Abstract
Both endogenous and exogenous factors cause skin aging. This study aimed to compare the differential expressions of collagen type I (COL I) and collagen type VI (COL VI) in skins with biological aging and photoaging. In order to comprehend the impact of solar radiation in the dermis and the expression of COL I and COL VI, we studied the expression and their detection in healthy skin and in skin that had been characterized by aging. The hematoxylin and eosin staining protocol was performed in tissue paraffin blocks and they were then stained immunohistochemically with rabbit monoclonal anti-COL I and anti-COL VI antibodies. A total of 201 slides were studied with an Olympus BX 41 microscope, and the expressions of COL I and COL VI in the dermis were scored on a scale of 1 to 5, and then positively and statistically analyzed with IBM SPSS Statistics software. The results show that solar elastosis changes the structure of the skin’s collagen and solar elastosis was observed in the skin tissues with photoaging without appearing to be affected by its appearance in relation to age. Solar radiation divides the collagen fibers more rapidly than normal biological aging and replaces the collagen fibers of the skin. COL I and COL VI are expressed differently along the dermis of healthy skin tissue and the skin tissue subject to photoaging.
1. Introduction
The human body produces collagen using a complete range of nutrients that it takes from a balanced diet containing vitamins, trace elements, enzymes, coenzymes, and amino acids. The most important vitamin, which is the most basic structural element with which collagen is built, is vitamin C. Collagen is primarily produced by fibroblasts, while the basic structural unit of collagen is tropocollagen [1].
Collagen has the particularity of making insoluble fibers with high resistance to degeneration. The collagen fibers are thicker and rougher in the deeper layers of the dermis than the most superficial layers where collagen fibers are thinner and more relaxed. In youthful skin, the collagen fibers are intact and the skin is hydrated and elastic. It is durable and adapts to daily damage from various facial expressions. Over time, collagen levels are reduced, the collagen grid fades and the connective tissue is not as compact. The skin loses its elasticity and tone as its collagen support decreases [2].
Collagen consists of a triple helix consisting of two identical polypeptide chains and an additional chain that differs slightly in its chemical composition from the other two and is linked together by hydrogen bonds. The most common motifs in the collagen amino acid sequence are glycine–proline–X and glycine–X–hydroxyproline, where X is any amino acid other than glycine, proline, or hydroxyproline. Collagen communicates with cells through integrin receptors and mediates cell movement as well as adhesion [3]. There are many collagen types but one of the most basic types of collagen in the skin is collagen type I (COL I). COL I is the main component of the skin and its assessment is related to quantitative studies in dermatopathology. Repeated and chronic exposure to UV radiation upregulates MMP [4] and induces the marks of photoaging. The consequences of damage to collagen and MMP are exacerbated by the fact that in damaged cells, the synthesis of new collagen is reduced. Research results indicate that photo-exposed skins show an accumulation of fragmented collagen and its irregular (nonuniform) distribution along the dermis. Collagen depletion in heavily photo-exposed skin is normal, but these skin lesions can also occur during the natural aging process [5].
Collagen type VI is another type of collagen that is expressed in a significant amount in skin. COL VI is nonfibrillar collagen expressed in many connective tissues and involved in extracellular matrix (ECM) organization. Findings from studies on COL VI expression in the dermis indicate that it is a key regulator of dermal matrix assembly, fibroblast composition, and behavior, and may play a vital role in wound healing and tissue regeneration. COL VI‘s expression is homogeneously distributed along the dermis [4]. COL VI interacts with COL V and is associated with mucopolysaccharides.
Additionally, COL V and COL VI maintain the acellular dermal matrix (ADM) architecture [5]. The skin nerves are located mainly in the dermis, and COL VI plays a neuroprotective role [6].
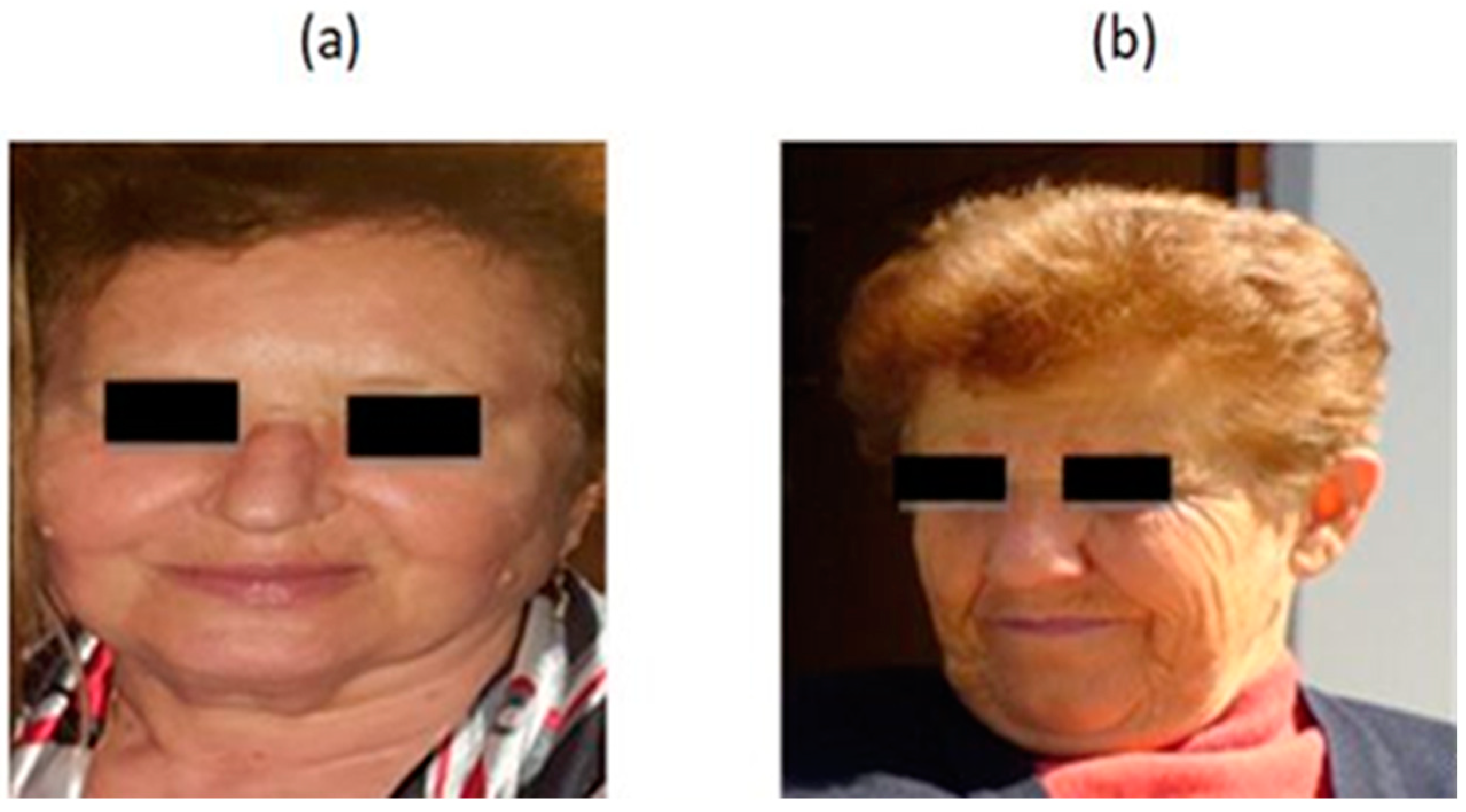
The factors that affect collagen by causing it to decline, or even its destruction, are divided into two categories, endogenous and exogenous factors [6,7]. As a result, skin aging is divided into endogenous and exogenous. Endogenous aging is a continuous process throughout life [8]. Further, skin aging is classified into biological aging and photoaging. Biological aging is the result of normal cell aging, with the normal appearance of aging characteristics. On the contrary, photoaging is the result of premature aging cells and the premature appearance of aging characteristics. The following figure is just a clinical example, and it is not part of the study (Figure 1).

Figure 1.
(a) Biological aging. A 72 year-old-woman, who lives in an urban city, uses suncream, drinks water >1.5 L per day, and eats fruits and vegetables daily. (b) Photoaging. A 68-year-old woman who lives in a village in Greece. She is a farmer, does not use suncream and does not like to eat fruits and vegetables. The woman in (b) is younger than the woman in picture (a), but her skin has signs of premature aging (deep wrinkles, dull skin, intense relaxation. (Archival material by courtesy of Dr. Foteini Biskanaki, 2020).
Every time we smile or we have anger, we practice pressure on the collagen of our skin. The result of these expressions is the appearance of fine lines and wrinkles on the face. From the age of 60, the reduction in collagen is even greater. It is expressed in the form of relaxation and severe wrinkles and is called skin aging. The characteristics of chronological or biological aging are the reduction in the rate of skin cell proliferation, the thinning of the skin, and the reduction in elastin and collagen. There is also a moderate increase in metalloproteinases (MMPs) and collagen degradation as well as a decrease in the proliferation rate of fibroblasts. The small veins below the skin expand without losing their architecture. Finally, fine wrinkles, brown–yellow spots, and sagging skin appear [9].
Both UVA and UVB have been associated with the onset of photoaging as well as various types of skin cancer. They are harmful to the skin either through their direct interaction with biological molecules or through their interaction with atmospheric oxygen and the formation of free radicals [10,11]. The characteristics of photoaging are the irregular shape of keratinocytes and melanocytes, the increase in the rate of proliferation of epidermal cells, the thickening of the keratin layer, the reduction in Langerhans cells, the increase in the activity of metalloproteinases and the degradation of collagen. Additionally, the concentration of poor-quality collagen with reduced functionality, the increase in elastin production, the accumulation of short, dysfunctional elastin fibers (elastic material), and the thickening of the dermis all increase the metabolic activity of fibroblasts in mast cells and cytokines that are associated with inflammation. Finally, the small veins under the skin dilate and become tortuous; the skin becomes rough with deep wrinkles with localized increases in melanin synthesis [12].
Endogenous factors are related to DNA, genes, and heredity. Over time, the production of collagen is reduced within the skin; the properties of elastin are altered and slow down the renewal of the epidermal cells. This process usually begins in the middle of the third decade of life, that is, around the age of 25 years old. From this age onwards, collagen levels in the body decrease by 1.5% per year [9]. Fibroblasts slow down the production of collagen resulting in the deterioration of the quality of the collagen contained in the connective tissue. The reasons for the productivity of fibroblasts being slowed down are hormonal. Menopause stops estrogen production. Estrogens play an important role in regulating fibroblasts. Chronological aging accompanies menopause and results in collagen loss and skin thinning. The rate at which the functions are altered is controlled by each person’s genes, while its speed is different from person to person and depends on the genetic material each person inherited from their parents.
Exogenous factors depend on the environmental factors to which each person is exposed during life. The most serious exogenous factor in premature skin aging is sun exposure. Sunlight consists of UVA and UVB rays. When the skin is exposed to the sun, UVA rays penetrate deep into the skin and cause significant changes at the level of the skin. A percentage of 80% UVB radiation is absorbed by the epidermis and 20% UVA radiation by the dermis. About 20% of UVA that penetrates the skin is responsible for the destruction of capillaries and fibroblasts, the denaturation of proteins, and other skin cells. The destruction of fibroblasts by solar radiation results in the suppression of collagen and elastin production, while the denaturation of proteins results in the breakdown of the collagen chain and the production of chemical compounds, such as free radicals [13].
UV radiation causes oxidative stress in skin cells, and as a result, cells damaged by oxidized lipids activate complement systems and cause inflammation, which leads to infiltration and the activation of macrophages. Activated macrophages release uterine metalloproteinases (MMPs), which break down the extracellular matrix [14]. The complement system is inactivated by repeated UV exposure, thereby damaging the epidermal–dermis junction, where macrophages are deposited and overloaded with oxidized lipids. Overloaded macrophages release proinflammatory cytokines and reactive oxygen species (ROS), which cause chronic inflammation and long-term damage to the dermis [15]. Additionally, smoking causes serious biochemical changes in our bodies, and among other harmful changes, smoking accelerates endogenous aging mechanisms. Cigarette smoke and air pollution cause oxidative stress that wears down skin proteins such as collagen and slows cell renewal. Stress and the intense modern lifestyle burden the body and accelerate endogenous aging factors increasing free radicals and tissue strain. The body in a state of stress secretes the hormone cortisol, which in excess destroys the collagen structure.
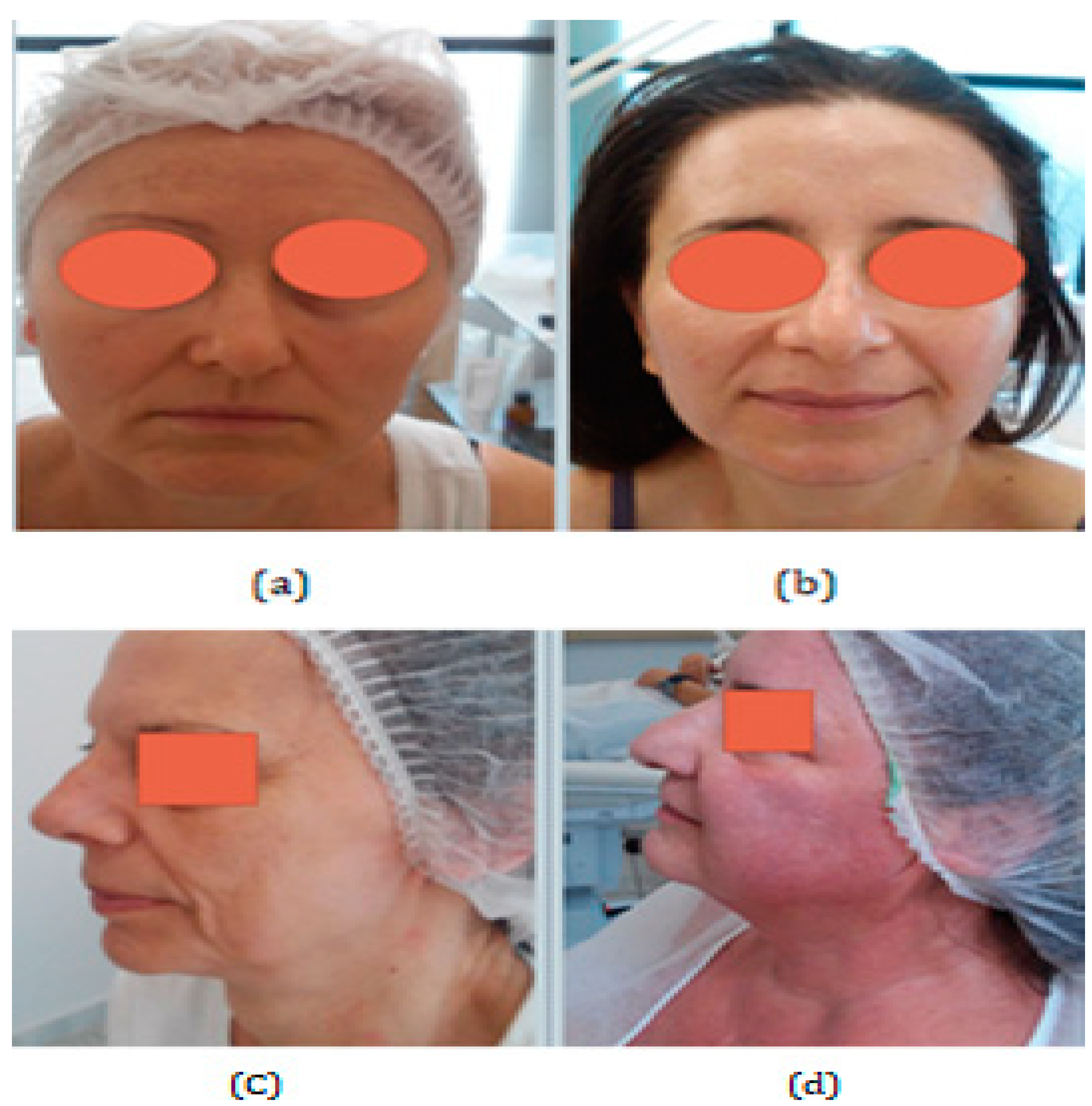
The following figure is just a clinical example, and it is not part of the study. It illustrates the differences between the subcategories of aging. In chronoaging, biological age coincides with cell age; in photoaging, the premature aging of cells, sagging, and deep wrinkles are observed. Irradiated skin has reduced collagen, so, for example, at the age of 54 years old, skin can appear to be like that of a 64-year-old [16] (Figure 2).
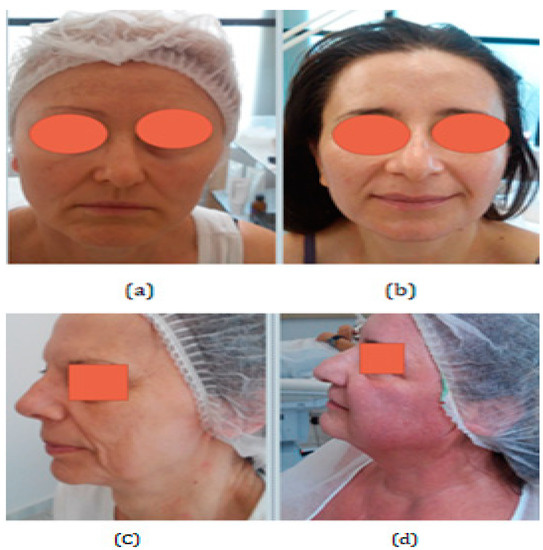
Figure 2.
(a) Biological aging in dry skin. The subject does not smoke but lives in a city with air pollution. (b) Biological aging with oily skin. The subject does not smoke, lives out of the city, uses moisturizing cream daily, drinks water, and eats fruits every day. (c) Photoaging with dry skin. The subject does not drink water and does not eat fruits daily. She lives in the city and does not use moisturizing cream daily. (d) Biological aging in sensitive and oily skin. All four women have the same age (54 years old), and use sun cream daily, but have different types of skin and different ways of life, and as a result, show different characteristics of aging. (Archival material by courtesy of Dr. Foteini Biskanaki, 2019).
This study is based on the different expressions of COL I and COL VI in the dermis between healthy (youthful skin and skin with biological aging) and pathological tissues (skins with photoaging—solar elastosis). The aim was to assess the impact of solar radiation on the expression of COL I and COL VI in the dermis of skins with aging.
2. Materials and Methods
2.1. Tissue Samples
Sixty-seven biopsies of skin (healthy skin and sun-damaged skin) were recovered from the First Department of Pathology of the Medicine School of the National and Kapodistrian University of Athens in Greece. Tissue samples (skins with biological aging: n = 44, skins with photoaging: n = 20, and healthy skins that were used as controls, youth skin: n = 3) were fixed in buffered formalin, embedded into paraffin blocks, and then stained with hematoxylin and eosin.
2.2. Antibοdies
Rabbit monoclonal anti-COL I antibody [EPR7785] IHC-P 1/1500 and anti-COL VI antibody [EPR17072] IHC-P 1/250 were used. Heat-mediated antigen retrieval was performed with Thermo Scientific Pierce Tris-EDTA (TE) buffer, pH 9, before commencing with IHC staining for protocollagen.
2.3. Immunohistochemistry Microscopy Analysis
The microscope slides were evaluated using an Olympus BX 41 microscope at magnifications of ×40 and ×100. The immunohistochemical report was performed by estimation with a visual evaluation of the percentage of COL I and COL VI expression on a positive scale of 1 to 5 (weak +, weak to moderate ++, moderate +++, moderate to severe ++++, and severe +++++) [17].
2.4. Statistical and Data Analysis
All of the collected data were entered into a database created in Excel. Data analysis was performed using IBM SPSS part number CC0RMEN Statistics for Windows, version 26.0. Frequencies were calculated for qualitative variables. Categorical variables were sex, age categories, body part, and type of lesion. They were studied using chi-square Χ2 and descriptive analysis in relation to (a) the type of lesion (body part), (b) the expression of COL I, (c) the expression of COL VI, and the degree of severity of solar elastosis. One sample t-test was applied to determine the different expressions of COL I and COL VI in tissues with aging (biological aging and photoaging). The Kolmogorov–Smirnov test was applied to check normality. This relationship was accessed by the Kruskal–Wallis test, providing the mean and standard deviation. Values of p < 0.05 were indicative of statistical significance.
3. Results
3.1. Characteristics of Tissue Samples
Forty-seven tissue samples were characterized as healthy (n = 44 biological aging and n = 3 youthful skin), taken from the abdomen (n = 4), face (n = 22), and breast (n = 21). In terms of the pathological specimens with solar elastosis (photoaging n = 20), 10 were from the face, 4 were from the back, 2 were from the abdomen, and 4 were from the extremities. The specimens were divided into three age groups (1st = 66–85 years old, 2nd = 46–65 years old, 3rd = 25–45 years old). The study focused on the expression of COL I and COL VI in two indexes. The first index was between the epidermis and solar elastosis (index A), and the second was along the dermis (index B).
3.2. Healthy Tissue Samples with Biological Aging
The IHC microscopy results analysis showed that the healthy tissues had a uniform expression of collagen type I along the dermis. COL I staining confirmed that the collagen fibers were thin and loose in the papillary dermis and thicker in the reticular dermis. The healthy samples with youthful skin and chronological aging appeared with collagen fibers that were thin and loose in the papillary dermis and thicker in the reticular dermis. The distance between collagen fibers was greater in samples with aging compared to youthful skin samples.
On the other hand, in the aging sample tissues, the keratin layer appeared with hyperplasia. Skin atrophy and a reduction in the number of skin components were also observed, and only the sebaceous glands were overgrown. The expression of COL I was weaker than in sample tissues from a younger age. The expression of COL I in healthy tissue samples with biological aging was uniform along the dermis and weaker than the expression in young skin. The expression of COL I in the healthy tissue samples with biological aging was uniform along the dermis and weaker than in the expression in young skin. Moderate to intense (=4) expression was observed in the 25–45 age group, and moderate expression (=3) in the 46–65 age group, with a percentage of 65.96%. However, in the 66–85 age group with biological aging, the expression of COL I was moderate (=3), and a small percentage (2.65%) showed a weak (=1) expression (in ages over 75 years old). Generally, the expression of COL I was weaker in tissues with biological aging than in youthful skin tissues.
The results from the expression of COL VI in the dermis showed that the healthy—youthful skin—had a uniform expression. The expression of COL VI in healthy tissue samples with biological aging was uniform along the dermis and weaker than the expression in young skin. Moderate expression was observed in the 46–65 and 66–85 age groups, with a percentage of 75% and 78%, respectively.
In summary, according to the results, in the samples of the 66–85 age group with biological aging, COL VI was expressed moderately at a percentage of 78%, while the expression of COL I was moderate at a percentage of 55%. Respectively, in the same group, the expression of COL VI was weak at a percentage of 22%, and for COL I, at a percentage of 56%. In the 46–65 age group, the expression of COL VI was moderate at a percentage of 75%, moderate to severe at 29%, and severe at 7%. On the other hand, the expression of COL I was moderate at a percentage of 96%. In both of these age groups, COL VI had a uniform expression, in contrast to COL I, which had a uniform expression in youthful skins, and over time, this uniform expression was lost.
3.3. Photoaging
Initially, the photoaging specimens were evaluated according to the severity of the solar elastosis per body part. Then, it was compared by the degree of expression of COL I and COL VI. The results of the average expression of COL I and COL VI per age group and body part are described in Table 1.

Table 1.
Average expression of COL I and COL VI between the epidermis and solar elastosis (index A), and along the dermis (index B), per body part. Results are expressed as the average of the degree of severity οf sοlar elastosis per body part.
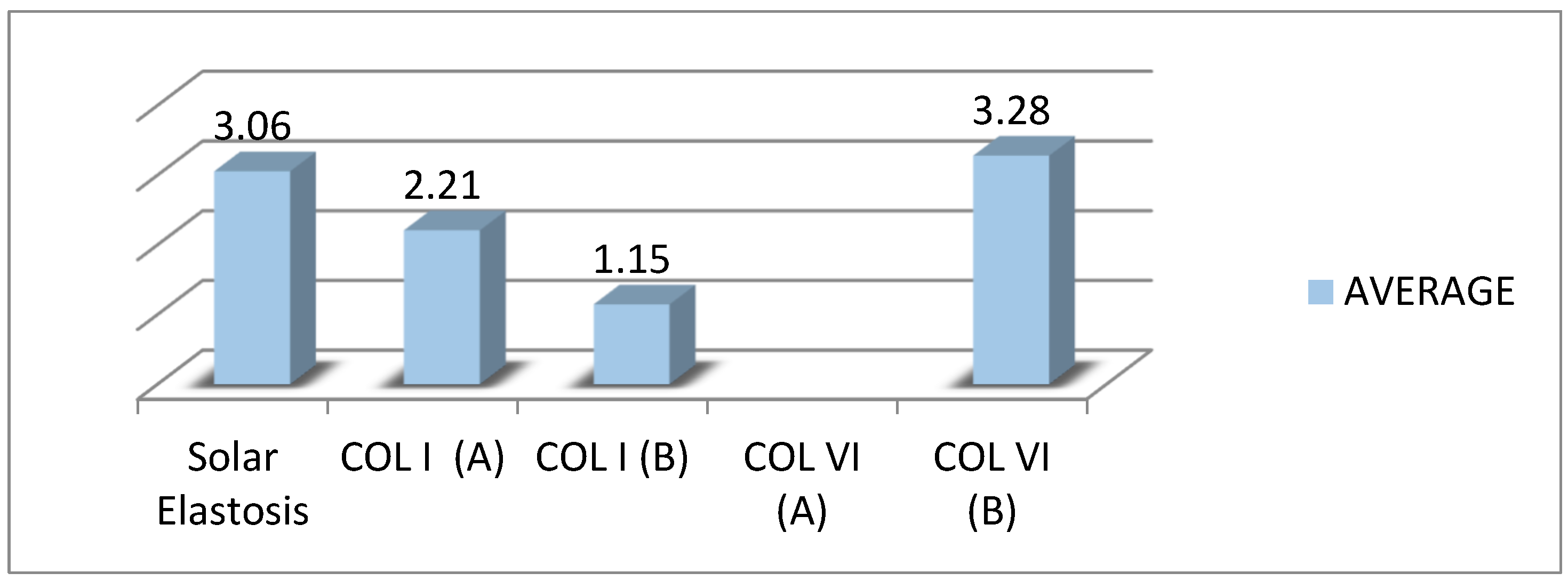
In photoaged tissue samples, the formation of a solar elastin island under the skin was observed replacing the collagen. The mean expression of COL I between the epidermis and solar elastosis (A score) was weak to moderate and weak along the dermis. Under the epidermis, it was observed that COL I was not expressed at all. The most severe solar elastosis was observed on the extremities, then on the back, and less so on the abdomen and face. Solar elastosis is represented as an abnormal band of elastin under the epidermis. The degree of solar elastosis had a negative correlation with COL I index A, in the order of 42.3% (when the degree of elastosis increases by one unit, the effect of collagen of elastosis decreases by one unit, and the effect of collagen increases by 0.42 of a unit).
On the other hand, there was no existing COL VI expression between the epidermis and solar elastosis (index A). Along the dermis (index B), the COL I expression was homogenously moderate and interrupted by solar elastosis. The degree of solar elastosis had a negative correlation with COL VI index B, in the order of 16% (when the degree of elastosis increases by one unit, the effect of collagen of elastosis decreases by one unit the effect of collagen increases by 0.16 of a unit) (Figure 3).
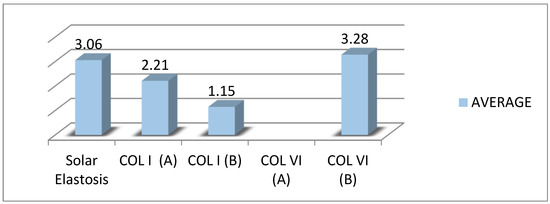
Figure 3.
The average expression of COL I and COL VI relative to the degree of solar elastosis.
3.4. Comparison of Biological Aging—Photoaging
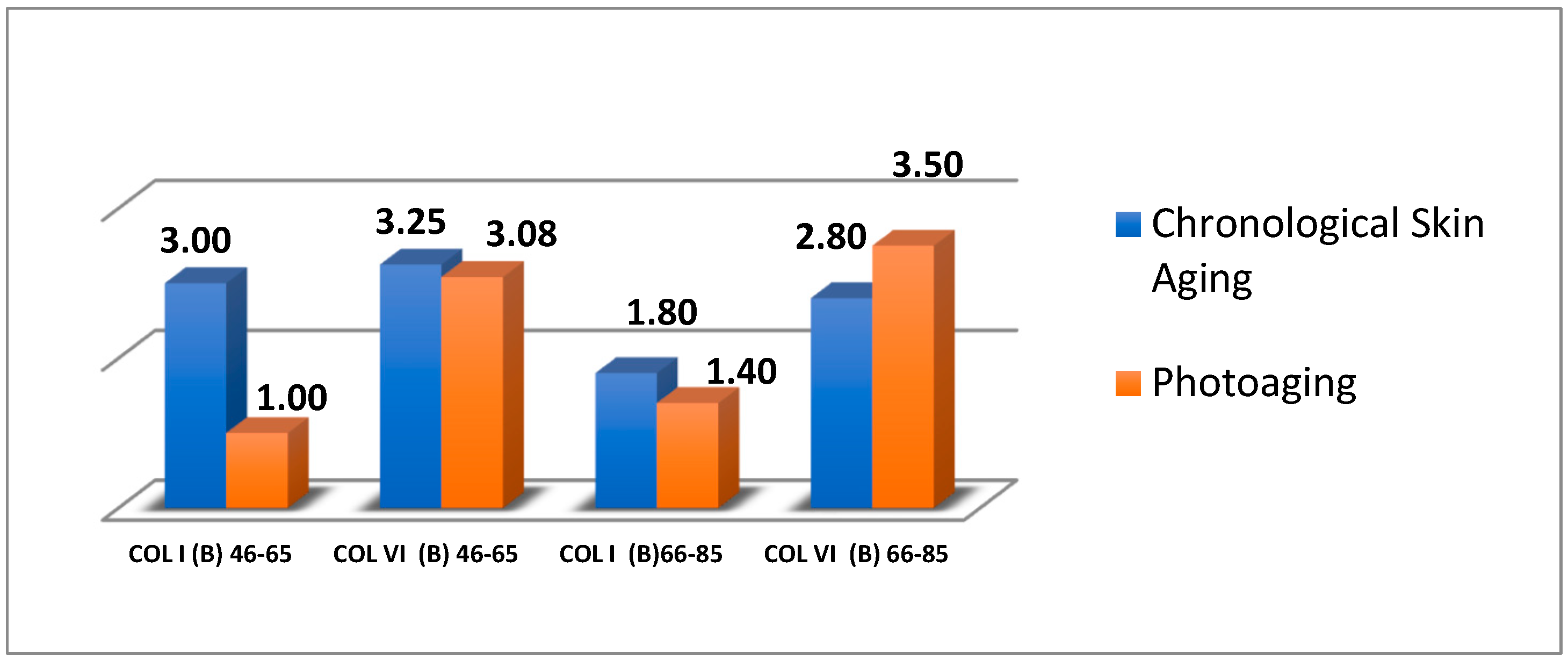
Comparing the expression of COL I and COL VI in a uniform sample (in age groups (a) 46–65 years old and (b) 66–85 years old, in the facial part) relative to index B, it was observed that:
- In the 46–65 age group with biological aging, the average expression of COLI and COLVI was moderate.
- In the 46–65 age group with photoaging, the average expression of COL I was weak, while the average expression of COL VI was almost the same as in the biological aging sample.
- In the 66–85 age group with biological aging, the average expressions of COL I and COL VI were even weaker than the previous age group.
- In the 66–85 age group with photoaging, the expression of COL I was even weaker than the samples with biological aging. On the other hand, the average expression of COL VI was more than moderate (avg. = 3.5) than in the samples with biological aging (avg. = 2.8) ( Figure 4 and Figure 5).
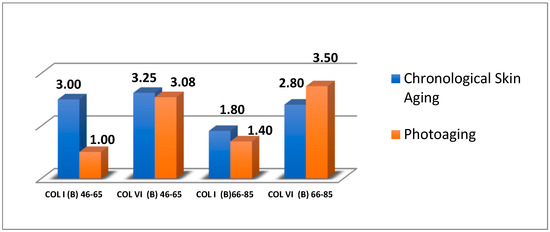 Figure 4. Average expression of COL I and COL VI (Index Β) by age group and type of aging.
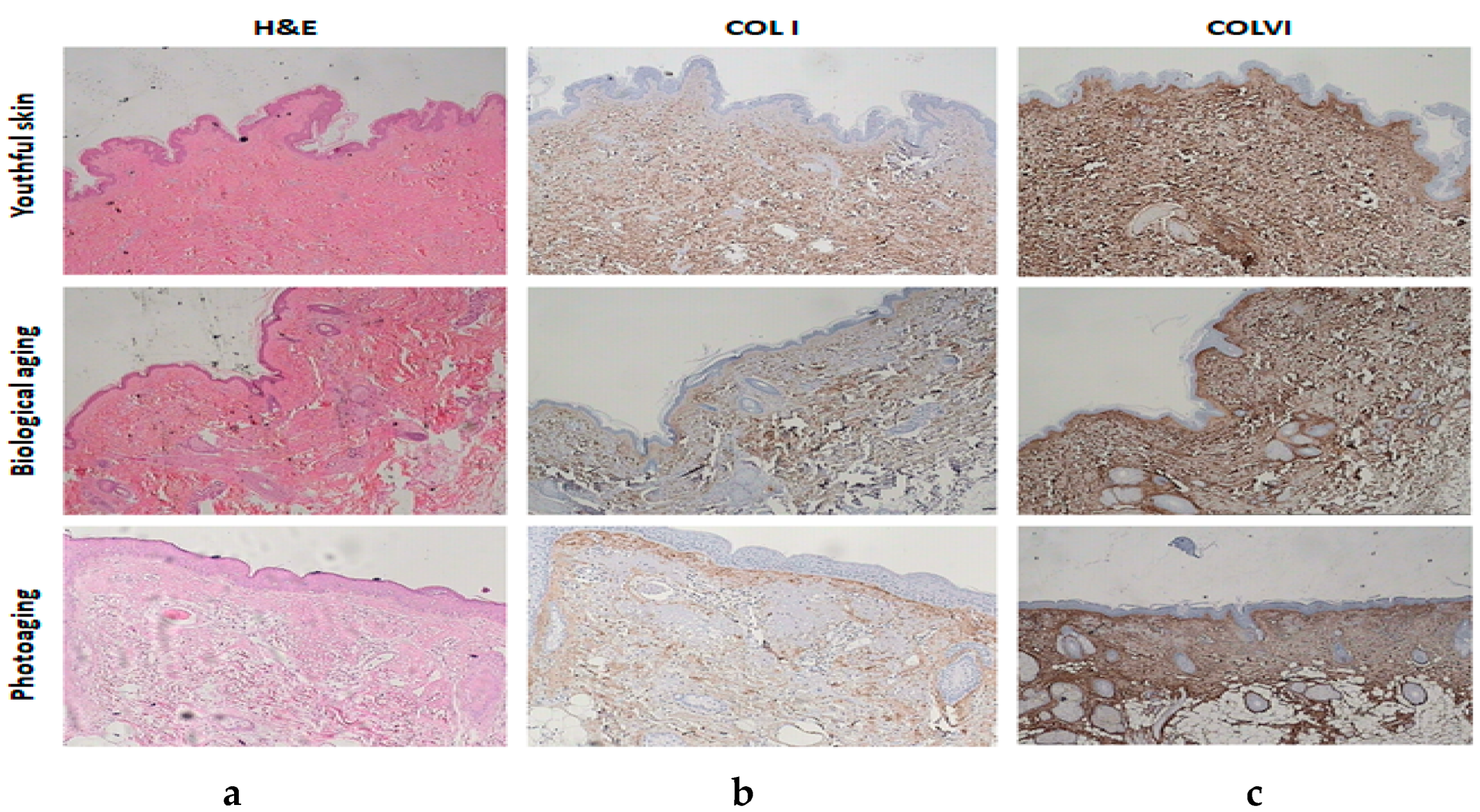
Figure 4. Average expression of COL I and COL VI (Index Β) by age group and type of aging.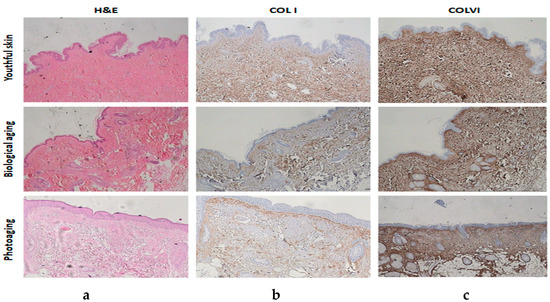 Figure 5. (a) Histochemical stainings of hematoxylin–eosin: youthful tissue, biological aging, and photoaging. (b) Anti-COL I stainings: youthful tissue, biological aging, and photoaging. (c) Anti-COL VI stainings: youthful tissue, biological aging, and photoaging.
Figure 5. (a) Histochemical stainings of hematoxylin–eosin: youthful tissue, biological aging, and photoaging. (b) Anti-COL I stainings: youthful tissue, biological aging, and photoaging. (c) Anti-COL VI stainings: youthful tissue, biological aging, and photoaging.
In summary, in healthy tissue samples characterized by biological aging, COL I was, on average, expressed more weakly than COL VI. In tissues that were characterized with photoaging, the expression of COL I appeared to reduce by half, and the expression of COL VI appeared to have doubled, on average.
It was observed that solar radiation negatively affects the expression of COL I in the dermis as opposed to the expression of COL VI, which increased.
4. Discussion
The primary cause of exogenous skin aging is long-term exposure to UV radiation. In order to understand the expression of COL l and COL VI in human skin with aging, their expression and localization in healthy skin and tissues with solar elastosis were studied. In healthy tissues (youthful skins and skins with biological aging), COL l staining confirmed that collagen fibers are thin and loose in the papillary dermis and thicker with homogeneity. However, over time, they become weaker and lose their homogeneity [16]. COL VI has a uniform distribution along the dermis and its expression is weaker than COL I expression at older ages, without losing its homogeneity.
Solar elastosis can change both the collagen and elastin in the skin. It appears superficially because, as it is known, UVA radiation is absorbed more by the epidermis (at a percentage of 80%) and less by the dermis (at a percentage of 20%) [17]. It was also confirmed in this study that in the dermis, UV radiation divides the collagen more rapidly than in biological aging [18]. The formation of an abnormal elastin stripe (solar elastosis) below the epidermis was observed in the skin with photoaging, which replaces (by covering) the collagen fibers of the skin [19]. These findings confirm that the elastotic material replaces collagen [20,21]. In our specimen, it was observed that solar elastosis was more severe in the areas of the back and extremities and less in the face. COL I was expressed by moderate and film-like distribution between the skin and solar elastosis, and it had a weak expression in the rest of the dermis. On the contrary, COL VI was moderately and homogeneously expressed throughout the thickness of the dermis (interrupted by the position of solar elastosis), and more intense in all age groups compared to COL I in biologically aging tissues in which it had a weak expression; moreover, its expression was reduced in the tissues with photoaging. This appears to reflect a particular stability of COL VI, which, unlike most extracellular molecules, remains unaffected by the process of biological aging and photoaging [19,21].
The severity of solar elastosis is not completely related to age. It was observed that in people over 75 years of age, solar elastosis was milder than at age 65, and we would expect a more severe reduction in aged skin. This could be a random finding observed in our sample (according to various factors, e.g., lifestyle, location, duration of sun exposure, etc.) [7]. However, the possible cause could be related to the ozone layer, as stratospheric ozone effectively absorbs UV radiation. As the ozone layer becomes thinner, the protective filter provided by the atmosphere gradually diminishes. Consequently, each year both humans and the environment are exposed to higher levels of UV radiation, with more severe adverse effects at younger ages than in the past [22].
Finally, previous studies emphasized the presence of COL VI in malignant cells by enhancing the production of primitive factors and causing epithelial–mesenchymal transition [16,18]. In addition, it affects the microenvironment of the tumor by increasing the mobility of macrophages and endothelial cells, thus prοmοting tumοr inflammatiοn and angiogenesis. The differential expression of COL VI has been cited as a promising diagnostic and prognostic parameter. It has also been reported that collagen VI is a promising open field of research because it promotes chemotherapy resistance and may be considered a possible biomarker for cancer diagnosis [12,23].
Author Contributions
Conceptualization, F.B. and E.R.; methodology, A.C.L.; software, E.R.; validation, F.B.; formal analysis, F.B.; investigation, F.B.; resources, V.K.; data curation A.C.L.; writing—original draft preparation, F.B.; writing—review and editing, E.R. and V.K.; visualization, A.C.L.; supervision, E.R. and A.C.L.; and project administration; F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the School of Medicine, the National and Kapodistrian University of Athens, Greece, 1718016667/2512018, 25/1/2018.
Informed Consent Statement
Patient consent was waived due to the sample was from archival material. However, informed consent was obtained from all subjects involved in the figures.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
We thank A. Kapranou and V. Papadopoulos for their help in the IHC analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Draelos, Z.D. Nutrition and enhancing youthful-appearing skin. Clin. Dermatol. 2010, 28, 400–408. [Google Scholar] [CrossRef]
- Theocharidis, G.; Drymoussi, Z.; Kao, A.P.; Barber, A.H.; Lee, D.A.; Braun, K.M.; Connelly, J.T. Type VI Collagen Regulates Dermal Matrix Assembly and Fibroblast Motility. J. Investig. Dermatol. 2016, 136, 74–83. [Google Scholar] [CrossRef]
- Di Lullo, G.A.; Sweeney, S.M.; Korkko, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the Ligand-binding Sites and Disease-associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.-Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Suzanne EG Fligiel Varani, J.; Subhash CDatta Kang, S.; Gary JFisher John, J. Voorheesw, Col Degradation in Aged/Photodamaged Skin In Vivo and After Exposure to Matrix Metalloproteinase-1 In Vitro. J. Investig. Dermatol. 2003, 120, 842–848. [Google Scholar]
- Garrone, R.; Lethias, C.; Le Guellec, D. Distribution of minor collagens during skin development. Microsc. Res. Tech. 1997, 38, 407–412. [Google Scholar] [CrossRef]
- Kobayasi, T.; Karlsmark, T. Type V and VI collagen for cohesion of dermal fibrillar structures. J. Submicrosc. Cytol. Pathol. 2006, 38, 103–108. [Google Scholar] [PubMed]
- Gregorio, I.; Braghetta, P.; Bonaldo, P.; Cescon, M. ColVI in healthy and diseased nervous system. Cell Transpl. 2018, 27, 729–738. [Google Scholar]
- Morita, A. Tobacco smoke causes premature skin aging. J. Dermatol. Sci. 2007, 48, 169–175. [Google Scholar] [CrossRef]
- Biskanaki, F.; Rallis, E.; Skouras, G.; Stofas, A.; Thymara, E.; Kavantzas, N.; Lazaris, A.C.; Kefala, V. Impact of Solar Ultraviolet Radiation in the Expression of Type I Collagen in the Dermis. Cosmetics 2021, 8, 46. [Google Scholar] [CrossRef]
- Montesu, M.A.; Onnis, G.; Gunnella, S.; Lissia, A.; Satta, R. Elastosis perforansserpiginosa: Causes and associated disorders. Eur. J. Dermatol. 2018, 28, 476–481. [Google Scholar]
- Weihermann, A.C.; Lorencini, Μ.; Brohem, C.A.; de Carvalho, C.M. The structure of elastin and its involvement in photosynthesis of the skin. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fisher, G.J.; Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qin, Z.; Alexander Wilks, J.; Balimunkwe, R.M.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Physical properties of the photodamaged human skin dermis: Rougher Colsurface and stiffer/harder mechanical properties. Exp. Dermatol. 2019, 28, 914–921. [Google Scholar] [CrossRef]
- Trudel, D.; Desmeules, P.; Turcotte, S.; Plante, M.; Grégoire, P.; Renaud, M.C.; Orain, M.; Bairati, I.; Têtu, B. Visual and automated assessment of matrix metalloproteinase-25 tissue expression for the evaluation of ovarian cancer prognosis. Modern Pathol. 2014, 27, 1394–1404. [Google Scholar] [CrossRef]
- Juher, F.T.; Pérez, B.E. An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing. Nutr. Hosp. 2015, 18, 62–66. [Google Scholar]
- Pain, S.; Berthélémy, N.; Naudin, C.; Degrave, V.; André-Frei, V. Understanding Solar Skin Elastosis-Cause and Treatment. J. Cosmet. Sci. 2018, 69, 175–185. [Google Scholar]
- Varvaresou, A.; Tsirivas, E.; Tsaoula, E.; Protopapa, E. Oxidative stress, photoaging and topical antioxidant protection. Rev. Clin. Pharm. Pharm. 2014, 18, 261–266. [Google Scholar]
- Biskanaki, F.; Rallis, E.; Skouras, G.; Kapranou, A.; Papadopoulos, V.; Protopapa, E.; Revelos, K.; Diamantopoulou, K.; Kefala, V.; Lazaris, A.C. Non-Melanoma Skin Cancer (NMSC) and Collagen Type VI: Histological study of the Damaging Effect of Solar Radiation on the Dermis. J. Community Med. Public Health Rep. 2021, 2. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, Μ.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, S.V.; Blaauw, B.; Cossu Vozzi, G.; et al. ColVI Regulates Satellite Cell Self-Renewal and Muscle Regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef] [PubMed]
- Kielty, C.M.; Lees, M.; Shuttleworth, C.A.; Woolley, D. Catabolism of intact type VI collagen microfibrils: Susceptibility to degradation by serine proteinases. Biochem. Biophys. Res. Commun. 1993, 191, 1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cescon, M.; Bonaldo, P. ColVI in cancer and its biological mechanisms. Trends Mol. Med. 2013, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).