Naidí (Euterpe oleracea Mart.), a Colombian Pacific Fruit with Potential Use in Animal Feed: A Systematic Review
Abstract
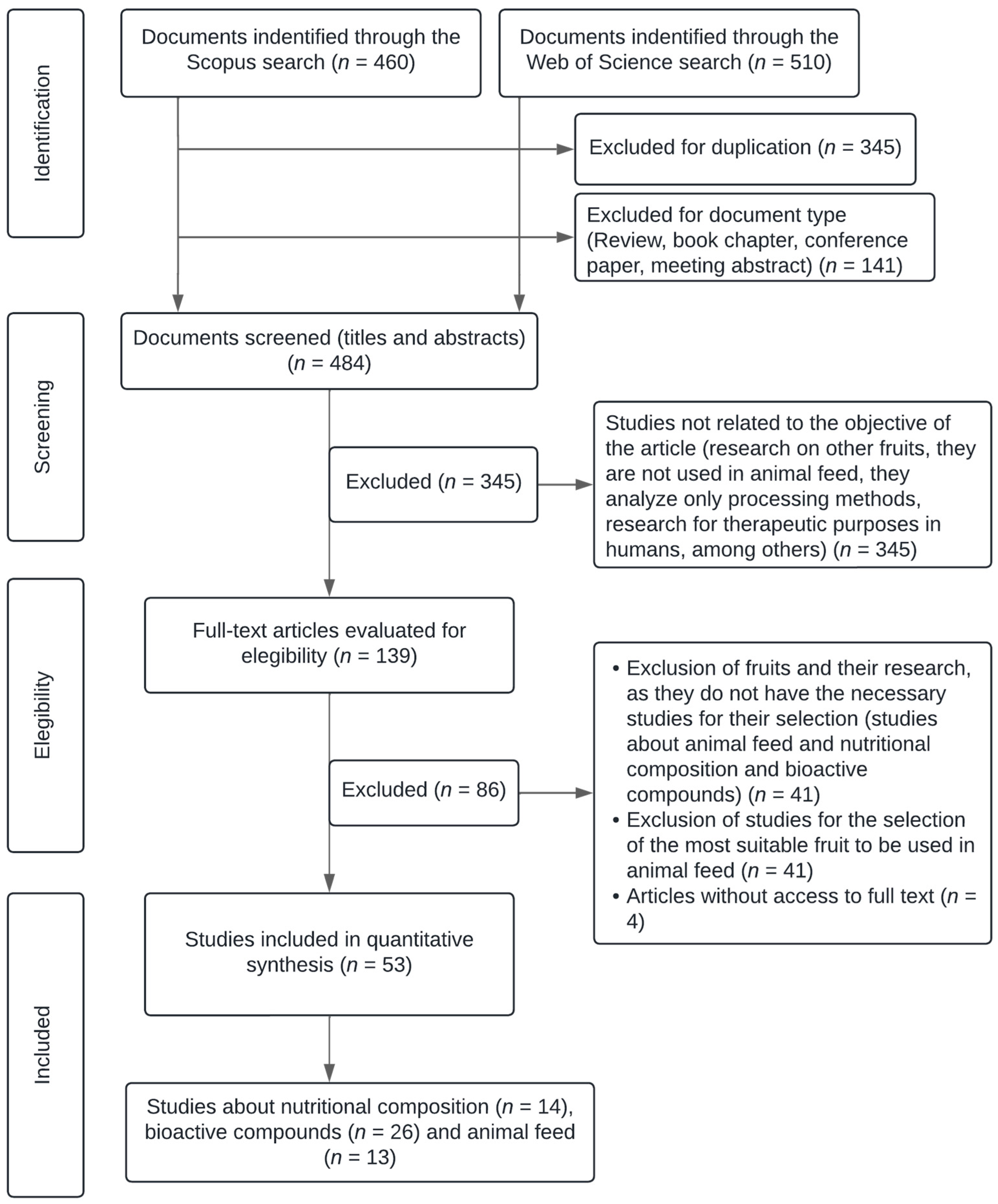
1. Introduction
2. Methods and Materials
2.1. Review Planning
2.2. Study Inclusion and Exclusion Criteria
- Investigations of different types of fruits.
- Fruits were not used for animal feed.
- The evaluation focused only on processing methods.
- Research with therapeutic purposes in humans.
- Ultra-processed products were evaluated.
- Focus on leaves, trees, and roots, not fruits or fruit by-products.
- Research was conducted in fields other than those of interest, such as nutritional composition, content of bioactive compounds, and animal feed.
2.3. Documentation and Review Analysis
3. Results and Discussion
3.1. Proximal Analysis
| Raw Material | Proximal Analysis | Source | ||||||
|---|---|---|---|---|---|---|---|---|
| Moisture (g/100 g DM) | Protein (g/100 g DM) | Fat (g/100 g DM) | Ashes (g/100 g DM) | Total Fiber (g/100 g DM) | Energy (kcal/100 g DM) | Total Carbohydrates (g/100 g DM) | ||
| Freeze-dried Pulp | 5.23 | 8.89 | 39.14 | - | 19.87 | - | - | [22] |
| 5.68 ± 0.14 | 9.19 ± 0.01 | 49.14 ± 0.36 | 5.16 ± 0.09 | 20.29 ± 0.14 | 591.8 ± 4.12 | 36.6 ± 0.06 | [24] | |
| 1.87 ± 0.116–2.76 ± 0.06 | 6.55 ± 0.072–9.188 ± 0.07 | 43.74 ± 1.087–52.96 ± 0.067 | 3.68 ± 0.055–4.894 ± 0.037 | 6.845 ± 0.113–10.997 ± 0.085 | - | - | [21] | |
| 4.92 ± 0.12 | 8.13 ± 0.63 | 40.75 ± 2.75 | 3.68 ± 0.08 | - | 489.39 | 42.53 ± 3.56 | [23] | |
| - | 10.54 ± 0.47 | 42.79 ± 0.31 | 3.03 ± 0.11 | 13.02 ± 0.71 | - | - | [31] | |
| - | 10.54 ± 0.27 | 42.79 ± 0.18 | 3.02 ± 0.06 | 13.01 ± 0.40 | 642.19 | 43.65 ± 0.37 | [30] | |
| 3.16 ± 0.14 | 9.34 ± 0.88 | 51.17 ± 0.00 | 3.75 ± 0.12 | - | - | 21.21 ± 1.00 | [26] | |
| Pulp | 1.8 | 7.6 ± 0.34 | 43.1 ± 0.05 | 1.03 | 19.4 | - | - | [20] |
| - | 12 ± 0 | 48 ± 4 | 4 ± 0 | - | - | 36 ± 4 | [25] | |
| - | 14.5 ± 0.4 | 24.0 ± 1.0 | 2.2 ± 0.4 | - | - | 38.4 | [29] | |
| - | 15.9 ± 0.3 | 33.1 ± 1.4 | 2.2 ± 0.1 | 20.0 | - | 48.8 | [28] | |
| - | 1.57 | 42.6 | 3.78 | - | - | 52.03 | [27] | |
| Pulp flour | 8 | 13.7 ± 0.3 | 22.9 ± 1.0 | 1.0 ± 0.2 | 20.5 ± 0.9 | - | 41.9 | [29] |
| Seed Flour and insoluble residues | 2.59 | 9.42 | 6.98 | 2.64 | - | - | - | [33] |
| Peel | 22.16 | - | 7.07 | 0.6 | 66.6 | - | - | [22] |
| Filter residues | 8.2 | 5.8 ± 0.88 | 1.2 ± 0.21 | 0.67 | 75.5 | - | - | [20] |
| Freeze-dried seed | 7.91 ± 0.01 | 4.89 ± 0.03 | 2.75 ± 0.01 | 1.36 ± 0.01 | - | - | - | [34] |
| Seeds | 32.93 | - | 1.75 | 2.09 | 66.64 | - | - | [22] |
| 8.5 | 9.3 ± 1.52 | 3.5 ±0.08 | 0.96 | 73 | - | - | [20] | |
| 9.3 | 4.06 | 0.29 | 3.06 | - | 417 | - | [35] | |
| Seed with mesocarp | 43.01 ± 0.07 | 2.86 ± 0.03 | 0.78 ± 0.21 | 1.27 ± 0.04 | 80.52 ± 0.56 | 441.7 ± 2.0 | - | [36] |
| Seed without mesocarp | 31.14 ± 0.05 | 3.78 ± 0.10 | 1.42 ± 0.19 | 1.29 ± 0.01 | 77.20 ± 0.78 | 430.4 ± 1.0 | - | |
| Whole berry | - | 4.95 ± 0.09–5.36 ± 0.55 | 8.74 ± 0.18–12.15 ± 0.11 | 0.12 ± 0.01–0.13 ± 0.01 | 51.55 ± 0.05–53.10 ± 0.15 | - | 31.57 ± 0.01–33.59 ± 0.17 | [37] |
3.2. Mineral Content
3.3. Vitamins
3.4. Fatty Acid Profile
3.5. Chemical Constitution of Euterpe oleracea
3.6. Quantitative Analysis of Polyphenols and Other Phenolic Compounds, and Antioxidant Capacity
- 1.
- Fresh pulp: TPC values were very close to the average values found for freeze-dried pulp, ranging from 4.34 to 61.66 ± 0.06 mg GAE/g DM [29,43,61,68,73,74]. Only [74] report the presence of flavonoids in the fresh pulp (3.52 ± 0.05). The anthocyanin content (TAC) differs greatly among the different authors. The lowest value is 0.62 mg Cyn-glu/g DM [74]), from values around 2.0 mg Cyn-glu/g DM [65,73] to 17.74 to 19.06 mg Cyn-glu/g DM. The latter values exceed those found for freeze-dried pulp. These variations are to be expected, given that the values reported are for pulps processed by hand and for commercial pulps, which vary in total solids content. In addition, the content of various compounds is influenced by climatic conditions, variety, harvest time and fruit maturity stage [69]. As an example, the results obtained by [76] can be mentioned. They recorded anthocyanin content values between 88 and 211 mg/L in fruits harvested from the same palm in different years. From the information collected, it can be deduced that the pulp is an important source of anthocyanins, which are related to potent antioxidant effects [29,37,65]. Like carotenoids, anthocyanins are natural pigments that enhance the color of the final product, a quality that is of interest in some animal feed production systems [30,31,77]. Non-anthocyanidin flavonoids are present in high concentrations in naidí pulp. These flavonoids are recognized for their beneficial effects on fish growth performance, feed efficiency, antioxidant activity, and immunity ([78]. Of the other types of pulp reviewed, the highest carotene values were found in the fresh pulp, with recorded values ranging from 176.1 mg/g DM [46] to 391.82 mg/g DM [47]. B-carotene and lycopene were identified [74]. Carotenes have potent antioxidant effects [67].
- 2.
- Freeze-dried pulp: For TPC values from 14.05 ± 0.02 mg GAE/g DM [24] to 77.51 ± 0.54 mg GAE/g DM were found [26]. Most authors found values near to 30 mg GAE/g DM [20,25,46,47,60,72]. TFC values oscillate between 4.0 and 17.48 mg GAE/g DM [20,46,47,64,71]. For TAC, values from 0.03 mg Cyn-glu/g DM [60] to 7.0 mg Cyn-glu/g DM were found [26,46,47]. Variations in anthocyanin values, especially those that are very low, may be related to a possible degradation of these components due to poor storage and transport conditions prior to sample analysis [20]. B-carotene, α-carotenelutein [67] and zeaxanthin [62,67] were found in freeze-dried pulp.
- 3.
- Defatted pulp: TPC 22 ± 2 mg GAE/g DM and TAC 16.0 ± 2.0 mg Cyn-glu/g DM (Sanabria & Sangronis, 2007) [28].
- 4.
- Defatted freeze-dried pulp: TPC between 96.43 ± 0.46 and 150.20 ± 1.32 mg GAE/g DM. TAC from 13.24 ± 0.55 to 13.84 ± 0.64 mg Cyn-glu/g DM [26]. TAC values are similar to those reported in defatted pulp.
- 5.
- Defatted pulp flour: Values of TPC and TAC of 16 ± 3.2 and 9.6 ± 2.2 mg GAE/g DM, respectively [29].
- 6.
- Defatted freeze-dried pulp flour: TPC from 88.4 ± 0.4 mg GAE/g DM [59].
- 7.
- Peel, freeze-dried filtrate residues (peel and pulp fibers) and seed flour: The TPC values are low due to the nature of the sample (1.32, 1.6 y 1.14 mg GAE/g DM, respectively) [20,22,33]. Only in the study of [20] an analysis of the total flavonoid content (TFC) in the freeze-dried peel fiber residues was performed. The result was a value of 0.82 mg GAE/g DM.
- 8.
- Berry: There is only one paper where values for TPC, TFC, and TAC were reported, showing variations in the content of bioactive compounds of the same species from two different locations [37]. Additionally, during maturation, TPC and TFC decrease while TAC increases. This increase is due, at least in part, to the presence of non-phenolic precursors. Ref. [75] provided only the TAC value, but it could not be expressed in terms of MD because of the lack of information about berry moisture.
- 9.
- Pulp oil: It can be a source of fatty acids and antioxidant compounds in animal feed, which contribute to the efficiency of production systems under stressful conditions and to the improvement of quality [32]. Ref. [26] reported a significant concentration of carotenoids, which was related to a remarkable antioxidant and anti-inflammatory activity reported in lactating ewes by [32]. In addition, ref. [26] quantified the carotenoids in pulp oil in a study comparing different extraction methods. They obtained values ranging from 246.22 ± 2.51 to 277.09 ± 3.65. These values are quite close to those of fresh pulp, given the nonpolar nature of both carotenoids and oil.
- 10.
- Oil from insoluble pulp residues: only a TPC value of 1.25 ± 0.042 mg GAE/g DM was found [50], this is low due to the lipophilic nature of the sample.
- 11.
- Seed: Two studies were found. These reported TPC values of 4.37 [22] and 17.7 mg GAE/g DM [20]. This part of the plant is considered a significant source of procyanidins and flavonoids of the flavan-3-ols type, which exhibit high antioxidant properties [34,70]. The use of this part of the plant is due to the high concentrations of insoluble fiber, but the biological potential of the compounds it contains could be exploited [35,36]. Due to the high presence of tannins, which give it an astringent flavor, the inclusion of the seed in animal feed should be limited as it can affect the palatability of the feed [20].
- 12.
3.7. Antioxidant Capacity
3.8. Animal Feed
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The Contribution of Fisheries and Aquaculture to the Global Protein Supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; McCoskey, S. Does Global Meat Consumption Follow an Environmental Kuznets Curve? Sustain. Sci. Pract. Policy 2013, 9, 26–36. [Google Scholar] [CrossRef]
- Schader, C.; Muller, A.; El-Hage Scialabba, N.; Hecht, J.; Isensee, A.; Erb, K.H.; Smith, P.; Makkar, H.P.S.; Klocke, P.; Leiber, F.; et al. Impacts of Feeding Less Food-Competing Feedstuffs to Livestock on Global Food System Sustainability. J. R. Soc. Interface 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012; Available online: https://www.fao.org/economic/esa (accessed on 4 May 2025).
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Steinfeld, H. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006; Available online: https://www.fao.org/4/a0701e/a0701e00.htm (accessed on 4 May 2025).
- Maiolo, S.; Parisi, G.; Biondi, N.; Lunelli, F.; Tibaldi, E.; Pastres, R. Fishmeal Partial Substitution within Aquafeed Formulations: Life Cycle Assessment of Four Alternative Protein Sources. Int. J. Life Cycle Assess 2020, 25, 1455–1471. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products; Makkar, H., Ed.; FAO: Rome, Italy, 2013; Available online: https://www.fao.org/4/i3273e/i3273e00.htm (accessed on 17 February 2025).
- Patel, A.; Temgire, S.; Borah, A. Agro-Industrial Waste as Source of Bioactive Compounds and Their Utilization: A Review. Pharma Innov. 2021, 10, 192–196. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Ruiz-Canizales, J.; Villegas-Ochoa, M.A.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Use of By-Products from the Food Industry. An Approach to the Circular Economy. Rev. Iberoam. Tecnol. Postcosecha 2022, 23, 92–99. Available online: https://www.redalyc.org/journal/813/81373798002/81373798002.pdf (accessed on 23 May 2025).
- Gutiérrez-Espinosa, M.C.; Merino, M.C. Manual Práctico Para La Preparación de Alimentos Balanceados Artesanales Para Piscicultura; MADR, AUNAP, FAO: Bogotá, Colombia, 2021. Available online: https://www.aunap.gov.co/documentos/Fomento/manuales/Manual-preparacion-de-alimentos-artesanales.pdf (accessed on 24 May 2025).
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe Oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15, 989. [Google Scholar] [CrossRef]
- Borja-Rentería, J.J.; Ledezma Rentería, E.; Copete, J.C. Reproductive biology of Euterpe oleracea Mart., a palm with high nutritional fruits for the communities from Colombian Pacific region. Rev. Cienc. 2024, 27, 1–18. [Google Scholar] [CrossRef]
- Rojano, B.A.; Zapata Vahos, I.C.; Alzate Arbeláez, A.F.; Mosquera Martínez, A.J.; Cortés Correa, F.B.; Gamboa Carvajal, L. Polyphenols and Antioxidant Activity of the Freeze-Dried Palm Naidi (Colombian Açai) (Euterpe Oleracea Mart). Rev. Fac. Nal. Agr. Medellín 2011, 64, 6213–6220. [Google Scholar]
- da Silveira, J.T.; da Rosa, A.P.C.; de Morais, M.G.; Victoria, F.N.; Costa, J.A.V. An Integrative Review of Açaí (Euterpe Oleracea and Euterpe Precatoria): Traditional Uses, Phytochemical Composition, Market Trends, and Emerging Applications. Food Res. Int. 2023, 173, 113304. [Google Scholar] [CrossRef]
- Montenegro-Gómez, S.P.; Rosales-Escarria, M. Fruit Naidi (Euterpe Oleracea) and Perspective Food Security in Colombia. Entramado 2015, 11, 200–207. [Google Scholar] [CrossRef]
- Vallejo, M.I.; Valderrama, N.; Bernal, R.; Galeano, G.; Arteaga, G.; Leal, C. Current Status and Perspectives of Palm Heart Production from Euterpe Oleracea Mart. (Arecacea), at the Pacific Coast of Colombia. Colomb. For. 2011, 14, 191–212. Available online: http://www.scielo.org.co/pdf/cofo/v14n2/v14n2a05.pdf (accessed on 26 May 2025). [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Zuluaga-Hernández, C.D.; Hincapié, C.A.; Osorio, M. Non-Conventional Ingredients for Tilapia (Oreochromis Spp.) Feed: A Systematic Review. Fishes 2023, 8, 556. [Google Scholar] [CrossRef]
- Buratto, R.T.; Cocero, M.J.; Martín, Á. Characterization of Industrial Açaí Pulp Residues and Valorization by Microwave-Assisted Extraction. Chem. Eng. Process. Process Intensif. 2021, 160, 108269. [Google Scholar] [CrossRef]
- Carvalho, A.V.; Ferreira Ferreira da Silveira, T.; Mattietto, R.d.A.; Padilha de Oliveira, M.d.S.; Godoy, H.T. Chemical Composition and Antioxidant Capacity of Açaí (Euterpe Oleracea) Genotypes and Commercial Pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vallejo, M.C.; Poveda-Giraldo, J.A.; Cardona Alzate, C.A. Valorization Alternatives of Tropical Forest Fruits Based on the Açai (Euterpe Oleracea) Processing in Small Communities. Foods 2023, 12, 2229. [Google Scholar] [CrossRef]
- Menezes, E.M.D.S.; Torres, A.T.; Srur, A.U.S. Lyophilized Açaí Pulp (Euterpe Oleracea Mart) Nutritional Value. Acta Amaz. 2008, 38, 311–316. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Ribeiro, A.E.C.; Oliveira, É.R.; Garcia, M.C.; Soares Júnior, M.S.; Caliari, M. Structural and Physicochemical Properties of Freeze-Dried Açaí Pulp (Euterpe Oleracea Mart.). Food Sci. Technol. 2020, 40, 282–289. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; De Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; De Andrade Mattietto, R.; Friedrich, M.; Da Matta, V.M.; Marx, F. Chemical Characterization and Evaluation of Antioxidant Properties of Açaí Fruits (Euterpe Oleracea Mart.) during Ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.M.M.; Campos, A.L.D.B.S.; Pires, F.C.S.; Ferreira, M.C.R.; Silva, A.P.D.S.E.; Menezes, E.G.O.; Ramos, I.N.D.F.; Khayat, A.S.; Rêgo, J.D.A.R.D.; Carvalho Junior, R.N.D. Evaluation of Bioactive Compounds and Antioxidant and Cytotoxic Effects of Oil and Pulp without Açaí Fat (Euterpe Oleracea) Obtained by Supercritical Extraction. Foods 2024, 13, 2819. [Google Scholar] [CrossRef]
- do Nascimento, R.J.S.; Couri, S.; Antoniassi, R.; Freitas, S.P. Fatty Acids Composition of Açaí Pulp Oil Obtained by Enzymatic Technology and Hexane. Rev. Bras. Frutic. 2008, 30, 498–502. [Google Scholar] [CrossRef]
- Sanabria, N.; Sangronis, E. Caracterización Del Acai o Manaca (Euterpe Oleracea Mart.): Un Fruto Del Amazonas. Organo Of. Soc. Latinoam. Nutr. 2007, 57, 94–98. Available online: https://www.alanrevista.org/ediciones/2007/1/art-13/ (accessed on 26 May 2025).
- Sangronis, E.; Sanabria, N. Impact of Solar Dehydration on Composition and Antioxidant Properties of Acai (Euterpe Oleraceae Mart.). Organo Of. Soc. Latinoam. Nutr. 2011, 61. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0004-06222011000100010 (accessed on 26 May 2025).
- da Silva, T.V.N.; dos Santos, C.F.; dos Santos, J.M.L.; Schmitz, M.J.; Ramírez, J.R.B.; Torres, M.F.; Barbas, L.A.L.; Sampaio, L.A.; Verde, P.E.; Tesser, M.B.; et al. Effects of Dietary Inclusion of Lyophilized Açai Berries (Euterpe Oleraceae) on Growth Metrics, Metabolic and Antioxidant Biomarkers, and Skin Color of Juvenile Tambaqui (Colossoma Macropomum). Aquac. Int. 2023, 31, 1031–1056. [Google Scholar] [CrossRef]
- Silva, S.M.; Ramos, P.B.; Buitrago, J.R.; da Silva, T.V.N.; Simião, C.S.; Colombo, G.M.; Schmitz, M.; Tesser, M.B.; Prentice, C.; Wasielesky, W.; et al. Zootechnical Performance, Biochemical Response, and Chromaticity in Pacific White Shrimp (Litopenaeus Vannamei) (Boone, 1931) after the Inclusion of Lyophilized Açaí (Euterpe Oleracea) in the Diet. Aquac. Int. 2020, 28, 1563–1577. [Google Scholar] [CrossRef]
- Dos Santos, D.D.S.; Klauck, V.; Campigotto, G.; Alba, D.F.; Dos Reis, J.H.; Gebert, R.R.; Souza, C.F.; Baldissera, M.D.; Schogor, A.L.B.; Santos, I.D.; et al. Benefits of the Inclusion of Açai Oil in the Diet of Dairy Sheep in Heat Stress on Health and Milk Production and Quality. J. Therm. Biol. 2019, 84, 250–258. [Google Scholar] [CrossRef]
- Sousa, M.C.S.; Galli, G.M.; Bottari, N.B.; Alba, D.F.; Leal, K.W.; Lopes, T.F.; Druzian, L.; Schetinger, M.R.C.; Gloria, E.M.; Mendes, R.E.; et al. Fumonisin-(Fusarium Verticillioides)-Contaminated Feed Causes Hepatic Oxidative Stress and Negatively Affects Broiler Performance in the Early Stage: Does Supplementation with Açai Flour Residues (Euterpe Oleraceae) Minimize These Problems? Microb. Pathog. 2020, 146, 104237. [Google Scholar] [CrossRef]
- Melo, P.S.; Selani, M.M.; Gonçalves, R.H.; Paulino, J.d.O.; Massarioli, A.P.; de Alencar, S.M. Açaí Seeds: An Unexplored Agro-Industrial Residue as a Potential Source of Lipids, Fibers, and Antioxidant Phenolic Compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar] [CrossRef]
- Silva, R.C.; Coelho, G.d.J.; Sousa, M.B.F.; Caldas, C.S.; Maciel, R.P.; Mezzomo, R.; Gomes, D.Í.; Neta, E.R.D.S.; Tavares, F.B.; Alves, K.S.; et al. Intake, Digestibility, and Behavior of Horses Fed Açaí Kernel (Euterpe Oleracea Mart.) as a Substitute of Mombaça Grass (Megathyrsus Maximus). Trop. Anim. Health Prod. 2024, 56, 361. [Google Scholar] [CrossRef]
- Arruda, J.d.C.B.; da Fonseca, L.A.B.; Pinto, L.C.P.; Pinheiro, H.C.d.O.; Monteiro, B.T.O.; Manno, M.C.; Lima, K.R.d.S.; de Lima, A.R. Açaí Seed Bran in the Feed of Slow-Growth Broilers. Acta Amaz. 2018, 48, 298–303. [Google Scholar] [CrossRef]
- Flor-Unda, O.; Guanochanga, F.; Samaniego, I.; Arias, V.; Ortiz, B.; Rosales, C.; Palacios-Cabrera, H. Physicochemical Characterization and Antioxidant Capacity of Açaí (Euterpe Oleracea) in Ecuadorian Region. Foods 2024, 13, 3046. [Google Scholar] [CrossRef]
- Ferreira Pereira, J.A.; Cotta Coutinho, Í.A.; Soares, E.L.; Soares, A.A.; de Souza Caetano, A.P.; de Assis de Paiva Campos, F. Morphoanatomical and Histochemical Studies of the Seed Development of Euterpe Oleracea (Arecaceae). Rodriguesia 2021, 72. [Google Scholar] [CrossRef]
- de Lima, A.C.P.; Bastos, D.L.R.; Camarena, M.A.; Bon, E.P.S.; Cammarota, M.C.; Teixeira, R.S.S.; Gutarra, M.L.E. Physicochemical Characterization of Residual Biomass (Seed and Fiber) from Açaí (Euterpe Oleracea) Processing and Assessment of the Potential for Energy Production and Bioproducts. Biomass Convers Biorefin 2021, 11, 925–935. [Google Scholar] [CrossRef]
- Gomes, D.I.; Véras, R.M.L.; Alves, K.S.; Detmann, E.; Oliveira, L.R.S.; Mezzomo, R.; dos Santos, R.B.; de Sousa Barcelos, S. Performance and Digestibility of Growing Sheep Fed with Açai Seed Meal-Based Diets. Trop. Anim. Health Prod. 2012, 44, 1751–1757. [Google Scholar] [CrossRef]
- Santos, G.A.; Carvalho, A.A.C.; Oliveira, A.P.; Naozuka, J.; Matta, F.V.; Felipe-Sotelo, M.; Ward, N.I.; Corrêa, N.C.F.; Nomura, C.S. Bioaccessibility of Essential Elements in Açaí (Euterpe Oleracea Mart.) Pulp. ACS Food Sci. Technol. 2021, 1, 874–883. [Google Scholar] [CrossRef]
- Alves, B.S.F.; Pereira Junior, J.B.; Carvalho, F.I.M.; Dantas Filho, H.A.; Fernandes Dantas, K.G. Mineral Composition of Amazonian Fruits by Flame Atomic Absorption Spectrometry Using Multivariate Analysis. Biol. Trace Elem. Res. 2019, 189, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Minighin, E.C.; de Souza, K.F.; Valenzuela, V.d.C.T.; Silva, N.d.O.C.e.; Anastácio, L.R.; Labanca, R.A. Effect of in Vitro Gastrointestinal Digestion on the Mineral Content, Phenolic Compounds, and Antioxidant Capacity of Commercial Pulps of Purple and White Açaí (Euterpe Oleraceae Mart.). J. Food Sci. Technol. 2020, 57, 1740. [Google Scholar] [CrossRef] [PubMed]
- Kinupp, V.F.; de Barros, I.B.I. Protein and Mineral Contents of Native Species, Potential Vegetables, and Fruit. Ciência Tecnol. Aliment. 2008, 28, 846–857. [Google Scholar] [CrossRef]
- de Oliveira, A.P.; Naozuka, J. Effects of Iron Enrichment of Adzuki Bean (Vigna Angularis) Sprouts on Elemental Translocation, Concentrations of Proteins, Distribution of Fe-Metalloproteins, and Fe Bioaccessibility. J. Braz. Chem. Soc. 2017, 28, 1937–1946. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- de Souza, M.C.; Figueiredo, R.W.; Maia, G.A.; Alves, R.E.; Brito, E.S.; Moura, C.F.H.; Rufino, M.S.M. Bioactive Compounds and Antioxidant Activity on Fruits from Different Açaí (Euterpe Oleracea Mart) Progenies. Acta Hortic. 2009, 455–458. [Google Scholar] [CrossRef]
- da Costa, P.A.; Ballus, C.A.; Teixeira-Filho, J.; Godoy, H.T. Phytosterols and Tocopherols Content of Pulps and Nuts of Brazilian Fruits. Food Res. Int. 2010, 43, 1603–1606. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai (Euterpe Oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- de Moura, L.B.; Cavalcante, J.G.; Nascimento, E.T.d.S.; e Silva, I.C.; Salaro, A.L.; Barbas, L.A.L.; Veras, G.C.; Campelo, D.A.V. Dietary Euterpe Oleracea Essential Oil, the Amazon Açaí, as Feed Additive to Amazonian Ornamental Fish, during Post-Larvae Growing Stage: A Preliminary Study. Fishes 2022, 7, 369. [Google Scholar] [CrossRef]
- Santos, O.V.; Lemos, Y.S.; da Conceição, L.R.V.; Teixeira-Costa, B.E. Lipids from the Purple and White Açaí (Euterpe Oleracea Mart) Varieties: Nutritional, Functional, and Physicochemical Properties. Front. Nutr. 2024, 11, 1385877. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ucar, Y.; Takadaş, F.; Durmus, M.; Köşker, A.R.; Polat, A. Comparision of Green and Conventional Extraction Methods on Lipid Yield and Fatty Acid Profiles of Fish Species. Eur. J. Lipid Sci. Technol. 2018, 120. [Google Scholar] [CrossRef]
- Schmid, A.; Collomb, M.; Sieber, R.; Bee, G. Conjugated Linoleic Acid in Meat and Meat Products: A Review. Meat Sci. 2006, 73, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Omega-3 Long-Chain Polyunsaturated Fatty Acids and Aquaculture in Perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Schmitz, M.J.; Colombo, G.M.; Simião, C.d.S.; Ortiz, C.R.; Costa, L.D.F.; da Silva, T.V.N.; Ramos, P.B.; Yunes, J.S.; Wasielesky, W.; Tesser, M.B.; et al. Modulation of Nodularin Toxicity in Shrimp Litopenaeus Vannamei (Boone, 1931) Fed with Dietary Açai (Euterpe Oleracea) Inclusion. Fish Shellfish. Immunol. 2020, 103, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Heck, K.L.; Yi, Y.; Thornton, D.; Zheng, J.; Calderón, A.I. A Comparative Metabolomics Analysis of Açaí (Euterpe Oleracea Mart.) Fruit, Food Powder, and Botanical Dietary Supplement Extracts. Phytochem. Anal. 2025, 36, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Thomasi, S.S.; Forti, D.; De Benedicto, C.; Da, T.; Alves, C.; Bellete, B.S.; Venâncio, T.; De Andrade, R.; Antonio, M.; Ferreira, G.; et al. Chemical Constituents of Açai Berry Pulp (Euterpe Oleracea Mart.) by LC-UV-BPSU/NMR and LC-UV-SPE/NMR. Nat. Prod. Res. 2024, 39, 4317–4324. [Google Scholar] [CrossRef]
- Silva, A.P.d.S.; de Camargo, A.C.; Lazarini, J.G.; Franchin, M.; Sardi, J.d.C.O.; Rosalen, P.L.; de Alencar, S.M. Phenolic Profile and the Antioxidant, Anti-Inflammatory, and Antimicrobial Properties of Açaí (Euterpe Oleracea) Meal: A Prospective Study. Foods 2023, 12, 86. [Google Scholar] [CrossRef]
- Xiong, J.; Matta, F.; Grace, M.; Ann Lila, M.; Ward, N.; Felipe-Sotelo, M.; Esposito, D. Phenolic Content, Anti-Inflammatory, and Dermal Wound Repair Properties of Industrially Processed and Non-Processed Acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic Composition and Antioxidant Activity of Açai (Euterpe Oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Valenzuela, D.; Guachamin, A.; Méndez, G.; Heredia-Moya, J.; Vera, E. Bioactive Compound Profiling and Antioxidant Activity of Phytelephas Tenuicaulis and Other Amazonian Fruits. Foods 2024, 13, 2151. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Insfran, D.; Brenes, C.H.; Talcott, S.T. Phytochemical Composition and Pigment Stability of Açai (Euterpe Oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef]
- Gallori, S.; Bilia, A.R.; Bergonzi, M.C.; Barbosa, W.L.R.; Vincieri, F.F. Polyphenolic Constituents of Fruit Pulp of Euterpe Oleracea Mart. (Açai Palm). Chromatographia 2004, 59, 739–743. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical Composition and Thermal Stability of Two Commercial Açai Species, Euterpe Oleracea and Euterpe Precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Previtalli-Silva, H.; Hardoim, D.d.J.; Banaggia, R.d.L.; Moragas-Tellis, C.J.; Chagas, M.D.S.d.S.; Behrens, M.D.; Dias-Silva, T.d.S.; Calabrese, K.d.S.; Cardoso, F.d.O. Antioxidant and Anti-Inflammatory Activity of Euterpe Oleracea Mart. (Açaí) Seed Bioproducts. Heliyon 2024, 10, e40510. [Google Scholar] [CrossRef]
- Torma, P.D.C.M.R.; Brasil, A.V.S.; Carvalho, A.V.; Jablonski, A.; Rabelo, T.K.; Moreira, J.C.F.; Gelain, D.P.; Flôres, S.H.; Augusti, P.R.; Rios, A.d.O. Hydroethanolic Extracts from Different Genotypes of Açaí (Euterpe Oleraceae) Presented Antioxidant Potential and Protected Human Neuron-like Cells (SH-SY5Y). Food Chem. 2017, 222, 94–104. [Google Scholar] [CrossRef]
- Stafussa, A.P.; Maciel, G.M.; Bortolini, D.G.; Maroldi, W.V.; Ribeiro, V.R.; Fachi, M.M.; Pontarolo, R.; Bach, F.; Pedro, A.C.; Haminiuk, C.W.I. Bioactivity and Bioaccessibility of Phenolic Compounds from Brazilian Fruit Purees. Future Foods 2021, 4, 100066. [Google Scholar] [CrossRef]
- Vera de Rosso, V.; Hillebrand, S.; Cuevas Montilla, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of Anthocyanins from Acerola (Malpighia Emarginata DC.) and Açai (Euterpe Oleracea Mart.) by HPLC-PDA-MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Martins, G.R.; Amaral, F.R.L.D.; Brum, F.L.; Mohana-Borges, R.; de Moura, S.S.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.; Figueiredo, N.G.; da Silva, A.S. Chemical Characterization, Antioxidant and Antimicrobial Activities of Açaí Seed (Euterpe Oleracea Mart.) Extracts Containing A- and B-Type Procyanidins. LWT 2020, 132, 109830. [Google Scholar] [CrossRef]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian Fruit Pulps as Functional Foods and Additives: Evaluation of Bioactive Compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of Açaí (Euterpe Precatoria Mart.) Fruit Pulp, Superior Antioxidant and Anti-Inflammatory Properties to Euterpe Oleraceae Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Neves, L.T.B.C.; Campos, D.C.D.S.; Mendes, J.K.S.; Urnhani, C.O.; de Araújo, K.G.M. Quality of Fruits Manually Processed of Açaí (Euterpe Oleracea Mart.) and Bacaba (Oenocarpus Bacaba Mart.). Rev. Bras. Frutic. 2015, 37, 729–738. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Ávila, S.; Ito, V.; Nogueira, A.; Wosiacki, G.; Haminiuk, C.W.I. The Association between Chromaticity, Phenolics, Carotenoids, and In Vitro Antioxidant Activity of Frozen Fruit Pulp in Brazil: An Application of Chemometrics. J. Food Sci. 2014, 79, C510–C516. [Google Scholar] [CrossRef] [PubMed]
- Rogez, H.; Pompeu, D.R.; Akwie, S.N.T.; Larondelle, Y. Sigmoidal Kinetics of Anthocyanin Accumulation during Fruit Ripening: A Comparison between Açai Fruits (Euterpe Oleracea) and Other Anthocyanin-Rich Fruits. J. Food Compos. Anal. 2011, 24, 796–800. [Google Scholar] [CrossRef][Green Version]
- Lichtenthäler, R.; Rodrigues, R.B.; Maia, J.G.S.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total Oxidant Scavenging Capacities of Euterpe Oleracea Mart. (Açaí) Fruits. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.B.; Colombo, G.M.; Schmitz, M.J.; Simião, C.S.; Machado, K.d.S.; Werhli, A.V.; Costa, L.D.F.; Yunes, J.S.; Prentice, C.; Wasielesky, W.; et al. Chemoprotection Mediated by Açaí Berry (Euterpe Oleracea) in White Shrimp Litopenaeus Vannamei Exposed to the Cyanotoxin Saxitoxin Analyzed by in Vivo Assays and Docking Modeling. Aquat. Toxicol. 2022, 246, 106148. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla Fruticosa L. and Its Quality Assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction Techniques and Analysis of Anthocyanins from Food Sources by Mass Spectrometry: An Update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O. Nutritional Immunity of Fish Intestines: Important Insights for Sustainable Aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Colombo, G.M.; dos Santos Simião, C.; Schmitz, M.J.; Pedrosa, V.F.; Romano, L.A.; Tesser, M.B.; Ramos, P.B.; Wasielesky, W.; Monserrat, J.M. The Role of Açaí (Euterpe Oleracea Mart. 1824) as a Chemoprotective Agent in the Evaluation of Antioxidant Defence, Oxidative Damage and Histology of Juvenile Shrimp Litopenaeus Vannamei (Boone, 1931) Exposed to Ammonia. Aquac. Res. 2020, 51, 1551–1566. [Google Scholar] [CrossRef]
- Colombo, G.M.; dos Santos Simião, C.; Ramírez, J.R.B.; de Sousa Araujo, A.C.; Gomes, R.M.M.; Buitagro, S.A.M.; Wasielesky, W.; Monserrat, J.M. Bioflocs Enriched with Lyophilized Açaí (Euterpe Oleracea) Improved the Survival and Weight Gain of Litopenaeus Vannamei Post-Larvae Cultivated in the BFT System. Aquaculture 2023, 566, 739230. [Google Scholar] [CrossRef]
- Colombo, G.M.; Marreiro Gomes, R.M.; Muñoz Buitrago, S.A.; Buitrago Ramírez, J.R.; de Sousa Araujo, A.C.; Silva Oliveira, F.P.; Pedrosa, V.F.; Romano, L.A.; Tesser, M.; Wasielesky, W.; et al. Effects of Lyophilized Açaí (Euterpe Oleracea) Supplementation on Oxidative Damage and Intestinal Histology in Juvenile Shrimp Penaeus Vannamei Reared in Biofloc Systems. Animals 2023, 13, 3282. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. The Triple Defensive Barrier of Phenolic Compounds against the Lipid Oxidation-Induced Damage in Food Products. Trends Food Sci. Technol. 2016, 54, 165–174. [Google Scholar] [CrossRef]
- Huber, P.C.; Almeida, W.P.; Fátima, Â.d. Glutathione and Related Enzymes: Biological Roles and Importance in Pathological Processes. Quim. Nova 2008, 31, 1170–1179. [Google Scholar] [CrossRef]
- Ventura-Lima, J.; de Castro, M.R.; Acosta, D.; Fattorini, D.; Regoli, F.; de Carvalho, L.M.; Bohrer, D.; Geracitano, L.A.; Barros, D.M.; Marins, L.F.F.; et al. Effects of Arsenic (As) Exposure on the Antioxidant Status of Gills of the Zebrafish Danio Rerio (Cyprinidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 538–543. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.V.N.; Gomes, R.M.M.; Torres, M.F.; Barbas, L.A.L.; Sampaio, L.A.; Monserrat, J.M. Water Quality and Oxidative Stress in Fish Colossoma Macropomum Fed with Dietary Amazonian Fruit Euterpe Oleracea Mart. after Transport Simulation. Chem. Ecol. 2024, 40, 351–368. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Dadar, M.; Khalili, M.; Cerezuela, R.; Esteban, M.Á. Effect of Dietary Supplementation of Palm Fruit Extracts on the Transcriptomes of Growth, Antioxidant Enzyme and Immune-Related Genes in Common Carp (Cyprinus Carpio) Fingerlings. Aquac. Res. 2017, 48, 3684–3692. [Google Scholar] [CrossRef]
- Vanderzwalmen, M.; Eaton, L.; Mullen, C.; Henriquez, F.; Carey, P.; Snellgrove, D.; Sloman, K.A. The Use of Feed and Water Additives for Live Fish Transport. Rev. Aquac. 2019, 11, 263–278. [Google Scholar] [CrossRef]
- Copete, J.C.; Torres, M.C.; Castellano, C. Population Structure, Fruit Production and Uses of the Palm Naidí (Euterpe Oleracea Mart.) in Colombian Pacific. Bioetnia 2021, 18, 5–15. [Google Scholar] [CrossRef]
| Raw Material | Mineral Content (mg/100 g DM) | Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | Cu | Mn | P | Fe | Zn | ||
| Pulp | 102.78 ± 4.28–153.20 ± 10.28 | 4182.50 ± 151.40–4712.75 ± 144.68 | 1114.49 ± 46.25–2521.03 ± 82.43 | 739.74 ± 2.36–1221.25 ± 33.93 | 0.63 ± 0.27–1.00 ± 0.28 | 196.63 ± 3.99–671.51 ± 2.25 | - | - | - | [43] |
| 6.8 ± 0.7 | 930 ± 9.9 | 423 ± 1.2 | 172 ± 0.3 | - | 13.3 ± 0.1 | 186 ± 1.5 | 7.8 ± 0.2 | 2.1 ± 0.0 | [25] | |
| - | 1136.2 ± 16.6 | 455.1 ± 12.1 | 223.4 ± 5.4 | 2.08 ± 0.01 | 59.1 ± 1.2 | 156.5 ± 1.9 | 4.4 ± 0.1 | 3.13 ± 0.07 | [41] | |
| 66 ± 30 | 697 ± 132 | 373 ± 7 | 112 ± 6 | 1 ± 0.1 | 9 ± 21 | 200 ± 11 | 23 ± 2 | 6 ± 1 | [29] | |
| 9 ± 1 | 466 ± 40 | 182 ± 12 | 112 ± 6 | 1 ± 1 | 13 ± 1 | 92 ± 5 | 15 ± 7 | 2 ± 1 | [28] | |
| Freeze-dried pulp | 28.5 | 900 | 330 | 124.4 | 2.15 | 10.71 | 54.5 | 4.5 | 2.82 | [23] |
| <0.14 | 71.1 ± 0.3 | 379.4 ± 0.7 | 129.3 ± 0.1 | 1.62 ± 0.03 | 53.43 ± 0.02 | - | 6.05 ± 0.02 | 1.62 ± 0.01 | [42] | |
| Fatty Acids | Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [20] | [50] | [27] | [32] | [51] | [23] | [52] | [34] | [50] | |
| Pulp Oil (g/100 g) | Lyophilized Pulp Oil (g/100 g) | Seed Oil (g/100 g) | Insoluble Filter Residue Oil (g/100 g) | ||||||
| C12:0—Lauric | - | - | 0.07 | - | 2.9 | - | - | 8.74 | - |
| C14:0—Myristic | - | - | 0.13 | - | 4.6 | - | - | 22.86 | - |
| C16:0—Palmitic | 11.4 | 23.0 ± 0.1 | 26.18 | 11.3 | 16.1 | 25.56 | 21.89 ± 1.33 | 16.27 | 17.4 |
| C18:0—Stearic | 4.1 | 1.3 ± 0.0 | 1.81 | 1.81 | 3.2 | 1.84 | 1.86 ± 0.57 | 1.37 | 3.2 |
| Saturated fatty acids (SFA) | 15.5 | 24.3 | 28.3 | 13.11 | 26.8 | 27.4 | 23.75 | 49.24 | 20.6 |
| C16:1—Palmitoleic | 3.7 | 5.0 ± 0.1 | 4.88 | - | - | 3.54 | 3.06 ± 0.7 | 0.61 | 0.3 |
| C18:1—Oleic | 58.5 | 54.4 ± 0.2 | 52 | 37.4 | 51.3 | 52.7 | 62.45 ± 3.07 | 26.79 | 69.2 |
| C18:1 (cis 11)—Vaccenic acid | - | - | 3.45 | - | - | - | - | - | - |
| Monounsaturated (MUFA) | 62.2 | 59.4 | 60.33 | 37.4 | 51.3 | 56.24 | 62.453 | 27.4 | 69.5 |
| C18:2—Linoleic | 22.3 | 16.0 ± 0.0 | 7.28 | 44.6 | 14.6 | 0.95 | 10.26 ± 1.13 | 22.52 | 8.4 |
| C18:3—Linolenic | - | 0.8 ± 0.1 | 0.55 | 3.45 | - | - | 0.49 ± 0.13 | 0.84 | 1.1 |
| Polyunsaturated (PUFA) | 22.3 | 16.8 | 7.83 | 48.05 | 14.6 | 0.95 | 10.74 | 23.36 | 9.5 |
| Unsaturated fatty acids (UFAs) | 84.5 | 76.2 | 68.16 | 85.45 | 65.9 | 57.19 | 76.25 | 50.76 | 79 |
| PUFA/SFA | 1.44 | 0.69 | 0.27 | 3.66 | 0.54 | 0.03 | 0.45 | 0.46 | 0.46 |
| n-6/n-3 | - | 20 | 13.23 | 12.95 | - | - | 20.93 | 26.81 | 7.63 |
| Classification | Compounds | Sample Type | Source | |
|---|---|---|---|---|
| Anthocyanins | Cyanidin-3,5-hexoside-pentoside, Cyanidin 3-O-glucoside, Cyanidin 3-O-rutinoside, Pelargonidin 3-O-glucoside, Pelargonidin 3-O-rutinoside, Peonidin 3-O-glucoside, Peonidin 3-O-rutinoside, Delphinidin 3-O-rutinoside, Malvidin 3-O-glucoside, Cyanidin 3-O-sambubioside, cyanidin 3-(acetyl) hexose, petunidin 3-O-(6”-p-coumaroyl-glucoside) | Pulp, freeze-dried pulp, defatted freeze-dried pulp, defatted freeze-dried pulp flour, seeds | [21,25,58,59,60,61,63,64,65,66,67,68,69] | |
| Hydroxybenzoic acids and their conjugates | Protocatechuic acid hexoside, Protocatechuic acid, p-Hydroxybenzoic acid, Vanillic acid, Syringic acid, Gallic acid, 4-Hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, ellagic acid | Pulp, freeze-dried pulp, defatted freeze-dried pulp, insoluble pulp residue oil | [21,25,50,58,61,62,63,65] | |
| Hydroxycinnamic acids and their conjugates | Hydroxyferuloyl quinic acid, Synapoyl deoxyhexoside, Caffeoylquinic acid, 5-Caffeoylquinic acid, 4-Caffeoyl shikimic acid, p-Coumaroyl hexoside, Caffeic acid, Feruloyl sinapic acid isomer 1, Ferulic acid conjugate 1, Caffeoyl shikimic acid isomer 1, Feruloyl sinapic acid isomer 2, Caffeoyl shikimic acid isomer 2, Sinapoyl hexoside, Ferulic acid conjugate 2, p-coumaric acid, chlorogenic acid, feluric acid, p-Coumaric acid ethyl ester, p-Coumaric acid 4-O-glucoside. | Pulp, freeze-dried pulp, defatted freeze-dried pulp, defatted freeze-dried pulp flour, seeds, insoluble pulp residue oil | [21,25,50,59,61,62,63,65,66,68] | |
| non-anthocyanin flavonoids | Flavan-3-ols | (+) Catechin, (-) Epicatechin, (+)-Gallocatechin 3-O-gallate. | Pulp, freeze-dried pulp, seeds, freeze-dried seeds, insoluble pulp residue oil | [34,50,61,63,65,66,70] |
| Flavones | Orientin, Isovitexin, Homoorientin, Vitexin, Luteolin, Scoparin, Chrysoeriol, Chrysoeriol 7-O-glucoside, Apigenin di-glucoside, 6,8-di-C-hexosyl apigenin (vicenin-2), 6-C-glycosyl luteolin (isoorientin), 6-C-glycosyl apigenin (isovitexin), Rhamnocitrin, isoorientin, Luteolin 7-O-glucoside, luteolin di-glucoside, luteolin hexoside, velutin | Pulp, freeze-dried pulp, defatted freeze-dried pulp, defatted freeze-dried pulp flour, seeds | [21,25,58,59,61,64,65,66,68,70] | |
| Flavanones (Flavanonol) | Taxifolin deoxyhexose isomer 1, Taxifolin deoxyhexose isomer 2, taxifolin, Taxifolin 3-O-glucoside, Eriodictyol 7-O-glucoside I, Taxifolin deoxyhexose. | Pulp, freeze-dried pulp, defatted freeze-dried pulp, defatted freeze-dried pulp flour | [59,61,64,65,68] | |
| Flavonols | Rutin, Isorhamnetin rutinoside, Kaempferol rutinoside, Quercetin hexoside, Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside, Kaempferol rutinoside, Quercetin hexoside. | Pulp, freeze-dried pulp, defatted freeze-dried pulp, defatted freeze-dried pulp flour, seeds | [59,61,66,68] | |
| Dehydroflavonols | Dihydrokaempferol isomer 1, Dihydrokaempferol isomer 2, dihydrokaempferol-3-glucoside | Pulp, freeze-dried pulp | [58,61] | |
| Procyanidins | Procyanidin B1, Procyanidin B2, Procyanidin A, Procyanidin dimer, Procyanidin trimer, Procyanidin trimer C1, Procyanidin Dimer B1 | Pulp, freeze-dried pulp, seeds, freeze-dried seeds, insoluble pulp residue oil | [34,50,60,65,66,70] | |
| Carotenoids | β-carotene, α-carotene, lycopene, Lutein, Zeaxanthina, Zeinoxanthin | Pulp, freeze-dried pulp | [62,67] | |
| Phytosterols | Campesterol, Stigmasterol, b-Sitosterol + sitostanol, D5-Avenasterol +D7-stigmasterol | Pulp oil | [48] | |
| Tocopherols | α-Tocopherol | Pulp oil | ||
| Fatty acids and their conjugates | linolenic acid, 1,2-di-O-α-linolenoyl-3-O-β-D-galactopyranosyl-sn-glycerol. | Pulp | [58] | |
| Aminoacids and nucleosides | Valine, uridine, adenosine | Pulp | ||
| Organic acids and derivates | citric acid, Quinic acid, Malic acid, Tartaric acid, p-Coumaroyl tartaric acid, p-Coumaroyl malic acid. | Pulp, freeze-dried pulp | [58,62,68] | |
| Other compounds | Tachioside, isotachioside, guaiacylglycerol, syringylglycerol, dimethoxy-1,4-benzoquinone, koaburaside, eurycorymboside B, 7,8-dihydroxy dihydrodehydroconiferyl alcohol-9-O-β-D-glucopyranoside, isolariciresinol-9-O-β-D-glucopyranoside, 5-methoxyisolariciresinol-9-O-β-D-ucopyranoside, 9,12-octadecadienoic acid (Z,Z)-2-hydroxy-1-(hydroxymethyl) ethyl ester, Prodelphinidin trimer, 3,7-Dimethylquercetin, 5,4′ -Dihydroxy-7,3′,5′ -trimethoxyflavone. | Pulp, seeds | [58,66] | |
| Sample Type | Polyphenols, Flavonoids and Anthocyanins Contents | Antioxidant Capacity | Source | |||||
|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g DM) | TFC (mg GAE/g DM) | TAC (mg Cyn-glu/g DM) | DPPH | FRAP | ABTS | ORAC | ||
| Freeze-dried pulp | 65.1 | - | - | 4.2297 (μmol TE/g DM) | - | - | - | [22] |
| 28.6 | 5.46 | 2.93 | - | - | - | 897.60 (μmol TE/g DM) | [20] | |
| 14.05 ± 0.02 | - | 1.27 ± 0.06 | 41.5 ± 0.75 (% inhibition) | - | - | - | [24] | |
| 28.80 ± 4.39–58.83 ± 2.18 | - | 0.03 ± 0.00–0.11 ± 0.00 | - | - | - | - | [60] | |
| - | - | - | 17.86 ± 1.89–71.54 ± 0.69 (EC50 g pulp/g DPPH DM) | - | 923.11 ± 3.62–1413.44 ± 8.72 (μmol TE/g DM) | 409.78 ± 8.39–1842.22 ± 57.13 (μmol TE/g DM) | [21] | |
| 18.08 ± 0.28 | 6.72 ± 0.40 | 15.74 ± 1.01 (mg TE/g DM) | - | 13.67 ± 118 (mg AAE/g DM) | - | [71] | ||
| 31.2 ± 2.6 | - | - | 133.4 ± 11.2 (μmol TE/g DM) | - | - | 1014.0 (μmol TE/g DM) | [72] | |
| 34.37 ± 1.54 | - | - | - | - | 2.78 ± 0.10 (μmol TE/100 g DM) | 24.0 (EC50 mg DM/100 mL) | [25] | |
| - | 4 | 0.5 | - | - | - | - | [64] | |
| 28.55 ± 2.77 | 12.7 ± 1.26 | 6.98 ± 1.89 | 4264 ± 1381 (EC50 g pulp/g DPPH FM) | 32.1 ± 6.5 (μmol Fe2SO4/g) | 15.1 ± 4.1 (μmol TE/g) | - | [46] | |
| 33.2–43.46 | 11.57–17.48 | 4.59–8.99 | - | - | - | - | [47] | |
| 77.51 ± 0.54 | - | 6.94 ± 0.02 | 289.08 ± 6.83 (µmol TE/g DM) | 500.35 ± 8.66 (μmol Fe2SO4/g DM) | 385.92 ± 8.90 (µmol TE/g DM) | - | [26] | |
| 15.23 | - | - | - | - | 16 (µmol TE/g DM) | - | [62] | |
| Pulp | 61.66 ± 0.06 | - | - | 168.89 ± 6.03 (μmol TE/g DM) | - | 348.40 ± 2.44 (μmol TE/g DM) | - | [68] |
| 19.62 ± 0.13–36.67 ± 0.18 | - | - | 1986.66 ± 87.41–3167.14 ± 217.18 (EC50 g pulp/g DPPH FM) | 24.69 ± 1.90–74.34 ± 1.47 (μmol Fe2SO4/g FM) | 11.49 ± 0.13–55.05 ± 2.83 (μmol TE/g FM) | - | [43] | |
| 47.86 ± 18.80 | - | 4.58 ± 3.28 | 210.49 ± 30.71 (μmol TE/g DM) | - | 24.7 ± 10.6 (μmol TE/100 g DM) | - | [61] | |
| 35.09 | - | 2.7 | - | - | - | - | [73] | |
| 4.34 ± 0.1 | 3.52 ± 0.05 | 0.62 | 67.92 ± 0.89 (% inhibition) | 32,177.78 ± 1673.76 (μmol TE/kg) | - | - | [74] | |
| 50 ± 1 | - | 7.3 ± 1.0 | 88.03 ± 0.32 (% inhibition) | - | - | - | [29] | |
| - | - | 2.06 ± 0.083 | - | - | - | 87.4 ± 4.4 (μmol TE/g) | [65] | |
| - | - | 17.74–19.06 | - | - | - | - | [69] | |
| - | - | - | - | - | - | 48.6 (µmol TE/mL) | [63] | |
| Defatted pulp | 22 ± 2 | - | 16.0 ± 2.0 | 87.82 ± 0.20 (% inhibition) | - | - | - | [28] |
| Defatted freeze-dried pulp | 96.43 ± 0.46–150.20 ± 1.32 | - | 13.24 ± 0.55–13.84 ± 0.64 | 362.71 ± 5.07–414.99 ± 5.02 (µmol TE/g DM) | 653.13 ± 9.97–746.25 ± 3.82 (μmol Fe2SO4/g DM) | 554.53 ± 7.68–644.23 ± 7.23 (µmol TE/g DM) | - | [26] |
| Defatted freeze-dried pulp flour | 88.4 ± 0.4 | - | - | - | 986.0 ± 22.0 (μmol Fe2SO4/g DM) | 820.0 ± 36.4 (μmol TE/g) | 975.7 ± 69.0 (μmol TE/g) | [59] |
| Defatted pulp flour | 16 ± 3.2 | - | 9.6 ± 2.2 | 79.97 ± 0.04 (% inhibition) | - | - | - | [29] |
| freeze-dried filtrate residues | 1.6 | 0.82 | - | - | - | - | 78.70 (μmol TE/g DM) | [20] |
| Seed flour and filtrate residues | 1.14 | - | - | 84.6 (EC50 μg/mL) | - | - | - | [33] |
| Peel | 1.32 | - | - | 39.81 (μmol TE/100 g DM) | - | - | - | [22] |
| Seeds | 4.37 | - | - | 65.92 (μmol TE/100 g DM) | - | - | - | |
| 17.7 | 2.42 | - | - | - | - | 652.63 (μmol TE/g DM) | [20] | |
| - | - | - | 16.95 ± 0.18 (EC50 mg/L) | - | 3835.44 ± 73.50 (μmol TE/g) | 4082.16 ± 58.55 (μmol TE/g) | [70] | |
| Freeze-dried seeds | 64.58 ± 1.89 | - | - | 622.81 ± 67.56 (μmol TE/g DM) | - | 763.09 ± 17.27 (μmol TE/g) | - | [34] |
| 6.88 ± 1.11–9.94 ± 0.76 | - | 0.11 ± 0.00–0.17 ± 0.00 | - | - | - | - | [60] | |
| Berry | - | - | 1.44 mg Cyn-glu/g FM | - | - | - | - | [75] |
| 33.20 ± 2.18–51.50 ± 2.17 | 28.84 ± 3.19–33.03 ± 0.39 | 90.16 ± 1.53–99.59 ± 0.65 | - | 315.43 ± 4.96–430.94 ± 9.23 (µmol TE/g) | 402.41 ± 10.38–463.22 ± 24.92 (µmol TE/g) | - | [37] | |
| Pulp oil | - | - | - | 2.02 ± 0.07–2.55 ± 0.14 (µmol TE/g DM) | 5.50 ± 9.97–15.25 ± 1.69 (μmol Fe2SO4/g DM) | 2.05 ± 0.04–2.60 ± 0.05 (µmol TE/g DM) | - | [26] |
| Insoluble pulp residue oil | 1.25 ± 0.042 | - | - | - | - | - | 21.5 ± 1.7 (μmol TE/mL) | [50] |
| Raw Material | Animal Species | Use Specifications | Results | Source |
|---|---|---|---|---|
| Freeze-dried Naidí (FdN) | White shrimp (Litopenaeus vannamei) | (Postlarvae) Inclusion of 5–80 mg/L in the culture water, every 24 h for 27 days. Feeding 3 times/day with commercial feed. | Productive: ↑ WG, Su y ↓ FCR at 20 mg/L concentration. immunity: modulation of antioxidant capacities (↓ ACAP, ↔ LPO, ↓ GSH y ↑ protein integrity) in shrimps. | [85] |
| (Juveniles) Inclusion of 10% FdN, 2 times/day for 35 days. | Productive: WG, SGR, Su, FCR, PER (↔) did not differ from the control, 85% replacement of fish oil. Immunitary: ↑ of polyphenol content in the hepatopancreas, ↑ antioxidant capacity in the hepatopancreas and muscle (↓ LPO, ↑ GSH, ↑ GST, ↓ of histopathological changes). Economic: potential improved shelf life of the final products. | [84] | ||
| (Juveniles) Inclusion of 2.5–10% FdN, 2 times/day for 43 days. | Productive: ↔ productive parameters with respect to the control diet, considering the complete substitution of fish oil with the inclusion of 10%. ↑ flavonoids in diets and bioflocs at inclusion levels of 5 and 10%. ↑ in the reddish coloration of fresh and cooked shrimp. Economic: added value of products due to higher coloring. Environmental: alternative to fish oil use. Immunity: ↑ flavonoids in the gills at higher inclusion levels. | [31] | ||
| (Juveniles) Inclusion of 10% FdN, 2 times/day for 30 days. | Productive: zootechnical parameters between treatments (↔). Economic: ↑ added value due to reddening of cooked shrimp Immunity: ↑ of the antioxidant capacity. In the gills (↑ GST), in the muscle and hepatopancreas (↓ LPO). | [77] | ||
| Inclusion of 5–80 mg/L FdN in culture water, every 24 h for 30 days. The shrimp were fed commercial feed twice daily. | Productive: ↑ in muscle protein content and shrimp survival in the 5 mg/L treatment. Immunitary: ↓ of lipidic peroxidation in gills and hepatopancreas. 20 mg/L dose had the best performance for biochemical and hystologic parameters (↑ height and area of intestinal microvilli). Higher doses led to prooxidant effects. | [86] | ||
| Inclusion of 10% FdN, 2 times/day during 30 days. | Economic and Environmental: Replace fish oil with an alternative raw material that is a substitute for traditional ingredients. Immunitary: ↑ of the antioxidant capacity in hepatopancreas and gills (↑ GSH) y ↓ LPO in the muscle. | [56] | ||
| Juvenile black cachama (Colossoma macropomum) | Inclusion of 6.3–100 g/kg FdN in the concentrate, 4 times/day during 30 days | Productive: ↑ in growing yield (↑WG, ↑LT, ↑SGR) y ↑ in feed utilization indexes (↑FCR, ↑PER, ↓FC) in treatments with 50 y 100 gr FdN, with regard to control. Economic: ↑ in cyan coloration of dorsal region and ↓ triglycerides and cholesterol (hypolipidemic effect). Both can add value in the market. Immunity: ↔ bioactive compound content and antioxidant capacity in carcasses between treatments. ↑ACAP in intestine and a possible ↓ LPO in the same organ. The concentration of 50 g/kg showed the best performance in terms of zootechnical, antioxidant, metabolic and skin coloration parameters in juveniles. | [30] | |
| Inclusion of 6.3–100 g/kg FdN in the concentrate, 4 times/day during 30 days | Economic: addition, as a supplement to mitigate the stressful effect of transport and its aftermath, contributing to survival. Immunity: ↑ ACAP in the liver in the 12.5–100 g treatments for up to 12 h transport time. The addition of 6.3 g/kg was effective for ↓ LPO in liver. 50–100 g/kg keeps ↓ LPO in brain, gills and liver after log transportation. It is recommended to supplement with 50–100 g/kg of FdN for juvenile transport for up to 12 h. | [52] | ||
| Essential oil of naidí (EON) | Ornamental fishes: Pterophyllum scalare and Heros severus | Inclusion of 0.5–2% EON for H. severus and 0.5–4% EON for P. scalare, 4 times/day during 30 days. | Productive: The inclusion of up to 2.48% for P. scalare and 0.88% for H. severus of EON, ↑ WG, ↑ SGR y ↑ lot uniformity for weight. Batch uniformity parameters for length and survival rate had no differences (↔). | [51] |
| Crushed naidí seed (CNS) | Mares (no breed information) | CNS replacement of 18.75–75% of mombasa grass (dry matter). 80% forage and 20% concentrate, 2 times/day, 85 days. | Productive: ↑ Dry matter intake and digestibility of nutrients, such as neutral detergent fiber and total carbohydrates, are reduced. The physicochemical characteristics of the feed reduce feeding time. Economic and environmental: They are an economical, abundant source that reduces environmental pollution. | [35] |
| Naidí oil (NOi) | Lactating ewes (Lacaune) | Inclusion of 2% during 14 days. | Productive: ↑ milk production and production efficiency, ↓ milk fat content, ↔ lactose and protein content. | [32,33] |
| Economic: ↑ production and quality of products, generation of added value (nutraceuticals and shelf life). Immunity: ↑ ACAP y ↓ LPO in whey and milk, ↓ serum leukocyte count, blood glucose and globulin concentrations, ↓ de triglycerides and urea. | ||||
| Naidí residues flour (NRF) | Broiler chicks (Cobb 500) | Inclusion of 2% NRF, ad libitum for 20 days. | Productive: ↑ WG y CA, ↓ FCR. Environmental and economic: Use of agroindustrial residues as feed. immunity: ↑ serum albumin, ↓ AST y ↑ hepatic catalase (hepatoprotection), ↑ The intestinal villi/intestinal cells ratio promotes intestinal health and function. | [33] |
| Naidí seed bran without mesocarp (NSB) | Slow-growing chicks (French Red-Naked Neck) | Inclusion of 2–10% NSB, ad libitum during 28 days. | Productive: ↔ WG and viability with respect to control ↓ FCR and FC with respect to the other treatments. Economic: ↔ with respect to control (feasible alternative). Environmental: potentially positive impact from the use of waste as feed. | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavarro-Parra, E.J.; Hincapié, C.A.; Hincapié-Llanos, G.A.; Osorio, M.; Gañán-Rojo, P. Naidí (Euterpe oleracea Mart.), a Colombian Pacific Fruit with Potential Use in Animal Feed: A Systematic Review. Resources 2025, 14, 161. https://doi.org/10.3390/resources14100161
Chavarro-Parra EJ, Hincapié CA, Hincapié-Llanos GA, Osorio M, Gañán-Rojo P. Naidí (Euterpe oleracea Mart.), a Colombian Pacific Fruit with Potential Use in Animal Feed: A Systematic Review. Resources. 2025; 14(10):161. https://doi.org/10.3390/resources14100161
Chicago/Turabian StyleChavarro-Parra, Eduardo J., Carlos A. Hincapié, Gustavo Adolfo Hincapié-Llanos, Marisol Osorio, and Piedad Gañán-Rojo. 2025. "Naidí (Euterpe oleracea Mart.), a Colombian Pacific Fruit with Potential Use in Animal Feed: A Systematic Review" Resources 14, no. 10: 161. https://doi.org/10.3390/resources14100161
APA StyleChavarro-Parra, E. J., Hincapié, C. A., Hincapié-Llanos, G. A., Osorio, M., & Gañán-Rojo, P. (2025). Naidí (Euterpe oleracea Mart.), a Colombian Pacific Fruit with Potential Use in Animal Feed: A Systematic Review. Resources, 14(10), 161. https://doi.org/10.3390/resources14100161







