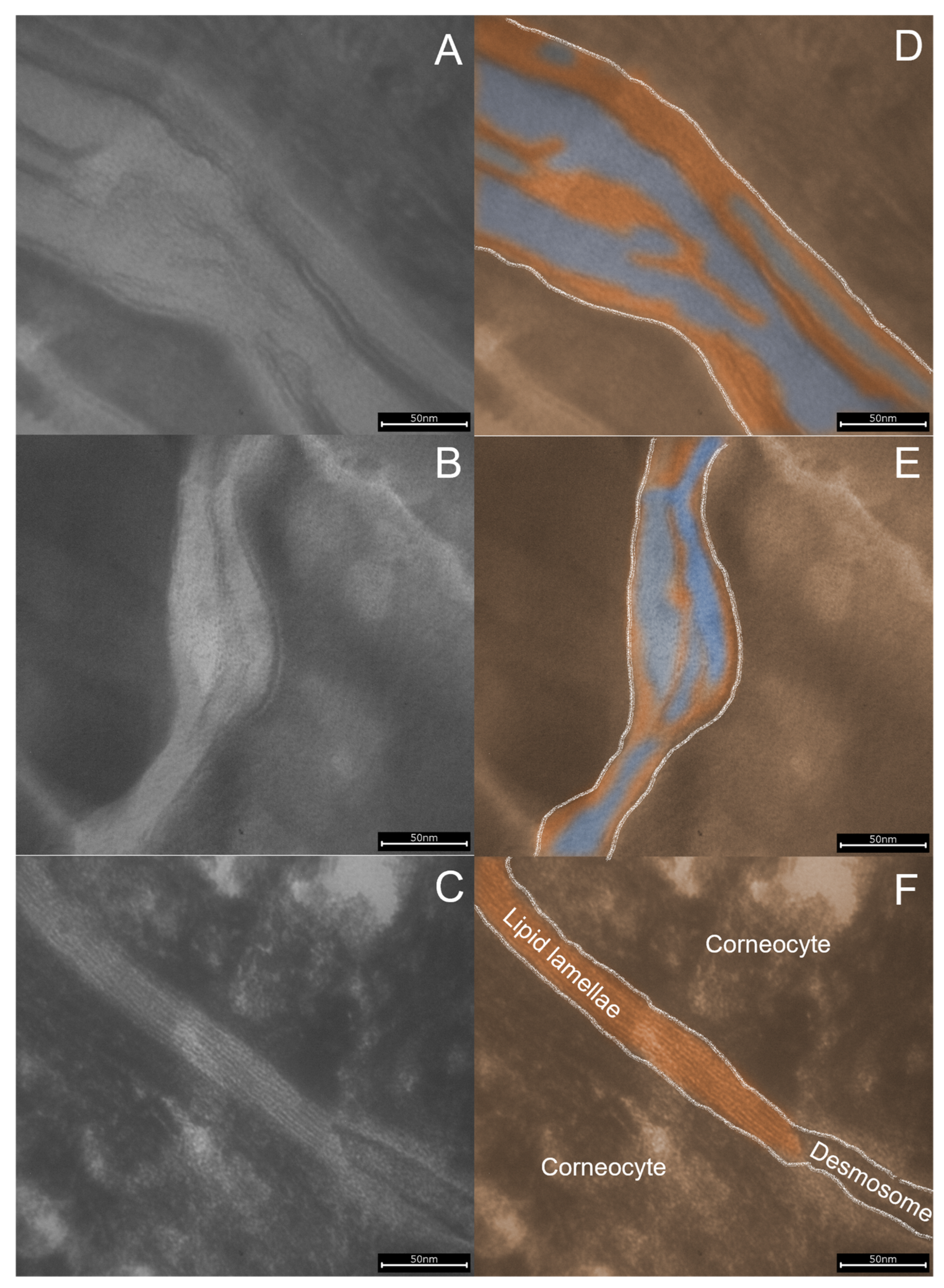
3.3. SC Lipid Lamellae Analysis Using Lipbarvis®
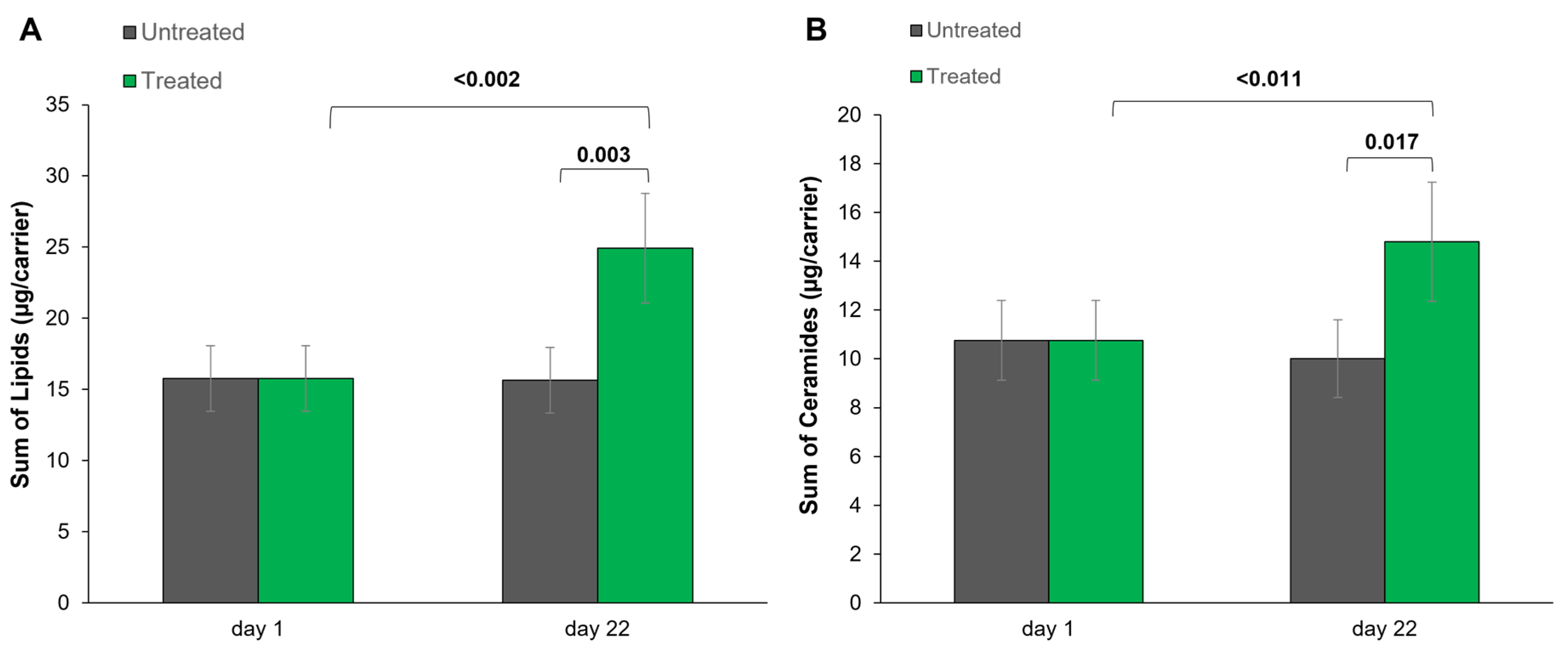
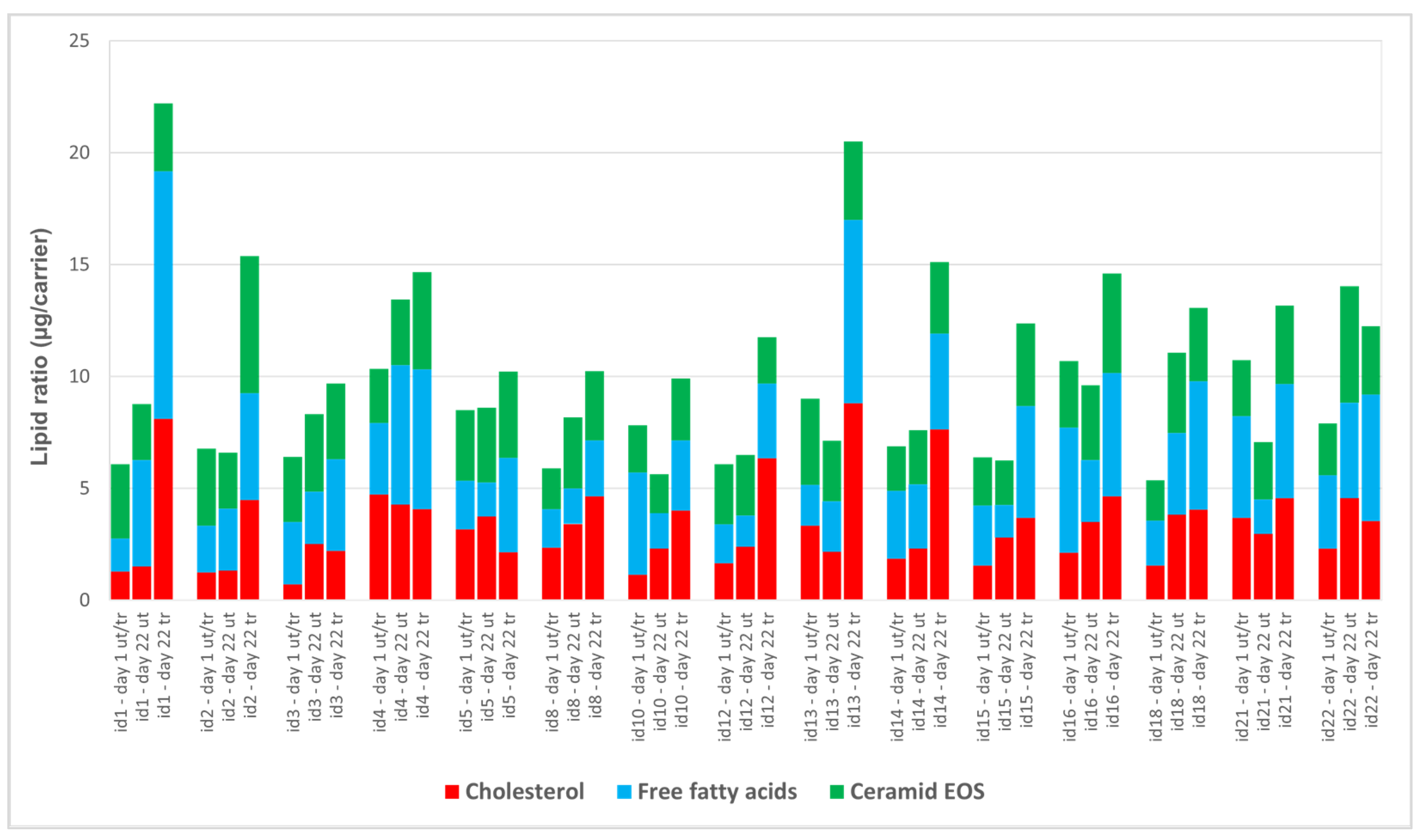
3.4. SC Lipid Spectrum and Profile Analysis via HPTLC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elias, P.M. Stratum corneum defensive functions: An integrated view. J. Investig. Dermatol. 2005, 125, 183–200. [Google Scholar] [CrossRef]
- Menon, G.K.; Kligman, A.M. Barrier functions of human skin: A holistic view. Ski. Pharmacol. Physiol. 2009, 22, 178–189. [Google Scholar] [CrossRef]
- Bosko, C.A. Skin barrier insights: From bricks and mortar to molecules and microbes. J. Drugs Dermatol. 2019, 18, s63–s67. [Google Scholar]
- Barco, D.; Giménez-Arnau, A. Xerosis: A dysfunction of the epidermal barrier. Actas Dermosifiliogr. 2008, 99, 671–682. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 4, 12–16. [Google Scholar] [CrossRef]
- Öhman, H.; Vahlquist, A. The pH gradient over stratum corneum differs in x-linked recessive and autosomal dominant ichthyosis: A clue to the molecular origin of the “acid skin mantle”? J. Investig. Dermatol. 1998, 111, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Eberlein-König, B.; Schäfer, T.; Huss-Marp, J.; Darsow, U.; Möhrenschläger, M.; Herbert, O.; Abeck, D.; Krämer, U.; Behrendt, H.; Ring, J. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef]
- Man, M.Q.; Xin, S.J.; Song, S.P.; Cho, S.Y.; Zhang, X.J.; Tu, C.X.; Feingold, K.R.; Elias, P.M. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Ski. Pharmacol. Physiol. 2009, 22, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Seidenari, S.; Giusti, G. Objective assessment of the skin of children affected by atopic dermatitis: A study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm. Venereol. 1995, 75, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Sparavigna, A.; Setaro, M.; Gualandri, V. Cutaneous pH in children affected by atopic dermatitis and in healthy children: A multicenter study. Ski. Res. Technol. 2009, 5, 221–227. [Google Scholar] [CrossRef]
- Knor, T.; Meholjić-Fetahović, A.; Mehmedagić, A. Stratum corneum hydration and skin surface pH in patients with atopic dermatitis. Acta Dermatovenerol. Croat. 2011, 19, 242–247. [Google Scholar] [PubMed]
- Elias, P.M.; Wakefield, J.S.; Man, M.Q. Moisturizers versus current and next-generation barrier repair therapy for the management of atopic dermatitis. Ski. Pharmacol. Physiol. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blaak, J.; Theiss, C.; Schleißinger, M.; Simon, I.; Schürer, N.Y.; Staib, P. A commercially available skin care lotion with a pH of 4.5 and 10% urea improves skin surface pH, stratum corneum hydration and epidermal barrier function in subjects with dry skin and atopic diathesis. J. Cosmet. Dermatol. Sci. Appl. 2020, 10, 116–133. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Fartasch, M.; Hornstein, O.P. Criteria of atopic skin diathesis. Dermatosen 1991, 39, 79–83. [Google Scholar]
- Oman, S.V.; Ritonja, A. Die Pufferkapazität kosmetischer und pharmazeutischer Emulsionen. Parfümerie Und Kosmet. 1984, 65, 186–189. [Google Scholar]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Ski. Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Rogiers, V. EEMCO Guidance for the assessment of transepidermal water loss in cosmetic science. Ski. Pharmacol. Appl. Ski. Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef]
- Parra, J.L.; Paye, M. EEMCO Guidance for the in vivo assessment of skin surface pH. Ski. Pharmacol. Appl. Ski. Physiol. 2003, 16, 188–202. [Google Scholar] [CrossRef]
- Dähnhardt-Pfeiffer, S.; Surber, C.; Wilhelm, K.P.; Dähnhardt, D.; Springmann, G.; Böttcher, M.; Förster-Holst, R. Noninvasive stratum corneum sampling and electron microscopical examination of skin barrier integrity: Pilot study with a topical glycerin formulation for atopic dermatitis. Ski. Pharmacol. Physiol. 2012, 25, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Akihito, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor of atopic dry skin. J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Blaak, J.; Dähnhardt, D.; Dähnhardt-Pfeiffer, S.; Bielfeldt, S.; Wilhelm, K.P.; Wohlfart, R.; Staib, P. A plant oil-containing pH 4 emulsion improves epidermal barrier structure and enhances ceramide levels in aged skin. Int. J. Cosmet. Sci. 2017, 39, 284–291. [Google Scholar] [CrossRef]
- Elias, P.M. The how, why and clinical importance of stratum corneum acidification. Exp. Dermatol. 2017, 26, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Schulte to Brinke, A.; Mehlich, A.; Doberenz, C.; Janssens-Böcker, C. Acidification of the skin and maintenance of the physiological skin pH value by buffered skin care products formulated around pH 4. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 44–57. [Google Scholar] [CrossRef]
- Danby, S.G.; Cork, M.J. pH in atopic dermatitis. Curr. Probl. Dermatol. 2018, 54, 95–107. [Google Scholar]
- Hatano, Y.; Man, M.Q.; Uchida, Y.; Crumrine, D.; Scharschmidt, T.C.; Kim, E.G.; Mauro, T.M.; Feingold, K.R.; Elias, P.M.; Holleran, W.M. Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Hatano, Y.; Zhang, W.; Fujiwara, S. Defective maintenance of pH of stratum corneum is correlated with preferential emergence and exacerbation of atopic-dermatitis-like dermatitis in flaky-tail mice. J. Dermatol. Sci. 2014, 74, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yoon, N.Y.; Lee, N.R.; Jung, M.; Kim, D.H.; Choi, E.H. Topical acidic cream prevents the development of atopic dermatitis- and asthma-like lesions in murine model. Exp. Dermatol. 2014, 23, 736–741. [Google Scholar] [CrossRef]
- Jang, H.; Matsuda, A.; Jung, K.; Karasawa, K.; Matsuda, K.; Oida, K.; Ishizaka, S.; Ahn, G.; Amagai, Y.; Moon, C.; et al. Skin pH is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J. Investig. Dermatol. 2016, 136, 127–135. [Google Scholar] [CrossRef]
- Blaak, J.; Wohlfart, R.; Schürer, N.Y. Treatment of aged skin with a pH 4 skin care product normalizes increased skin surface pH and improves barrier function: Results of a pilot study. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 50–58. [Google Scholar] [CrossRef]
- Blaak, J.; Kaup, O.; Hoppe, W.; Baron-Ruppert, W.; Langheim, H.; Staib, P.; Wohlfart, R.; Lütje, D.; Schürer, N.Y. A long-term study to evaluate acidic skin care treatment in nursing home residents: Impact on epidermal barrier function and microflora in aged skin. Ski. Pharmacol. Physiol. 2015, 28, 269–279. [Google Scholar] [CrossRef]
- Behm, B.; Kemper, M.; Babilas, P.; Abels, C.; Schreml, S. Impact of a glycolic acid-containing pH 4 water-in-oil emulsion on skin pH. Ski. Pharmacol. Physiol. 2015, 28, 290–295. [Google Scholar] [CrossRef]
- Angelova-Fischer, I.; Fischer, T.W.; Abels, C.; Zillikens, D. Accelerated barrier recovery and enhancement of the barrier integrity and properties by topical application of a pH 4 vs. a pH 5.8 water-in-oil emulsion in aged skin. Br. J. Dermatol. 2018, 179, 471–477. [Google Scholar] [CrossRef]
- Kilic, A.; Masur, C.; Reich, H.; Knie, U.; Dähnhardt, D.; Dähnhardt-Pfeiffer, S.; Abels, C. Skin acidification with a water-in-oil emulsion (pH 4) restores disrupted epidermal barrier and improves structure of lipid lamellae in the elderly. J. Dermatol. 2019, 46, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dähnhardt, D.; Surber, C.; Dähnhardt-Pfeiffer, S. Influence of topical formulations: Lipid lamella organization and lipid composition of stratum corneum as a surrogate marker for barrier integrity. Curr. Probl. Dermatol. 2018, 54, 166–172. [Google Scholar]
- Di Nardo, A.; Wertz, P.; Giannetti, A.; Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 1998, 78, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Rabionet, M.; Gorgas, K.; Sandhoff, R. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta 2014, 1841, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K. Lamellar Bodies: The key to cutaneous barrier function. J. Investig. Dermatol. 2012, 132, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Epilogue: Fixing the barrier—Theory and rational deployment. In Skin Barrier, 1st ed.; Elias, P.M., Feingold, K.R., Eds.; Taylor & Francis: New York, NY, USA, 2006; pp. 591–600. [Google Scholar]
- Loden, M. Urea as a moisturizing and barrier-enhancing ingredient. In Skin Moisturization, 2nd ed.; Rawlings, A.V., Leyden, J.J., Eds.; Informa Healthcare: London, UK, 2009; pp. 335–346. [Google Scholar]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Kohne, Z.; Majora, M.; Marini, A.; Jaenicke, T.; Rodriguez-Martin, M.; Trullas, C.; Hupe, M.; et al. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J. Investig. Dermatol. 2012, 132, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Celleno, L. Topical urea in skincare: A review. Dermatol. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef]
- Scheinfeld, N.S. Urea: A review of scientific and clinical data. Skinmed 2010, 8, 102–106. [Google Scholar]
- Pan, M.; Heinicke, G.; Bernado, S.; Tsui, C.; Levitt, J. Urea: A comprehensive review of the clinical literature. Dermatol. Online J. 2013, 19, 20392. [Google Scholar] [CrossRef] [PubMed]
- Blaak, J.; Staib, P. An updated review on efficacy and benefits of sweet almond, evening primrose and jojoba oils in skin care applications. Int. J. Cosmet. Sci. 2022, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.; Bopp, R.; Rippke, F.; Gloor, M. Effect of topically applied evening primrose oil on epidermal barrier function in atopic dermatitis as a function of vehicle. Arzneimittelforsch 1999, 49, 635–642. [Google Scholar] [CrossRef]
- Zeichner, J.; Berson, D.; Donald, A. The use of an over-the-counter hand cream with sweet almond oil for the treatment of hand dermatitis. J. Drugs Dermatol. 2018, 17, 78–82. [Google Scholar] [PubMed]
- Mehri, Z.; Afrasiabifar, A.; Hosseini, N. Improved itchy quality of life following topical application of sweet almond oil in patients with uremic pruritus: A randomized, controlled trial. Jundishapur J. Chronic Dis. Care 2018, 7, e68164. [Google Scholar] [CrossRef]
- Bordoni, A.; Biagi, P.L.; Masi, M.; Ricci, G.; Fanelli, C.; Patrizi, A.; Ceccolini, E. Evening primrose oil (Efamol) in the treatment of children with atopic eczema. Drugs Exp. Clin. Res. 1998, 14, 291–297. [Google Scholar]
- Chung, B.Y.; Kim, J.H.; Cho, S.I.; Ahn, I.S.; Kim, H.O.; Park, C.W.; Lee, C.H. Dose-dependent effects of evening primrose oil in children and adolescents with atopic dermatitis. Ann. Dermatol. 2013, 25, 285–291. [Google Scholar] [CrossRef]
- Lio, P. Rapid improvement and protective effects of an almond oil-based ointment for diaper dermatitis. J. Drugs Dermatol. 2016, 15, s86–s90. [Google Scholar]
- Wohlrab, J.; Gebert, A. pH and buffer capacity of topical formulations. Curr. Probl. Dermatol. 2018, 54, 123–131. [Google Scholar]

| Aqua, Urea, Glycerin, Prunus Amygdalus Dulcis Oil, Oenothera Biennis Oil, Tocopheryl Acetate, Cetearyl Alcohol, Panthenol, Glyceryl Stearate SE, Sodium Lactate, Phytosterols, Bisabolol, Rosmarinus Officinalis Leaf Extract, Citronellol, Benzyl Salicylate, Limonene, Geraniol, Linalool, Benzyl Alcohol, p-Anisic Acid, Parfum, Caprylyl Glycol, Lactic Acid, Sorbitan Oleate, Sodium Stearoyl Glutamate, Glycine Soja Oil, Ascorbyl Palmitate, Xanthan Gum, Triacetin, Tocopherol |
| Type of emulsion: o/w pH value: 4.5 ± 0.1 (adjusted by Lactic Acid/Sodium Lactate) Buffer Capacity (Lab. Sample No. 210819587): 1.54 (according to Oman et al. [15]) Content of urea [%]: 10.0 Plant Oils (sweet almond oil, SAO; evening primrose oil, EPO) are indicated in italics |
| Day 1 (t0) | Day 2 to 21 | Day 22 (t1) | |
|---|---|---|---|
| Preparation | |||
| Informed consent | ✕ | ||
| In-/Exclusion criteria | ✕ | ||
| Visual atopic scoring (modified according to EAS) | ✕ | ||
| Acclimatization (30 min) | ✕ | ✕ | |
| Parameters | |||
| Biophysical measurements (TEWL, SCH, ss-pH) | ✕ | ✕ | |
| Visual objective and subjective scoring (dryness, roughness, feeling of tension) | ✕ | ✕ | |
| SC lipid matrix analysis using Lipbarvis® (nICCL, Lipid composition and profile) | ✕ | ✕ | |
| Application | |||
| Test product usage (twice daily) | ✕ * | ✕ |
| Day 1 (t0) (Treated) | Day 22 (t1) (Treated) | Diff (Absolute val.) | Diff (%) | ||
|---|---|---|---|---|---|
| Subgroup 1 Basis ss-pH > 5.0 (n = 10) | ss-pH | 5.43 ± 0.31 | 4.79 ± 0.48 | −0.64 ± 0.28 | −11.91 ± 5.36 |
| SCH | 25.51 ± 8.78 | 38.24 ± 14.72 | 12.73 ± 8.98 | 50.56 ± 31.63 | |
| TEWL | 12.01 ± 3.21 | 11.01 ± 1.22 | −1.00 ± 2.82 | −4.30 ±19.13 | |
| Subgroup 2 Basis ss-pH ≤ 5.0 (n = 15) | ss-pH | 4.65 ± 0.28 | 4.47 ± 0.25 | −0.19 ± 0.29 | −3.81 ± 6.16 |
| SCH | 25.39 ± 7.74 | 38.99 ± 12.73 | 13.61 ± 8.14 | 55.82 ± 37.07 | |
| TEWL | 11.39 ± 2.02 | 12.24 ± 3.46 | 0.85 ± 3.44 | 8.97 ± 30.27 | |
| Parameter | Time Point | Code | Mean | Significance (p-Value) | |
|---|---|---|---|---|---|
| Comparison (Untreated t1 vs. Treated t1) | Comparison (t0 vs. t1) | ||||
| Skin dryness | Day 1 (t0) | untreated | 0.9 (±0.4) | --- | --- |
| treated | 0.9 (±0.6) | --- | |||
| Day 22 (t1) | untreated | 0.9 (±0.6) | <0.001 | 0.745 ns | |
| treated | 0.5 (±0.3) | <0.001 | |||
| Skin roughness | Day 1 (t0) | untreated | 0.7 (±0.4) | --- | --- |
| treated | 0.8 (±0.4) | --- | |||
| Day 22 (t1) | untreated | 0.7 (±0.5) | <0.001 | 0.998 ns | |
| treated | 0.3 (±0.3) | <0.001 | |||
| Feeling of tension | Day 1 (t0) | untreated | 0.8 (±0.7) | --- | --- |
| treated | 0.8 (±0.7) | --- | |||
| Day 22 (t1) | untreated | 0.6 (±0.7) | 0.008 | 0.144 ns | |
| treated | 0.2 (±0.6) | <0.001 | |||
| Time Point | Day 1 (t0) | Day 22 (t1) | Significance | ||
|---|---|---|---|---|---|
| Test Site | Untreated † | Treated † | Untreated | Treated | p-Value * (Untreated t1 vs. Treated t1) |
| nICCL (nm/1000 nm2) | 61.13 | 61.13 | 61.05 | 187.07 | 0.001 sig |
| Sum of Lipids (µg/carrier) | 15.77 | 15.77 | 15.64 | 24.92 | 0.003 sig |
| CHOL (µg/carrier) | 2.18 | 2.18 | 2.90 | 4.86 | 0.012 sig |
| FFA (µg/carrier) | 2.84 | 2.84 | 2.73 | 5.26 | 0.001 sig |
| Sum of CER (µg/carrier) | 10.75 | 10.75 | 10.01 | 14.80 | 0.017 sig |
| CER EOS (µg/carrier) | 2.63 | 2.63 | 2.94 | 3.56 | 0.081 ns |
| CER NP (µg/carrier) | 3.46 | 3.46 | 2.84 | 5.19 | 0.078 ns |
| CER AP (µg/carrier) | 4.66 | 4.66 | 4.23 | 6.05 | 0.025 sig |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaak, J.; Dähnhardt, D.; Bielfeldt, S.; Theiss, C.; Simon, I.; Wilhelm, K.-P.; Dähnhardt-Pfeiffer, S.; Staib, P. Improvement of Human Epidermal Barrier Structure and Lipid Profile in Xerotic- and Atopic-Prone Skin via Application of a Plant-Oil and Urea Containing pH 4.5 Emulsion. Cosmetics 2023, 10, 95. https://doi.org/10.3390/cosmetics10040095
Blaak J, Dähnhardt D, Bielfeldt S, Theiss C, Simon I, Wilhelm K-P, Dähnhardt-Pfeiffer S, Staib P. Improvement of Human Epidermal Barrier Structure and Lipid Profile in Xerotic- and Atopic-Prone Skin via Application of a Plant-Oil and Urea Containing pH 4.5 Emulsion. Cosmetics. 2023; 10(4):95. https://doi.org/10.3390/cosmetics10040095
Chicago/Turabian StyleBlaak, Jürgen, Dorothee Dähnhardt, Stephan Bielfeldt, Christiane Theiss, Isabel Simon, Klaus-Peter Wilhelm, Stephan Dähnhardt-Pfeiffer, and Peter Staib. 2023. "Improvement of Human Epidermal Barrier Structure and Lipid Profile in Xerotic- and Atopic-Prone Skin via Application of a Plant-Oil and Urea Containing pH 4.5 Emulsion" Cosmetics 10, no. 4: 95. https://doi.org/10.3390/cosmetics10040095
APA StyleBlaak, J., Dähnhardt, D., Bielfeldt, S., Theiss, C., Simon, I., Wilhelm, K.-P., Dähnhardt-Pfeiffer, S., & Staib, P. (2023). Improvement of Human Epidermal Barrier Structure and Lipid Profile in Xerotic- and Atopic-Prone Skin via Application of a Plant-Oil and Urea Containing pH 4.5 Emulsion. Cosmetics, 10(4), 95. https://doi.org/10.3390/cosmetics10040095






