The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Plant Extracts
2.2. Quantification of the Antioxidant Activity (In Vitro)
2.3. Preparation of the O/W Emulsions
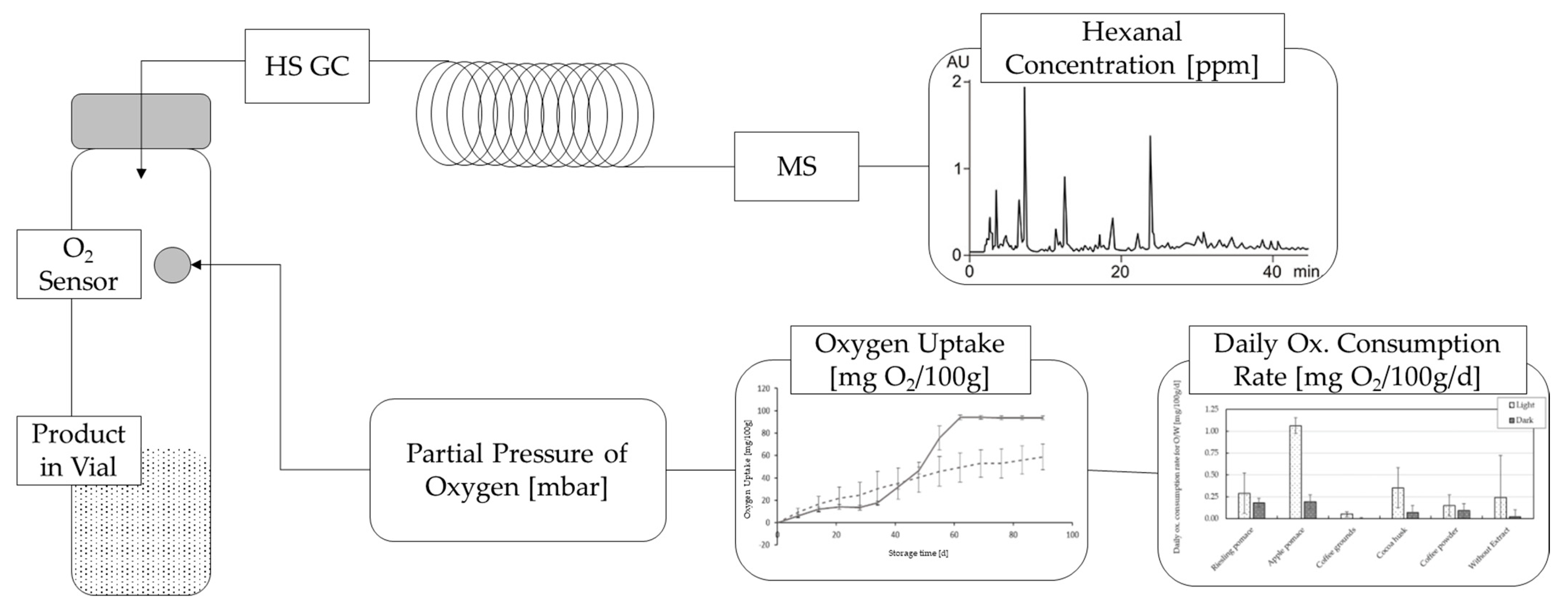
2.4. Quantification of the Oxygen Uptake and Oxidation Products
2.5. Data Analysis
3. Results
3.1. Determination of In Vitro Antioxidant Behavior of Plant Extracts
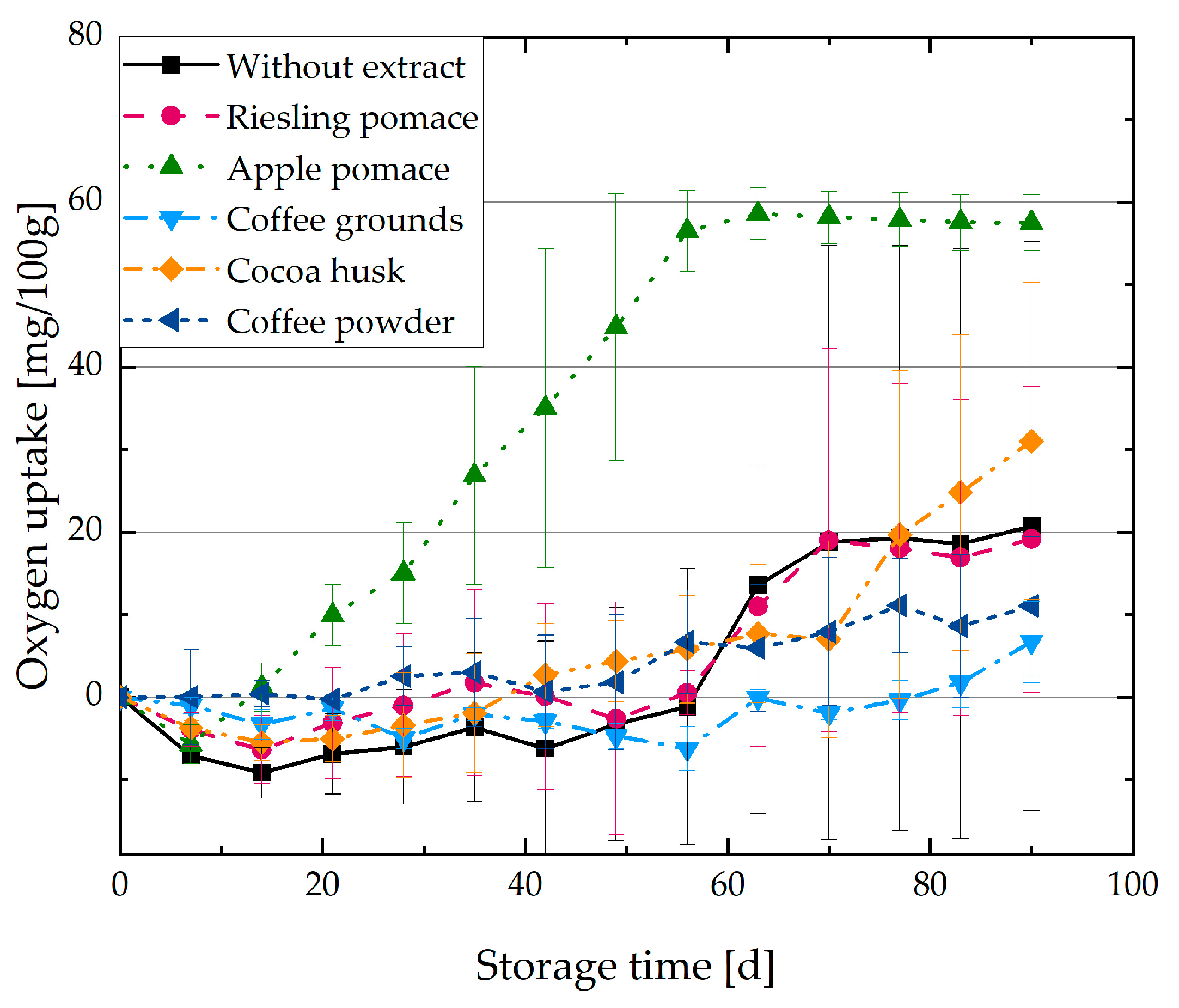
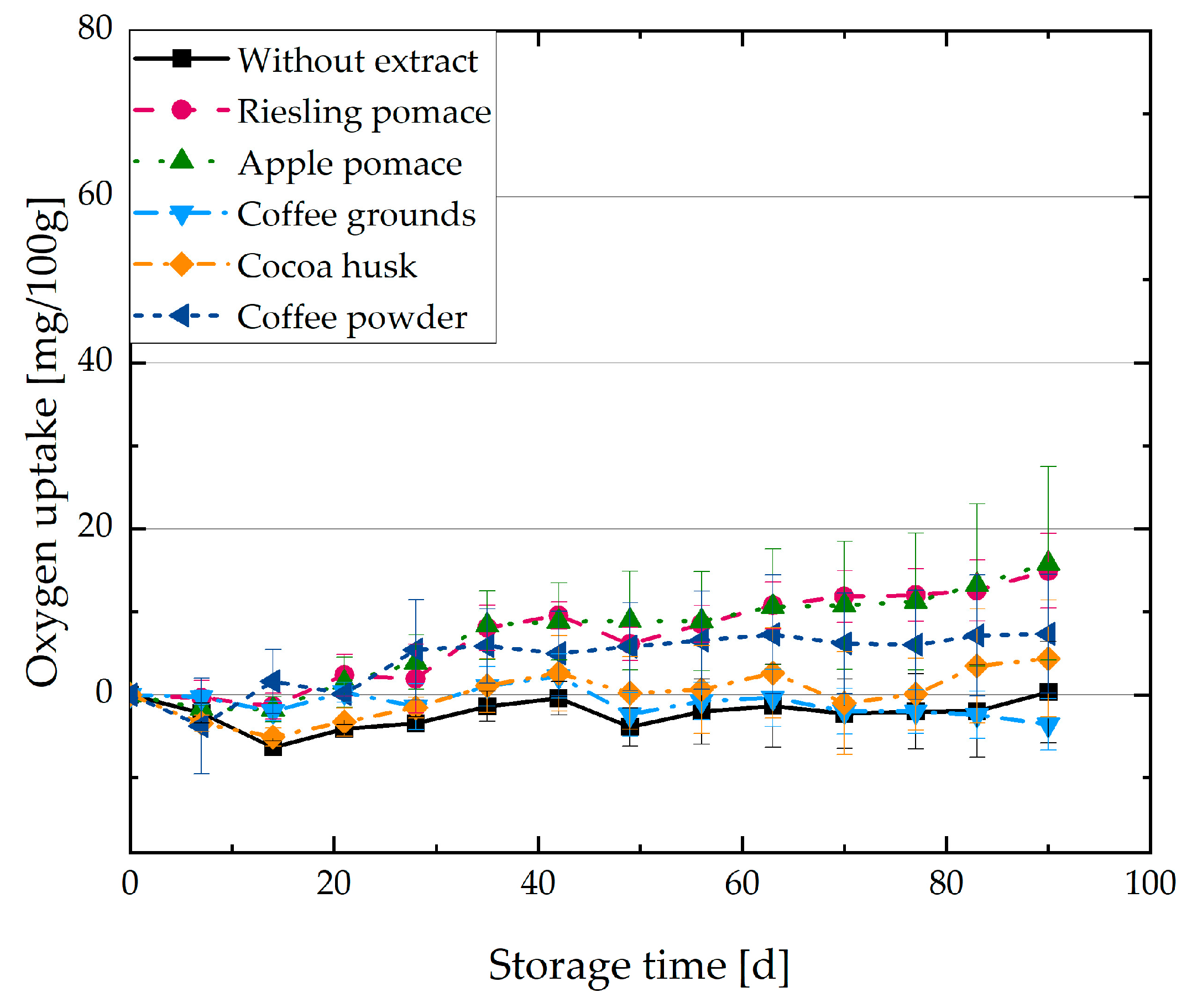
3.2. Oxygen Uptake of O/W Emulsions with Plant Extracts
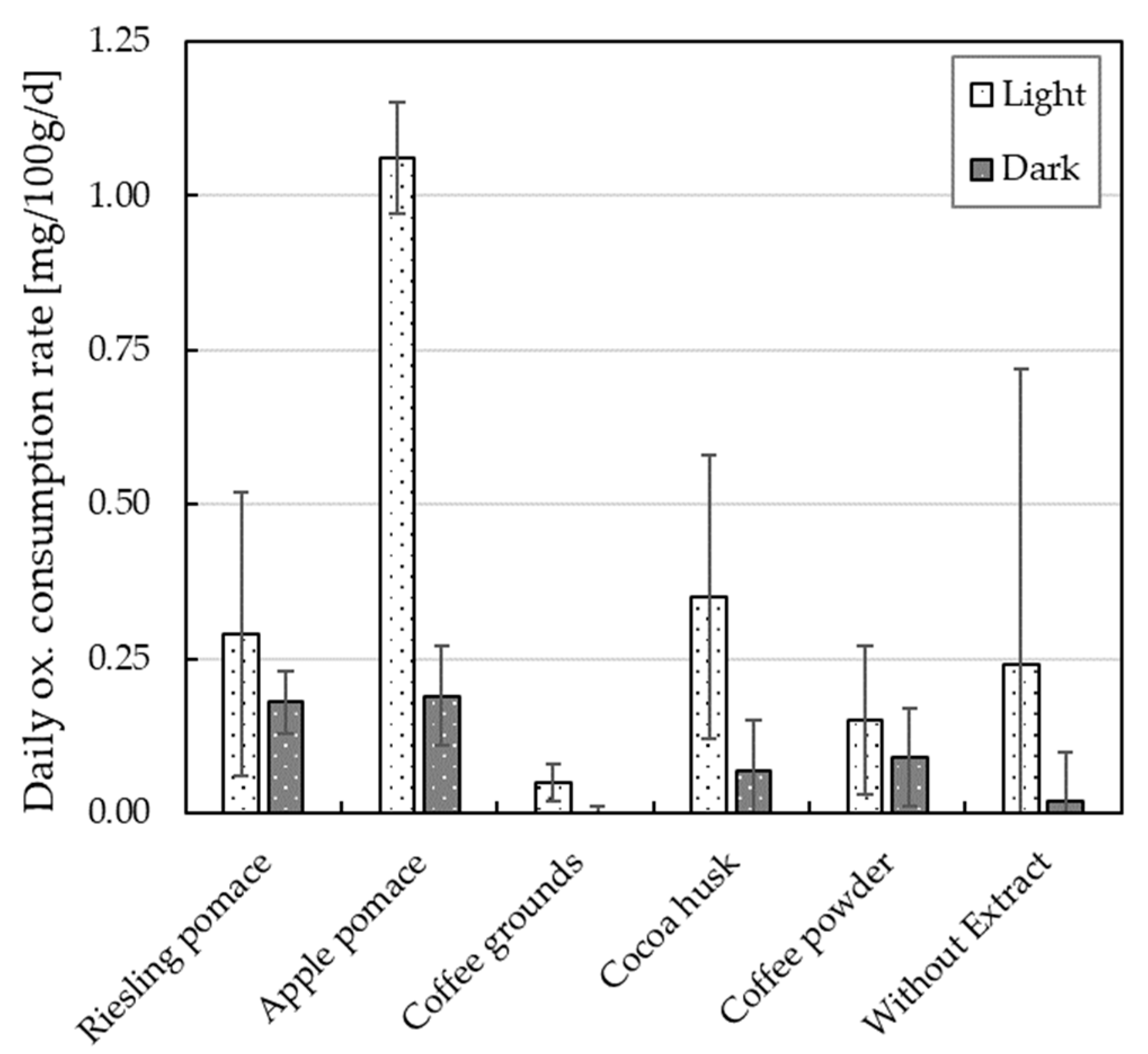
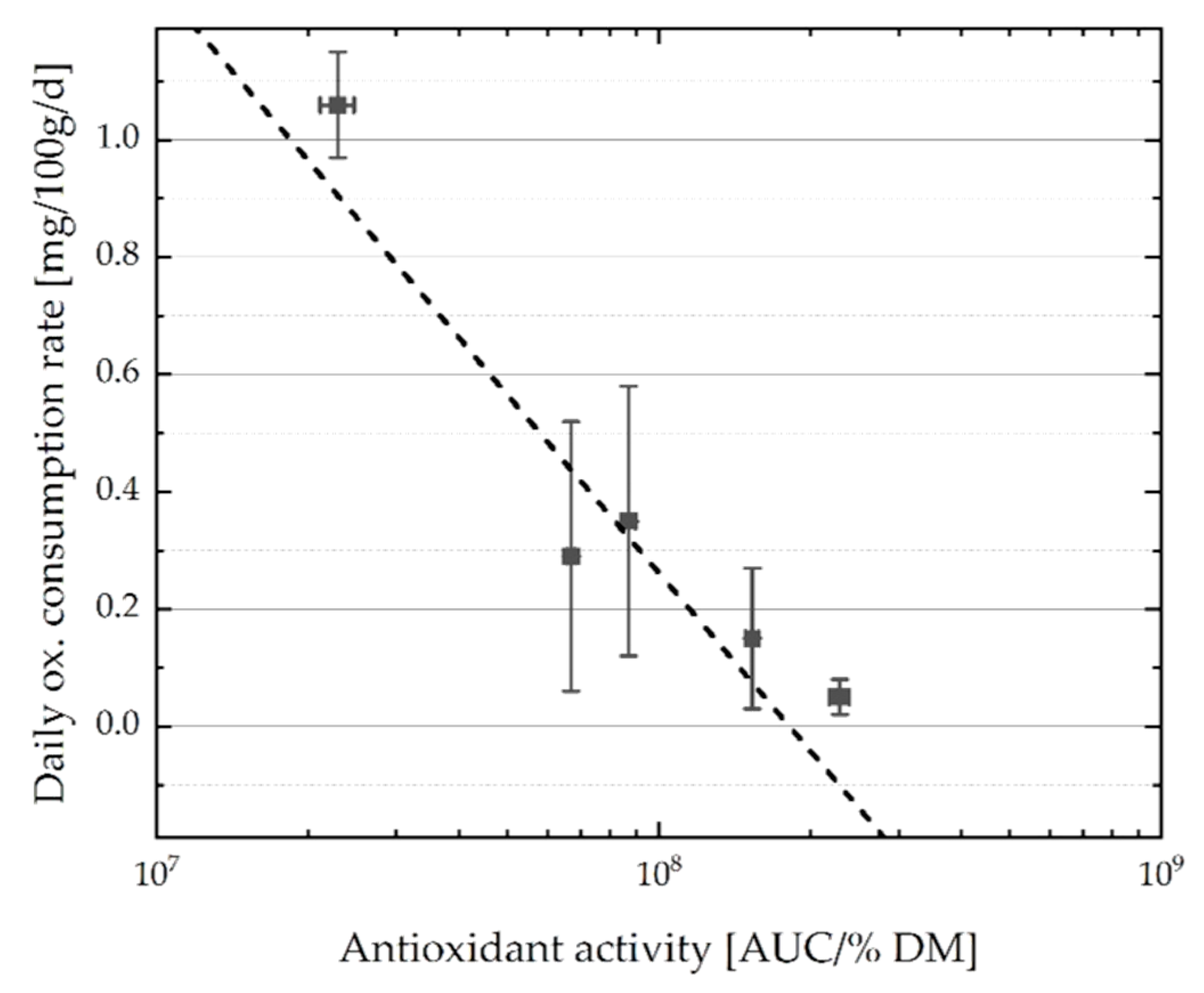
3.3. Daily Oxygen Consumption Rate of O/W Emulsions with Plant Extracts
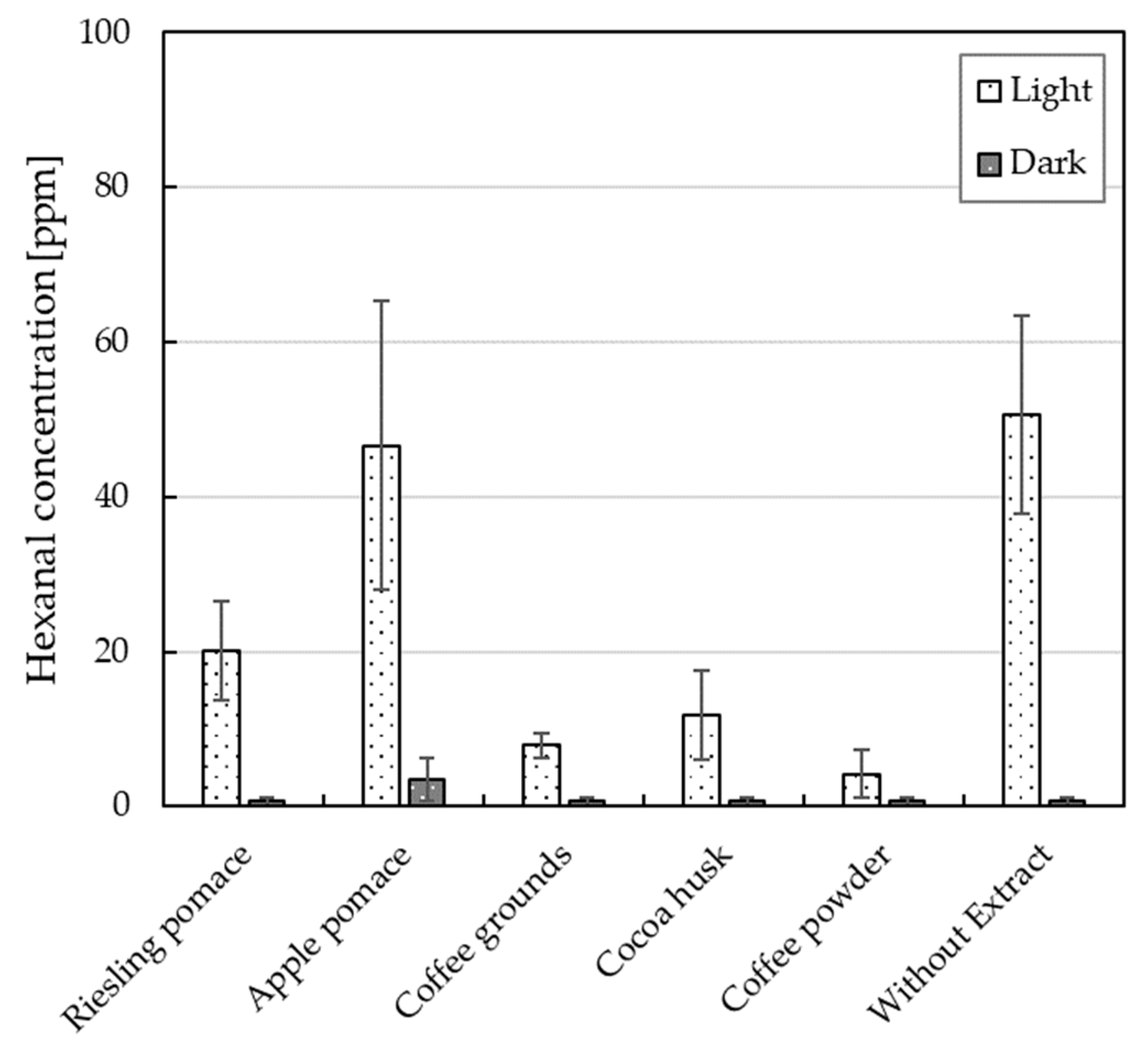
3.4. Hexanal Concentration of O/W Emulsions with Plant Extract
4. Discussion
4.1. Antioxidant Activity Results
4.2. Oxidation Kinetics
4.3. Hexanal Concentration
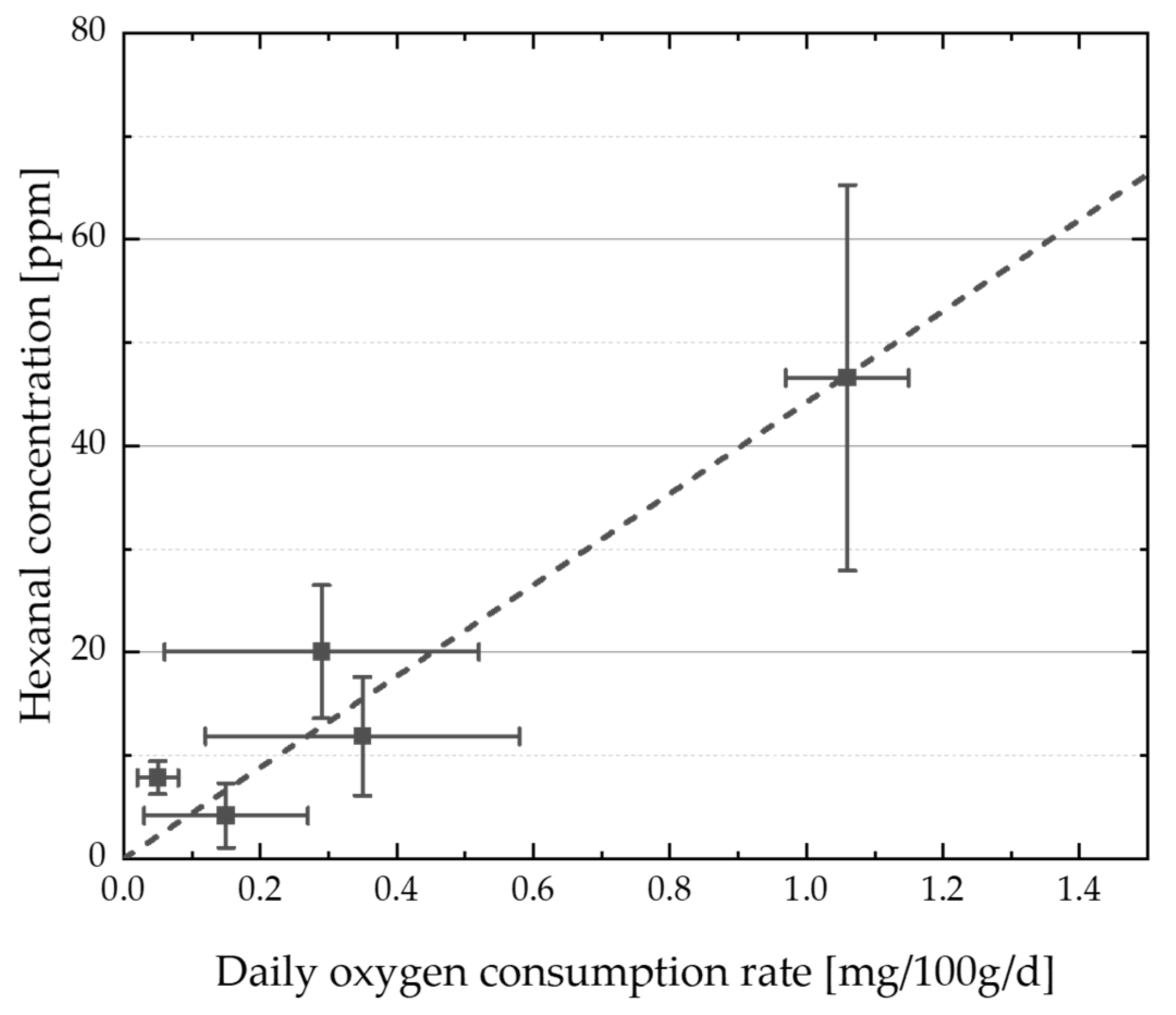
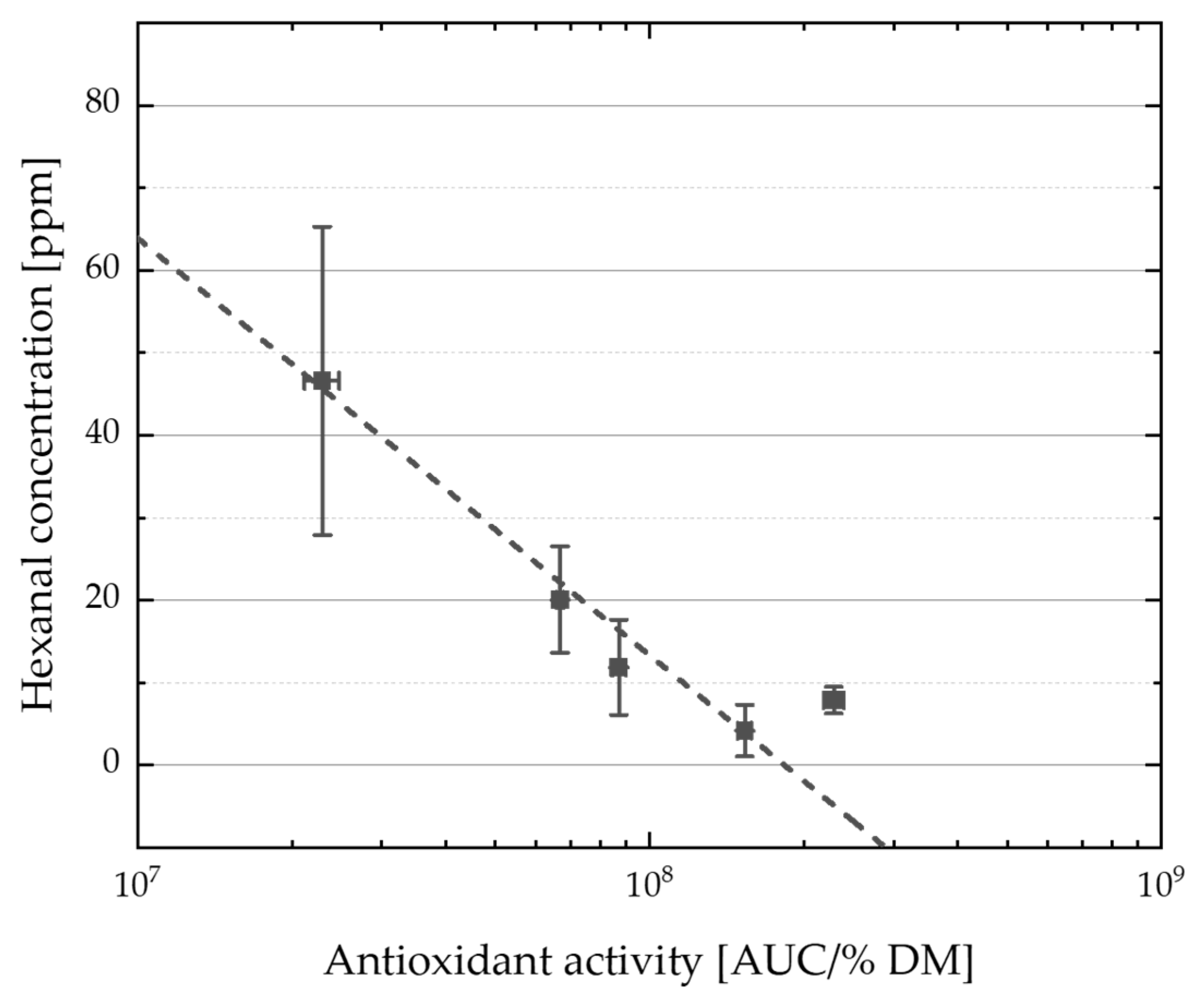
4.4. Correlations
4.5. The Role of the Emulsion on Oxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Amberg, N.; Fogarassy, C. Green consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Matić, M.; Puh, B. Consumers’purchase Intentions Towards Natural Cosmetics. Ekon. Vjesn./Econviews-Rev. Contemp. Bus. Entrep. Econ. Issues 2016, 29, 53–64. [Google Scholar]
- Rähse, W. Cosmetic Creams: Development, Manufacture and Marketing of Effective Skin Care Products; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Statista, Umsatz mit Naturkosmetik in Deutschland in den Jahren 2007 bis 2019: (in Millionen Euro). Available online: https://de.statista.com/statistik/daten/studie/201220/umfrage/umsatz-mit-naturkosmetik-in-deutschland/2020 (accessed on 19 January 2023).
- Morse, P.F.; Horrobin, D.F.; Manku, M.S.; Stewart, J.C.M.; Allen, R.; Littlewood, S.; Wright, S.; Burton, J.; Gould, D.J.; Holt, P.J.; et al. Meta-analysis of placebo-controlled studies of the efficacy of Epogam in the treatment of atopic eczema. Relationship between plasma essential fatty acid changes and clinical response. Br. J. Dermatol. 1989, 121, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, M.J.; Korting, H.C. Treatment of atopic eczema with evening primrose oil: Rationale and clinical results. Clin. Investig. 1992, 70, 167–171. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Fiadeiro, T.; Silva, M.; Soares, A.P. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998, 7, 213–216. [Google Scholar] [CrossRef]
- Williams, H.C. Evening primrose oil for atopic dermatitis. BMJ 2003, 327, 1358–1359. [Google Scholar] [CrossRef]
- Van Gool, C.J.A.W.; Zeegers, M.P.A.; Thijs, C. Oral essential fatty acid supplementation in atopic dermatitis—A meta-analysis of placebo-controlled trials. Br. J. Dermatol. 2004, 150, 728–740. [Google Scholar] [CrossRef]
- Nasrollahi, S.A.; Ayatollahi, A.; Yazdanparast, T.; Samadi, A.; Hosseini, H.; Shamsipour, M.; Akhlaghi, A.A.; Yadangi, S.; Abels, C.; Firooz, A. Comparison of linoleic acid-containing water-in-oil emulsion with urea-containing water-in-oil emulsion in the treatment of atopic dermatitis: A randomized clinical trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 21. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W. Lehrbuch der Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Reklamation.com. Reklamation—Weleda Granatapfel—Regenerierende Pflegelotion. Available online: https://reklamation.com/weleda/weleda-granatapfel-regenerierende-pflegelotion/ranzig-obwohl-noch-haltbar-bis-01-2019 (accessed on 19 January 2023).
- COSMOS-Standard AISBL. Cosmos Standard Internationale Zertifizierung für Kosmetik. Available online: https://www.cosmos-standard.org/about-the-cosmos-standard?lang=de (accessed on 19 January 2023).
- Ecocert. Ecocert Standard—Natural and Organic Cosmetics. Available online: https://ecocert.app.box.com/v/Ecocert-Standard (accessed on 19 January 2023).
- NATRUE. The International Natural and Organic Cosmetics Association, NATRUE-Label: Kriterien für Natur- und Biokosmetik: Version 3.9. 2021. Available online: https://www.natrue.org/uploads/2021/01/DE-NATRUE-Label_criteria_v3.9_January-2021.pdf (accessed on 19 January 2023).
- Mohd Fauzi, N.; Spickett, C.M. Lipid Oxidation. In Studies on Experimental Toxicology and Pharmacology; Humana Press: Cham, Switzerland, 2015; pp. 43–79. [Google Scholar]
- Baltes, W. Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Krist, S.; Buchbauer, G.; Klausberger, C. Lexikon der pflanzlichen Fette und Öle; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kranl, K.; Schlesier, K.; Bitsch, R.; Hermann, H.; Rohe, M.; Böhm, V. Comparing antioxidative food additives and secondary plant products–use of different assays. Food Chem. 2005, 93, 171–175. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Di Mambro, V.M.; Fonseca, M.J. Assays of physical stability and antioxidant activity of a topical formulation added with different plant extracts. J. Pharm. Biomed. Anal. 2005, 37, 287–295. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.d.O.; Gonçalves, F.C.d.S.; Eberlin, S.; Dávila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-enriched plant-extract-loaded emulsion: A novel phytocosmetic sunscreen formulation with antioxidant properties. Antioxidants 2019, 8, 443. [Google Scholar] [CrossRef]
- Khan, B.A.; Akhtar, N.; Khan, H.; Braga, V.D.A. Development, characterization and antioxidant activity of polysorbate based O/W emulsion containing polyphenols derived from Hippophae rhamnoides and Cassia fistula. Braz. J. Pharm. Sci. 2013, 49, 763–773. [Google Scholar] [CrossRef]
- Malinowska, P.; Gliszczyńska-Świgło, A.; Szymusiak, H. Protective effect of commercial acerola, willow, and rose extracts against oxidation of cosmetic emulsions containing wheat germ oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1553–1562. [Google Scholar] [CrossRef]
- Skowyra, M.; Falguera, V.; Azman, N.A.; Segovia, F.; Almajano, M.P. The effect of Perilla frutescens extract on the oxidative stability of model food emulsions. Antioxidants 2014, 3, 38–54. [Google Scholar] [CrossRef]
- Abdalla, A.E.; Roozen, J.P. Effect of plant extracts on the oxidative stability of sunflower oil and emulsion. Food Chem. 1999, 64, 323–329. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Balboa, E.M.; Soto, M.L.; Nogueira, D.R.; González-López, N.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crops Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef]
- Kargar, M.; Spyropoulos, F.; Norton, I.T. The effect of interfacial microstructure on the lipid oxidation stability of oil-in-water emulsions. J. Colloid Interface Sci. 2011, 357, 527–533. [Google Scholar] [CrossRef]
- Noon, J.; Mills, T.B.; Norton, I.T. The use of natural antioxidants to combat lipid oxidation in O/W emulsions. J. Food Eng. 2020, 281, 110006. [Google Scholar] [CrossRef]
- Fomuso, L.B.; Corredig, M.; Akoh, C.C. Effect of emulsifier on oxidation properties of fish oil-based structured lipid emulsions. J. Agric. Food Chem. 2002, 50, 2957–2961. [Google Scholar] [CrossRef]
- Dimakou, C.P.; Kiokias, S.N.; Tsaprouni, I.V.; Oreopoulou, V. Effect of processing and storage parameters on the oxidative deterioration of oil-in-water emulsions. Food Biophys. 2007, 2, 38–45. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Yi, J.; Liu, N.; Cao, Y.; Decker, E.A.; McClements, D.J. Impact of interfacial composition on lipid and protein co-oxidation in oil-in-water emulsions containing mixed emulisifers. J. Agric. Food Chem. 2018, 66, 4458–4468. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free. Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A.; Bernardi, M.; Cappiello, F.; Galgano, F.; Caruso, M.C.; Favati, F. Comparison of OXITEST and RANCIMAT methods to evaluate the oxidative stability in frying oils. Eur. Food Res. Technol. 2018, 244, 747–755. [Google Scholar] [CrossRef]
- Sun, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Sun, Y.E.; Wang, W.D.; Chen, H.W.; Li, C. Autoxidation of unsaturated lipids in food emulsion. Crit. Rev. Food Sci. Nutr. 2011, 51, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, H.J. Fettsäurezusammensetzung wichtiger pflanzlicher und tierischer Speiseöle und-fette. In Deutsches Lebensmittelbuch; Deutsche Gesellschaft für Fettwissenschaft: Frankfurt, Germany, 2011; Volume 116. [Google Scholar]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.N.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Lück, E. Chemische Lebensmittelkonservierung: Stoffe Wirkungen Methoden; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Springer, A.; Ziegler, H. The Role of Preservatives and Multifunctionals on the Oxidation of Cosmetic O/W Emulsions. Cosmetics 2022, 9, 59. [Google Scholar] [CrossRef]
- Böhner, N. Einfluss der Handelsbeleuchtung auf die Qualität lichtempfindlicher Lebensmittel am Beispiel von Brühwurst und Kaffeesahne. Ph.D. Thesis, Technische Universität München, München, Germany, 2019. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Šidák, Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- Moreira, D.P.; Monteiro, M.C.; Ribeiro-Alves, M.; Donangelo, C.M.; Trugo, L.C. Contribution of chlorogenic acids to the iron-reducing activity of coffee beverages. J. Agric. Food Chem. 2005, 53, 1399–1402. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Manzocco, L.; Lerici, C.R. Antioxidant properties of coffee brews in relation to the roasting degree. LWT-Food Sci. Technol. 1997, 30, 292–297. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Steinhart, H.; Luger, A.; Piost, J. Antioxidative effect of coffee melanoidins. In Proceedings of the 19th Colloque Scientifique International sur le Café, Trieste, Italy, 14–18 May 2001; Association Scientifique Internationale du Café (ASIC): Allenwinden, Switzerland, 2001; pp. 1–8. [Google Scholar]
- Fuster, M.D.; Mitchell, A.E.; Ochi, H.; Shibamoto, T. Antioxidative activities of heterocyclic compounds formed in brewed coffee. J. Agric. Food Chem. 2000, 48, 5600–5603. [Google Scholar] [CrossRef]
- Del Castillo, M.D.; Ames, J.M.; Gordon, M.H. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 2002, 50, 3698–3703. [Google Scholar] [CrossRef]
- Nebesny, E.; Budryn, G. Antioxidative activity of green and roasted coffee beans as influenced by convection and microwave roasting methods and content of certain compounds. Eur. Food Res. Technol. 2003, 217, 157–163. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.H.; Shi, Y.; Duan, C.Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef]
- Tomas-Barberán, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerdá, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef]
- van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and antiradical activity of coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef]
- Pollini, L.; Juan-García, A.; Blasi, F.; Mañes, J.; Cossignani, L.; Juan, C. Assessing bioaccessibility and bioavailability In Vitro of phenolic compounds from freeze-dried apple pomace by LC-Q-TOF-MS. Food Biosci. 2022, 48, 101799. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Rawls, H.R.; Van Santen, P.J. A possible role for singlet oxygen in the initiation of fatty acid autoxidation. J. Am. Oil Chem. Soc. 1970, 47, 121–125. [Google Scholar] [CrossRef]
- Martínez, M.L.; Penci, M.C.; Ixtaina, V.; Ribotta, P.D.; Maestri, D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT-Food Sci. Technol. 2013, 51, 44–50. [Google Scholar] [CrossRef]
- Malviya, A.; Vrabec, J. Henry’s law constant of nitrogen, oxygen, and argon in ternary aqueous alcoholic solvent mixtures. J. Chem. Eng. Data 2019, 65, 1189–1196. [Google Scholar] [CrossRef]
- Tokunaga, J. Solubilities of oxygen, nitrogen, and carbon dioxide in aqueous alcohol solutions. J. Chem. Eng. Data 1975, 20, 41–46. [Google Scholar] [CrossRef]
- PreSens. Available online: https://www.presens.de/products/detail/oxygen-sensor-spot-sp-pst3-nau (accessed on 19 January 2023).
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. A new pyrogallol coated oxygen scavenging film and their effect on oxidative stability of soybean oil under different storage conditions. Food Sci. Biotechnol. 2017, 26, 1535–1543. [Google Scholar] [CrossRef]
- Springer, A.; Kiese, S.; Platzer, M.; Pazurik, B. Protection Against Oxidation COSSMA (2022), Nr. 7–8, pp. 24–27. Available online: https://www.cossma.com/ingredients/article/protection-against-oxidation-36616.html?L=1%27&cHash=84ed45b02692340935c37dd2791c7668 (accessed on 19 January 2023).
- Farhoosh, R. Initiation and propagation kinetics of inhibited lipid peroxidation. Sci. Rep. 2021, 11, 6864. [Google Scholar] [CrossRef]
- Matissek, R.; Steiner, G.; Fischer, M. Lebensmittelanalytik, 5th ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Bachofen, R. Gas metabolism of microorganisms. Experientia 1991, 47, 508–513. [Google Scholar] [CrossRef]
- Fritsch, C.W.; Gale, J.A. Hexanal as a measure of rancidity in low fat foods. J. Am. Oil Chem. Soc. 1977, 54, 225–228. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of secondary and tertiary volatile compounds resulting from the lipid oxidation of rapeseed oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef]
- Petersen, K.D.; Kleeberg, K.K.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Comparison of analytical and sensory lipid oxidation parameters in conventional and high-oleic rapeseed oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1193–1203. [Google Scholar] [CrossRef]
- Palamand, S.R.; Dieckmann, R.H. Autoxidation of n-hexanal. Identification and flavor properties of some products of autoxidation. J. Agric. Food Chem. 1974, 22, 503–506. [Google Scholar] [CrossRef]
- Khan, M.A.; Shahidi, F. Oxidative stability of stripped and nonstripped borage and evening primrose oils and their emulsions in water. J. Am. Oil Chem. Soc. 2000, 77, 963–969. [Google Scholar] [CrossRef]
- Richards, A.; Wijesundera, C.; Salisbury, P. Evaluation of oxidative stability of canola oils by headspace analysis. J. Am. Oil Chem. Soc. 2005, 82, 869–874. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Ohloff, G. Riechstoffe und Geruchssinn: Die molekulare Welt der Düfte; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Rosenow, P.; Destler, E.; Springer, A. The Search for Suitable Packaging for Cosmetics—A Case Study. Sofw J. 2022, 10, 5. [Google Scholar]
- Mancuso, J.R.; McClements, D.J.; Decker, E.A. The effects of surfactant type, pH, and chelators on the oxidation of salmon oil-in-water emulsions. J. Agric. Food Chem. 1999, 47, 4112–4116. [Google Scholar] [CrossRef]
- An, S.; Lee, E.; Choe, E. Effects of solubility characteristics of sensitiser and pH on the photooxidation of oil in tuna oil-added acidic O/W emulsions. Food Chem. 2011, 128, 358–363. [Google Scholar] [CrossRef]
- Lagaly, G.; Schulz, O.; Zimehl, R. Dispersionen und Emulsionen: Eine Einführung in die Kolloidik feinverteilter Stoffe einschließlich der Tonminerale; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chaiyasit, W.; McClements, D.J.; Weiss, J.; Decker, E.A. Impact of surface-active compounds on physicochemical and oxidative properties of edible oil. J. Agric. Food Chem. 2008, 56, 550–556. [Google Scholar] [CrossRef]
- Chaiyasit, W.; Elias, R.J.; McClements, D.J.; Decker, E.A. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 2007, 47, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Uluata, S.; McClements, D.J.; Decker, E.A. How the multiple antioxidant properties of ascorbic acid affect lipid oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2015, 63, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Boersma, M.G.; de Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; van Zanden, J.J.; van der Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]

| Ingredient | Step | Content (g/100 g) |
|---|---|---|
| Canola (Brassica napus) Oil | 1 | 22.8 |
| Xanthan Gum | 1 | 0.2 |
| Glyceryl Stearate SE | 2 | 7.0 |
| Plant Extract | 2 | See Table 2a |
| Added Ethanol (96%) | 2 | See Table 2b |
| Citric acid | 2 | 0.2 |
| Water | 2 | Add to 100 |
| Ingredient | Dry Mass in Extract (%) | a: Extract Content in Emulsion (g/100 g) | Ethanol in Plant Extract (g/100 g) | b: Content Added Ethanol (g/100 g) |
|---|---|---|---|---|
| Riesling (Vitis vinifera) pomace extract | 3.41 | 5.27 | 3.69 | 11.78 |
| Apple (Malus domestica) pomace extract | 4.75 | 3.79 | 2.65 | 12.86 |
| Coffee (Coffea arabica) grounds extract | 0.78 | 20.51 | 14.36 | 0.67 |
| Cocoa (Theobroma cacao) husk extract | 0.89 | 20.23 | 14.16 | 0.88 |
| Coffee (Coffea arabica) powder extract | 1.58 | 11.39 | 7.98 | 7.32 |
| Parameter | Value |
|---|---|
| Headspace conditioning | 30 min 60 °C |
| GC column | Optima WAXplus, Thickness of 0.25 μm, diameter of 0.25 mm, length of 30 m, polar |
| Column oven temperature | 35 °C |
| Carrier gas | Helium 1.4 mL/min |
| Temperature gradient | 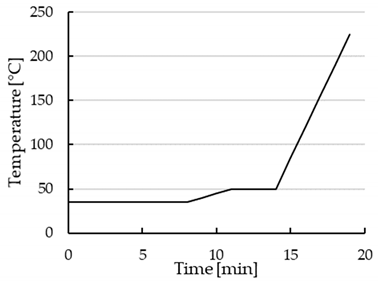 |
| Ionization | Electron impact ionization, ion source temperature of 200 °C, 70 eV |
| Mass spectrometry | Detection: quadrupole, interface temperature of 230 °C, scan mode of 35–350 m/z, speed of 1666/min |
| Riesling Pomace | Apple Pomace | Coffee Grounds | Cocoa Husk | |
|---|---|---|---|---|
| Apple pomace | <0.001 *** | |||
| Coffee grounds | <0.001 *** | <0.001 *** | ||
| Cocoa husk | <0.001 *** | <0.001 *** | <0.001 *** | |
| Coffee powder | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** |
| Riesling Pomace | Apple Pomace | Coffee Grounds | Cocoa Husk | Coffee Powder | Without Extr. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | Light | Dark | Light | |||
| Riesling pomace | Light | ||||||||||||
| Dark | 1.000 | ||||||||||||
| Apple pomace | Light | 0.001 *** | <0.001 *** | ||||||||||
| Dark | 1.000 | 0.946 | <0.001 *** | ||||||||||
| Coffee grounds | Light | 0.997 | 1.000 | <0.001 *** | 1.000 | ||||||||
| Dark | 0.915 | 1.000 | <0.001 *** | 1.000 | 1.000 | ||||||||
| Cocoa husk | Light | 1.000 | 1.000 | 0.004 *** | 1.000 | 0.939 | 0.649 | ||||||
| Dark | 0.999 | 1.000 | <0.001 *** | 1.000 | 0.999 | 1.000 | 0.974 | ||||||
| Coffee powder | Light | 1.000 | 1.000 | <0.001 *** | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| Dark | 1.000 | 1.000 | <0.001 *** | 1.000 | 1.000 | 1.000 | 0.989 | 1.000 | 1.000 | ||||
| Without Extract | Light | 1.000 | 1.000 | <0.001 *** | 1.000 | 1.000 | 0.990 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| Dark | 0.983 | 1.000 | <0.001 *** | 1.000 | 0.999 | 1.000 | 0.847 | 1.000 | 1.000 | 1.000 | 0.999 | ||
| Riesling Pomace | Apple Pomace | Coffee Grounds | Cocoa Husk | Coffee Powder | Without Extr. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | Light | Dark | Light | |||
| Riesling pomace | Light | ||||||||||||
| Dark | 0.118 | ||||||||||||
| Apple pomace | Light | 0.006 *** | <0.001 *** | ||||||||||
| Dark | 0.304 | 1.000 | <0.001 *** | ||||||||||
| Coffee grounds | Light | 0.847 | 1.000 | <0.001 *** | 0.997 | ||||||||
| Dark | 0.111 | 1.000 | <0.001 *** | 1.000 | 0.999 | ||||||||
| Cocoa husk | Light | 0.998 | 0.917 | <0.001 *** | 0.997 | 1.000 | 0.898 | ||||||
| Dark | 0.109 | 1.000 | <0.001 *** | 1.000 | 0.999 | 1.000 | 0.911 | ||||||
| Coffee powder | Light | 0.379 | 1.000 | <0.001 *** | 1.000 | 1.000 | 1.000 | 0.999 | 1.000 | ||||
| Dark | 0.116 | 1.000 | <0.001 *** | 1.000 | 0.999 | 1.000 | 0.922 | 1.000 | 1.000 | ||||
| Without Extract | Light | 0.001 *** | <0.001 *** | 1.000 | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | ||
| Dark | 0.113 | 1.000 | <0.001 *** | 1.000 | 0.999 | 1.000 | 0.905 | 1.000 | 1.000 | 1.000 | <0.001 *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; Ziegler, H.; Bach, K. The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions. Cosmetics 2023, 10, 40. https://doi.org/10.3390/cosmetics10020040
Springer A, Ziegler H, Bach K. The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions. Cosmetics. 2023; 10(2):40. https://doi.org/10.3390/cosmetics10020040
Chicago/Turabian StyleSpringer, Arielle, Helena Ziegler, and Katrin Bach. 2023. "The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions" Cosmetics 10, no. 2: 40. https://doi.org/10.3390/cosmetics10020040
APA StyleSpringer, A., Ziegler, H., & Bach, K. (2023). The Influence of Antioxidant Plant Extracts on the Oxidation of O/W Emulsions. Cosmetics, 10(2), 40. https://doi.org/10.3390/cosmetics10020040






