Valorization of the Invasive Red Lionfish (Pterois volitans L.) as a Natural and Promising Source of Bioactive Hydrolysates with Antioxidant and Metal-Chelating Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample
2.3. Protein Hydrolysate Preparation
2.4. Degree of Hydrolysis (DH)
2.5. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Amino Acid Analysis
2.7. Antioxidant Activity of Protein Hydrolysates
2.7.1. Free Radical Scavenging Activity
2.7.2. Copper- and Iron-Chelating Activity
2.8. Statistical Design and Analysis
3. Results
3.1. Proximal Composition
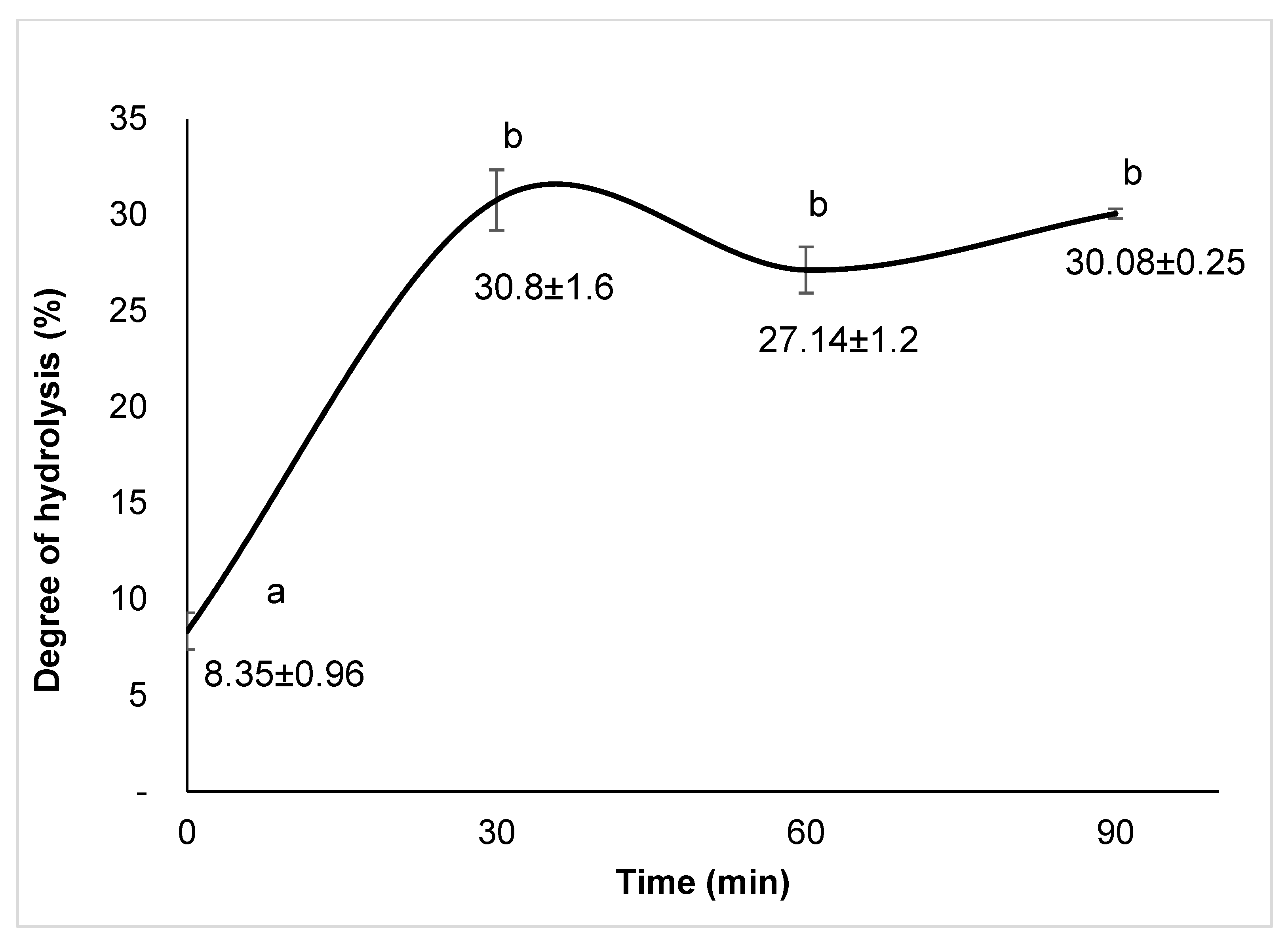
3.2. Degree of Hydrolysis (DH)
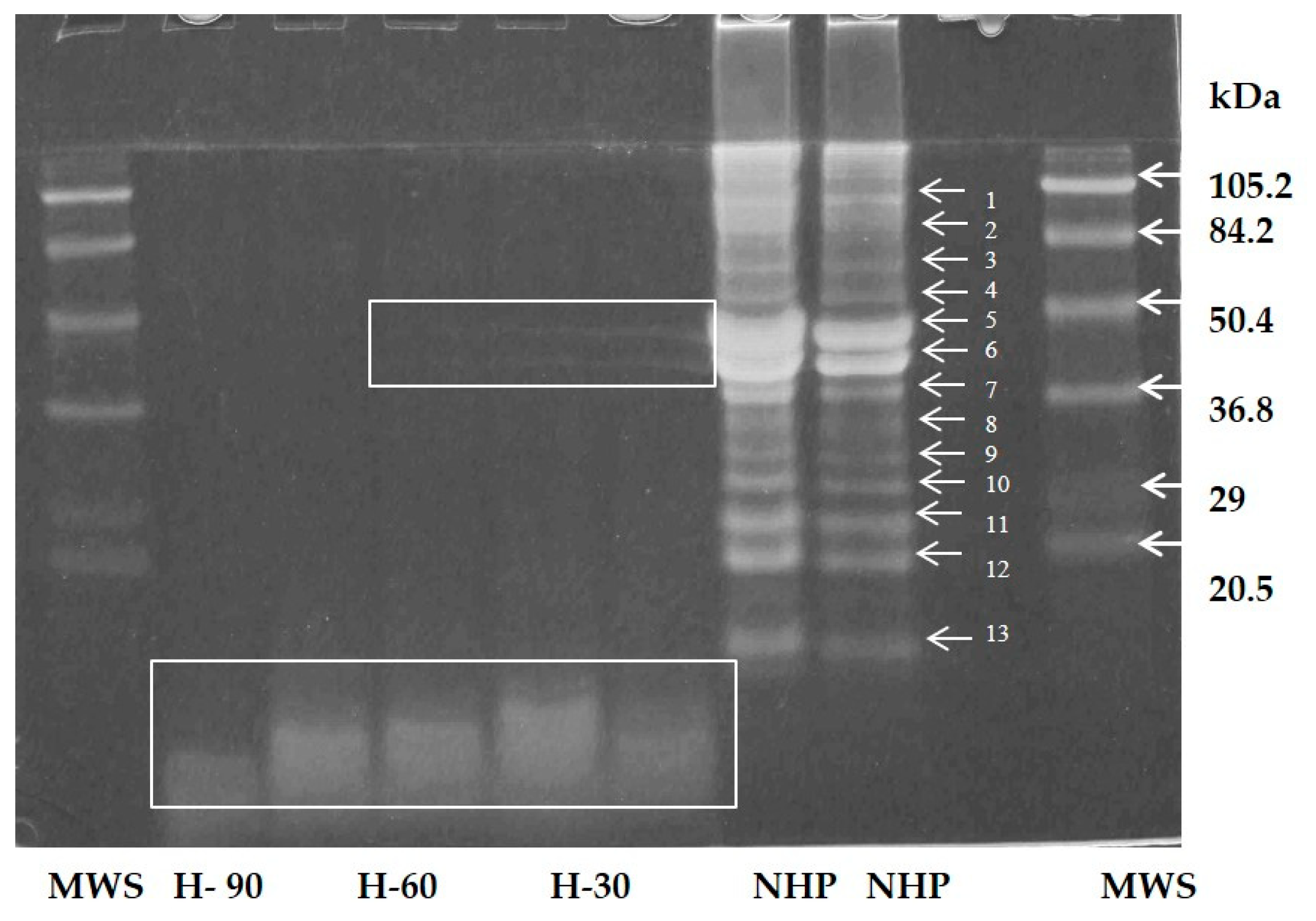
3.3. Electrophoretic Profile
3.4. Amino Acid Profiles
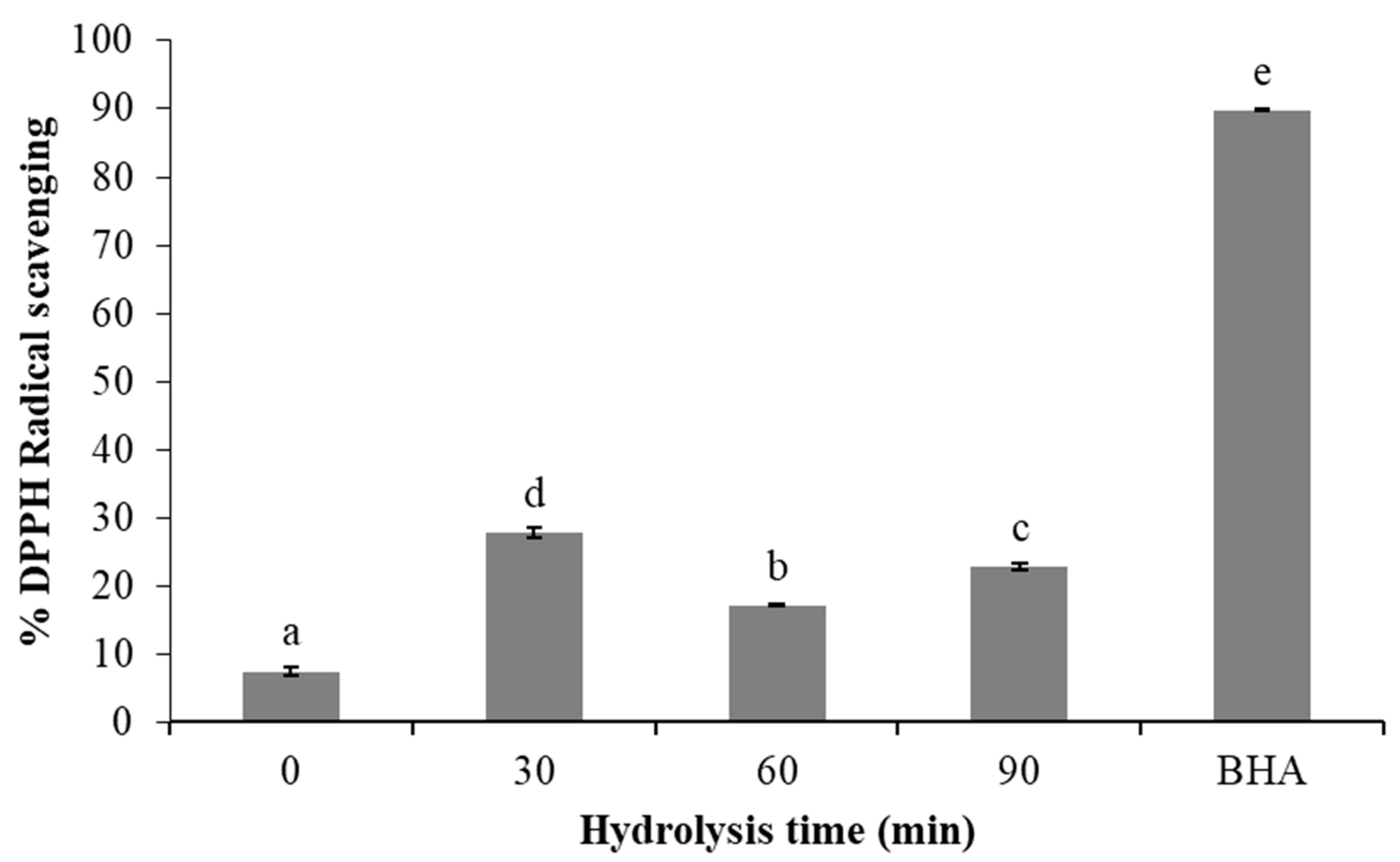
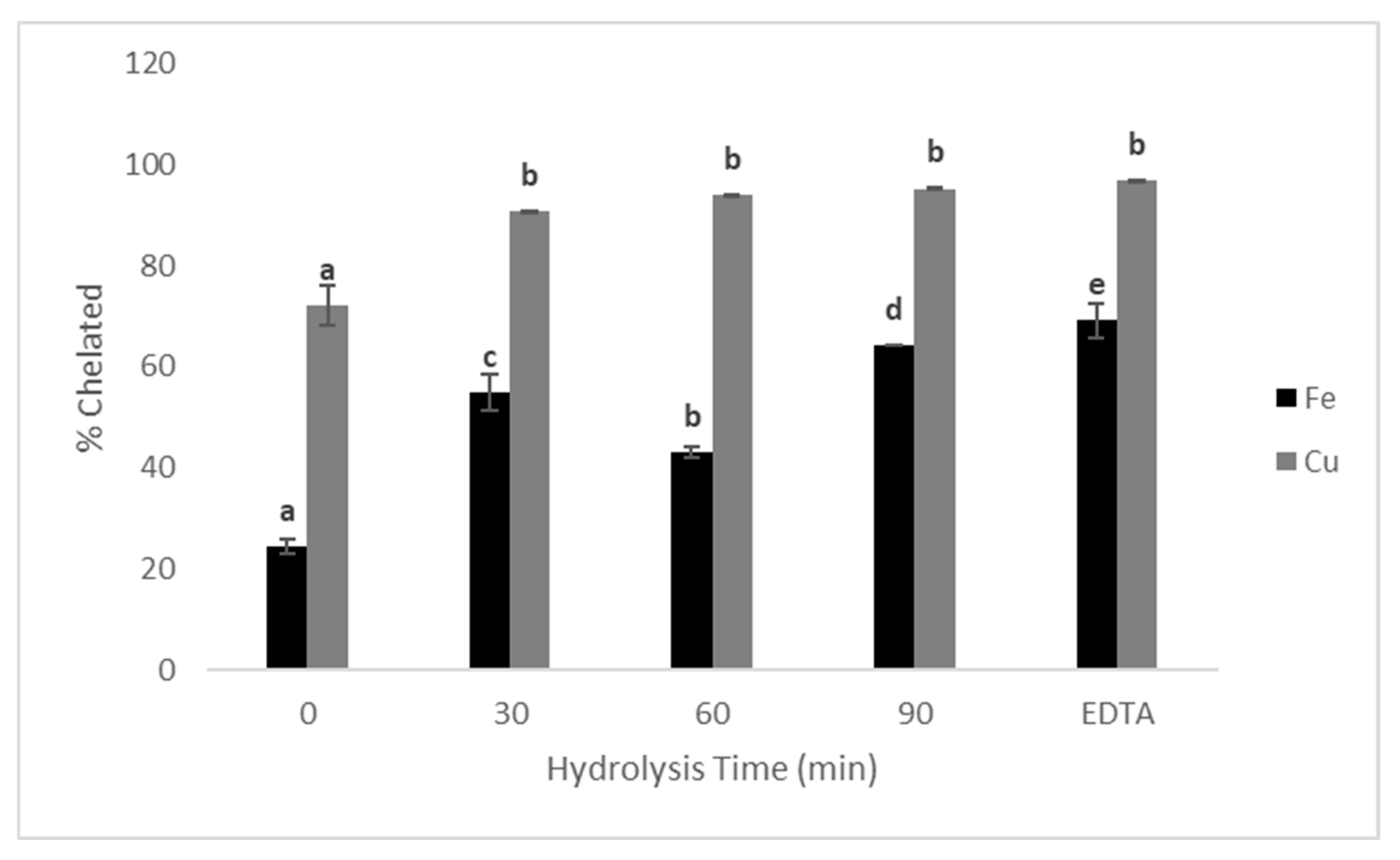
3.5. Antioxidant and Chelating Activity of Protein Hydrolysates
4. Discussion
4.1. Proximate Composition
4.2. Degree of Hydrolysis
4.3. Electrophoretic Profile
4.4. Amino Acid Profiles
4.5. Antioxidant and Chelating Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Río, L.; Navarro-Martínez, Z.M.; Cobián-Rojas, D.; Chevalier-Monteagudo, P.P.; Angulo-Valdes, J.A.; Rodriguez-Viera, L. Biology and ecology of the lionfish Pterois volitans/Pterois miles as invasive alien species: A review. PeerJ 2023, 11, e15728. [Google Scholar] [CrossRef] [PubMed]
- Bumbeer, J.; da Rocha, R.M.; Bornatowski, H.; Robert, M.d.C.; Ainsworth, C. Predicting impacts of lionfish (Pterois volitans) invasion in a coastal ecosystem of southern Brazil. Biol. Invasions 2017, 20, 1257–1274. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Caballero-Vázquez, J.A.; Guerra-Infante, F.M.; López-Hurtado, M. Analysis of the chemical composition of the lionfish Pterois volitans as a food strategy for its control. Lat. Am. J. Aquat. Res. 2019, 47, 841–844. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Tak, Y.; Kaur, M.; Amarowicz, R.; Bhatia, S.; Gautam, C. Pulse Derived Bioactive Peptides as Novel Nutraceuticals: A Review. Int. J. Pept. Res. Ther. 2021, 27, 2057–2068. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, B.; Li, B.; Wang, C.; Luo, Y. Preparation and identification of peptides and their zinc complexes with antimicrobial activities from silver carp (Hypophthalmichthys molitrix) protein hydrolysates. Food Res. Int. 2014, 64, 91–98. [Google Scholar] [CrossRef]
- Guo, L.; Hou, H.; Li, B.; Zhang, Z.; Wang, S.; Zhao, X. Preparation, isolation and identification of iron-chelating peptides derived from Alaska pollock skin. Process Biochem. 2013, 48, 988–993. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Li, B.; Hou, H.; Zhang, Z.; Zhao, X.; FitzGerald, R.J. Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci. Technol. 2014, 37, 92–105. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Chel, L.; Cua, D.; Betancur, D.; Chuc, A.; Aranda, I.; Gallegos, S. Antioxidant and chelating activities from Lion fish (Pterois volitans L.) muscle protein hydrolysates produced by in vitro digestion using pepsin and pancreatin. Emir. J. Food Agric. 2020, 32, 62–72. [Google Scholar] [CrossRef]
- Chel, L.; Estrella, Y.; Betancur, D.; Aranda, I.; Castellanos, A.; Gallegos, S. Antioxidant, chelating, and angiotensin-converting enzyme inhibitory activities of peptide fractions from red lionfish (Pterois volitans L.) muscle protein hydrolysates. Int. Food Res. J. 2020, 27, 224–233. [Google Scholar]
- AOAC. International. Official Methods of Analysis—AOAC, 22nd ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2023. [Google Scholar]
- Megías, C.; Pedroche, J.; Yust, M.M.; Girón, J.; Alaiz, M.; Millán, F.; Vioque, J. Affinity purification of copper chelating peptides from chickpea protein hydrolysates. J. Agric. Food Chem. 2007, 55, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Schägger, H.; Von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Alaiz, M.; Navarro, J.L.; Girón, J.; Vioque, E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J. Chromatogr. 1992, 591, 181–186. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Saiga, A.I.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Food and Agriculture Organization. Dietary Protein Quality Evaluation in Human; Report of an FAO Expert Consultation 92; Food and Agriculture Organization: Auckland, New Zealand, 2011; pp. 27–30. ISSN 0254-4725. Available online: https://www.fao.org/ag/humannutrition/35978-02317b979a686a57aa4593304ffc17f06.pdf (accessed on 10 December 2024).
- Arias-González, J.; González-Gándara, C.; Cabrera, J.L.; Christensen, V. Predicted impact of the invasive lionfish Pterois volitans on the food web of a Caribbean coral reef. Environ. Res. 2011, 111, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Yarnpakdee, S.; Senphan, T.; Halldórsdóttir, S.M.; Kristinsson, H.G. Fish Protein Hydrolysates: Production, Bioactivities and Applications. In Antioxidants and Functional Components in Aquatic Foods; Kristinsson, H.G., Ed.; Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Fodor, S.; Zhang, Z. Rearrangement of terminal amino acid residues in peptides by protease-catalyzed intramolecular transpeptidation. Anal. Biochem. 2006, 356, 282–290. [Google Scholar] [CrossRef] [PubMed]
- García, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo, F.G.; Guadix, A.; Guadix, E.M. Antioxidant activity of protein hydrolysates obtained from discarded Mediterranean fish species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Pacheco, R.; Mazorra, M.A.; Ramírez, J.C. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 2008, 109, 782–789. [Google Scholar] [CrossRef]
- Pratama, I.S.; Putra, Y.; Pangestuti, R.; Kim, S.-K.; Siahaan, E.A. Bioactive peptides-derived from marine by-products: Development, health benefits and potential application in biomedicine. Fish. Aquat. Sci. 2022, 25, 357–379. [Google Scholar] [CrossRef]
- Pérez, J.A.; Olivera, L.; Gómez, J.A.; Hernández, B. Release of multifunctional peptides by gastrointestinal digestion of sea cucumber (Isostichopus badionotus). J. Funct. Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- Galla, N.R.; Pamidighantam, P.R.; Akula, S.; Karakala, B. Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channa striatus and Labeo rohita. Food Chem. 2012, 135, 1479–1484. [Google Scholar] [CrossRef]
- Rehbein, H.; Kündiger, R.; Yman, I.M.; Ferm, M.; Etienne, M.; Jerome, M.; Craig, A.; Mackie, I.; Jessen, F.; Martínez, I.; et al. Species identification of cooked fish by urea isoelectric focusing and sodium dodecylsulfate polyacrylamide gel electrophoresis: A collaborative study. Food Chem. 1999, 67, 33–339. [Google Scholar] [CrossRef]
- Mackie, I.M. Métodos de Identificación de Especies de Pescado Fresco o Procesado. In Tecnología del Procesado del Pescado, 2nd ed.; Hall, G.M., Ed.; Acribia: Zaragoza, Spain, 2001; pp. 175–181. [Google Scholar]
- Larraín, M.A.; Abugoch, L.; Quitral, V.; Vinagre, J.; Segovia, C. Capillary zone electrophoresis as a method for identification of golden kinglip (Genypterus blacodes) species during frozen storage. Food Chem. 2002, 76, 377–384. [Google Scholar] [CrossRef]
- Chai, T.T.; Law, Y.C.; Wong, F.C.; Kim, S.K. Enzyme-Assisted Discovery of Antioxidant Peptides from Edible Marine Invertebrates: A Review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Jiménez, A.; Alaiz, M.; Vioque, J.; Rupérez, P. Health-promoting activities of ultra-filtered okara protein hydrolysates released by in vitro gastrointestinal digestion: Identification of active peptide from soybean lipoxygenase. Eur. Food Res. Technol. 2010, 230, 655–663. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Kumar, A.G.; Basha, R.K. Optimization of enzymatic hydrolysis of tilapia (Oreochromis Spp.) scale gelatine. Int. Aquat. Res. 2015, 7, 27–39. [Google Scholar] [CrossRef]
- Jafarpour, A.; Gregersen, S.; Gomes, M.R.; Marcatili, P.; Olsen, T.H.; Jacobsen, C.; Overgaard, M.T.; Sørensen, A.D.M. Biofunctionality of enzymatically derived peptides from codfish (Gadus morhua) frame: Bulk in vitro properties, quantitative proteomics, and bioinformatic prediction. Mar. Drugs 2020, 18, 599. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Sharma, D.; Gite, S.; Tuohy, M.G. Exploring the Physicochemical Characteristics of Marine Protein Hydrolysates and the Impact of In Vitro Gastrointestinal Digestion on Their Bioactivity. Mar. Drugs 2024, 22, 452. [Google Scholar] [CrossRef]
- Shekoohi, N.; Carson, B.P.; Fitzgerald, R.J. Antioxidative, Glucose Management, and Muscle Protein Synthesis Properties of Fish Protein Hydrolysates and Peptides. J. Agric. Food Chem. 2024, 72, 21301–21317. [Google Scholar] [CrossRef]
- Sabeena, K.; Lystbæk, L.; Hauch, H.; Jacobsen, C.; Jakobsen, G.; Johansson, I.; Flemming, J. Antioxidant activity of Cod (Gadus morhua) protein hydrolysates: In vitro assays and evaluation in 5% fish oil-in-water emulsion. Food Chem. 2014, 149, 326–334. [Google Scholar] [CrossRef]
- Peng, X.; Kong, B.; Xia, X.; Liu, Q. Reducing and radical-scavenging activities of whey protein hydrolysates prepared with Alcalase. Int. Dairy J. 2010, 20, 360–365. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhao, Y.; Zeng, M. Enzymatic preparation and characterization of iron-chelating peptides from anchovy (Engraulis japonicus) muscle protein. Food Res. Int. 2012, 48, 435–441. [Google Scholar] [CrossRef]
- Mazorra, M.A.; Ramírez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Xiong, Y.L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, A.; Khatua, K.; Shanbhag, V.; Comba, P.; Datta, A. Cu2+ selective chelators relieve copper-induced oxidative stress in vivo. Chem. Sci. 2018, 9, 7916–7930. [Google Scholar] [CrossRef] [PubMed]
| Amino Acids | Hydrolysis Time (min) | FAO [23] | |||
|---|---|---|---|---|---|
| Essentials | NHP | H30 | H60 | H90 | |
| Ile | 4.54 ± 0.09 | 4.54 ± 0.03 | 4.38 ± 0.01 | 4.59 ± 0.03 | 3.0 |
| Leu | 8.53 ± 0.18 | 9.02 ± 0.06 | 8.36 ± 0.04 | 8.62 ± 0.15 | 6.1 |
| Lys | 9.88 ± 0.34 | 10.69 ± 0.01 | 10.31 ± 0.02 | 10.24 ± 0.10 | 4.8 |
| Met | 2.24 ± 0.22 | 1.36 ± 0.04 | 2.55 ± 0.04 | 2.25 ± 0.57 | 2.3 * |
| Cys | 0.046 ± 0.02 | 0.15 ± 0.00 | 0.18 ± 0.05 | 0.08 ± 0.01 | |
| Phe | 3.95 ± 0.08 | 3.61 ± 0.01 | 3.52 ± 0.00 | 3.55 ± 0.02 | 4.1 ** |
| Tyr | 2.80 ± 0.19 | 2.71 ± 0.01 | 2.83 ± 0.03 | 2.89 ± 0.04 | |
| Thr | 4.31 ± 0.02 | 4.68 ± 0.01 | 4.56 ± 0.03 | 4.66 ± 0.00 | 2.5 |
| Val | 4.80 ± 0.12 | 5.11 ± 0.03 | 4.91 ± 0.00 | 5.08 ± 0.02 | 4.0 |
| His | 1.55 ± 0.01 | 1.74 ± 0.07 | 1.85 ± 0.00 | 1.76 ± 0.08 | 1.6 |
| Trp | 0.34 ± 0.03 | 0.29 ± 0.05 | 0.58 ± 0.07 | 0.53 ± 0.03 | 0.66 |
| Non-essentials | |||||
| Ala | 3.37 ± 0.02 | 3.52 ± 0.12 | 3.53 ± 0.03 | 3.53 ± 0.00 | |
| Arg | 11.76 ± 0.08 | 13.70 ± 0.07 | 12.88 ± 0.08 | 13.22 ± 0.11 | |
| Asp a | 10.64 ± 0.45 | 11.28 ± 0.19 | 11.13 ± 0.18 | 11.44 ± 0.05 | |
| Glu b | 16.02 ± 0.32 | 15.28 ± 0.06 | 14.64 ± 0.30 | 14.71 ± 0.12 | |
| Gly | 4.63 ± 0.01 | 5.42 ± 0.01 | 5.03 ± 0.02 | 5.14 ± 0.09 | |
| Ser | 3.38 ± 0.11 | 3.93 ± 0.04 | 4.01 ± 0.02 | 4.18 ± 0.11 | |
| Pro | 7.21 ± 0.17 | 3.17 ± 0.47 | 5.24 ± 0.74 | 3.981 ± 0.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chel-Guerrero, L.; Betancur-Ancona, D.; Chim-Chi, Y.A.; Sandoval-Peraza, V.M.; Gallegos Tintoré, S. Valorization of the Invasive Red Lionfish (Pterois volitans L.) as a Natural and Promising Source of Bioactive Hydrolysates with Antioxidant and Metal-Chelating Properties. Resources 2025, 14, 94. https://doi.org/10.3390/resources14060094
Chel-Guerrero L, Betancur-Ancona D, Chim-Chi YA, Sandoval-Peraza VM, Gallegos Tintoré S. Valorization of the Invasive Red Lionfish (Pterois volitans L.) as a Natural and Promising Source of Bioactive Hydrolysates with Antioxidant and Metal-Chelating Properties. Resources. 2025; 14(6):94. https://doi.org/10.3390/resources14060094
Chicago/Turabian StyleChel-Guerrero, Luis, David Betancur-Ancona, Yasser Alejandro Chim-Chi, Valentino Mukthar Sandoval-Peraza, and Santiago Gallegos Tintoré. 2025. "Valorization of the Invasive Red Lionfish (Pterois volitans L.) as a Natural and Promising Source of Bioactive Hydrolysates with Antioxidant and Metal-Chelating Properties" Resources 14, no. 6: 94. https://doi.org/10.3390/resources14060094
APA StyleChel-Guerrero, L., Betancur-Ancona, D., Chim-Chi, Y. A., Sandoval-Peraza, V. M., & Gallegos Tintoré, S. (2025). Valorization of the Invasive Red Lionfish (Pterois volitans L.) as a Natural and Promising Source of Bioactive Hydrolysates with Antioxidant and Metal-Chelating Properties. Resources, 14(6), 94. https://doi.org/10.3390/resources14060094







