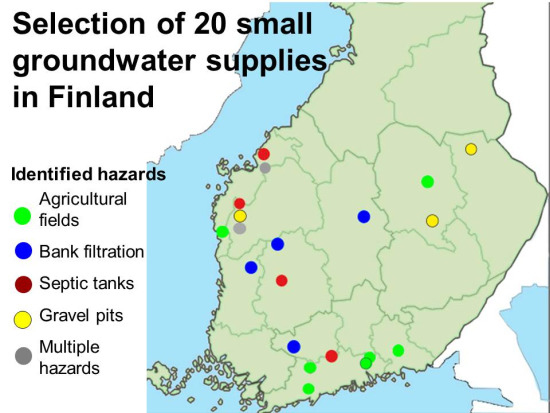
Drinking Water Quality and Occurrence of Giardia in Finnish Small Groundwater Supplies
Abstract
:1. Introduction
2. Results
2.1. Water Supplies
| Water Supply | Aquifer Type | Average Thickness of Vadose Zone (m) | Well Type 1 | Well Depth (m) | Well Maintenance 2 | Water Intake Constructed | Main Risk Factor | Water Treatment 3 |
|---|---|---|---|---|---|---|---|---|
| 1 | Glaciofluvial esker, unconfined, sand and gravel | 7 | Driven well | 8 | 3 | 1998 | Bank filtration | 1 |
| 2 | Glaciofluvial esker, semi-confined, silty till and sand | <5 | Dug well | NA | 3 | 1987 | Bank filtration | 1 |
| 3 | Ablation moraine, semi-confined, sandy till | <5 | Spring well | 2 | 3 | 1963 | Bank filtration | 0 |
| 4 | Glaciofluvial esker, unconfined, till and gravel | <5 | Dug well | 6 | 2 | 1978 | Bank filtration | 0 |
| 5 | Deep bedrock aquifer, confined | NA | Drilled well | NA | 1 | 1979 | Agriculture, sewage | 0 |
| 6 | Glaciofluvial esker, confined, clay and sand | NA | Spring well | 3 | 2 | 1960 | Agriculture | 0 |
| 7 | Ablation moraine, semi-confined, sandy till | <5 | Driven + dug well | 185 | 3 | NA | Agriculture | 2 |
| 8 | Glaciofluvial sand formation, confined, clay and sand | NA | Dug well | 4 | 2 | 1988 | Agriculture | 3 |
| 9 | Moraine, confined, clay and silty till | NA | Dug well | 10 | 1 | 1983 | Agriculture | 3 |
| 10 | Littoral sand, semi-confined, clay and till | NA | Dug + spring wells | 3 | 3 | 1940 | Agriculture | 0 |
| 11 | Glaciofluvial esker, unconfined, sand and silt | 3 | Driven well | 7 | 2 | 1988 | Agriculture | 1 |
| 12 | Glaciofluvial ice-marginal formation, semi-confined, sand and silt | 3 | Driven well | 10 | 3 | 1986 | Sewage | 3 |
| 13 | Glaciofluvial interlobate formation, semi-confined, sand, gravel and till | NA | Dug well | 3 | 1–2 | 1962 | Sewage | 0 |
| 14 | Littoral sand, semi-confined, till and sand | 2 | Dug well | 4 | 1 | 1978 | Sewage | NA |
| 15 | Moraine, semiconfined, till and sand | 3 | Spring wells | 5–6 | 3 | 1962 | Sewage | 0 |
| 16 | Littoral sand, semi-confined, till and sand | 1 | Dug wells | 2–4 | 1 | 1948 | Gravel mining | 0 |
| 17 | Deep bedrock aquifer and moraine, confined, till and gravel | NA | Dug well | NA | 3 | 1993 | Gravel mining | 1 |
| 18 | Ablation moraine, semi-confined, sandy till and gravel | 6 | Spring well | 4 * | 1 | 1992 | Gravel mining | 1 |
| 19 | Littoral sand, semi-confined, till and sand | <5 | Dug wells | NA | 1–2 | NA | Surface water runoff | 0 |
| 20 | Moraine, confined, till and sand | NA | Spring well | NA | 1–2 | NA | Flooding | 0 |
2.2. Microbiological Quality
| Water Supply | Risk Factor Category | Coliforms | E. coli | Intestinal Enterococci | HPC | Aeromonas | Giardia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFUs/1000 mL | CFUs/1000 mL | CFUs/1000 mL | CFUs/mL | CFUs/1000 mL | cysts/100 L | ||||||||
| Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | ||
| 1 | 1 | - | - | - | - | - | - | <1 | <1 | - | - | - | 1 |
| 2 | 1 | - | - | - | - | - | - | 2 | 0 | - | - | - | - |
| 3 | 1 | - | - | - | - | - | - | <1 | <1 | - | - | - | - |
| 4 | 1 | - | 830 | - | - | - | - | <1 | 18 | - | 9 | - | - |
| 5 | 2 | - | - | - | - | - | - | 3 | 14 | - | 12 | - | 1 |
| 6 | 2 | - | 10 | - | - | - | - | <1 | <1 | 1 | - | - | - |
| 7 | 2 | - | - | - | - | - | - | 1 | <1 | - | - | - | - |
| 8 | 2 | - | - | - | - | - | - | 83 | 1 | 33 | 5 | - | - |
| 9 | 2 | - | 10 | - | 2 | - | - | 3 | 5 | - | 2 | - | - |
| 10 | 2 | - | - | - | - | - | - | 6 | <1 | - | - | - | - |
| 11 | 2 | - | - | - | - | - | - | <1 | 1 | - | - | - | - |
| 12 | 3 | - | 50 | - | 40 | - | - | <1 | 8 | - | - | - | - |
| 13 | 3 | - | - | - | - | - | - | 3 | 0 | 15 | 3 | - | 1 |
| 14 | 3 | - | - | - | - | - | 4 | 296 | 10 | - | - | - | 2 |
| 15 | 3 | - | 70 | - | - | - | 5 | 30 | 25 | 5 | - | - | - |
| 16 | 4 | - | - | - | - | - | - | 267 | 1 | - | - | - | - |
| 17 | 4 | - | - | - | - | - | - | 14 | 8 | - | - | - | - |
| 18 | 4 | - | - | - | - | - | 2 | 200 | 214 | - | - | - | - |
| 19 | 5 | - | - | - | - | - | - | 4 | 8 | - | - | - | - |
| 20 | 5 | - | 150 | - | - | - | - | <1 | 45 | - | - | - | - |
2.3. Chemical Parameters
2.4. Association of Recognized Risk Factors with Microbiological and Chemical Parameters
| Water Supply | T (°C) | pH | EC (µS/cm) | TOC (mg/L) | Color mgPt/mL | Turbidity (FTU) | P (µg/L) | Fe (µg/L) | Mn (µg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | |
| 1 | 7.1 | 10.2 | 5.6 | 6.2 | 92 | 93 | 1.7 | 2.0 | 5 | 5 | 0.50 | 1.10 | 7 | 11 | 440 | 780 | 25 | 32 |
| 2 | 2.2 | 8.4 | 6.1 | 6.5 | 48 | 53 | 5.7 | 1.0 | 5 | <5 | 1.05 | 1.33 | 16 | 18 | 120 | 170 | 11 | 8.2 |
| 3 | 4.8 | 6.5 | 6.2 | 6.3 | 41 | 46 | 1.2 | 1.0 | <5 | <5 | 0.16 | 0.15 | 28 | 27 | <20 | <20 | <5 | <5 |
| 4 | 0.7 | 17.8 | 6.9 | 7.6 | 44 | 95 | 1.8 | 4.1 | 15 | 10 | 0.15 | 0.15 | 8 | 12 | 55 | 110 | <5 | 56 |
| 5 | 7.5 | 7.3 | 7.5 | 6.9 | 328 | 337 | 1.1 | 1.2 | <5 | 5 | 0.22 | 0.08 | 8 | 13 | 26 | 23 | 25 | 28 |
| 6 | 7.9 | 8.1 | 7.5 | 7.6 | 391 | 421 | 0.9 | 1.0 | <5 | <5 | 0.06 | 0.52 | 23 | 24 | <20 | <20 | 130 | 180 |
| 7 | 6.6 | 8.0 | 6.5 | 7.0 | 259 * | ns | 1.2 | 1.3 | <5 | <5 | 0.06 | 0.04 | 9 | 11 | <20 | 53 | 12 | 12 |
| 8 | 7.8 | 6.5 | 6.6 | ns | 178 * | ns | 3.7 | 3.3 | 5 | <5 | 0.69 | 0.35 | 11 | 12 | 150 | 83 | 79 | 46 |
| 9 | 6.8 | 5.7 | 6.5 | ns | 215 * | ns | 1.3 | 1.2 | <5 | <5 | 0.80 | 0.70 | 14 | 9 | 71 | 126 | <5 | 7 |
| 10 | 3.5 | 7.9 | 5.6 | 6.0 | ns | ns | 1.1 | 0.8 | <5 | <5 | 0.04 | 0.02 | 15 | 16 | <20 | <20 | <5 | <5 |
| 11 | 5.6 | 5.7 | 6.0 | 5.9 | 46 | 153 | 1.4 | 1.4 | <5 | <5 | 0.94 | 0.30 | 13 | 7 | 390 | 240 | 59 | 51 |
| 12 | 5.0 | 7.0 | ns | 6.5 | 148 * | 150 | 0.8 | 0.9 | <5 | <5 | 0.52 | 0.92 | 5 | 11 | 38 | 220 | <5 | <5 |
| 13 | 5.2 | 8.0 | 7.0 | 6.9 | 214 | 196 | 1.0 | 1.0 | 5 | <5 | 0.39 | 0.26 | 10 | 15 | 35 | 31 | <5 | <5 |
| 14 | 5.4 | 8.5 | ns | 5.4 | 12 | 202 | 5.3 | 5.2 | 20 | 20 | 3.7 | 3.46 | 20 | 21 | 440 | 120 | <5 | <5 |
| 15 | 4.3 | 8.5 | 6.1 | 6.0 | 231 | 479 | 11.0 | 10.8 | 40 | 40 | 0.73 | 0.79 | 182 | 108 | 180 | 150 | 100 | 110 |
| 16 | 1.5 | 9.7 | 5.5 | 5.9 | ns | 30 | 3.5 | 2.8 | 10 | 5 | 0.15 | 0.12 | 8 | 9 | <20 | <20 | <5 | <5 |
| 17 | 3.3 | 7.4 | 7.2 | 6.3 | 150 | 148 | 1.7 | 1.2 | <5 | <5 | 0.20 | 0.24 | 15 | 15 | <20 | <20 | <5 | <5 |
| 18 | 4.7 | 6.9 | 6.2 | 6.3 | 81 | 79 | 0.5 | 0.6 | <5 | <5 | 0.16 | 0.16 | 21 | 12 | <20 | <20 | <5 | <5 |
| 19 | 4.0 | 6.1 | 6.3 | 5.1 | 173 | ns | 1.8 | 1.5 | <5 | <5 | 0.18 | 0.16 | 16 | 15 | 31 | 29 | <5 | <5 |
| 20 | 3.5 | 7.1 | 5.1 | 5.0 | ns | ns | 1.8 | 1.8 | <5 | <5 | 0.12 | 0.15 | 12 | 13 | <20 | 21 | <5 | <5 |
2.5. Associations within Microbiological and Chemical Parameter Results at Spring and Autumn
3. Discussion
4. Experimental Section
4.1. Selection and Characterization of the Water Supplies
4.2. Sampling and Analysis
4.3. Detection of Enteric Pathogens
4.4. Analyses of Indicator Bacteria
4.5. Chemical Analyses
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Richardson, H.Y.; Nichols, G.; Lane, C.; Lake, I.R.; Hunter, P.R. Microbiological surveillance of private water supplies in England—The impact of environmental and climate factors on water quality. Water Res. 2009, 43, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, T.; Karinen, P.; Miettinen, I.T.; Lettojärvi, H.; Heikkilä, A.; Maunula, R.; Aula, V.; Kuronen, H.; Vepsäläinen, A.; Nousiainen, L.L.; et al. Microbial contamination of groundwater at small community water supplies in Finland. Ambio 2011, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Lapinlampi, T.; Raassina, S. SY541 Vesihuoltolaitokset 1998–2000; Finnish Environment Centre (SYKE): Helsinki, Finland, 2002. (In Finnish) [Google Scholar]
- Isomäki, E. Pienet pohjavesilaitokset Suomessa. Vesitalous 2006, 3, 11–16. (In Finnish) [Google Scholar]
- Decree of the Ministry of Social Affairs and Health Relating to the Quality and Monitoring of Water Produced by Small Water Supplies 401/2001, Finlex Data Bank; Edita Publishing Oy: Helsinki, Finland, 2001. Available online: http://www.finlex.fi/fi/laki/alkup/2001/20010401 (accessed on 20 August 2015). (In Finnish)
- Decree of the Ministry of Social Affairs and Health Relating to the Quality Requirements and Monitoring of the Household Water 461/2000, Finlex Data Bank, Edita Publishing Oy: Helsinki, Finland, 2000. Available online: http://www.finlex.fi/fi/laki/alkup/2000/20000461 (accessed on 20 August 2015). (In Finnish)
- European Union. Council directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, L330, 32–54. [Google Scholar]
- Hulsmann, A. Small Systems Large Problems—A European Inventory of Small Water Systems and Associated Problems; Report of Web-based European Knowledge Network on Water WEKNOW/ENDWARE, European Commission; KWR Watercycle Research Institute: Nieuwegein, The Netherlands, 2005; p. 41. [Google Scholar]
- Lahermo, P.; Tarvainen, T.; Hatakka, T.; Backman, B.; Juntunen, R.; Kortelainen, N.; Lakomaa, T.; Nikkarinen, M.; Vesterbacka, P.; Väisänen, U.; et al. Tuhat kaivoa—Suomen kaivovesien fysikaalis-kemiallinen laatu vuonna 1999; (Summary: One thousand wells—The physical-chemical quality of Finnish well waters in 1999); Report of Investigation 155; Geological Survey of Finland: Espoo, Finland, 2002; p. 92. Available online: http://tupa.gtk.fi/julkaisu/tutkimusraportti/tr_155.pdf (accessed on 20 August 2015).
- Zacheus, O. Talousveden valvonta ja laatu vuonna 2008. Yhteenveto viranomaisvalvonnan tuloksista; Avauksia 18/2010; Terveyden ja hyvinvoinnin laitos (THL): Helsinki, Finland, 2010; p. 67. Available online: http://urn.fi/URN:NBN:fi-fe201205085417 (accessed on 20 August 2015). (In Finnish)
- Zacheus, O.; Miettinen, I.T. Increased information on waterborne outbreaks through efficient notification system enforces actions towards safe drinking water. J. Water Health 2011, 9, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, I.T.; Zacheus, O.; von Bonsdorff, C.H.; Vartiainen, T. Waterborne epidemics in Finland in 1998–1999. Water Sci. Technol. 2001, 43, 67–71. [Google Scholar] [PubMed]
- Pitkänen, T. Review of Campylobacter spp. in drinking and environmental waters. J. Microbiol. Methods 2013, 95, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Rimhanen-Finne, R.; Hänninen, M.L.; Vuento, R.; Laine, J.; Jokiranta, T.S.; Snellman, M.; Pitkänen, T.; Miettinen, I.; Kuusi, M. Contaminated water caused the first outbreak of giardiasis in Finland, 2007: A descriptive study. Scand. J. Infect. Dis. 2010, 42, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Simonen, A. The Precambrian in Finland; Bulletin 304; Geological Survey of Finland: Espoo, Finland, 1980; p. 58. Available online: http://tupa.gtk.fi/julkaisu/bulletin/bt_304.pdf (accessed on 20 August 2015).
- Borrell, N.; Acinas, S.G.; Figueras, M.J.; Martinez-Murcia, A.J. Identification of Aeromonas. clinical isolates by restriction fragment length polymorphism of PCR-amplified 16S rRNA genes. J. Clin. Microbiol. 1997, 35, 1671–1674. [Google Scholar] [PubMed]
- Corkal, D.; Schutzman, W.C.; Hilliard, C. Rural water safety from the source to the on-farm tap. J. Toxicol. Environ. Health A 2004, 67, 1619–1642. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Nichols, G.L.; Swan, A.; de Louvois, J. A survey of the microbiological quality of private water supplies in England. Epidemiol. Infect. 2000, 124, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Hörman, A.; Korpela, H.; Sutinen, J.; Wedel, H.; Hänninen, M.L. Meta-analysis in assessment of the prevalence and annual incidence of Giardia spp. and Cryptosporidium spp. infections in humans in the Nordic countries. Int. J. Parasitol. 2004, 34, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Hörman, A.; Rimhanen-Finne, R.; Maunula, L.; von Bonsdorff, C.H.; Torvela, N.; Heikinheimo, A.; Hänninen, M.L. Campylobacter spp., Giardia spp., Cryptosporidium, noroviruses and indicator organisms in surface water in southwestern Finland, 2000–2001. Appl. Environ. Microbiol. 2004, 70, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rimhanen-Finne, R.; Vuorinen, A.; Marmo, S.; Malmberg, S.; Hanninen, M.L. Comparative analysis of Cryptosporidium, Giardia and indicator bacteria during sewage sludge hygienization in various composting processes. Lett. Appl. Microbiol. 2004, 38, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Wright, F.; Nichols, G.L.; Reacher, M.; Rutter, M. Outbreaks of infectious disease associated with private drinking water supplies in England and Wales 1970–2000. Epidemiol. Infect. 2003, 130, 469–479. [Google Scholar] [PubMed]
- Von Hertzen, L.; Laatikainen, T.; Pitkänen, T.; Vlasoff, T.; Makela, M.J.; Vartiainen, E.; Haahtela, T. Microbial content of drinking water in Finnish and Russian Karelia—Implications for atopy prevalence. Allergy 2007, 62, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Casemore, D. Towards a US national estimate of the risk of endemic waterborne disease—Sero-epidemiologic studies. J. Water Health 2006, 4, 121–163. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Coote, B.G.; Ashbolt, N.J.; Stevenson, I.M. Relationships between indicators, pathogens and water quality in an estuarine system. Water Res. 1996, 30, 2045–2054. [Google Scholar] [CrossRef]
- Cizek, A.R.; Characklis, G.W.; Krometis, L.A.; Hayes, J.A.; Simmons, O.D., III; di Lonardo, S.; Alderisio, K.A.; Sobsey, M.D. Comparing the partitioning behavior of Giardia and Cryptosporidium with that of indicator organisms in stormwater runoff. Water Res. 2008, 42, 4421–4438. [Google Scholar] [CrossRef] [PubMed]
- LeChevallier, M.W.; Norton, W.D.; Lee, R.G. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl. Environ. Microbiol. 1991, 57, 2610–2616. [Google Scholar] [PubMed]
- Hänninen, M.L.; Siitonen, A. Distribution of Aeromonas. phenospecies and genospecies among strains isolated from water, foods or from human clinical samples. Epidemiol. Infect. 1995, 115, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, M.L.; Oivanen, P.; Hirvelä-Koski, V. Aeromonas. speciesin fish, fish-eggs, shrimp and freshwater. Int. J. Food Microbiol. 1997, 34, 17–26. [Google Scholar] [CrossRef]
- Altwegg, M.; Steigerwalt, A.G.; Altwegg-Bissig, R.; Luthy-Hottenstein, J.; Brenner, D.J. Biochemical identification of Aeromonas. genospecies isolated from humans. J. Clin. Microbiol. 1990, 28, 258–264. [Google Scholar] [PubMed]
- Hirotani, H.; Sese, C.; Kagawa, H. Correlations of Aeromonas. hydrophila with Indicator Bacteria of Water Qualityand Environmental Factorsin a Mountain Stream. Water Environ. Res. 1999, 7, 132–138. [Google Scholar] [CrossRef]
- Kersters, I.; van Vooren, L.; Huys, G.; Janssen, P.; Kersters, K.; Verstraet, W. Influence of temperature and process technology on the occurrence of Aeromonas. species and hygienic indicator organisms in drinking water production plants. Microb. Ecol. 1995, 30, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, M.L.; Haajanen, H.; Pummi, T.; Wermundsen, K.; Katila, M.L.; Sarkkinen, H.; Miettinen, I.; Rautelin, H. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol. 2003, 69, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA; EPA-821-R-99–006; United States Environmental Protection Agency: North Chelmsford, MA, USA, 1999. [Google Scholar]
- Rimhanen-Finne, R.; Hörman, A.; Ronkainen, P.; Hänninen, M.L. An IC-PCR method for detection of Cryptosporidium and Giardia in natural surface waters in Finland. J. Microbiol. Methods 2002, 50, 299–303. [Google Scholar] [CrossRef]
- Homan, W.L.; Gilsing, M.; Bentala, H.; Limper, L.; van Knapen, F. Characterization of Giardia duodenalis by polymerase-chain-reaction fingerprinting. Parasitol. Res. 1998, 84, 707–714. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Stardardization. Water Quality—Detection and Enumeration of Thermotolerant Campylobacter Species; ISO 17995; International Organization for Stardardization: London, UK, 2005. [Google Scholar]
- Gilgen, M.; Germann, D.; Luthy, J.; Hubner, P. Three-step isolation method for sensitive detection of enterovirus, rotavirus, hepatitis A virus, and small round structured viruses in water samples. Int. J. Food Microbiol. 1997, 37, 189–199. [Google Scholar] [CrossRef]
- Maunula, L.; Piiparinen, H.; von Bonsdorff, C.H. Confirmation of Norwalk-like virus amplicons after RT-PCR by microplate hybridization and direct sequencing. J. Virol. Methods 1999, 83, 125–134. [Google Scholar] [CrossRef]
- Finnish Standard Association. Water Quality—Membrane Filter Technique for the Enumeration of Total Coliform Bacteria; SFS 3016; Finnish Standard Association: Helsinki, Finland, 2001. [Google Scholar]
- International Organization for Stardardization. Water Quality—Enumeration of Culturable Micro-Organisms. Colony Count by the Inoculation in a Nutrient Agar Culture Medium; ISO 6222; International Organization for Stardardization: London, UK, 1999. [Google Scholar]
- International Organization for Stardardization. Water Quality—Detection and Enumeration of Intestinal Enterococci. Part 2: Membrane Filtration Method; ISO 7899-2; International Organization for Stardardization: London, UK, 2000. [Google Scholar]
- Havelaar, A.H.; During, M.; Versteegh, J.F. Ampicillin-dextrin agar medium for the enumeration of Aeromonas. species in water by membrane filtration. J. Appl. Bacteriol. 1987, 62, 279–287. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Water Quality—Examination and Determination of Colour; ISO 7887-4; International Organization for Standardization: London, UK, 1994. [Google Scholar]
- International Organization for Standardization. Water Quality—Determination of Turbidity; ISO 7027; International Organization for Standardization: London, UK, 1999. [Google Scholar]
- Finnish Standards Association. Metal Content of Water, Sludge and Sediment Determined by Flameless Atomic Absorption Spectrometry. Atomization in a Graphite Furnace. Special Guidelines for Aluminium, Cadmium, Cobolt, Chromium, Copper, Lead, Manganese, Nickel and Iron; SFS Method 5502; Finnish Standards Association: Helsinki, Finland, 1990. [Google Scholar]
- International Organization for Standardization. Water Quality—Determination of Nitrite Nitrogen and Nitrate Nitrogen and the Sum of both by Flow Analysis (CFA and FIA) and Spectrometric Detection; ISO 13395; International Organization for Standardization: London, UK, 1996. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitkänen, T.; Juselius, T.; Isomäki, E.; Miettinen, I.T.; Valve, M.; Kivimäki, A.-L.; Lahti, K.; Hänninen, M.-L. Drinking Water Quality and Occurrence of Giardia in Finnish Small Groundwater Supplies. Resources 2015, 4, 637-654. https://doi.org/10.3390/resources4030637
Pitkänen T, Juselius T, Isomäki E, Miettinen IT, Valve M, Kivimäki A-L, Lahti K, Hänninen M-L. Drinking Water Quality and Occurrence of Giardia in Finnish Small Groundwater Supplies. Resources. 2015; 4(3):637-654. https://doi.org/10.3390/resources4030637
Chicago/Turabian StylePitkänen, Tarja, Tiina Juselius, Eija Isomäki, Ilkka T. Miettinen, Matti Valve, Anna-Liisa Kivimäki, Kirsti Lahti, and Marja-Liisa Hänninen. 2015. "Drinking Water Quality and Occurrence of Giardia in Finnish Small Groundwater Supplies" Resources 4, no. 3: 637-654. https://doi.org/10.3390/resources4030637
APA StylePitkänen, T., Juselius, T., Isomäki, E., Miettinen, I. T., Valve, M., Kivimäki, A.-L., Lahti, K., & Hänninen, M.-L. (2015). Drinking Water Quality and Occurrence of Giardia in Finnish Small Groundwater Supplies. Resources, 4(3), 637-654. https://doi.org/10.3390/resources4030637