1. Introduction
Currently, anti-ageing skin care products attract the attention of dermatologists throughout the world [
1]. Face masks are efficient alternatives to skin treatment procedures performed in a beauty salon. These masks are usually manufactured on the basis of synthetic cloths impregnated with active ingredients and require the use of chemical additives (emulsifiers, preservatives, etc.). Bioactive biopolymer masks that release various compounds on a desired skin area can be an alternative to cosmetic products for preventing the first signs of ageing [
2].
Natural polymer chitin and its derivative chitosan are promising compounds for developing skin care products [
3] thanks to the following characteristics: antimicrobial and antioxidant activities, regenerative and cell-penetrating properties, the absence of toxicity, and immunogenicity. Chitosan is used in the cosmetic industry, in preparation of drug delivery systems, as a chelating agent that increases the stability of a formulation, and as an agent that promotes healing, stimulates tissue growth, and inhibits fibrosis [
4,
5].
In addition, it has been demonstrated that chitosan macromolecules (in addition to anti-ageing, anti-inflammatory, and antioxidant properties) are able to absorb ultraviolet rays [
6]. Chitosan oligosaccharides reduce the ageing of the skin on the back of hairless mice after exposure to ultraviolet irradiation for 10 weeks [
7]. Morganti and colleagues [
8] have developed a safe, effective, and eco-friendly anti-ageing cosmetic mask for humans with the use of natural ingredients (chitin nanofibers, lignin, and metalloproteinase). Solubility of chitosan in diluted solutions of acids and good film- and fiber-forming properties facilitate chitosan processing and preparation of chitosan-based materials of various structures and geometrical shapes [
9,
10,
11,
12,
13,
14,
15,
16]. It is known that chitosan is well-soluble in the majority of acids. However, only a limited number of acids can be used in cosmetology, e.g., lactic acid, succinic acid, salicylic acid, and glycolic acid. Alpha hydroxy acids (AHA, lactic, succinic, and glycolic acids) stimulate the synthesis of collagen and elastin fibers that are responsible for skin elasticity and resiliency, facilitate deep moisturizing of the skin, and have anti-ageing effects. Beta hydroxy acids (BHA, e.g., salicylic acid) decrease pore size and possess antibacterial and anti-inflammatory properties [
17].
Since the materials for medicine and cosmetology are obtained from weakly acidic aqueous solutions of chitosan, investigations of the structure and properties of salt forms of this polymer attract much attention. In early works [
11,
18,
19], phase states of the chitosan/acetic acid/water system have been studied, rheological properties of solutions of this polymer in acetic acid and their stability have been investigated, and deformation and thermal properties of chitosan in its salt form have been described.
Traditionally, the reduction of polymer solubility and swelling is achieved by treatment with crosslinking agents (which are commonly toxic) [
20,
21]; therefore, even the presence of small traces of crosslinking agents in a material is extremely undesirable. Due to the presence of highly reactive amino groups in chitosan macromolecules, a non-reagent method for hydrophobization and reduction of chitosan solubility can be proposed; this method consists of thermal treatment of chitosan in its salt form.
Thermally modified chitosan films retain a high ability to absorb metals and organic compounds from aqueous solutions without the use of additional agents. These chitosan materials may be used as biocompatible and bioresorbable carriers for pharmaceutical substances in medicine and cosmetology [
11,
22,
23].
The aim of this work was to develop the method of obtaining thermally modified chitosan and chitosan/chitin nanofibrils composite films from solutions of lactic and acetic acids to study sorption, thermal, and mechanical properties of the products as well as their structure and biocompatibility. Acetic acid was chosen because it is widely used as a solvent for chitosan. Lactic acid is a popular and efficient component of various skin care formulations.
The obtained materials are biocompatible and environmentally safe; thus, thermally modified chitosan films can be used as a basis for the production of disposable face masks. Face masks for one-time use are very popular all over the world, and their consumption increases every year. However, they are currently manufactured on the basis of non-woven fabrics with prolonged biodegradation time.
2. Materials and Methods
Films were prepared on the basis of shrimp chitosan (CS) produced by Biolog Heppe GmbH (Landsberg, Germany) with molecular mass (1.64–2.1) × 105 and deacetylation degree equal to 92%; chitin nanofibrils (CNfs) were obtained from Morganti R&D lab (Rome, Italy). The concentration of chitin nanofibrils in their aqueous dispersion was 20 mg/mL.
Chitosan was stirred in water for 30 min until swelling occurred. Then, a solution of acetic (AA) or lactic acid (LA) was introduced into the mixture at constant stirring; the acid concentration in the common solution was brought up to 2 wt.%. The polymer concentration in this chitosan/acid solution was 4.0 wt.%. The solution was stirred for 90 min, then 0.1 wt.% of a non-ionogenic surfactant (Tween-80) was added to improve the productivity of the preparation of films on hydrophobic substrates. The resulting solution was filtered and deaerated in a vacuum chamber for 24 h at 10 kPa.
To prepare composite films, CNfs were dispersed in water for 4 min with the use of an IL10-0.63 INLAB ultrasonic dispergator (Saint Petersburg, Russia) at a frequency of 40 kHz and power output of 190 W (similar to the procedure described in our previous publications) [
24]. The amount of chitosan that provided the chitosan:chitin ratio equal to 95:5 was added to the obtained dispersion; the total concentration of polymers in the solution was 4%.
The films were obtained on the device MSK-AFA-IIID-Automatic Thick Film by spinning chitosan solutions through a slit die onto a substrate followed by drying at room temperature for 24 h. The film thickness was equal to 35 ± 10 µm.
Thermal modification of films was performed at temperatures that did not exceed the temperature of chitosan destruction (
T = 120 °C,
t = 3 h) [
19,
24]. The mass loss after annealing of samples Δ
W was equal to 12–14%.
Fourier-transform infrared (FTIR) spectra of films (resolution: 4 cm−1; number of scans: 40) were obtained with a “Vertex70” spectrometer (“Bruker”, Billerica, MA, USA) using a ZnSe attenuated total reflectance (ATR) accessory (“Pike”). The FTIR-ATR spectra were corrected for the wavelength dependence of the depth of light penetration.
The thermal properties of films were studied by thermogravimetric analysis (TGA) with the aid of an NETZSCH TG 209 F1 instrument. A sample was placed into an open cubicle (Al2O3), which was mounted to the sample holder. The experiments were carried out in the temperature range from 30 to 800 °C in an inert atmosphere (argon) at a heating rate of 10 °C/min. The flow rate of the inert gas passing through a sample was 40 mL/min; the flow rate of the protective gas passing through a thermobalance was 25 mL/min. The sample mass was 2–3 mg.
The surface morphology of the films was investigated by atomic force microscopy (AFM) with a SOLVER PRO scanning probe microscope in the contact mode.
The X-ray structural analysis was performed using a DRON-3M diffractometer (“Bourevestnik” Scientific Development and Production Center, Saint Petersburg, Russia) in the reflection mode (Bragg-Brentano geometry); the Cu/Kα radiation was used (λ = 0.154 nm, nickel β-filter). Samples were placed into quartz cuvettes (without averaging rotation). The diffractograms were registered in a step mode in the angular range 2θ = 10–75°; the exposure time at a given point was 1 s. The data processing and peak identification were performed with the use of the DifWin software package (“Bourevestnik”, ver.2006) and the ICDD PDF-2 2022 database (Newtown Square, PA, USA)
The swelling degree was determined gravimetrically. Before measurements, samples were kept in a vacuum chamber for 24 h until a constant weight was achieved. The dried samples were exposed to distilled water and removed from water after fixed periods of time: 5, 10, 20, 30, 60, and 300 s. The samples were squeezed out with two layers of filter paper and weighed using ER-182A Electronic Analytical Balance. The swelling degree
Q, g/g, was calculated according to the following formula:
where
m1 is the mass of a sample exposed to water (g);
m0 is the mass of a dry sample (g).
The mechanical properties of samples were studied with an Instron 5943 instrument; the base length was 10 mm, and the sample extension rate was 10 mm/min. Before the extension tests, chitosan film samples were kept in a desiccator at a relative air humidity of 66% for not less than 24 h.
Cytotoxicity studies of thermally modified chitosan and composite films containing chitin nanofibrils obtained from solutions in lactic and acetic acids were performed.
Film samples (mass: 1 mg) were sterilized with ozone for 90 min and incubated in the complete DMEM F12 nutrient medium (Biolot, Saint Petersburg, Russia) containing 1% of L-glutamine, 1% of antibiotics (penicillin 100 units/mL, streptomycin 100 µg/mL), 1% of fungizone (amphotericin B, 25 µg/mL), and 10% of fetal bovine serum (Gibco, Billings, MT, USA). One milliliter of the complete nutrient medium was taken per 1 mg of a sample. After incubation for 24 h, the conditioned medium was taken and used for the cultivation of human dermal fibroblasts and estimation of their viability.
Fibroblasts were inoculated into the wells of a 96-well plate (8000 per well) and exposed for 48 h. Then the medium with unattached cells was removed, and the conditioned medium (100 µL) was added to the wells; the cells were cultivated for 48 h. When the incubation period ended, the medium was removed, and the DMEM/F12 medium containing MTT was introduced (50 µL per well). The cells were cultivated in a CO2 incubator at 37 °C for 2 h. After removal of the supernatant, the formazan crystals formed by metabolically viable cells were dissolved in dimethylsulfoxide (50 µL per well), and optical density at 570 nm was measured using a plate spectrophotometer. The calculations involved polynomial regression analysis in Microsoft Excel (Microsoft Office Professional Plus 2016). The optical density of the control sample (cells in the common nutrient medium) was taken as 100%.
The morphology of the cells was estimated with the use of an inverted light microscope (Carl Zeiss, Jena, Germany).
3. Results and Discussion
In the course of thermal modification, the properties of chitosan films change due to the decomposition of salts, formation of crosslinked structures, and amidation of chitosan in the salt form. It has been demonstrated [
22] that the amidation degree and the crosslinking processes occurring during thermal modification are determined not only by the temperature and duration of treatment but also by the strength and volatility of the acids used as solvents for chitosan. Ageev et al. [
11] have also studied chitosan by X-ray diffraction and electron microscopy and shown that preparation of chitosan films leads to its partial amorphization and a decrease in crystallite size; thus, thermal treatment facilitates the formation of a less ordered crystalline structure.
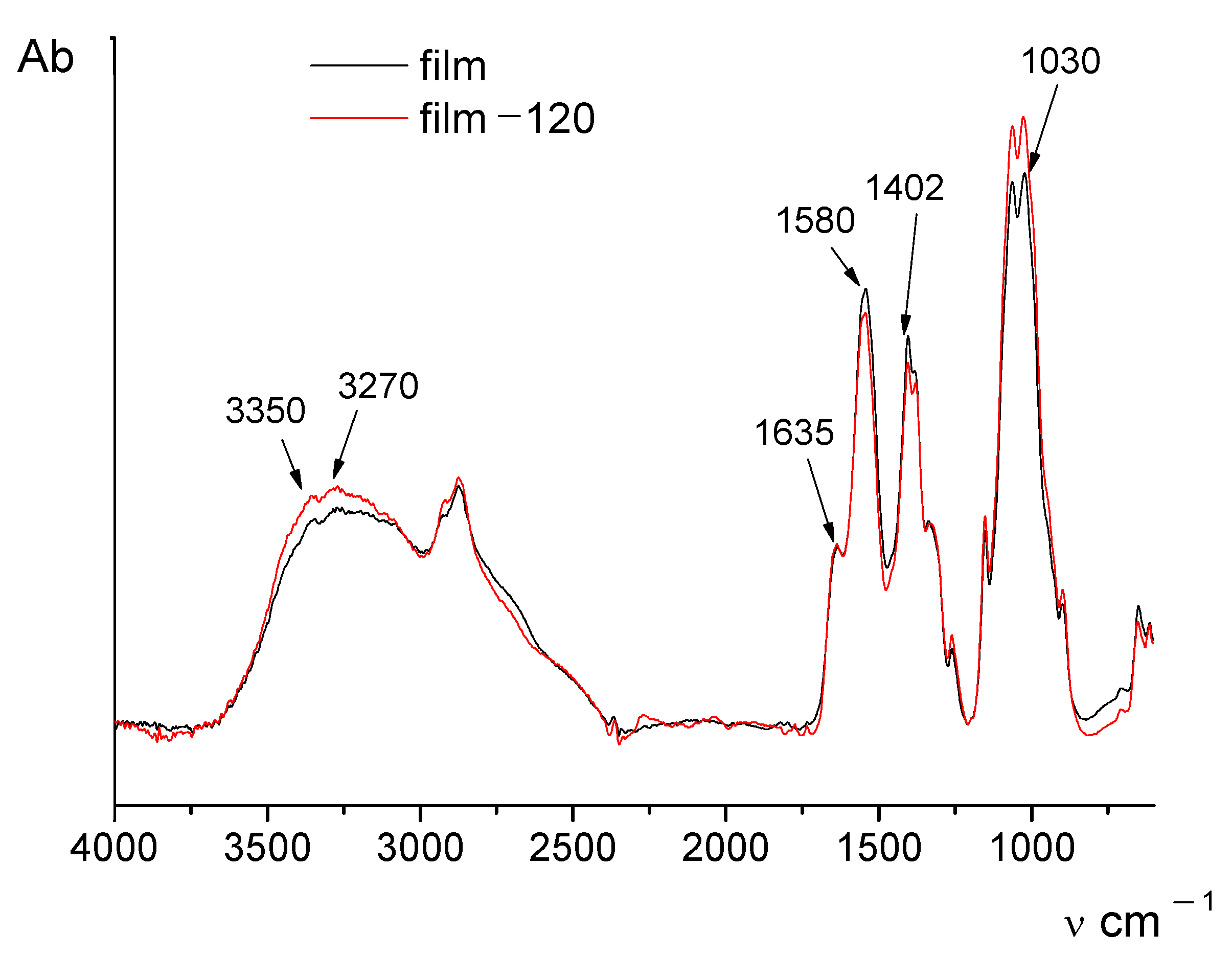
The IR spectrum of chitosan in the salt form contains the following characteristic absorption bands (
Figure 1): the wide band at 3500–3100 cm
−1 (primary and secondary OH groups); 3350 and 3270 cm
−1 (protonated amino groups); 1635 cm
−1 (CO groups of Amide I); and 1320 cm
−1 (Amide III). The wide band with the maximum at 1580 cm
−1 has a complex structure and consists of three overlapped absorption bands related to Amide II, protonated amino groups, and COO– groups (acetate counterions). The presence of acetate counterions is also indicated by the appearance of the absorption band at 1410 cm
−1. The area between 1100 cm
−1 and 890 cm
−1 includes the bands assigned to vibrations of glycosidic ring.
It is seen from
Figure 1 that the IR spectra of the initial and thermally treated films differ only slightly; however, the intensity of the band near 1580 cm
−1 decreases after thermal treatment.
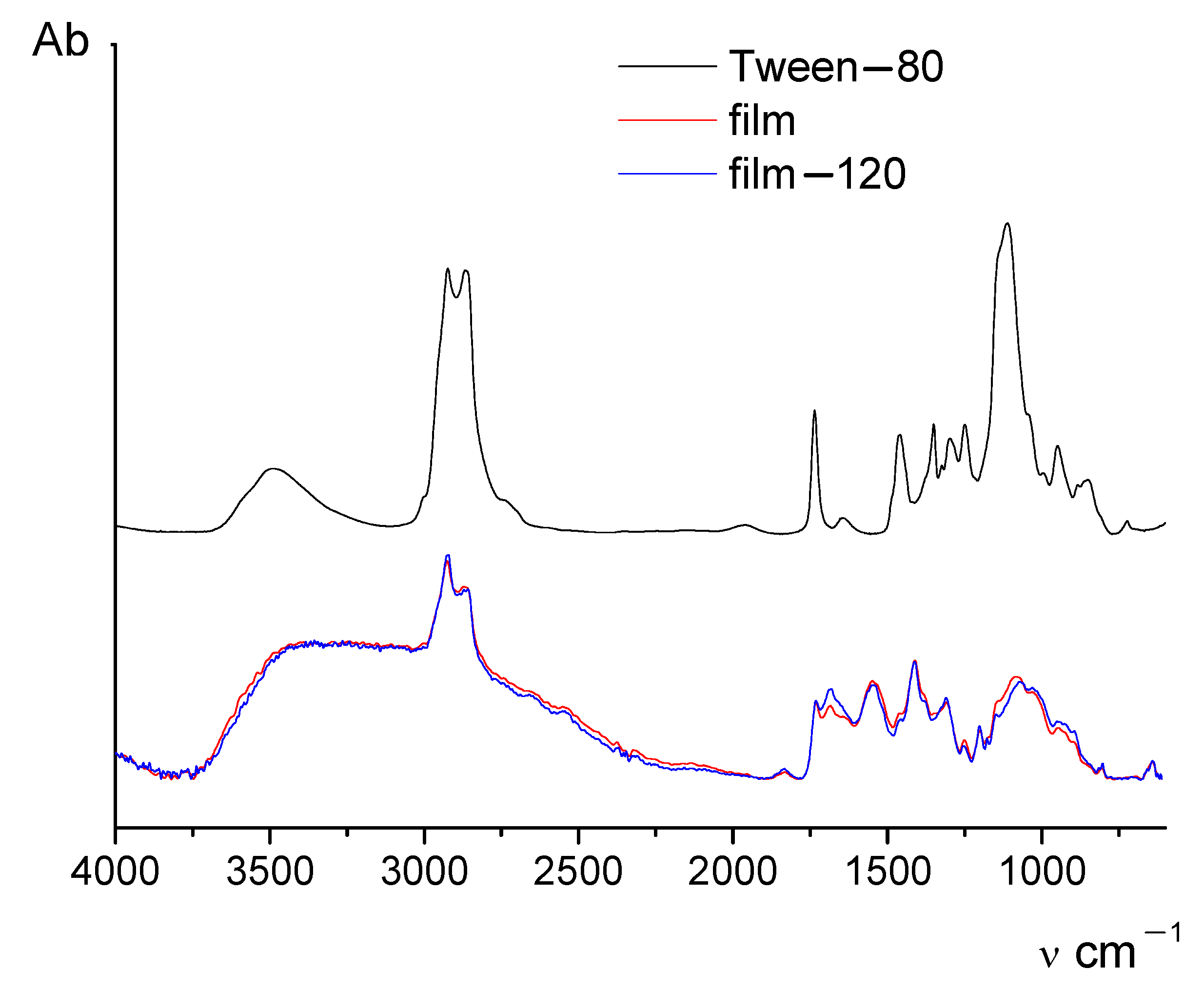
Figure 2 shows IR spectra of the initial and thermally treated chitosan films containing 5 wt.% of CNfs and 0.1% of surfactant Tween-80, and the IR spectrum of the pure surfactant. It is evident that the addition of Tween-80 to the chitosan solution leads to certain changes in the IR spectra of films. To estimate the influence of thermal treatment on the structure of chitin/chitosan composite films, the ratio between integral intensities of the bands at 1580 cm
−1 and 1030 cm
−1 was used. The presence of Tween-80 does not have any significant influence on the positions and intensities of these peaks. The results are given in
Table 1.
Figure 3 presents the difference spectrum obtained by subtraction of the spectrum of the initial film from that of heated film. The difference spectrum contains the bands at 3300 cm
−1 (N-H—stretching), 1680 cm
−1 (C=O- stretching), 1540 cm
−1 (N-H-bending), and 1320 cm
−1 (C-N- stretching) that indicate the presence of amide group.
In paper [
22], the main reaction that proceeds during the thermal treatment of films of chitosan carboxylic acid salts (salts of acetic and lactic acids) is the amidation reaction that leads to the ordering of the structure, compactization of a film, and the formation of azomethine crosslinks.
Analysis of IR spectra (
Table 1) showed that the addition of nanofibrils accelerates the process of thermal transformation in the composite films. This is apparent from the observed increase in the intensity ratio of 1030 cm
−1\1580 cm
−1 peaks in the spectrum of the thermally treated film (as compared to the spectrum of the initial sample and the spectrum of a chitosan film without chitin nanofibrils).
A decrease in the residual content of acids and the acid/chitosan molar ratio that occurs during thermal treatment causes the compactization of chitosan structure, crosslinking of macromolecules, and, as a consequence, to a reduction in solubility of chitosan films in the salt form.
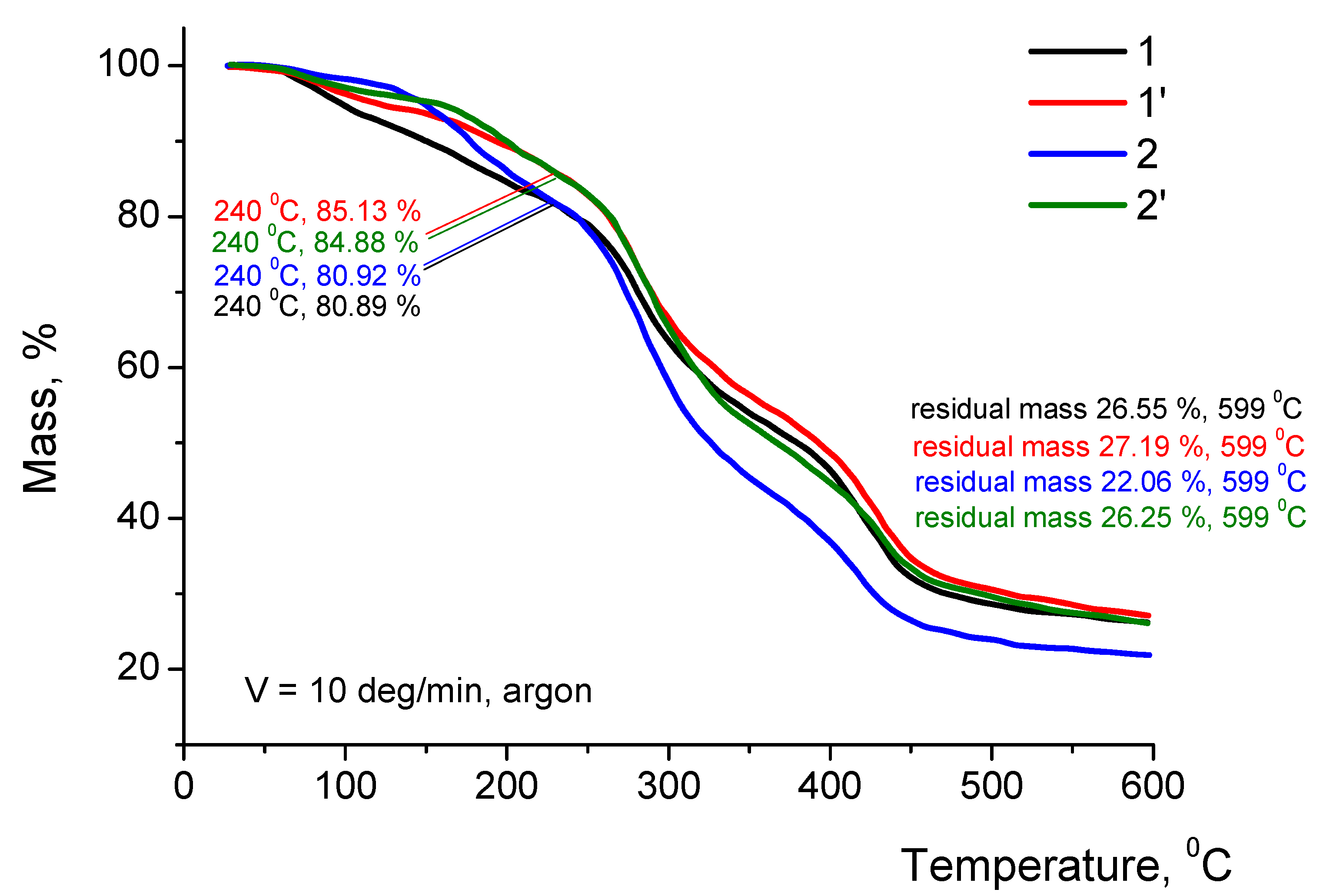
The TGA curves of chitosan films in the salt form obtained before and after thermal modification differ insignificantly (by 5%) in residual mass in the 50–600 °C temperature range (
Figure 4). In all TGA curves, the area corresponding to chitosan destruction lies in the 260–360 °C range. Behavior of the films in the salt form after modification depends on strength and volatility of an acid used for dissolving chitosan; this indirectly confirms differences in the amidation degrees and the formation of crosslinked structures during thermal modification. After TGA experiments, the residual masses at 240 °C and 600 °C were determined (
Figure 4).
In the process of thermal modification of chitosan films, the polymer is transformed into a non-soluble material but retains high sorption ability in aqueous solutions.
The study of swelling of various chitosan-based samples in water (
Figure 5) revealed that swelling of all thermally modified films (prepared from solutions in different acids) is restricted. Swelling occurs fast, and the maximum swelling degree is achieved 1 min after the beginning of the experiment. This property of the obtained chitosan films is beneficial for their use in medicine and cosmetology since saturation of these films with a necessary solution would not take much time. The introduction of a hydrophobic component in the form of chitin nanofibrils naturally leads to a decrease in the swelling degree of composite films but has no significant effect on the rate of this process.
The data discussed above confirm the results of IR spectroscopy studies, which demonstrated that the addition of chitin nanofibrils accelerates the process of thermal transformation in the films and, correspondingly, leads to the compactization of the chitosan structure.
In our previous works [
25,
26], it has been demonstrated that chitin nanofibrils have a sufficiently large specific surface area and efficiently adsorb chitosan from water solution. Owing to a high thermodynamic affinity between chitin and chitosan, the latter is adsorbed on the surface of chitin nanofibrils, which leads to the formation of a new spatial arrangement of chitosan chains and denser packing of macromolecules.
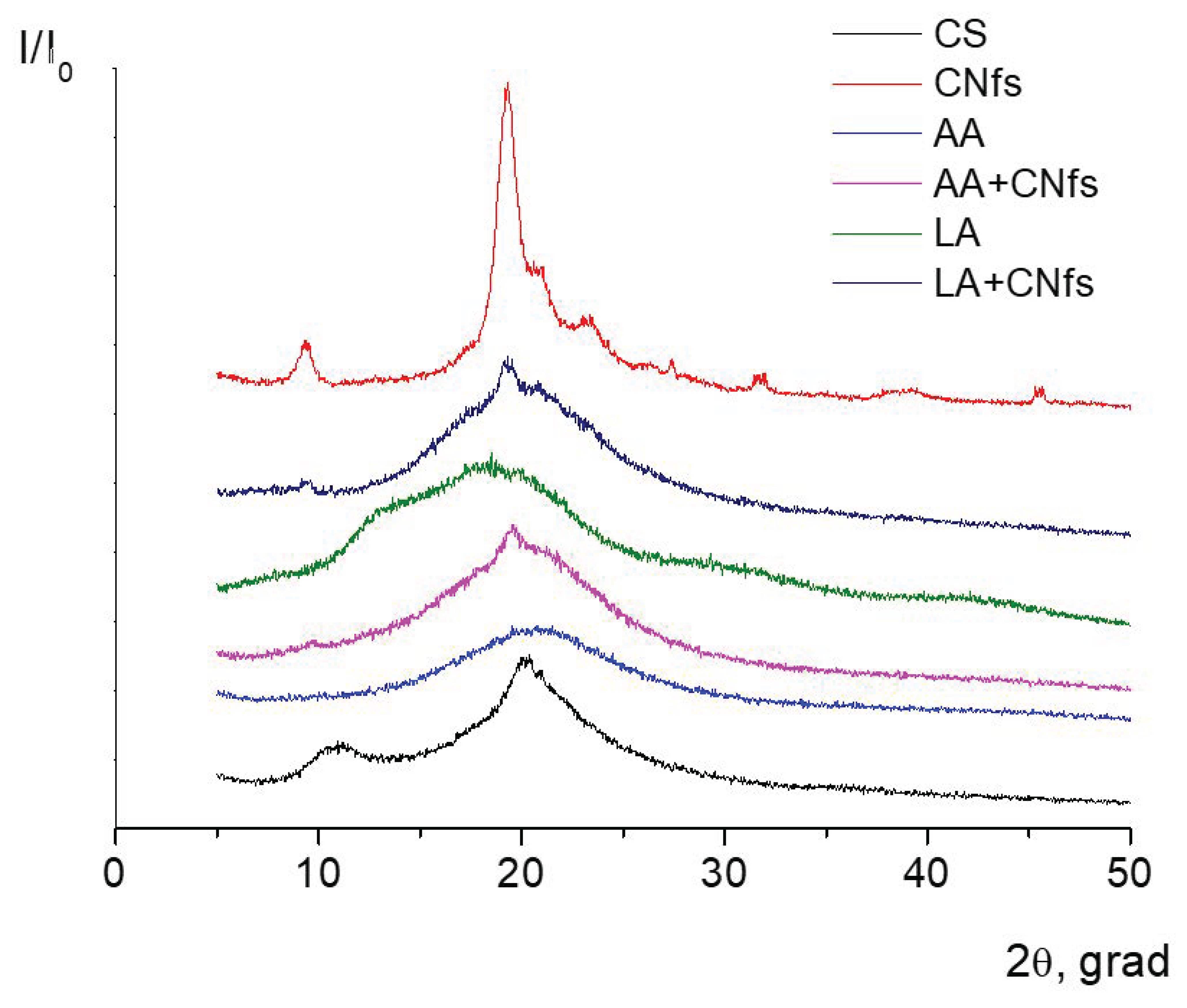
Figure 6 presents diffractograms of thermally modified chitosan and composite films containing chitin nanofibrils.
It is seen from the presented results that the diffractograms of chitosan films prepared on the basis of polymer solutions in acetic or lactic acids typically contain a wide diffraction maximum at 2θ = 20° (
Figure 6), which is characteristic of amorphous polymers. Diffraction patterns of the films containing chitin nanofibrils include chitin reflections of low intensity, which is caused by a small amount (5 wt.%) of nanofibrils in the composite.
Good compatibility between chitin nanofibrils and macromolecules of the chitosan scaffold was demonstrated; this can be explained by similarities in the chemical structure of these two polymers. The diffraction pattern of the chitosan film prepared from a polymer solution in lactic acid contains a new peak at 2θ = 15°, which confirms the presence of an anhydrous (dehydrated) form of chitosan in this film. The appearance of dehydrated chitosan macromolecules causes a decrease in the sorption capability of this chitosan film.
Mechanical characteristics of chitosan and composite films filled with chitin nanofibrils (obtained from solutions in different acids) are given in
Table 2.
Introduction of chitin nanofibrils facilitates an increase in the mechanical strength of the composite films obtained from solutions in different acids; this result also confirms the conclusion regarding acceleration of thermal transformation in chitosan films in the presence of chitin nanofibrils.
The films prepared on the basis of polymer solution in acetic acid possess higher strength and elasticity than the samples obtained from solutions in lactic acid. This increase in strength is explained by the high volatility of acetic acid (as compared to that of lactic acid) [
22]. This film has a homogeneous amorphous structure (
Figure 6). It should be noted that thermal treatment facilitates the compactization of film structure and increases the homogeneity of the amorphous phase, as was demonstrated in [
11]. The presence of an amorphous structure explains the high sorption capacity of the film obtained from the acetic acid solution (
Figure 4). Thus, the high elasticity of the film is related to the plasticizing effect of an absorbed substance (water) in the polymer scaffold. As a consequence, we observe a reduction in the number of steric hindrances that prevent the moving of macromolecule segments during the uniaxial extension of films.
The AFM images of film surfaces were used to calculate a statistical parameter for each sample: the arithmetic average roughness (
Ra). This parameter was measured at five different areas of each sample; the image size was equal to 5 × 5 µm. The measurement error for
Ra (the standard deviation,
SD) was calculated; the obtained results are presented in
Table 3.
The AFM images of Samples 1 and 2 were taken both in the initial state and after exposure to distilled water for 8 s (these samples are denoted in
Table 3 as 1b and 2b). Then water was removed from the sample by compressed air jet; the film was exposed at room temperature for 15 min (to achieve sufficient rigidity of the surface) and then studied by AFM.
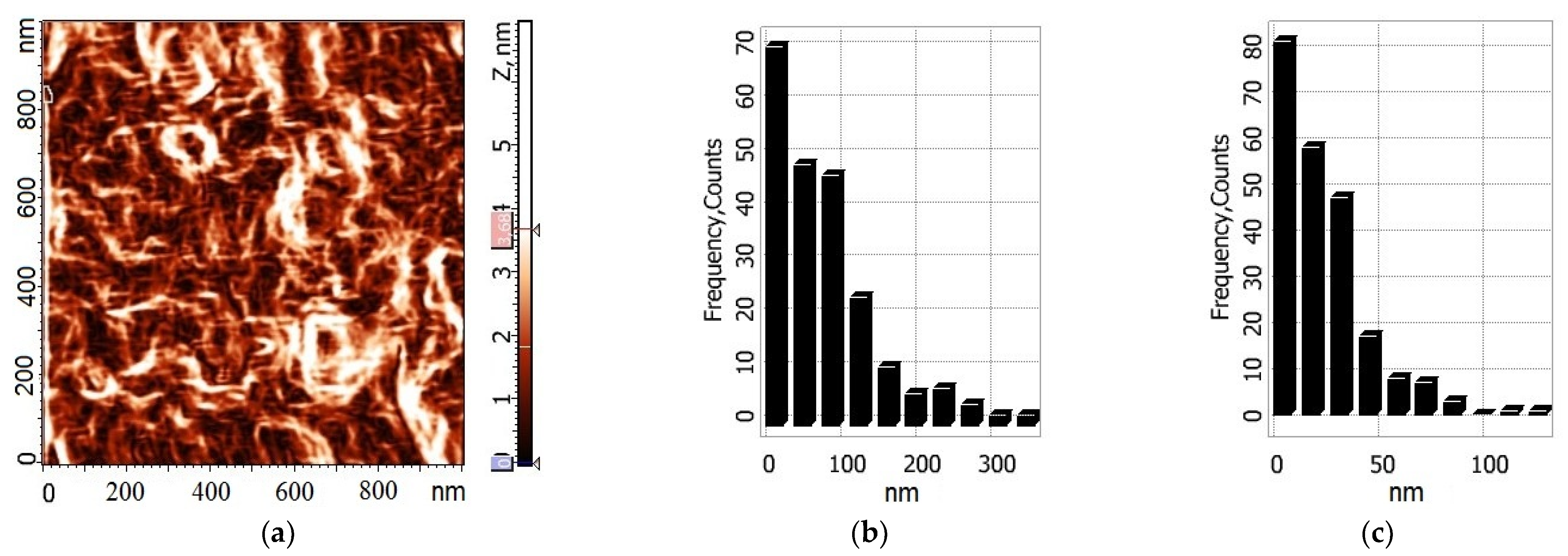
The roughness values calculated for the chitosan films prepared with the use of acetic and lactic acids are close; for the corresponding chitin-containing samples, the values of these parameters are higher. The introduction of chitin nanofibrils leads to a 5-fold increase in the surface roughness of the films obtained from lactic acid. Thus, the AFM data confirm the above-mentioned influence of chitin nanofibrils on the rate of thermal modification (namely, different degrees of amidation and formation of crosslinked structures in the films obtained from polymer solutions in acetic or lactic acids).
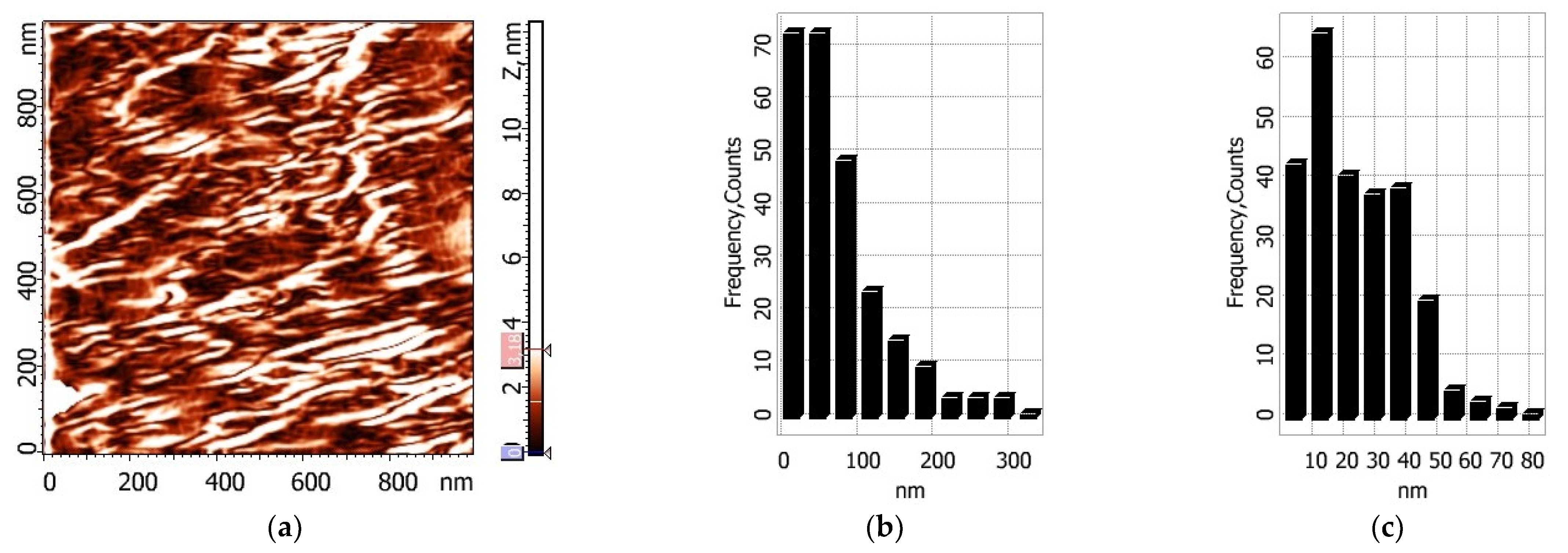
Two-dimensional images of the surfaces of composite films and histograms showing the size distributions of particles on them are presented in
Figure 7 and
Figure 8. It is seen that upon swelling of thermally treated chitosan film in water, a regular structure appears (
Figure 8); the dimensions of structural elements remain within the same range.
From the results obtained, it may be deduced that thermal treatment of the films does not provide the transformation of 100% of chitosan molecules into the modification insoluble in water. Indeed, the films swell easily, and the residual molecules of unbound (non-crosslinked) chitosan can interact with skin cells. In this work, fibroblasts were selected for performing in vitro studies because they are the main cells of derma providing a synthesis of extracellular matrix and, therefore, skin elasticity and resilience. Deterioration of these parameters leads to the appearance of the first signs of skin ageing. Acetic and lactic acids were used as solvents for chitosan. After incubation of the films in the complete nutrient medium, the liquid acquired a yellowish tinge, which indicates a decrease in the pH value (acidification). It should be noted that the most intense yellow coloring was observed after incubation of the films obtained with the use of lactic acid. The presence of chitin nanofibrils did not have any influence on the medium color. On the contrary, the color of the nutrient medium remained virtually the same after incubation of the films obtained with the use of acetic acid; this is explained by the comparatively low boiling point of acetic acid and its complete evaporation during thermal treatment in the corresponding temperature range.
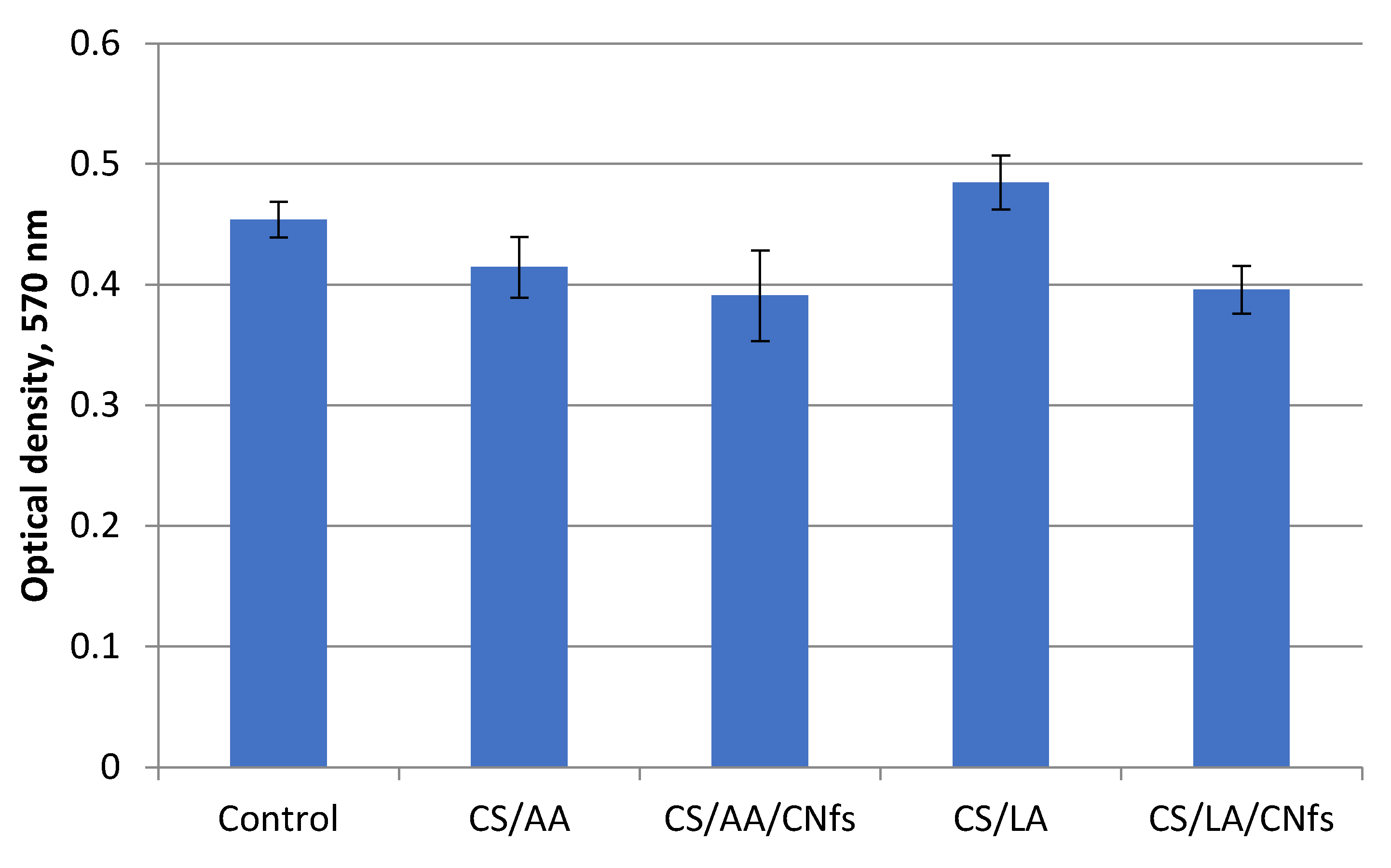
The results of the MTT test show only an insignificant decrease in the viability of dermal fibroblasts cultivated in the presence of the conditioned medium (the liquid obtained after exposure of films to the nutrient medium) for all types of films (
Figure 9). However, let us call attention to the enhanced viability of the cells cultivated in the presence of the conditioned medium after incubation of the films containing traces of lactic acid. It is not only higher than the viability of cells in other cases but also exceeds the viability of the control cells. This increase in cell viability in the presence of lactic acid is explained by the participation of this acid in mammalian metabolism and, in particular, metabolic processes in the human body.
It should be emphasized that lactic acid and lactate are metabolized in high amounts during wound healing; they play an important role due to high levels of released cytokines and growth factors and neovascularization that proceeds after the activation of the immune system. This leads to an intensification of metabolism and, possibly, to hypoxia [
27]. Therefore, lactate, as an energy substrate, may satisfy high energy needs in the process of wound healing. In addition, the accumulated lactate increases the pH value (it neutralizes the alkaline medium that appears due to a decrease in the carbon dioxide levels and high content of oxygen). As a consequence, cells can propagate and differentiate in the optimized physiological pH range [
28]. In addition, the literature data indicate that lactate may stimulate collagen synthesis in the extracellular matrix by fibroblasts [
29,
30]. It has been demonstrated that lactate stimulates vasculogenic stem cells via the redox system [
31].
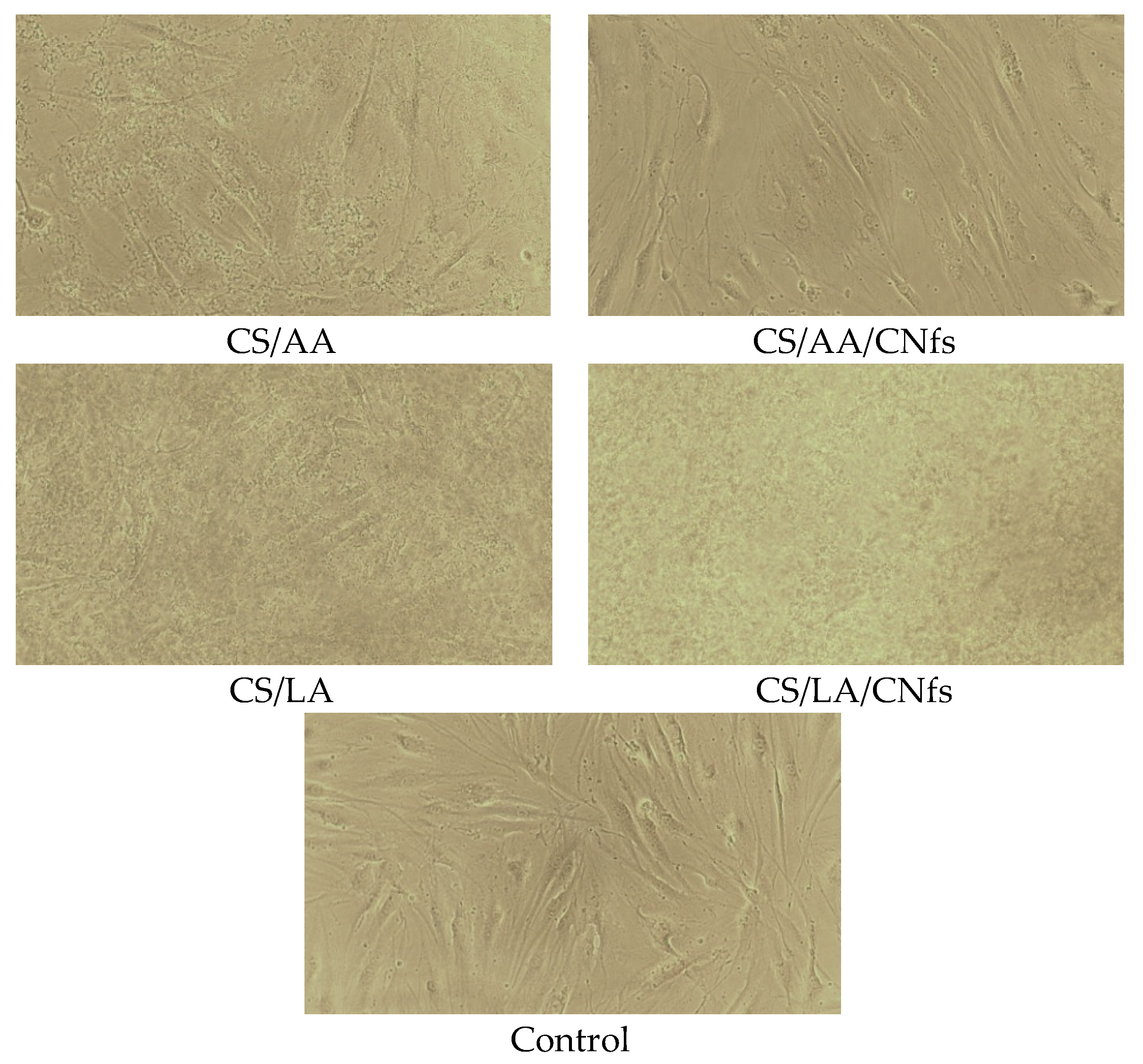
From the analysis of cell morphology after cultivation for one day, it was inferred that the presence of residual acids and chitosan molecules soluble in an aqueous medium virtually does not affect cell morphology (
Figure 10). All cells have elongated (spindle-shaped) forms typical of fibroblasts. Some differences were observed in the samples in the presence of the films without chitin nanofibrils, irrespective of the nature of the used acid. In all cases, a foreign suspension was observed; apparently, it consisted of non-crosslinked chitosan. Despite the presence of relatively high amounts of this suspension, the viability of cells decreased only slightly. When the experiments involved composite chitin/chitosan films, no suspension was visible since chitin nanofibrils facilitate crosslinking of chitosan and prevent its release from films.