Abstract
Neodymium and dysprosium are two rare earth elements (REEs), out of a group of 17 elements. Due to their unique properties, REEs gained increasing importance in many new technologies, like wind turbines, batteries, etc. However, the production of REEs requires high material and energy consumption and is associated with considerable environmental burdens. Due to the strong dependency of European industry on Chinese REE exports, this paper presents a possible European production chain of REEs based on the mineral eudialyte found in Norra Kärr (Sweden). This European production is compared to a Chinese route, as China produces more than 85% of today’s REEs. Bayan Obo as the largest REE deposit in China is considered as the reference system. Using the life cycle assessment method, the environmental impacts of both production lines are assessed. This study presents newly-estimated data of a possible Swedish eudialyte-based production route for Europe. Results for the new eudialyte process route show reduced environmental burdens, although the total REE content in eudialyte is much smaller than in the Bayan Obo deposit. Especially, the results for dysprosium from eudialyte outreach those for Bayan Obo due to the higher content of heavy rare earth elements.
1. Introduction
Today, the production of rare earth elements (REE) mainly takes place in China with approximately 85% of the total world mine production of 124,000 t of rare earth oxide (REO) equivalents in 2015 [1]. Hence, European industries are totally dependent on imports of REEs. In addition, the use of REEs will increase in the future due to its decisive role in new technologies, such as wind turbines, batteries, lighting or medical techniques. The setup of an additional exclusively European supply chain is therefore economically and socially interesting. Although some European deposits were already identified [2], hardly any mining activities have taken place so far. The mineral exploration company Tasman Metals Ltd has set out a prefeasibility study for a Swedish mine in Norra Kärr [3]. The deposit contains resources of REE, yttrium (Y) and zirconium (Zr) with eudialyte as the main REE ore mineral.
Eudialyte is a rare, nine member ring cyclosilicate mineral, which forms in alkaline igneous rocks. Although REEs are not main components of the crystallite structure, they occur in considerable amounts due to substitution. Depending on the mineral type, REE concentrations from <1 up to 10% [4] have been found so far. A huge advantage compared to conventional RE minerals is the high share of heavy rare earth elements (HREEs) (up to 50% of total rare earth oxide (TREO)). The very low concentration of radioactive elements (only a few g/t eudialyte have been proven in experimental analysis) is a further major benefit. Moreover, eudialyte comprises also other high-tech materials, e.g., niobium, tantalum or zirconium. Therefore, other associated economically-important minerals, like nepheline, microcline, aegirine or catapleiite, can be mined together with eudialyte. Today, 191 eudialyte deposits are known worldwide. Beside Norra Kärr, eight further deposits (2 in Russia, 3 in Canada, 2 in Greenland, 1 in Malawi) show high potential for future mining of REEs [5,6]. They are all situated outside Europe. Thus, mining of a Swedish eudialyte deposit at Norra Kärr would reduce Chinese REE market power and increase the security of supply for the European industry. Particularly, it is already close to an existing infrastructure of electricity, transport and supply industry, which can be used without further effort.
Conventional RE production from bastnasite or monazite is known to require high material and energy amounts. Gupta and Krishnamurthy [7] provide very detailed process descriptions for several processing facilities and ore types. Environmental consequences, e.g., radioactive loaded dust and tailings, or impacts on human health are often addressed, especially in connection with Chinese RE production. While several studies address these impacts in a more general way [8,9,10,11], specific life cycle assessments (LCAs) considering Chinese supply chains exist [12,13,14,15]. Information about waste water and sludge treatment, as well as infiltration from tailings is almost completely missing. Navarro and Zhao [16] found out that many studies either rely on old data (1990) from the Mountain Pass mine in the USA or on deduced data for Bayan Obo. Furthermore, a recent LCA on monazite can be found [17]. Additionally, Zhao [18] has started to look into adsorption clays as a provider of HREEs. Schmidt [19] provides specific data for a refinery site in Malaysia. Schüler et al. [20] and Binnemans et al. [21] widen the scope by comparing primary production to recycling of REEs.
This paper focuses on the comparison of the environmental impacts of a hypothetical European supply chain in Sweden to those of today’s Bayan Obo route in Inner Mongolia. To show expected differences for light and heavy rare earth components, neodymium (Nd) and dysprosium (Dy) are chosen as representatives for each group. Data for the Swedish process chain are based on [3], but are mostly newly-developed in a joint four-year project of Rheinisch Westfälische Technische Hochschule (RWTH Aachen University), Siemens AG and Forschungszentrum Jülich. Using the LCA approach, major environmental effects can be identified. Furthermore, those processes can be distinguished that contribute most to these effects. A comparative quantitative assessment of environmental impacts is required in order to estimate, e.g., the smaller REE content of the ore at Norra Kärr compared to Bayan Obo versus the influence of stricter European environmental regulations. European environmental legislation inhibits uncontrolled release into the environment to prevent damages to humans and nature [22,23,24].
2. Method-Life Cycle Assessment
LCA is an adequate method for a holistic evaluation of the environmental effects of a product system. It is well established, internationally acknowledged and defined in the ISO standards 14040 [25] and 14044 [26]. Within LCA, all energy and material flows that occur during raw material extraction, production, operation and end of life of products or systems are quantified and evaluated in terms of environmental impacts. LCA distinguishes between four stages:
- The goal and scope definition describes the main purpose of the analysis. The investigated system is described, and the functional unit is defined, which is the basis for the comparison. The considered environmental effects are selected.
- In the life cycle inventory (LCI), all relevant inputs and outputs (resources, material, energy, emissions and waste) of the investigated system are collected.
- During the life cycle impact assessment (LCIA), the gathered inputs and outputs of the system are translated into environmental effects, so-called impact categories. In order to gain a better understanding of the relative importance of an environmental effect, an additional normalization step (optional in LCA) can complete the LCIA. Each effect calculated for the life cycle is benchmarked against the known total effect of a reference system, such as the total impacts of a specific region (e.g., EU, world, specific country) or the contribution of a single person to this impact. Therefore, every impact category is translated into relative contributions. Thereby, the different environmental impacts (e.g., global warming, acidification, eutrophication) become comparable.
- The interpretation, as the final step, summarizes the results from the LCI and LCIA.
The software used for modelling and analyzing the whole production chain of Nd and Dy is GaBi6 (GaBi6.0, thinkstep, Leinfelden-Echterdingen, Germany) [27]. Many of the data for the eudialyte process chain are based on laboratory experiments or modeling from project partners at RWTH Aachen University [28,29,30,31]. Basic process data for Bayan Obo are collected from the literature and are validated in a sound evaluation by experts from RWTH Aachen University. Data for tailings and sludge treatment are obtained as described in Section 3.1.4 and Section 3.2.4. It must be pointed out that RE production in Bayan Obo has been optimized for several years. In contrast to this, the production route of eudialyte has only been tested at the laboratory scale with no overall optimization or any reuse of chemicals or water. Data for auxiliary processes (e.g., construction, maintenance and disposal of mining operation units, chemical plants or trucks/trains, as well as energy supply and chemical production used for the main production chain of REE) come from either the GaBi6 [27] or Ecoinvent 2.2 database [32]. When regional information is available, general processes from the databases are adjusted to regional conditions. This is the case for electricity production (electricity generation mix), transport distances and the production of bulk chemicals (hydrochloric acid, caustic soda, sulfuric acid).
2.1. Goal and Scope Definition
The goal of this investigation is to assess environmental impacts related to the production of Nd and Dy metals from the eudialyte deposit at Norra Kärr and to compare them to those of today’s conventional production routes from the iron ore mine in Bayan Obo. Assuming differences for light and heavy rare earth components, Nd and Dy are chosen as representatives for each group. The functional unit of the investigation is therefore “1 kg Nd” and “1 kg Dy”, respectively.
The selection of impact categories is widely based on International Reference Life Cycle Data System (ILCD) recommendations [33] as implemented into GaBi6 and is listed in Table 1. For all impact categories, the ReCiPe 1.08 midpoint (H) method [34] is used to carry out a normalization step based on a consistent reference unit (“ReCiPe person equivalent, world” describes the average annual share of a person contributing to an environmental impact). No environmental effect is prioritized in its importance, so that no weighing between impact categories is necessary.

Table 1.
Overview of investigated ReCiPe impact categories.
3. Neodymium and Dysprosium Production
For this study, a hypothetical production chain, located in Europe, is developed and assessed. It starts with mining, beneficiation, production of REOs and, finally, production of RE metals, all assumed to be located in Norra Kärr. Data for the different processes are derived from literature, are deduced from existing production plants or are based on laboratory experiments. The considered systems are shown in Figure 1 and Figure 2 and described briefly in the following. The environmental effects are compared to those of the conventional Bayan Obo production routes, which are presented in Figure 3 and Figure 4.
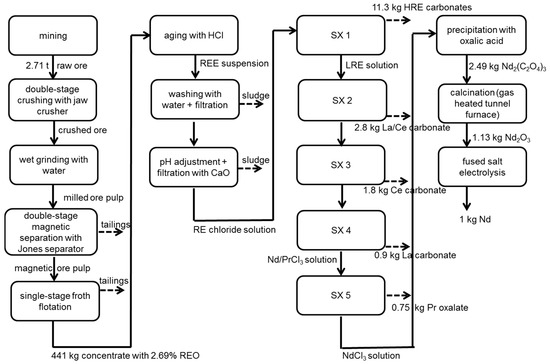
Figure 1.
Process chain of the production of 1 kg Nd based on eudialyte without allocation. SX, solvent extraction.
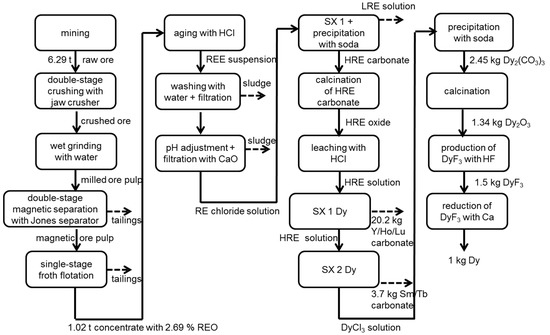
Figure 2.
Process chain of the production of 1 kg Dy based on eudialyte without allocation.
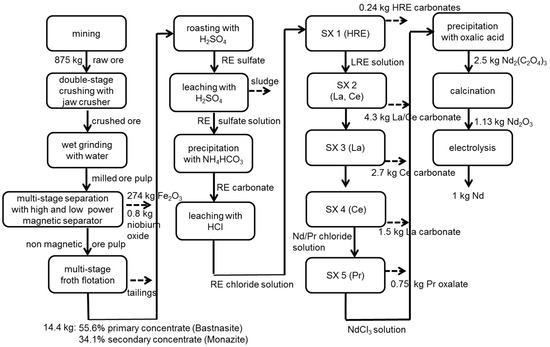
Figure 3.
Process chain of the production of 1 kg Nd at Bayan Obo without allocation.
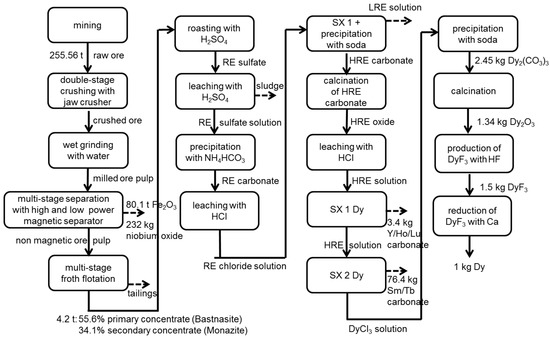
Figure 4.
Process chain of the production of 1 kg Dy at Bayan Obo without allocation.
3.1. European Eudialyte System
3.1.1. Mining
The European process chain starts with open pit mining at Norra Kärr, considering ore composition, mining rate, stripping rate and REO content as proposed in the prefeasibility study from 2015 [3]. This process comprises drilling and blasting of ore and excavated materials, as well as transport and dumping of materials. According to the prefeasibility study [3], the mining rate is 1.15 × 106 t/a, with a stripping ratio of 0.73:1 and an expected lifetime of 20 years. The primary energy demand for mining RE is set to 26.26 MJ/t moved rock, analogous to the demand for phosphate rock mining [35,36]. If taking the stripping rate into account, energy demand for Norra Kärr amounts to 45.43 MJ/t ore. Electricity and diesel demands are allocated according to the use of equipment [37]. The amount for blasting agents and lubricating oil is adapted to the moved rock amount [38,39]. Due to a lack of data, dust emissions must be assumed. Using the dust ratio of open pit mining to crushing from a gold mine [10] as the reference, a dust amount of 0.8 kg/t moved rock is calculated. Due to a planned sprinkler installation [3], this value can be reduced by 50% [40]. The distribution of dust particle size is adopted from Althaus et al. [41] with 50% > 10 µm, 45% 2.5–10 µm and 5% < 2.5 µm. The radioactive dust is assumed to have the same ThO2 and U3O8 concentration as the raw ore [42]. Thus, the ThO2 and U3O8 concentrations in the dust amount to 0.0026% and 0.0018%, respectively. With 1 g ThO2 having 3566 becquerel (Bq) Th232 and 1 g U3O8 having 10,470 Bq U238, these amount to 37.1 Bq Th232/t ore and 75.4 Bq U238/t ore.
Table 2 shows the ore composition of an average Norra Kärr eudialyte. The share of included REEs is gathered from measured data on two eudialyte samples from Norra Kärr by laser ablation inductively-coupled plasma mass spectrometry (LA-ICP-MS) and then averaged with ten additional data for the Norra Kärr deposit taken from [37] (Table 3). The LA-ICP-MS was conducted by the project partners from RWTH Aachen University.

Table 2.
Ore composition of Norra Kärr.

Table 3.
Rare earth element (REE) composition at Norra Kärr ore in % (total REO content: 0.59%; Table 2).
3.1.2. Beneficiation
To separate eudialyte from other ore components, several process steps are necessary. This includes crushing, grinding, magnetic separation, flotation and, finally, filter pressing. The ore is crushed, using a double-stage jaw crusher, to a particle size of 20–40 mm first. The amount of dust emitted during crushing is estimated by expert experience from RWTH Aachen University. After that, the ore is ground to <250 µm in a conventional wet grinding process. The energy demand and steel abrasion is calculated using the bond work index and abrasion index described in [37,38]. To remove nonmagnetic parts of the ore, magnetic separation is used. The magnetic ore pulp containing eudialyte is then further ground to <160 µm before it is fed to a froth flotation cell. In the single-stage flotation cell, the ore is enriched from 0.59% TREO to 2.69% TREO in the concentrate with a yield of 62%. The type and amount of flotation chemicals (oxalic acid, Na3PO4, H2SO4 and Flotinor SM15®) are identified in experiments [28]. The energy demand for magnetic separation [43] and flotation [44] are calculated by means of product information of supplier. For the determination of water demand, recirculation is considered.
3.1.3. Cracking and Separation
In an aging process, the concentrate is mixed with hydrochloric acid [29]. To prevent gelation, every mineral particle must be moisturized with acid. Gangue and minor elements are separated by washing and filtration. Adding lime milk to the filtrate, to adjust to a pH of four, causes iron, zirconia and aluminum separation. After that, several solvent extraction steps are necessary to separate each REE. For calculation, nine real solvent extraction steps (SX) are aggregated to 5 SX to reduce the complexity of modeling. The extraction process and the chemicals used are based on industrial extraction processes [45] and are adapted to the REE composition in eudialyte. As extraction agents, amines and phosphoric acid substituted by organic ligands are used. The subsequent precipitation is carried out using oxalic acid in the case of a Nd and soda in the case of a Dy process chain. The resulting oxalates and carbonates are then calcined in a tunnel furnace at 900 °C. The combustion is calculated in ASPEN PLUS® (Aspen Plus® V8.6, Aspen Technology, Bedford, MA, USA). For Dy production, thermal decomposition, leaching of heavy rare earth oxide with hydrochloric acid and further solvent extractions to separate Dy from other HREEs are assumed [29].
3.1.4. Tailings and Sludge Treatment for Beneficiation, Cracking and Separation
All waste waters from hydrometallurgical processes are purified, and the resulting sludge is then stored in a tailing storage facility, which is also used for tailings from beneficiation. Data for construction and operation are taken from the prefeasibility study [3]. The composition of flotation tailings is assumed to be analogous to raw ore components, without the REO amount separated during beneficiation and flotation chemicals. The composition of leaked particles from cracking and separation process tailings is based on an analysis of slurry from leaching [29] and on literature data for similar processes [19]. To quantify the ecological effects caused by sludge treatment, continuous infiltration of sludge water with solute particles is estimated. The solubility of most particles like metal oxides, sulfates, fluorides and CaCO3 is assumed to be 0.05%. For CaCl2 (74%) and Ca(OH)2, a higher solubility (1.7%) is assumed. Further exceptions are phosphorus pentoxide (P2O5) and strontium oxide (SrO) with 5% solubility considered. The amount of solids and chemicals infiltrating from tailings, causing pollution of ground water for Sweden, is estimated based on U.S. standards [46]. An amount of 82 L/m2·a from an American rare earth mine [46] is used. The yearly infiltration rate adds up to 5.49 × 104 m3 and is allocated to the different sludge stored.
3.1.5. RE Metal Production
The usually used fused-salt electrolysis is assumed for Nd reduction from oxide. Based on Cheng et al. [47], Vogel, Flerus, Stoffner and Friedrich [30] and Vogel and Friedrich [31] have developed three scenarios considering different production capacities for China (Table 4), as today‘s metal production mainly takes place in China. As no metal reduction exists in Sweden so far, the Chinese best practice scenario (Scenario 3) is chosen for Norra Kärr. For each scenario, energy demand and amounts of electrolyte, anodes and cathodes are estimated from the literature [47,48,49,50]. One component of the electrolyte is NdF3. For its production, a process described in [7] is assumed, where neodymium oxide and ammonium fluoride react in a resistance furnace at 300 °C. The energy demand is adopted from analogous production of LiF [51]. In Scenarios 2 and 3 additional dust recycling for lithium fluoride (LiF) and neodymium fluoride (NdF3) is considered (Table 4). The demand of chemicals and emissions are calculated according to the reaction equation with 20% stoichiometric excess. Emissions of CO and CO2 are based on Keller [51] and CF4 emissions on an aluminum melting process from the 1990s [52]. Furthermore, a wet scrubber with an efficiency of 96% to reduce fluoride emissions is assumed [47]. The production of Dy is carried out by the reduction of dysprosium fluoride (DyF3) with calcium. DyF3 is produced analogous to NdF3. The amount of Ca and the composition of slurry are based on the literature [53,54].

Table 4.
Main inputs and outputs of the electrolysis scenarios assumed for Bayan Obo and Norra Kärr (per 1 kg Nd).
3.2. Conventional Chinese System
3.2.1. Mining
The raw ore in Bayan Obo is mined in an open pit mining process, including drilling, blasting, loading, transportation and dumping. Because the mine was originally an iron ore mine, data regarding energy demand and the facility are used from an iron mine [38] and modified to site-specific parameters (e.g., mining rate 10 × 10−6 t/a, stripping ratio 1:1) [55]. For Bayan Obo, 0.8 kg dust/t moved rock are estimated because no sprinkler systems are installed. The distribution of the dust particle size is the same as at Norra Kärr. Thus, the ThO2 and U3O8 concentrations in the dust amount to 0.032% and 0.002%, respectively. These amount to 9133 Bq Th232/t ore and 168 Bq U238/t ore.
Due to the inhomogeneity of the huge orebody [56,57], a weighted average is assumed for the main orebody considering four ore types (Table 5).

Table 5.
Ore composition in Bayan Obo (main orebody).
The composition of REO (Table 6) represents an average of orebodies at Bayan Obo [6].

Table 6.
REO composition in Bayan Obo ore in % (total REO content: 6.22%; Table 5).
3.2.2. Beneficiation
Beneficiation of ore takes place at Baotou. Several beneficiation processes of the Bayan Obo deposit are described in Zhang [58]. The considered process represents common technology and includes: crushing, grinding, magnetic separation and flotation. Products of this process chain segment are a primary iron concentrate, as well as monazite and bastnasite concentrates.
The crushed ore is transported 150 km by train from the mine at Bayan Obo to the processing plant at Baotou. There, it is ground to a size of <74 µm by conventional wet grinding with an integrated hydro cyclone classifier [58]. Separation of ferrous magnetite is carried out by low and high power magnetic separation. The ore pulp of magnetic separation passes through a multi-stage flotation resulting in a bastnasite and monazite concentrate with 55.6% and 34.1% REO, respectively [28]. The yields for bastnasite and monazite concentrate amount to 12.6% and 6%, respectively [58].
The calculation of the energy demand for crushing and grinding is again based on the bond work index and abrasion index. Producer information is used for the calculation of energy demand for magnetic separation [43] and flotation [44]. The dust emissions during crushing are estimated by experts from RWTH Aachen University. Due to the lack of information regarding flotation chemicals (sodium silicate, fatty acid, other organic chemicals), the amounts used are deduced from older sources [59]. Anyhow, flotation at Baotou has an approximately six-times lower yield than the process at Norra Kärr, resulting in a higher ore demand.
3.2.3. Cracking and Separation
After flotation, both concentrates are roasted using sulfuric acid (98%) in a rotary kiln [60]. The demand of natural gas is calculated in ASPEN PLUS®; the amount of sulfuric acid and the energy demand are estimated by Zhang [58] and Krüger [61]. Flue gas from the roasting process is cleaned by spray adsorption, and waste water is purified using quick lime [62]. The remaining pollutant concentration of cleaned flue gas is based on Chinese emission standards for the rare earth industry [63]. CO2 emissions are calculated stoichiometrically considering the reaction of bastnasite with sulfuric acid, as well as the combustion of natural gas in the rotary kiln.
After roasting, RE sulfates are leached with water and sulfuric acid and then filtrated. By the addition of water and ammonium bicarbonate, RE carbonates are precipitated. By further addition of water and hydrochloric acid, precipitated RE carbonates are converted into RE chloride solution. The demands of ammonium bicarbonate and sulfuric acid are based on the content of ammonia and sulfate in the waste water [62]. The amount of hydrochloric acid considers a pH 4 adjustment and the assumption of an entire reaction of carbonate into CO2.
The subsequent solvent extraction steps are the same as assumed for Norra Kärr. The energy demand, the chemicals used and the waste water produced are adapted to the REE composition at Bayan Obo. Furthermore, subsequent processes for the production of Nd and Dy metals are the same as for eudialyte. Only the electricity and natural gas mixes differ. However, flue gas cleaning of electrolysis and fluoride production has lower efficiencies than in Sweden (Table 4).
3.2.4. Tailings and Sludge Treatment for Beneficiation, Cracking and Separation
All tailings from beneficiation and sludge from roasting off-gas treatment and the hydrometallurgic processes, except the leaching sludge, are led to a tailing pond. This is a fifty-year-old and 11 km2-wide lake for waste water, with a 20 m-thick sludge layer [64]. A theoretical infiltration rate of 1.0 × 10−7 m/s [3] based on sludge particle size is reduced to 5 × 10−8 m/s, because these high amounts could not be verified in laboratory tests. This leads to a yearly infiltration rate of 34.68 × 10−6 m3/a. The sludge composition is based on literature data [19,55]. The tailings composition is, as described in Section 3.1.4, assumed to be analogous to raw ore components without the REO amount separated, as well as iron and niobium.
Sludge from the leaching process after roasting is stored in an open radioactive storage facility. A plain foil separates the sludge from soil [55]. Based on Bonaparte and Gross [65], a conservative infiltration rate of 219 L/m2·a is chosen. Considering the size of the facility, an infiltration rate of 2.1 × 10−4 m3/a is gained.
3.3. Process Chain Modelling
According to all assumptions and data, each single process/box in Figure 1, Figure 2, Figure 3 and Figure 4 is modelled analogously to Figure 5, serving as an example for all other single processes/boxes. In Figure 5, all inputs necessary for the mining process are shown. As outputs and all emissions into the environment are accounted for. Inputs and outputs are related to the main output of this specific process (here, 1 t eudialyte mined). In this example, for the mining of 1 t eudialyte, 0.156 kg blasting agent, 2.7 kWh electricity, 0.19 tkm by truck, 0.4 m3 rock transported by front loader and hydraulic shovel, etc., are needed (Figure 5). Afterwards, all single processes are combined to an entire process chain, and all inputs and outputs are added accordingly using GaBi6.
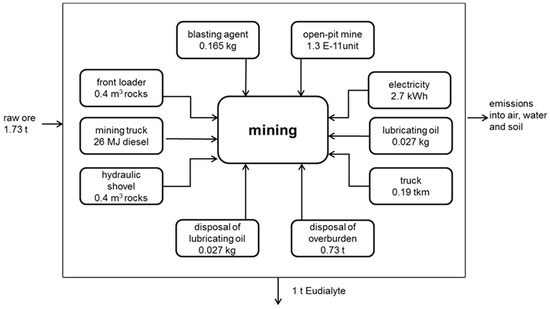
Figure 5.
Mining of eudialyte.
3.4. Allocation Procedure
Since the production of REE is a multi-product system due to the strong paragenesis of REEs, environmental burdens have to be allocated appropriately. This means that, for example, the energy demand or particulate matters emitted during mining are divided between products, considering the causer principle. Different allocation methods exist, addressing this causer principle. In this study, an allocation method based on the mass of produced REEs combined with their market prices [66] (from an international metals market analysis and pricing index company from 28 July 2015) are considered, typically used for multi-component ores. As the ores have different compositions and components, the allocation factor has to be assessed individually for each site. Table 7 shows prices assumed (third column) and the resulting allocation factors for the separated products calculated for each SX step. Figure 1, Figure 2, Figure 3 and Figure 4 show which separated products leave the main production chain (horizontal). The allocation is performed between these separated products and the remaining products forwarded along the main process chain (vertical). The allocation factors add up to 100% at each allocation step. Allocation factors for lanthanum, cerium, neodymium and praseodymium are very similar for the Bayan Obo and Norra Kärr production routes due to similar element percentages in the raw ores. However, the amount of HREEs in eudialyte is much higher, and therefore, the resulting allocation factors vary widely. For Bayan Obo, an additional allocation step is necessary due to the separation of iron and niobium oxides during magnetic separation (Figure 3 and Figure 4).

Table 7.
Allocation factors for Nd and Dy at Norra Kärr (NK) and Bayan Obo (BO).
4. Life Cycle Inventory, LCI
In LCI, the main energy and material inputs and outputs are summed up (selected inputs and outputs in Table 8) after every single process of Figure 1, Figure 2, Figure 3 and Figure 4 has been assembled with corresponding data. The values for the two REs, as well as for both production sites are very different regarding the amounts and types of material and energy necessary. For example, the Dy production at Norra Kärr uses the highest amount of energy resources, process water, caustic soda, hydrochloric acid and transport service due to the highest amount of raw ore mined (allocated as described above). The high process water amount at Norra Kärr is mainly related to data from an up-scaled laboratory facility, with no recycling. However, for a large-scale site, recycling is expected. On the other hand, most of the emissions released have their highest values at Bayan Obo (e.g., HF, non-methane volatile organic compound (NMVOC), heavy metals into air, radioactive emissions into air) due to poor process control, as well as hardly existing waste and sludge treatment. Due to the roasting process, the facilities at Bayan Obo use great amounts of sulfuric acid, but lower amounts of hydrochloric acid than at Norra Kärr. However, hydrochloric acid is the main input at Norra Kärr due to the aging process instead of roasting. The amount of lithium fluoride (LiF) is almost 1000-times higher for Nd production in Bayan Obo than at Norra Kärr due to the assumed better process control and re-use of LiF and NdF3 by dust recycling during electrolysis at Norra Kärr. Ammonia, calcium chloride and calcium are mainly used for the production of Dy fluoride and reduction to Dy. Therefore, the amounts of these three chemicals are higher for Dy production than for Nd. Altogether, almost all inputs and outputs for Dy production are higher than for Nd following the allocation described above.

Table 8.
Selected main inputs and outputs of the process chains per kg Nd and Dy.
5. Life Cycle Impact Assessment
The gathered inputs and outputs are allocated to specific environmental effects. To classify the importance of various effects, each is related to the average annual share a person contributes to this environmental impact worldwide, in the normalization step. Hence, Figure 6 shows the summarized results for each production chain in terms of normalized impacts (inclusive error bars; see Section 6, Table 10) in person equivalents related to the functional unit. The production of Nd and Dy at Norra Kärr is much less polluting than at Bayan Obo, although the amounts of raw ore needed per kg Nd and Dy are higher at Norra Kärr (Figure 1, Figure 2, Figure 3 and Figure 4). The impacts for the Swedish Nd production only amount to approximately 40% of the impacts occurring at Bayan Obo. Especially for Dy, the results of production at Norra Kärr show a considerable advantage against production at Bayan Obo due to the higher content of HREEs in the eudialyte mineral. The impacts for the Norra Kärr Dy production only amount to approximately 20% of the impacts of Bayan Obo.
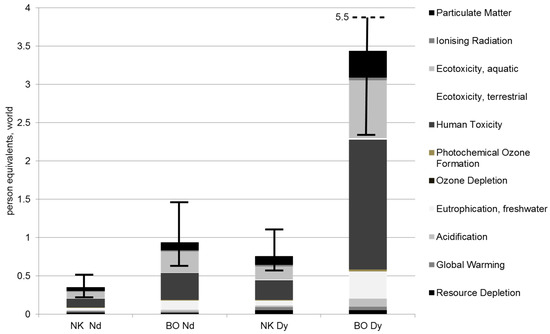
Figure 6.
Normalized impacts for the production of 1 kg Nd and Dy at Norra Kärr and Bayan Obo.

Table 10.
Share of data qualities (DQ) for different impact categories for the 4 process chains in %.
If the values are plotted in relative numbers (%) (Figure 7), the importance of different environmental impacts becomes even more visible. The crucial impact categories are human toxicity, ecotoxicity (aquatic), particulate matter and eutrophication followed by resource depletion and global warming at both production sites (Figure 7). The share of human toxicity amounts to 33%–49% of the total impacts. Human toxicity is predominately caused by emissions during the production of chemicals (e.g., hydrochloric acid, hydrofluoric acid, extracting agents) used for aging, precipitation and solvent extraction. Furthermore, heavy metal emissions into water during waste water treatment and hydrofluoric acid emissions during the production of DyF3 contribute to this effect. The latter are much lower for Dy production in Sweden due to an assumed enhanced flue gas cleaning during DyF3 production.
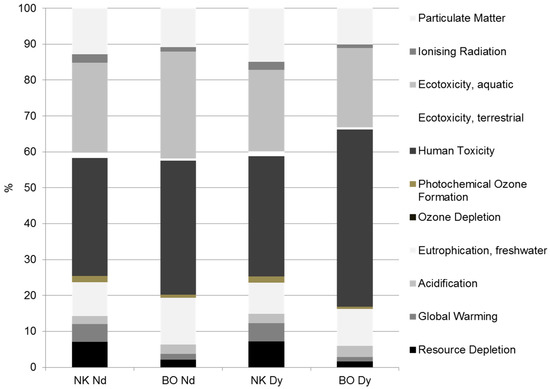
Figure 7.
Comparison of relative impacts for the production of 1 kg Nd and Dy at Norra Kärr and Bayan Obo.
Aquatic ecotoxicity follows with a share of 22%–30% of the total impacts and is largely caused by heavy metal emissions into water during waste water and sludge treatment. The share of particulate matter lies between 10% and 15% and is higher for Norra Kärr production, although absolute values are much smaller. The reason for the smaller relative share of particulate matter at Bayan Obo is the particularly high importance of human toxicity. Although Bayan Obo has a lower stripping rate and therefore less raw ore has to be broken per kg Nd and Dy, the dust emissions per kg broken ore are much higher due to the higher hardness grade of Bayan Obo ore (iron ore mine). Furthermore, most of the Chinese electrolysis has much higher dust emissions (Figure 8) than electrolysis at Norra Kärr due to the assumed optimized process control there (Table 4). The share of eutrophication accounts for 9%–13% and is mostly induced by phosphoric emissions (e.g., P2O5, phosphor, phosphate) into water during waste water and sludge treatment. The share of other impacts on the total environmental burdens is smaller than 5% each. Compared to other LCA studies [8,12,14,17,32,67], the GWP values of this approach (per kg Nd oxide measured in kg CO2 eqv.) are higher (15%–75%) due to the consideration of numerous additional upstream and downstream chains (e.g., waste water and sludge treatment) in contrast to other studies. Although mining of REE is always associated with thorium, ionizing radiation is hardly detected. The reason for that is the assumed safe storage of tailings in ponds. Only if a damaging event (e.g., earthquake, dam failure) occurs would radioactive emissions be released.
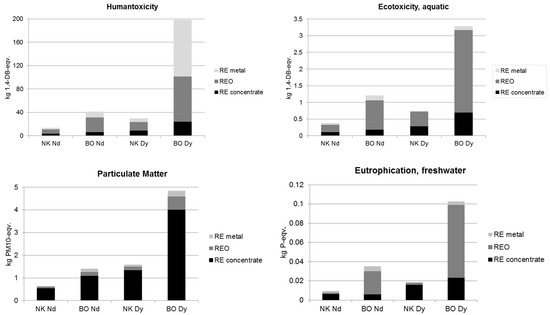
Figure 8.
Environmental impacts of Nd and Dy production at Norra Kärr and Bayan Obo.
Figure 8 shows the share of three process chain segments for four critical impact categories: 1. mining-flotation (REO concentrate); 2. roasting/aging-calcination (REO); 3. Nd/Dy reduction (RE metal). In case of human toxicity, ecotoxicity and eutrophication, the share of REO production (all processes between aging/roasting and calcination) is the highest due to the high amount of chemicals used, the emissions into water, as well as the waste and sludge treatment processes. In total, the shares of all waste water and sludge treatment processes along the whole process chain on the total human toxicity, ecotoxicity (aquatic) and eutrophication impacts add up to approximately 30%–50%. For particulate matter, the first process chain segment (all processes between mining and flotation) is most important due to dust emission mostly during crushing. In the case of human toxicity, also particularly high HF emissions during DyF3 production for the reduction process are the main causer in the third process chain segment for Dy production at Bayan Obo.
6. Data Quality
To determine the quality of results, each single process of the process chain is assigned with a data quality indicator (DQ) between DQ1 and DQ5. Each quality level is associated with data variation in analogy to uncertainty in cost data. The American Association of Cost Estimation developed a classification system, following a study of the Canadian Institute for Mining [68], adjusting variety levels for costs. Depending on the maturity level of the project, an expected accuracy level (AL) is defined [68]. DQ1 (AL −10%–15%) is the best quality assigned to measured data. However, it was not given to any process because no measures were conducted at the production sites at Norra Kärr or Bayan Obo. Processes whose data are gathered from the literature, describing specific site conditions, are assigned with DQ2 (AL −15%–20%). These are for example mining processes, which are very well understood, and detailed mining parameters for Bayan Obo, as well as for Norra Kärr exist. For many processes, data are gathered either by stoichiometric calculation under ideal conditions or are up-scaled from the laboratory scale. Typically, this is the case for the newly-developed and adjusted beneficiation processes, conventional precipitation or calcination processes, but also energy production processes. Those processes have the data quality DQ3 (AL −20%–30%). If data are estimated analogous to the older process description or similar processes, DQ4 (AL −30%–50%) is given. Many transport processes can be found here. DQ5 (AL −50%–100%), as the worst quality, is allocated to those processes, which are only roughly estimated, either by use of data from databases for global average processes or by a much reduced process description. This is often the case for upstream processes, such as the production of operation material, or downstream processes, such as waste treatments. An overview of data quality for each process of the main production processes at Bayan Obo and Norra Kärr can be found in Table 9. Additionally, an average is shown for all upstream and downstream processes within the processing stage. As can be seen, processes for Norra Kärr are assumed to be slightly better. This reflects the general lack of original data for the Bayan Obo process chain. Especially data for Chinese upstream or downstream processes from databases are not available, and only global averages can be used.

Table 9.
Assumed data qualities for the main processes with their upstream and downstream processes.
To determine the impacts of data quality on the results, Table 10 presents the average data qualities for each impact category of the four process chains. Most occurring DQs are marked by the darkest shade. The impacts of the eudialyte-based process chains are mostly derived from processes with DQ2 and DQ3, whereas impacts of the Bayan Obo process chains largely originate from processes with DQ3 and DQ4. For example, 78% of data used for the assessment of resource depletion are characterized by DQ2 in the case of Norra Kärr for Nd and Dy, respectively, but only 2% of the data in the case of Bayan Obo Dy (zero for Nd). The different impact categories present no consistent pattern regarding data quality. Generally, toxicity categories rely on processes with low data qualities. However, as discussed before, those are categories that contribute most to the overall effects.
For the overall production chain of each line, the following average variations are calculated: Norra Kärr (NK)/Nd −25–+43, NK/Dy −24–+40, Bayan Obo (BO)/Nd −32–+58, BO/Dy −33–+60. The corresponding error bars are shown in Figure 6. The differences between Nd and Dy produced along a production chain are small in contrast to the process chains at different sites. The results for the European production are associated with a lower variation than the results of Bayan Obo. Overall, the data quality of this study is moderate due to the lack of knowledge of Chinese production sites and due to the fictitious process chain based on eudialyte.
7. Interpretation and Conclusion
From a technical point of view, the setup of a solely European supply route is technically possible. Several specific studies have proven this approach [28,29,30,31]. Besides the desired reduction of economic dependency from a Chinese supply, also environmental advantages can be achieved by replacing the production route. Especially due to a better emission control, as well as waste and sludge treatment caused by a stricter environmental legislation in Sweden, normalized total values of the effects per kg LREEs can be reduced by approximately 60%. This effect becomes even more prominent for HREEs. Here, a reduction of approximately 80% can be reached in comparison to the Bayan Obo production. With the high share of HREEs, the Norra Kärr ore has an additional advantage over bastnasite- and monazite-based Bayan Obo ore. However, it has to be kept in mind that most of the Chinese Dy originates not from Bayan Obo, but other sources, mostly ion adsorption clays in southern China. Their production is not considered in this study because even less technical information is available than for Bayan Obo and only the first LCA data have been published most recently [18].
Even with this very different composition of the two ores and different production routes necessary, the environmental effects that are stressed are the same. Although absolute figures vary significantly between production sites and also between RE-metals processed, human toxicity is always the dominant environmental impact, followed by aquatic ecotoxicity, particulate matter and eutrophication. While particulate matter is mostly related to emissions during mining and beneficiation, human toxicity and ecotoxicity, as well as eutrophication are caused during the beneficiation and separation of REOs. Here, waste water, as well as waste and sludge treatment processes are the biggest polluter. Furthermore, during production of chemicals, emissions with human toxicity impacts occur. For Dy, reduction of DyF3 is necessary. Its production in China causes high amounts of toxic emissions for humans. A flue gas treatment as assumed for the Swedish DyF3 production can reduce this effect drastically. Stricter environmental legislation on waste treatment, but also on emissions control in Sweden reduces absolute environmental effects caused directly during processing. However, also the supply of operating materials, as in the case of chemicals or energy supply, is more environmentally friendly and, therefore, contributes to the high overall difference between the two production sites.
This enormous difference is striking when the different levels of maturity are considered, as well. Although Bayan Obo was originally an iron ore mine, RE production has been optimized for several years now. The production route for eudialyte has only been tested at the laboratory scale with no overall optimization of processes or any reuse option of chemicals. Therefore, the environmental performance could be further improved by process optimization and adjustment. On the other hand, data sources and quality for China are assumed to be much worse than up-scaled data from the laboratory scale for eudialyte. Whether promised improvement and stricter control of environmental performance, as announced by the Chinese government, have already been put into action is not known. The figures used in this study are all based on the few studies available.
So far, the assessment of the European process chain is focused on the production of REEs only. As in the case of (additional) iron production at Bayan Obo, other minerals occur in the Norra Kärr ore. With additional production of further products, expenses from mining and beneficiation can be allocated to more products, causing the specific amount per kg products to decrease. The other process chain segments stay the same.
However, it is most likely that a European production route with its high standards will yield considerable higher costs. Furthermore, the much higher demand for chemicals due to lower ore quality will determine production costs. As prices for Chinese Nd and Dy have decreased drastically in the last few years, it is doubtful that the production at Norra Kärr will start soon. Eudialyte remains an option to decrease supply dependency with high environmental benefits, but market conditions will regulate its use. Beside costs, also the missing social acceptance of new mining activities might hinder further implementation of European activities. The solely environmental perspective of this paper is therefore addressing only one aspect of decision making and needs further broadening by economic and social assessment.
Acknowledgments
We kindly thank the Siemens AG for their significant financial support in the years 2012–2015 allowing us to conduct the collaborative research center “rare earths—green mining and separation” combining five institutes of RWTH Aachen University and Forschungszentrum Jülich.
Author Contributions
While the expertise of Andrea Schreiber, Josefine Marx, Petra Zapp and Jürgen-Friedrich Hake lies in the field of LCA application and evaluation, the expertise of Daniel Voßenkaul and Bernd Friedrich is on metallurgical aspects.
Conflicts of Interest
The authors declare no conflict of interests.
References
- USGS Mineral Yearbook. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/index.html#myb (accessed on 14 October 2016).
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- GBM. Amended and Restated Prefeasibility Study–NI 43-101–Technical report for the Norra Kärr Rare Earth Element Deposit; GBM Minerals Engineering Consultants Limited: London, UK, 2015. [Google Scholar]
- Voncken, J.H.L. The rare earth element—An introduction. In The Series: Springer Briefs in Earth Sciences; Springer Nature: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Hudson Institute of Mineralogy. Available online: www.mindat.org/min-1420.html (accessed on 27 June 2016).
- Friedrichs, P.; Meyer, F.M. REE database management system: Evaluation of REE deposits and occurrences. J. Sustain. Metall. 2016. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earth; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- McLellan, B.; Corder, G.; Ali, S. Sustainability of rare earths—An overview of the state of knowledge. Minerals 2013, 3, 304–317. [Google Scholar] [CrossRef]
- Arpacioglu, C.B.; Er, C. Estimation of fugitive dust impacts of open pit mines on local air quality. In Proceedings of the 18th International Mining Congress and Exhibition, Antalya, Turkey, 10–13 June 2003.
- Golev, A.; Scott, M.; Erskine, P.D.; Ali, S.H.; Ballantyne, G.R. Rare earths supply chains: Current status, constraints and opportunities. Resour. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Koltun, P.; Tharumarajah, A. Life cycle impact of rare earth elements. ISRN Metall. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Adibi, N.; Lafhaj, Z.; Gemechu, E.D.; Sonnemann, G.; Payet, J. Introducing a multi-criteria indicator to better evaluate impacts of rare earth materials production and consumption in life cycle assessment. J. Rare Earths 2014, 32, 288–292. [Google Scholar] [CrossRef]
- Zaimes, G.G.; Hubler, B.J.; Wang, S.; Khanna, V. Environmental life cycle perspective on rare earth oxide production. ACS Sustain. Chem. Eng. 2015, 3, 237–244. [Google Scholar] [CrossRef]
- Sprecher, B.; Xiao, Y.; Walton, A.; Speight, J.; Harris, R.; Kleijn, R.; Visser, G.; Kramer, G.J. Life cycle inventory of the production of rare earths and the subsequent production of ndfeb rare earth permanent magnets. Environ. Sci. Technolo. 2014, 48, 3951–3958. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Zhao, F. Life cycle assessment of the production of rare earth elements for energy applications: A review. Front. Energy Res. 2014, 2, 1–17. [Google Scholar] [CrossRef]
- Browning, C.; Northey, S.; Haque, N.; Bruckard, W.; Cooksey, M. Life cycle assessment of rare earth production from monazite. In Rewas 2016: Towards Materials Resource Sustainability; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 81–88. [Google Scholar]
- Vahidi, E.; Navarro, J.; Zhao, F. An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Resour., Conserv. Recycli. 2016, 113, 1–11. [Google Scholar] [CrossRef]
- Schmidt, G. Description and Critical Environmental Evaluation of the Ree Refining Plant Lamp Near Kuantan/Malaysia—Radiological and Non-Radiological Environ-Mental Consequences of the Plant’S Opera-Tion and Its Wastes; Öko-Institut e. V. Freiburg: Darmstadt, Berlin, 2013. [Google Scholar]
- Schüler, D.; Buchert, M.; Liu, R.; Dittrich, S.; Merz, C. Study on Rare Earths and Their Recycling; Öko-Institut e.V. Freiburg: Darmstadt, Berlin, 2011. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Keith-Roach, M.; Grendfelt, B.; Hoglund, L.O.; Kousa, A.; Pohjolainen, E.; Magistrati, P.; Aggelatou, V.; Olivieri, N.; Ferrari, A. Legislation and best practice in the emerging european rare earth element industry. In Proceedings of the 1st European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014.
- European Comission. Reference Document on Best Available Techniques for Management of Tailings and Waste-Rock in Mining Activities; European Commission, Joint Research Centre: Seville, Spain, 2009. [Google Scholar]
- Keith-Roach, M.; Grundfelt, B.; Kousa, A.; Pohjolainen, E.; Magistrati, P.; Aggelatou, V.; Olivieri, N.; Ferrari, A. Past experience of environmental, health and safety issues in REE mining and processing industries and an evaluation of related EU and international standards and regulations. In Final Report of the EuRare Project; Kemakta Konsult AB: Stockholm, Sweden; Geological Survey of Finland: Espoo, Finland; Fen Minerals A/S: Trondheim, Norway; Institute of Geology & Mineral Exploration: Achamae, Greece; D’Appolonia S.p.A: Genoa, Italy, 2015. [Google Scholar]
- ISO 14040: Environmental Management—Life Cycle Assessment—Principles and Framework; International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044: Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Organization for Standardization: Geneva, Switzerland, 2006.
- GaBi 6.0 (Ganzheitliche Bilanzierung) Software. Available online: https://www.thinkstep.com/software/gabi-lca (accessed on 16 September 2016).
- Stark, T.; Silin, I.; Wotruba, H. Mineral processing of eudialyte ore from Norra Kärr. J. Sustain. Metall. 2016. [Google Scholar] [CrossRef]
- Voßenkaul, D.; Birich, A.; Müller, N.; Stoltz, N.; Friedrich, B. Hydrometallurgical processing of eudialyte bearing concentrates to recover rare earth elements via low-temperature dry digestion to prevent the silica gel formation. J. Sustain. Metall. 2016. [Google Scholar] [CrossRef]
- Vogel, H.; Flerus, B.; Stoffner, F.; Friedrich, B. Reducing greenhouse gas emission from the neodymium oxide electrolysis part I: Analysis of the anodic gas formation. J. Sustain. Metall. 2016. [Google Scholar] [CrossRef]
- Vogel, H.; Friedrich, B. Reducing greenhouse gas emission from the neodymium oxide electrolysis part II: Basics of a process control avoiding PFC emission. J. Sustain. Metall. 2016. under review. [Google Scholar]
- Ecoinvent. Datenbank für Ökobilanzdaten. Swiss Center for Life Cycle Inventory—Version 2.2; Ecoinvent: St. Gallen, Switzerland, 2012. [Google Scholar]
- European Commission—Joint Research Centre—Institute for Environment and Sustainability. International Reference Life Cycle Data System (ILCD) Handbook—Recommendations for Life Cycle Impact Assessment in the European Context, 1st ed.; Publications Office of the European Union: Luxemburg, The Grand Duchy of Luxembourg, 2011. [Google Scholar]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.A.J.; De Schryver, A.; Struijs, J.; Van Zelm, R. ReCiPe 2008—A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level. In Report I: Characterisation factors; Ministerie van Volkshuisvesting, Ruimtelijke Ordening en Milieubeheer: Bilthoven, The Netherlands, 2009. [Google Scholar]
- Althaus, H.-J.; Chudacoff, M.; Hischier, R.; Jungbluth, N.; Osses, M.; Primas, A. Lifecycle Inventories of Chemicals. Phosphate Rock. Final Report Ecoinvent data v 2.0 No. 8; EMPA Dübendorf, Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- Kippenberger, C.-M. Stoffmengenflüsse und Energiebedarf bei der Gewinnung ausgewählter mineralischer Rohstoffe—Auswertende Zusammenfassung; Schweizerbart Science: Stuttgart, Germany, 1999. [Google Scholar]
- Gates, P.A.; Harlacher, C.; Reed, G. Perliminary Economic Assessment NI 43-101 Technical Report for the Norra Kärr (REE-Y-Zr) Deposit Gränna Sweden; Pincock Allen & Holt: Lakewood, CO, USA, 2012. [Google Scholar]
- Classen, M.; Althaus, H.J.; Blaser, S.; Scharnhorst, W.; Tuchschmid, M.; Jungbluth, N.; Emmenegger, M.F. Life Cycle Inventories of Metals.Part II Iron and Steel. Final report ecoinvent data v 2.0 No. 10; EMPA Dübendorf, Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- Ruhrberg, M. Entwicklung Eines Betriebsübergreifenden Ressourcenmanagementsystems Für Metallische Rohstoffe Am Beispiel Des Kupferbergbaus; Mainz: Aachen, Germany, 2002. [Google Scholar]
- Jansen, D. Aktuelle ergebnisse—feinstaub aus tagebauen. In BUND Aktuell; Bund für Umwelt und Naturschutz Deutschland: Düsseldorf, Switzerland, 2006. [Google Scholar]
- Althaus, H.-J.; Chudacoff, M.; Hirschler, R.; Jungbluth, N.; Osses, M.; Primas, A. Lifecycle Inventories of Chemicals. Rare Earth Oxide Production from Bastnasite. Final Report Ecoinvent Data v 2.0 No. 8; EMPA Dübendorf, Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- IAEA. Radiation Protection and Norm Residue Management in the Production of Rare Eath From Thorium Containing Mineral; IAEA: Wien, Austria, 2011. [Google Scholar]
- Magnetic Separators. Available online: www.mbecoalandmineral.in/magnetic_separator.php (accessed on 4 April 2016).
- Outotec OK-R and OK-U flotation. Available online: www.outotec.com/ImageVaultFiles/id_794/d_1/cf_2/OTE_Outotec_OK-R_and_OK-U_flotation_cells_eng_web.PDF (accessed on 4 April 2016).
- Det Norsk Veritas. Technical Report No. EP029020, Quantitative Risk Assessment Study of Proposed Advanced Material Plant within the Gebeng Industrial Estate, Kuantan, Pahang; Det Norsk Veritas: Kuala Lumpur, Malaysia, 2010. [Google Scholar]
- California Regional Water Quality Control Board. Lahontan Regional Water Quality Control Board: Revised Waste Discharge Requirements and Revised Monitoring and Reporting Program for Molycorp Minterals LLC, Mountain Pass Mine and Mill Operations 2010, Board Order No. R6V-2010-0047; California Regional Water Quality Control Board: San Bernardino County, CA, USA, 2010.
- Cheng, Y.; Liang, Y.; Tao, D. Clean production technology in electrolysis process in a rare earth plant. Chin. Rare Earth 2011, 32, 92–96. [Google Scholar]
- Pang, S. Development on molten salt electrolytic methods and technology for preparing rare earth metals and alloys in China. Chin. J. Rare Met. 2011, 35, 440–450. [Google Scholar]
- Zhang, Z. Present situation and latest progress of process for producing metallic neodymium by electrolysis of neodymium oxide with fluoride salts. Non ferr. Smelt. 2001, 30, 23–25. [Google Scholar]
- Liu, K. Analysis of anodic gases in neodymium electrolysis. Chin. J. Nonferr. Met. 2001, 11, 1118–1121. [Google Scholar]
- Keller, R. Electrolytic production of neodymium with and without emission of greenhouse gases. Electrochem. Soc. Proc. 1998, 97–28, 143–145. [Google Scholar] [CrossRef]
- Chase, R.; Gibson, R.; Marks, J. PFC emissions performance for the global primary aluminum industry. In Proceedings of the Light Metals 2005, 134th TMS Annual Meeting, San Francisco, CA, USA, 13–17 February 2005.
- Sharma, R.A. Metallothermic reduction of rare earth fluorides. U.S. Patent US5,314,526, 24 May 1994. [Google Scholar]
- Velu, P.T.; Reddy, R.G. Calciothermic reduction of neodymium fluoride. Light Met. 2005, 2005, 1155–1159. [Google Scholar]
- Wu, Q.F.; Liu, H.; Ma, C.H.; Zhao, S.P.; Zhu, X.H.; Xiong, S.Q.; Wang, H.Y. The use and management of NORM residues in processing Bayan Obo ores in China. In Naturally Occuring Radioactive Material; IAEA: Marrakesh, Morocco, 2010; pp. 65–78. [Google Scholar]
- Castor, S.B.; Hedrick, J.B. Rare earth elements. Ind. Miner. Rocks 2006, 7, 769–792. [Google Scholar]
- Drew, L.J.; Qingrun, M.; Weijun, S. The Bayan Obo iron-rare-earth-niobium deposits, Inner Mongoia, China. Lithos 1990, 26, 43–65. [Google Scholar] [CrossRef]
- Zhang, J.; Edwards, C. A review of rare earth minaral processing technology. Can. Institut. Min. 2012, 79, 38–52. [Google Scholar]
- Yu, Y.C.Q.; Deng, Y. Industrial trail production practice in application of new technology of magnetic separation and flotation to the improvement of production lines of low and medium grade oxide ores at the concentrating mill, baotou iron and steel complex. Min. Metall. Eng. 1992, 1, 10–14. [Google Scholar]
- Bouorakima, A. Production of rare earth oxides. In Assessment Of The Environmental Impacts In Two Chinese Mines; University College London: London, UK, 2011. [Google Scholar]
- Krüger, J. Sachbilanz Zink; Institut für Metallhüttenkunde und Elektrometallurgie der RWTH Aachen: Aachen, Germany, 2001. [Google Scholar]
- Xu, Y.; Guo, W.; Ma, Y.; Xu, H.; Hu, W.; Qiao, J. Treatment and utilization of wastewater of baotou rare earth concentrate hydrometallurgy process. Chin. Rare Earth 2008, 29, 82–85. [Google Scholar]
- Ministry of Environmental Protection. Emission Standards of Pullutants from Rare Earth Industry; Ministry of Environmental Protection: Beijing, China, 2011.
- Huang, X.; Cao, G.; Liu, J.; Prommer, H.; Zheng, C. Reactive transport modeling of thorium in a cloud computing environment. J. Geochem. Explor. 2014, 144, 63–73. [Google Scholar] [CrossRef]
- Bonaparte, R.; Gross, B.A. Field behavior of double-liner systems. waste containment systems: Construction, regulation, and performance. ASCE Geotech. Spec. Publ. 1990, 26, 52–83. [Google Scholar]
- Asian Metals. Available online: www.asianmetals.com (accessed on 28 July 2015).
- Nuss, P.; Eckelman, M.J. Life cycle assessment of metals: A scientific synthesis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.; Hollmann, J.K.; Zwaigenbaum, G. Cost Estimate Classification System—As Applied in the Mining and Mineral Processing Industries; AACE International Recommended Practice No. 47R-11: Morgantown, WV, USA, 2012. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
