Abstract
The purpose of this study was to evaluate the production of biogas and the digestate obtained by means of the anaerobic digestion of sargassum, and its anaerobic co-digestion with municipal solid waste, while considering the effect of particle size and the carbon–nitrogen ratio (C:N) on methane generation. Physicochemical analyses of both Sargassum and the digestate were performed, including ultimate analysis and heavy metal content. The highest methane yield (92.62 mL CH4/gVS) was achieved with a 2 mm particle size and a C:N ratio of 15. Digestate characterization revealed the presence of arsenic and zinc, indicating the need for additional treatment before agricultural use. The agronomic potential of Sargassum digestate was assessed by comparing it with livestock waste, humus, and garden soil in tomato seedling growth trials. The Sargassum-based digestate significantly enhanced seedling biomass and development, supporting its potential as a sustainable soil amendment. Overall, the findings confirm the viability of Sargassum as a feedstock for biogas and biofertilizer production, emphasizing the importance of contaminant monitoring to ensure environmental safety. This study supports the integration of Sargassum into circular economy strategies and regenerative agricultural systems.
1. Introduction
When tackling organic waste management, anaerobic digestion (AD) is a strategic solution with two clear advantages: the generation of biogas as a renewable energy source and the production of digestate, a valuable subproduct with great potential for promoting sustainability within agriculture. It was discovered that the performance and efficiency of AD are determined by operational parameters such as pH, temperature, organic load rate, hydraulic retention time, and pressure [1]. Furthermore, the monitoring of factors like volatile fatty acids, the availability of micro and essential trace elements, as well as maintaining strict anaerobic conditions is essential for both the stability of the process and optimal biogas production [2]. The carbon–nitrogen ratio (C:N) is a key factor, as its balance promotes microbial activity and optimizes biogas production, whereas imbalances in this ratio can limit like volatile fatty acids generation and hinder the efficiency of the process [3,4]. Raw material particle size also has a significant influence on AD, making it an important factor that must be considered. Any reduction in particle size increases the surface area available for microbial action, thereby favouring hydrolysis and improving methane production [5,6,7,8,9,10,11]. It has been noted that a reduction in the particle size of rice straw increases methane performance from 107 mL/gVS to 197 mL/gVS due to greater decomposition of the cellulose [8,9]. Likewise, food waste ground into fine particles has shown greater methane performance and rates [10,11]. However, the optimal size does vary depending on the substrate. For certain waste, a size of 25 µm gave rise to a methane production increase of between 3 and 30% compared to larger particles [5]. In fruit and vegetable waste, the optimum size was 742.3 µm [12], whereas in other studies, particles smaller than 2 mm increased methane production by 19% [13] and sizes of 2.2 μm showed the greatest production rate [7].
The residual digestate of AD comprises two main fractions: liquid and solid. The liquid fraction is highlighted by its high content in nutrients that are easily available to plants and rapidly absorbed. Studies have shown that liquid digestate contains elevated concentrations of organic material, nutrients and ions, with particle sizes ranging from dissolved material to large suspended particles [14,15]. It has been reported that between 60 and 96% of organic material is found in the form of suspended particles > 1.2 μm, whereas nitrogen is mostly associated with particles < 100 μm and potassium is found in a dissolved state [14]. On the other hand, the solid fraction of the digestate provides organic material that improves soil structure and promotes root development. It contains essential nutrients such as nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg), which are essential for vegetable growth and soil fertility [16,17,18,19,20]. In comparison to undigested manure, the digestate shows a greater available nitrogen content, which improves crop growth and has the potential to partially replace mineral nitrogen fertilizers. However, due to its low mineralization rate, complementing it with mineral nitrogen may be necessary in order to optimize its effectiveness [21,22]. The use of digestate increases the organic material of the soil, improving its long-term structure and fertility [18,19,23]. Likewise, it stimulates microbial activity, which is essential for nutrient cycling [16,17,20,24,25]. Furthermore, it promotes microbial diversity and the growth of beneficial microbial communities, including those capable of decomposing contaminants such as hydrocarbons [24,25]. Several studies have shown that the digestate influences the chemical properties of the soil by modifying its pH and increasing its electrical conductivity, which in turn affects the availability of nutrients. It also contributes to the increase in total organic carbon and mobile humic acids, favouring the soil’s long-term stability and fertility [18,19,22,23].
The application of digestate was proven to be so effective that in some cases it even exceeded mineral fertilizers in terms of crop performance, by improving fruit quality with its greater content of bioactive compounds such as phenols and flavonoids [16,23]. Furthermore, it stimulates the development of above-ground and root biomass, which reflects healthy plant growth [17,18,23]. Its use also reduces dependency on chemical fertilizers, favouring a circular economy by recycling organic waste as agricultural supplies and contributing to the mitigation of greenhouse gas emissions like nitrous oxide [26,27,28,29]. Despite its recognized potential as a fertilizer, the digestate’s composition varies widely depending on the raw materials used and the operating conditions of the AD, which can lead to the presence of contaminants such as pathogens, heavy metals, and other potentially hazardous substances [30,31]. Therefore, its application must be suitably managed in order to maximize its agronomic benefits and minimize any possible negative environmental impacts [32,33]. Some associated environmental risks are the greater potential for ammonia emission in comparison to undigested manure and the accumulation of heavy metals in the soil [34]. To optimize the use of digestate it is essential to establish a personalized fertilization plan that considers both the soil’s characteristics and the crop’s needs, to ensure a suitable balance of nutrients and reduce any environmental risks [35]. The effectiveness of digestate as a fertilizer depends on the weather conditions, as well as soil characteristics such as the pH and organic matter content [16,23,36]. Microbial activity is reduced in cold climates, where temperatures below 15 °C can limit the availability of nitrogen to winter crops. Its application in conjunction with biodegradable wadding gives rise to synergistic effects on the quality and performance of the fruit, whereas in organic agricultural systems, its combination with biochar increases the productivity and quality of tomatoes [37].
The liquid and solid fractions of the digestate were shown to improve plant growth and strengthen the nutritional quality of crops like tomatoes by increasing their levels of vitamin C, flavonoids, phenolic compounds, and antioxidant activity [31,36]. Furthermore, its combination with mineral fertilizers was shown to be an effective substrate for tomato plant seedlings, with the best results obtained using a mixture of solid digestate, vermiculite, pearlite, and activated carbon at a ratio of 16:1:2:1 [38]. Furthermore, the integration of the digestate with reduced rates of phosphatic and nitrogenous fertilizers either maintains or improves the performance of the tomatoes in comparison to complete chemical fertilization [39]. Tests carried out in fields and greenhouses indicate that the digestate and compost can match or even exceed synthetic fertilizers, improving antioxidant activity and the concentration of bioactive compounds in tomatoes [30,31]. Likewise, the use of digestate in fertigation systems via subsurface dripping optimizes the supply of nutrients and increases both the productivity and quality of the crops [40]. In open fields, the application of diluted digestate coming from manure and food waste has significantly improved tomato growth [41]. However, its effectiveness varies depending on the kind of digestate, the application method, and the growing conditions.
Meanwhile, since 2011, the spread of the Great Atlantic Sargassum Belt generates yearly washed-up deposits of sargassum that can last up to nine months. In the Central and Western Caribbean these build ups reach tens of thousands of cubic metres per kilometre each year. These macroalgae can transport contaminants, pathogenic agents, and exotic species to coastal areas. During its decomposition it releases toxic gases and leachate that present significant risk to human health and other life forms [42]. The AD of this kind of macroalgae has awoken growing interest due its potential for energy recovery and efficient biomass management, highlighting itself as a viable alternative for biomethane production [43,44,45]. However, it was discovered that the mono-digestion of sargassum shows low biomethane performance (79.68 mL/gVS), whereas its co-digestion with organic municipal solid waste substantially improves this production (up to 327.27 mL/gVS) and shortens reaction lag times [44,46]. This proves its potential as a substrate for AD and the need for suitable digestate management, something which is key to the sustainability of biogas systems. This becomes relevant in the context of an increase in the installation of plants aimed at giving value to biomass, which has considerably increased the generation of digestate [47]. The absence of efficient management of the accumulation of this digestate could threaten both the environmental and financial viability of this process, and limit its contribution to sustainable development [48,49].
Therefore, it is essential to have sustainable alternatives for its use, allowing not only for AD to be consolidated as a source of renewable energy, but also closing productive cycles by efficiently using resources and mitigating any environmental impacts. The digestate derived from the AD of macroalgae like sargassum has not been widely studied in comparison to conventional agricultural substrates in crops with high commercial value like Solanum lycopersicum. This limitation in the literature hinders the understanding of its potential as a sustainable agricultural amendment. This study evaluates the generation of biogas and digestate from the AD and anaerobic co-digestion (ACoD) of sargassum with an organic fraction of municipal solid waste (OFMSW). The effect of particle size and the C:N ratio on the production of methane was analyzed under controlled laboratory conditions, and both the sargassum and its digestate were physicochemically characterized. Subsequently, its performance was compared with conventional agricultural substrates such as livestock excrement, humus, and garden soil in the growth of tomato plant seedlings under nursery conditions. Parameters for crop development were evaluated, such as number of leaves, biomass, root length, and fresh weight. The findings of this research provide key information for the use of sargassum digestate as an alternative to traditional agricultural substrates, promoting the implementation of AD as a strategy for biomethane production and giving value to organic waste as part of sustainable agriculture.
2. Materials and Methods
2.1. Sargassum Sample
The collection of raw material was carried out along the north coast of Quintana Roo, at Punta Brava beach at Puerto Morelos from the 27–30 April 2021. The macroalgae was placed on plastic grilles, shaken to remove any remaining sand, and then packed into black plastic bags (polyethylene) until around 10 kg was collected. Then, the samples reduction method was carried out according to standard practice ASTM C702/C702M–18 [50]. The collected biomass was washed with distilled water to remove any remaining sand and placed into an ECOSHEL incubator/oven (Ecoshel, Denver, CO, USA) at 70 °C for 24 h to minimize any humidity present. It was then stored in polyethylene bags for subsequent use in the AD.
The organic solid waste was collected during the month of November from household waste in order to obtain a synthetic blend, a familiar composition of waste from different fruit and vegetables, to be subsequently co-digested with the sargassum (Table 1). The waste was washed with distilled water and dried in an ECOSHEL incubator/oven for 24 h and the peels were stored separately in air-tight sealed bags. The composition of the synthetic OFMSW used in this investigation is shown in Table 1.

Table 1.
Composition of the synthetic organic fraction of kitchen waste in dry weight.
2.2. Biomass Characterization
The following physicochemical analyses were carried out on the biomasses under study:
Determination of elements C, H, N, and S (ultimate analysis): The elements carbon (C), hydrogen (H), nitrogen (N), and sulphur (S) present in the digestate were quantified using a Thermoscientific Flash Smart chromatographic analyzer (Thermo Fisher Scientific, Waltham, MA, USA). This device is equipped with a Porapak PQS column and molecular sieve, using helium as the carrier gas (flow rate: 90 mL/min). The results obtained enabled the carbon–nitrogen ratios (C:N) to be determined, which are essential for the design and operation of the anaerobic reactors used in the digestion.
Determination of metals and metaloids: In order to identify the presence and concentration of metals and metaloids in the biomass, inductively coupled plasma optical emission spectroscopy (ICP-OES) was used, using a Thermoscientific device model ICAP 6500 (Thermo Fisher Scientific, UK), operated at a plasma power of 1150 W, with argon as the plasma gas. This analysis is relevant due to the possible presence of potentially toxic elements that could affect the quality of the substrate or generate environmental risks.
The determination of volatile solids (VS) of the sargassum samples was carried out using an STA 2500 Regulus Simultaneous Thermal Analyzer (Selb, Germany), with helium as a carrier gas and a heating rate of 10 K/min according to the standard of ASTM E1131 [51]. The data obtained was used to determine the inoculum–substrate ratio in the design of the anaerobic reactors.
The percentage humidity of the digestate was determined by weighing 5 g of the sample in a watch glass, using a Velab VE-204 analytical balance (Velab, Puebla, Mexico) for initial weight determination. Subsequently, the sample was dried in an Ecoshel oven/incubator (Ecoshel, Mexico) at a constant temperature of between 110 and 120 °C for one hour. After drying, it was left to cool for 10 min and weighed once more (final weight). This analysis is crucial for determining the storage and handling conditions of the digestate as a substrate.
The pH analysis of the digestate was performed by dissolving 1 g of sample in 10 mL of distilled water. The mixture was homogenized using an IKA C-MAG HS 7 magnetic stirrer (IKA, Staufen, Germany) and the pH was measured using a calibrated Hanna HI2211 potentiometer (Hanna Instruments, Woonsocket, RI, USA) previously calibrated with standard buffers (pH 4.0, 7.0, and 10.0). The measurement of this parameter is essential in determining the digestate’s suitability as a culture medium and its impact on the availability of nutrients.
2.3. Experiment: AD and ACoD of Sargassum
The adjustments to the carbon–nitrogen ratios (weight/weight) in the anaerobic digestors were carried out by creating a mixture. The proportions and components of the synthetic OFMSW for the ACoD and the mono-digestion of the sargassum are shown in Table 2.

Table 2.
Conditions for the content of AD or ACoD reactors.
The AD of sargassum and its ACoD with OFMSW were carried out in batch digestors using bottles of HDPE Nalgene 0.25 L, with a total load of 0.2 L, sealed with lids/PTFE septum/silicon (0.006 inches in thickness). The inoculum used was activated sludge from the South wastewater treatment plant of Chihuahua city that had been previously acclimatized to the digestion of sargassum, in an inoculum–substrate ratio of 2. The substrates were sargassum samples oven dried at 70 °C for 24 h, which were subsequently shredded and passed through three sieve sizes (5 mm, 2 mm, and 0.600 mm). A 3 × 3 experimental design was defined, giving a total of nine combinations with three replicas and a total of 27 biodigesters, which were identified as DS5, DS2, DS0.6, CDSF5, CDSF2, CDSF0.6, CDSF2.5, CDSF2.2, and CDSF2.06. Furthermore, three reactors were managed as a control or IC (inoculum control), which only contains inoculum. They were maintained at a temperature of 37 ± 5 °C in the Ecoshel oven/incubator for a 30-day period. Before closing the reactor, the pH of the mixture was measured using a Hanna potentiometer.
Based on the elemental chemical composition, the stoichiometric methane production of the digestors was estimated using the Buswell equation [52].
Equation (1)—Buswell equation, where n, a, b and c are the stoichiometric coefficients C, H, O, and N present in the biomass.
In order to quantify the methane produced for each reactor with different particle sizes, the biogas pressure was read before and after the measurements using a pressure sensor in the digestor. Subsequently, the biogas volume was measured using the volumetric displacement method that consisted of using an inverted test tube filled with water attached to a universal support inside a container of water. Using an intravenous infuser device, one end of the hose was placed inside the test tube and the other end, with a needle, was passed through the digester septum to transfer the gas. The daily biogas production was measured in cm3, and these data were used to establish a linear regression model describing the relationship between the final pressure and the purged volume. For the reactor operated with a particle size of 5 mm, the model obtained was y = 1.5849X − 3.4537 (R2 = 0.977), while for the reactor with a particle size of 2 mm, the model was y = 1.6042X − 4.891 (R2 = 0.985). In these equations, X represents the final pressure and y the corresponding purged volume. In contrast, methane production was not achieved in the reactor with a particle size of 0.600 mm, and therefore, this reactor was only considered for the estimation of initial system volumes. Graphs for linear regression are shown in the Supplementary Materials (Figures S1 and S2).
Methane in the biogas was quantified using a gas chromatograph device micro-GC fusion (INFICON, East Syracuse, New York, NY, USA) with a Molsieve 5A column and TDC detector, using helium as the carrier gas.
2.4. Experiment: Tomato Seedling Development
As part of the experimental phase, the tomato seeds (Solanum lycopersicum L.) were planted in individual pots with garden soil to ensure uniform initial germination conditions. Subsequently, five plant groups were established, with each one subjected to different treatments, as shown in Table 3. The digestate of the AD used was obtained from a 40-Litre reactor using the adapted inoculum and sargassum mono-digestion.

Table 3.
Experimental treatment for seedling development.
After the germination phase of 20 days, the plants of the five groups were individually transplanted into pots with approximately 2 kg of soil, maintaining the experimental groups with three repetitions each. The corresponding fertilizers were applied to each group a week after transplant, while the control group remained unfertilized, in order to continue with the experiment under the same conditions. The plants were maintained with constant conditions of light and were watered twice a week. They were monitored daily for 45 days to ensure consistency of the experimental variables and prevent external interferences that could affect the results. Once the tomato plants began to show general signs of growth, data was collected daily. The variables observed were the height of the plants and number of leaves. At the end of the experiment, the final measurements were taken for the root size, humid weight, and final stem size. This data was recorded systematically in two databases for later analysis: one database for the measurements of daily accumulated growth and the emergence of leaves, and another database for the final measurements of root size, humid weight and final size.
2.5. Statistical Analysis
The data collected was analyzed using software Jamovi 2.3.28, which enabled a statistical analysis of the results to be carried out. Software was used to compare the experimental groups of particle size and C:N in the AD, and in the agricultural experiment for the digestate, the groups of liquid fertilizer made from sargassum digestate, earthworm humus, solid fertilizer made from sargassum, and fertilizer from cow and horse digestate. A significance level of 95% was used in order to identify the differences between the treatments, i.e., α = 0.05. In order to evaluate the results of the comparison studies, an analysis of variance (ANOVA) was carried out as the main statistical tool. This approach enabled the significant differences between the applied treatments to be determined. ANOVA was selected for its robustness when comparing the means of several groups under controlled conditions, and provides a rigorous statistical analysis of the differences observed. Before using ANOVA, Shapiro–Wilk and Kolmogorov–Smirnov tests were carried out to verify the normality of the populations. Depending on the normality results, three non-parametrical Kruskal–Wallis ANOVAs were applied and two traditional ANOVAs were carried out for variables complying with the parametric assumptions. Furthermore, post hoc tests were carried out to identify specific comparisons between the groups, thereby ensuring a detailed and precise interpretation of the experimental results.
3. Results and Discussion
3.1. Biomass Characterization
The sargassum collected at Punta Brava beach located in Puerto Morelos belongs to a group of brown algae (Phaeophyceae), mainly consisting of the Sargassum natans and Sargassum fluitans species. According to Thompson et al. (2021) Sargassum natans represents between 80 and 90%, whereas Sargassum fluitans comprises between 10 and 20%. It was identified that both species arrived in the coastal area of Puerto Morelos during the spring and summer seasons [53].
Regarding the physicochemical characterization, Table 4 shows the values for C, N, H, O, and S obtained in the elemental analysis of Sargassum spp., the inoculum, the OFMSW, and the mean of the digestate. This information is crucial for the design and optimization of anaerobic reactors, as it enables the carbon–nitrogen ratio (C:N) of the substrates to be calculated and adjusted, either individually or in unison. However, the low nitrogen concentration in the sargassum’s composition could limit the generation of new bacterial cells due to its accelerated consumption. This could cause inhibition in the reactors if sargassum is used alone, without the addition of complementary substrates or inoculum. This would result in a significant decrease in biogas production. Furthermore, the presence of sulphur in the sargassum indicates that the biogas generated would contain hydrogen sulphide (H2S), which could impact the pH of the system, directly inhibit methane production, and in this manner affect the general efficiency of the AD process itself.

Table 4.
Analysis of sargassum elements, OFMSW, and inoculum.
The values of the elemental analysis are similar in some aspects, according to the findings of authors Farghali et al. (2021) and Thompson et al. (2020), where a low nitrogen content and the presence of sulphur is reported [54,55] (values per particle size can be seen in the Supplementary Materials, Table S1).
The elemental characterization of the recovered digestate was determined as percentages, 30 days after treatment by AD. Table 5 shows the results for carbon and nitrogen for the digestate, with comparisons between particles.

Table 5.
Characteristics of the digestate resulting from the anaerobic digestion of sargassum.
The results indicate that the digestate obtained from smaller particles displayed a greater nitrogen content compared to larger particle sizes, which suggests greater degradability of finer material. However, despite the differences in load of the digesters and the initial adjustments in the C:N ratios, no significant variations in the percentages of carbon or nitrogen were observed in the samples.
During AD, the carbon is used as the main energy source and for the synthesis of metabolites, which gives rise to the production of both methane (CH4) and carbon dioxide (CO2), whereas the nitrogen is used in microbial growth. Although no substantial variations were seen between treatments in the concentrations of carbon and nitrogen in the digestate, the accumulation of biogas did depend on the size of the processed particle (Figure 1). This difference may be explained by the lesser physical availability of the carbon and nitrogen in larger sized particles, which reduces their use by microorganisms, resulting in lower methane production. Furthermore, if the structure of the particle collapses, this can hinder the freeing of gases and generate conditions of microbial intoxication that slow down metabolism.
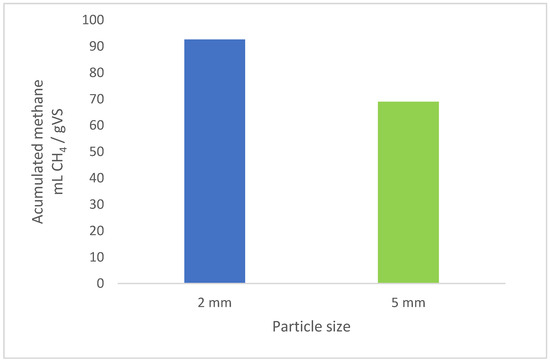
Figure 1.
Effect of particle size on anaerobic digestion of Sargassum spp., represented by the accumulated methane (2 mm, blue; 5 mm, green).
In this study, a fixed composition of synthetic OFMSW was selected to ensure experimental reproducibility and facilitate a controlled evaluation of the digestion process. However, in real conditions, the composition of OFMSW is highly variable, depending on seasonality, dietary habits, and regional waste management practices. Such variability can influence the C:N ratio, nutrient balance, and the presence of inhibitory compounds, ultimately affecting methane yield and process stability. Future studies should therefore investigate the effects of different OFMSW compositions on anaerobic digestion performance, in order to assess the robustness and adaptability of the process under practical scenarios.
3.2. Analysis of Metals and Metaloids in the Digestate
Table 6 shows the analysis of metals and metalloids detected in the substrates used: Sargassum spp., OFMSW inoculum, and mean for digestate. As previously noted, the sargassum species possess a remarkable ability to absorb metals, which implies that these elements interact on diverse levels during the AD processes. The influence of these will depend on their concentration and availability in the medium.

Table 6.
Metal analysis for the biomasses under study.
Elements such as phosphorus and potassium can be released during the system’s liquid phase via the hydrolysis processes, contributing to the maintenance of a buffer effect in the reactors. Furthermore, magnesium and sodium are usually found in the form of dissolved salts, and their concentrations are particularly high in the inoculum. Likewise, calcium, which is found in greater concentrations in the sargassum and OFMSW, can be released during the biochemical reactions and join the sodium, thereby altering osmotic pressure and affecting microbial viability.
This investigation did not detect any of the following elements: V, Cr, Co, Ni, Cd, Hg, and Pb. The Figure S3 in Supplementary Materials shows the zinc (Zn) concentrations detected in the digestate obtained from different C:N ratios. During the AD process, the heavy metals are released from the solid substrate towards the solid and liquid phases of the digestate. The results indicate that the larger particles (5 mm) displayed greater Zn concentrations in both digestates (zinc and arsenic values per particle size can be seen in the Supplementary Materials, Figures S3 and S4).
This was most evident in the digestate with an initial C:N ratio of 15, where a greater amount of Zn was accumulated at the end of the AD. In the other digestate analyzed, the Zn concentrations oscillated between 6.30 mg/kg and 16.03 mg/kg. These concentrations are found to be within the limits established by national standards such as NTEA-006-SMA-RS-2006 [56] and NOM-004-SEMARNAT-2002 [57], ensuring that the Zn present in the digestate does not present any risk for its application in agricultural soil. Within the suitable concentrations, Zn is an essential microelement for soil microorganisms, playing an important role in the biological and biochemical processes related to soil metabolism.
Furthermore, the Supplementary Materials (Figure S4) includes the As concentrations determined in the different digestate analyzed. The results show that the As concentrations are found to be above the limits set out by standard [56]. However, comparing them with the standard [57], these values are found to be within the permitted values, with a concentration range of between 18.96 mg/kg and 30.13 mg/kg. Under this regulatory framework, the digestate with an As content could be considered suitable for disposal at an authorized end disposal site.
As, unlike other trace elements, is not considered an essential element for soil, due to its toxicity. Its presence in the digestate is mainly attributed to its abundant presence in OFMSW and the capacity of the sargassum’s fronds, stipes, and gas bladders to capture and accumulate As during its passage across the ocean, due to the functional groups present in its structure [58]. According to Chávez-Vergara (2025) the As displays elevated concentrations both in fresh sargassum biomass and its waste during different stages of decomposition. The release of this metalloid through said waste represents a significant environmental risk, as its build up in coastal areas can contribute to the formation of brown tides and the intensification of eutrophication processes, negatively altering the ecological dynamic of the neritic zone [59]. This highlights the bio-accumulative nature of this marine species and the need to monitor heavy metals present in the digestate, resulting from ACoD. Although As is found in concentrations permitted by some standards, its toxicity level requires its elimination with prior treatments before considering any possible applications for the digestate, especially in agriculture. Methods such as adsorption, precipitation, or advanced separation techniques could be evaluated to guarantee the effective removal of this contaminant. It should be noted that the measurements were verified with an internal control of 0.5 ppm, which ensures the reliability of the data obtained in the analysis. These findings underline the importance of carrying out an exhaustive characterization of the digestate and the implementation of specific treatment strategies to mitigate the risks associated with toxic elements like As.
As concentrations exceeding the standard [56] highlight a potential environmental risk associated with digestate application. The mobility of As in agricultural soils is influenced by factors such as pH, redox conditions, and organic matter content, which in turn determine its bioavailability to plants and possible entry into the food chain. Although the present study did not evaluate these aspects in detail, it is important to consider that arsenic can be mobilized under reducing conditions or when competing ions are present. To mitigate these risks, passivation strategies such as the incorporation of biochar, iron oxides, or organic amendments have been reported to reduce As solubility and limit its uptake by crops. Future studies should therefore assess not only total concentrations but also speciation, mobility, and stabilization strategies to ensure the safe agricultural use of digestate.
As well as these parameters, the concentrations of elements considered as essential nutrients for plants were also analyzed, such as phosphorous (P) and potassium (K). Differences in the concentrations of these elements were identified which can be explained by various factors that occur during AD, such the consumption of nutrients by the bacteria to maintain the reactor, interchanges between the liquid and solid phases of the substrate, and possible precipitation of nutrients due to microbial metabolism [53,60,61]. Despite these variations, phosphorous and potassium concentrations were maintained within the values permitted by the standards, as shown in Table 7.

Table 7.
Physicochemical characteristics present in the digestate and its comparison with standard [56].
Standard [56] sets out specific criteria to guarantee that the digestate used as a soil improver has no negative effects on the same. Table 7 presents the values obtained in the physicochemical characterization of the digestate, including the C:N ratio and processed particle size, comparing them with the limits established by the standard. It was observed that only the digestate obtained by the particles of 0.6 mm and with a C:N ratio of 15 complied with the requirements set out by the standard. Particles of a greater size with the same C:N ratio displayed values that exceeded the permitted limit which disqualifies them as safe organic amendments for soil application. Furthermore, digestate with an initial C:N ratio of 20 did not comply with either the established values for the C:N ratio or the pH, indicating that they are unsuitable for use according to the standards.
3.3. Anaerobic Digestion of Sargassum
Elemental analysis of the sargassum enabled its empirical formula to be determined as C26H38O29N, which was used to estimate a theoretical maximum methane production of 274 mL CH4/gVS. This was considering the total conversion of volatile solids present in the substrate, in 250 mL reactors, with a total load of 200 mL without stirring. However, this estimate represents an overestimate of the real performance, given that complete conversion of the organic material is not achieved under experimental conditions. However, the values obtained are consistent with those reported in the literature [55].
Biogas production was evaluated according to particle size (5, 2, and 0.6 mm) and C:N ratio (15, 20, and 30) and it was discovered that particle size significantly influences the accumulation of biogas over 30 days, especially with a C:N ratio of 15. The greatest production was obtained with 2 mm particles, followed by 5 and 0.6 mm, Figure 2, whereas larger particles showed less efficiency due to the reduction in surface area available for AD.
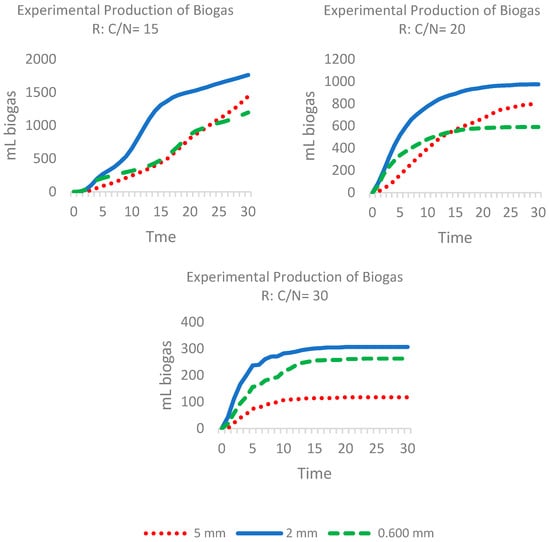
Figure 2.
Graphs showing the relationship between the generation of biogas and particle size with C:N ratio = 15, 20, and 30.
These results suggest that an excessive reduction in particle size could generate compaction and the accumulation of volatile fatty acids, thereby affecting the stability of the process. On increasing the C:N ratio to 20, the 2 mm particles maintained the best performance, favouring microbial activity, whereas smaller particles displayed limitations due to compaction. With a C:N ratio of 30, the biogas production was mainly restricted to the first 5 days of the process, indicating an accelerated initial decomposition followed by inhibition (Figure 2).
Analysis of biogas production according to the C:N ratio and particle size showed that a C:N ratio of 15 favours greater biogas accumulation, regardless of the particle size evaluated. However, C:N ratios of 20 and 30 showed a progressive decrease in biogas generation which suggests less efficiency in substrate decomposition. This pattern was consistent in the three particle sizes analyzed (5 mm, 2 mm, and 0.6 mm), confirming that a C:N ratio of 15 optimizes microbial activity and the conversion of organic material into biogas. However, higher C:N ratios could generate an imbalance in the availability of carbon and nitrogen, limiting the metabolism of the microorganisms involved in AD and reducing the efficiency of the process.
Figure 3 shows the accumulated methane production for a particle size of 2 mm and a C:N ratio of 15, with a performance of 92.62 mL CH4/gVS (blue line), whereas for 5 mm particles, 68.99 mL CH4/gVS was obtained under the same conditions (red line). However, these values are lower than those reported for other species of the Sargassum spp. genus, whose production ranges varied between 116.72 and 139.7 mL CH4/gVS [53,62]. Generally speaking, methane production for marine macroalgae oscillates between 120 and 280 mL CH4/gVS [55,63,64], indicating that the values obtained in this study are lower than the expected values.
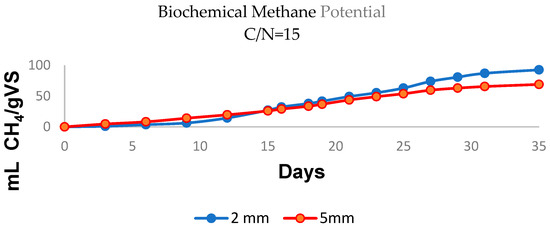
Figure 3.
Graphs showing the relationship between the generation of biogas and particle size with C:N ratio = 15 (2 mm, blue; 5 mm, red).
A graph of H2S generation in the experimental reactor with C:N = 15 can be seen in Figure 4; the H2S analysis via gas chromatography identified that higher concentrations were recorded within the first 6 days of the process, reaching a maximum peak on day 6. This indicates intense activity in sulphur bioconversion by the sulphate-reducing bacteria, which can affect methane production during this stage. From day 15, stabilization in the system was observed, as well as an increase in CH4 production, indicating favourable conditions for methanogenic archaea activity. However, H2S concentrations remained elevated, which suggests the need to implement technologies to purify and eliminate H2S in order to reduce health risks and prevent damage to the equipment [53].
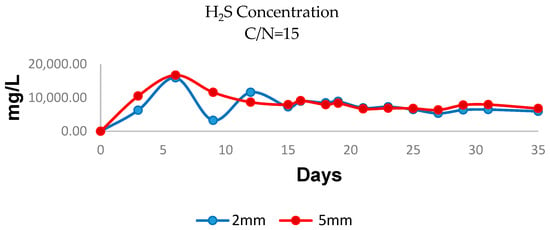
Figure 4.
Graphs showing the relationship between the generation of H2S in biogas and particle size with C:N ratio =15 (2 mm, blue; 5 mm, red).
In the present study, although the presence of H2S was qualitatively noted as a potential inhibitory factor, quantitative data on its concentration were not recorded. H2S is known to negatively affect anaerobic digestion by inhibiting methanogenic archaea, particularly at concentrations exceeding 200–250 ppm, where it interferes with enzymatic activity and disrupts the microbial community balance. Previous studies have reported that elevated H2S levels are often associated with substrates containing high sulphur content, such as marine biomass, leading to reduced methane yields [43,44]. The lack of continuous H2S monitoring in this study is recognized as a limitation; however, future work should incorporate gas composition analysis to establish correlations between H2S concentration and CH4 yield. Such analysis would provide valuable insights into process optimization and potential strategies for H2S mitigation (e.g., adsorption with iron salts, biological desulfurization), ultimately improving the efficiency of biogas production.
In general, these results reinforce the viability of sargassum as a substrate for AD and highlight the importance of particle size and C:N ratio in the efficiency of the process. Although screening does not significantly increase methane production, optimization of reactor parameters could improve the performances obtained.
The lower methane yield obtained in this study (92.62 mL CH4/gVS) compared to values reported in the literature (120–280 mL CH4/gVS) may be attributed to several limiting factors. First, the high sulphur content commonly associated with Sargassum spp. could have led to the release of hydrogen sulphide during anaerobic digestion, inhibiting microbial activity. In addition, the presence of heavy metals and arsenic in the biomass may have generated toxicity effects, further reducing methanogenic performance. Another possible factor is the adaptation of the microbial community, which may have required a longer acclimatization period to efficiently degrade the specific biochemical composition of Sargassum, characterized by complex polysaccharides and phenolic compounds. These aspects highlight the need for pretreatment strategies, microbial consortia optimization, and monitoring of inhibitory compounds to enhance methane production from this feedstock.
3.4. Experiment: Agriculture with the Digestate
Measurements carried out over 45 days enabled the effect of the digestate on crop growth to be evaluated in terms of final stem height, number of leaves, and final humid weight. The results indicate that the accumulated height of the stem oscillated between 5 and 8 cm, whereas the number of leaves varied between 2 and 4 (These daily values can be seen in the Supplementary Materials, Table S2). Table 8 shows the final values for final height, seedling size, and humid weight, enabling the effects of different substrates on plant development to be compared.

Table 8.
Final measurements of the seedlings using different substrates.
The plants cultivated in substrates enriched with digestate showed significantly greater growth in comparison to the controls. The final height of the stem was greater in those treated with digestate, which suggests greater availability and absorption of essential nutrients. The number of leaves also showed the differences between the treatments and control, with plants fertilized using digestate recording an increase compared to those that grew with other substrates. Furthermore, the final humid weight reflected greater accumulation of biomass in the plants treated with digestate, which indicates a positive impact on the efficiency of water and mineral absorption.
3.5. Statistical Analysis
In order to evaluate the distribution of data obtained in the experiment, normality tests were carried out for each of the response variables. Firstly, the Shapiro–Wilk test was applied to the response variables. The results indicated significance values greater than 0.05 in the case of biogas generation, following a normal distribution. For the variables accumulated growth, number of leaves, and final seedling size, the significance values were less than 0.05, which suggests that the data did not follow a normal distribution and therefore, the use of non-parametric statistical tests was required. Likewise, the Kolmogorov–Smirnov test was applied to the variables root size and final humid weight, obtaining significance values greater than 0.05, which indicates that these variables do follow a normal distribution, and consequently, they were analyzed using parametric tests.
To evaluate the interaction between the particle size, C:N ratio, and biogas generation, ANOVA was applied. The results indicated a value of p < 0.05, confirming the existence of significant differences between the evaluated treatments. In the multiple comparison post hoc tests, it was confirmed that greater biogas production was obtained with a particle size of 2 mm and C:N ratio of 15. The non-parametric ANOVA Kruskal–Wallis was used to evaluate the differences in the variables of accumulated weight, number of leaves, and final size of the seedlings among the different substrates evaluated, with a confidence level of 95%. The multiple comparisons indicated that the livestock liquid digestate showed the best performance in terms of accumulated growth, followed by the combination of solid sargassum digestate, liquid digestate, and humus. All of the treatments significantly exceeded the control. Regarding the number of leaves, the livestock liquid digestate showed a significantly superior performance compared to the other evaluated substrates. However, the combination of solid sargassum digestate and garden soil did not show any significant differences compared to the humus. Similarly, the sargassum liquid digestate showed similar behaviour to the humus, with no significant differences between them. For the variables final seedling size and root size, no significant differences were found between the treatments at a confidence level of 95%. However, significant differences were identified in the final humid weight. The post hoc tests revealed that the livestock liquid digestate presented significantly greater values compared to the control, the humus, and the combination of sargassum solid digestate and garden soil. In contrast, the sargassum liquid digestate displayed a similar behaviour to the other substrates evaluated. These values can be seen on graphs in the Supplementary Materials, Figures S5–S9.
Although the digestate groups outperformed the no-fertilizer control, the differences observed in relation to the commercial fertilizer (Group 3, humus) were less pronounced. This result suggests that, in the short term, digestate may provide similar agronomic benefits to those of conventional amendments. However, beyond crop yield, the use of digestate entails additional advantages that strengthen its practical relevance. From an economic perspective, digestate represents a low-cost input derived from the valorization of organic residues, reducing the expenses associated with waste management and decreasing dependence on external fertilizer markets. From an environmental standpoint, its application contributes to closing nutrient cycles, lowering greenhouse gas emissions linked to synthetic fertilizer production, and promoting circular economy models. Therefore, while the agronomic response may be comparable to that of humus, the integration of digestate into agricultural systems offers broader sustainability benefits that justify its implementation.
4. Conclusions
Anaerobic digestion of sargassum is a viable alternative for biogas production with performances similar to those reported for macroalgae. The optimal configuration identified (C:N ratio of 15 and particle size of 2 mm) maximized the generation of biogas and methane (92.62 mL CH4/gVS). However, the methane potential was less than other species of the Sargassum spp. genus, which suggests the need to optimize processing conditions and evaluate pretreatments to improve its efficiency.
The presence of elevated concentrations of H2S during the first few days of the process highlights the need to implement purification technologies to improve biogas quality and mitigate any environmental impacts. Furthermore, the analysis of metals and metalloids in the digestate confirmed the sargassum’s ability to accumulate these elements during its ocean journey. This underlines the importance of monitoring its presence in the raw material, in order to guarantee both the environmental and agronomic safety of the digestate.
The digestate generated showed agronomic potential, with positive effects on crop growth. In particular, the livestock liquid digestate promoted greater biomass accumulation and leaf development, possibly due to its nutritional contribution, which favours leaf expansion and photosynthetic activity. However, the possible presence of heavy metals must be considered before its application on agricultural soils, making the characterization of washed-up sargassum necessary in order to comply with environmental standards and avoid any risks involved.
These findings confirm the viability of sargassum as a substrate for biogas production and its digestate as an agriculturally sustainable biofertilizer. It is recommended that operational parameters are optimized, contaminants monitored, and digestate composition made suitable to guarantee its safe use on agricultural land, thereby promoting the sustainable use of sargassum.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/resources14100160/s1, Figure S1: Linear regression between final pressure and final volume used to calculate the initial biogas volume in the reactors from the initial pressure for 2 mm; Figure S2: Linear regression between final pressure and final volume used to calculate the initial biogas volume in the reactors from the initial pressure for 5 mm; Figure S3: Zinc concentration detected in digestate by particle size and C/N; Figure S4. Arsenic concentration detected in the digestate by particle size and C/N; Figure S5: Effect of treatments on the number of leaves recorded during the experimental period; Figure S6: Effect of treatments on the accumulated growth recorded during the experimental period; Figure S7: Effect of treatments on the wet weight recorded during the experimental period; Figure S8: Effect of treatments on the final size recorded during the experimental period; Figure S9: Effect of treatments on the root size recorded during the experimental period; Table S1: Characteristics of the digestate resulting from the anaerobic digestion of sargassum; Table S2: Daily measurements taken for 45 days with each of the treatments.
Author Contributions
Conceptualization, H.A.L.-A. and D.Q.-C.; methodology, D.Q.-C., F.J.Z.-D.d.l.S., L.C.N.-M. and H.A.L.-A.; software, H.A.L.-A. and D.Q.-C.; validation, A.P.-H., D.Q.-C., M.d.R.P.-P., F.J.Z.-D.d.l.S. and H.A.L.-A.; formal analysis, H.A.L.-A. and A.P.-H.; investigation, H.A.L.-A. and D.Q.-C.; resources, H.A.L.-A. and A.P.-H.; data curation, H.A.L.-A. and D.Q.-C.; writing—original draft preparation, H.A.L.-A.; writing—review and editing, A.P.-H., F.J.Z.-D.d.l.S., M.d.R.P.-P., L.C.N.-M. and H.A.L.-A.; visualization, A.P.-H., F.J.Z.-D.d.l.S., M.d.R.P.-P. and H.A.L.-A.; supervision, H.A.L.-A., L.C.N.-M. and A.P.-H.; project administration, H.A.L.-A. and A.P.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by SECIHTI–Ciencia de Frontera (No. 2023_G_1566), which contributed to the development of infrastructure and laboratory facilities.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or the Supplementary Materials.
Acknowledgments
We gratefully acknowledge the Centro de Investigación en Materiales Avanzados S. C. (CIMAV), Universidad Autónoma de Chihuahua (UACH) and Universidad La Salle de Chihuahua (ULSA), as well as the projects SECIHTI-SENER 243715, SECIHTI-SEMAR 305292, and SECIHTI-CIENCIA DE FRONTERA 2023_G_1566 for their support in the generation of infrastructure and laboratories.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| C:N | Carbon–nitrogen ratio |
| AD | Anaerobic digestion |
| OFMSW | Organic fraction of municipal solid waste |
| ACoD | Anaerobic co-digestion |
| DS | Sargassum: Digestion |
| CDSF | Co-digestion of sargassum and OFMSW, |
| TI | Control (inoculum) |
References
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Bajpai, P. Basics of Anaerobic Digestion Process. In Anaerobic Technology in Pulp and Paper Industry; Springer: Singapore, 2017; pp. 7–12. [Google Scholar]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of kinetic parameters with process parameters and operational conditions during anaerobic digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]
- Björnsson, L.; Murto, M.; Mattiasson, B. Evaluation of parameters for monitoring an anaerobic co-digestion process. Appl. Microbiol. Biotechnol. 2000, 54, 844–849. [Google Scholar] [CrossRef]
- Sebola, M.; Muzenda, E.; Tesfagiorgis, H. Effect of particle size on anaerobic digestion of different feedstocks. S. Afr. J. Chem. Eng. 2015, 20, 11–26. [Google Scholar]
- Parra-Orobio, B.A.; Torres-Lozada, P.; Marmolejo-Rebellón, L.F. Anaerobic digestion of municipal biowaste for the production of renewable energy: Effect of particle size. Braz. J. Chem. Eng. 2017, 34, 481–491. [Google Scholar] [CrossRef]
- Vigueras-Carmona, S.E.; Trujillo, M.; García Rivero, M.; Membrillo Venegas, I.; Zafra Jiménez, G. Effect of particle size on mesophilic anaerobic digestion of thermally pre-treated waste activated sludge. J. Biotech Res. 2016, 7, 11–19. [Google Scholar]
- Dai, X.; Hua, Y.; Dai, L.; Cai, C. Particle size reduction of rice straw enhances methane production under anaerobic digestion. Bioresour. Technol. 2019, 293, 122043. [Google Scholar] [CrossRef]
- Luo, L.; Qu, Y.; Gong, W.; Qin, L.; Li, W.; Sun, Y. Effect of Particle Size on the Aerobic and Anaerobic Digestion Characteristics of Whole Rice Straw. Energies 2021, 14, 3960. [Google Scholar] [CrossRef]
- Agyeman, F.; Tao, W. Anaerobic co-digestion of food waste and dairy manure: Effects of food waste particle size and organic loading rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Vian, J.; Velasco-Pérez, A.; Solar-González, R.; García-Herrera, T.; Puebla, H.; Vivar-Vera, G. Particle Size Effect on Anaerobic Digestion of Fruit and Vegetable Waste. Fermentation 2024, 10, 485. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Donoso-Bravo, A.; Torres-Lozada, P. Anaerobic digestion of food waste. Predicting of methane production by comparing kinetic models. Ing. Compet. 2017, 19, 219–227. [Google Scholar]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Cai, T.; Shao, L.; He, P. Resource potential of liquid digestate from food and kitchen waste digestion associated with particle size fractionation. Appl. Eng. Agric. 2015, 31, 661. [Google Scholar]
- Alburquerque, J.; Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Tan, F.; Zhu, Q.; Guo, X.; He, L. Effects of digestate on Biomass of a selected energy crop and soil properties. J. Sci. Food Agric. 2020, 101, 927–936. [Google Scholar] [CrossRef]
- Zoui, O.; Baroudi, M.; Drissi, S.; Abouabdillah, A.; Abd-Elkader, O.; Plăvan, G.; Bourioug, M. Utilization of Digestate as an Organic Manure in Corn Silage Culture: An In-Depth Investigation of Its Profound Influence on Soil’s Physicochemical Properties, Crop Growth Parameters, and Agronomic Performance. Agronomy 2023, 13, 1715. [Google Scholar] [CrossRef]
- Šlepetienė, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskienė, I.; Amaleviciute-Volunge, K.; Šlepetys, J.; Cesevičienė, J. The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag. 2019, 102, 441–451. [Google Scholar] [CrossRef]
- García-López, A.; Delgado, A.; Anjos, O.; Horta, C. Digestate Not Only Affects Nutrient Availability but Also Soil Quality Indicators. Agronomy 2023, 13, 1308. [Google Scholar] [CrossRef]
- Clements, L.; Salter, A.; Banks, C.; Poppy, G. The usability of digestate in organic farming. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2012, 66, 1864–1870. [Google Scholar] [CrossRef]
- Horta, C.; Carneiro, J.P. Use of digestate as organic amendment and source of nitrogen to vegetable crops. Appl. Sci. 2021, 12, 248. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holátko, J.; Sudoma, M.; Kintl, A.; Vopravil, J.; Ryant, P.; Škarpa, P.; Radziemska, M.; Látal, O.; Brtnický, M. Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth. Agronomy 2021, 11, 2041. [Google Scholar] [CrossRef]
- Gielnik, A.; Péchaud, Y.; Huguenot, D.; Cébron, A.; Riom, J.; Guibaud, G.; Esposito, G.; Van Hullebusch, E. Effect of digestate application on microbial respiration and bacterial communities’ diversity during bioremediation of weathered petroleum hydrocarbons contaminated soils. Sci. Total Environ. 2019, 670, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sánchez, M.; Garcia-Romera, I.; Cajthaml, T.; Tlustoš, P.; Száková, J. Changes in soil microbial community functionality and structure in a metal-polluted site: The effect of digestate and fly ash applications. J. Environ. Manag. 2015, 162, 63–73. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, M. Nutrient recovery from anaerobic digestate: Fertilizer informatics for circular economy. Environ. Res. 2024, 245, 117953. [Google Scholar] [CrossRef]
- Pranckietienė, I.; Navickas, K.; Venslauskas, K.; Jodaugienė, D.; Buivydas, E.; Žalys, B.; Vagusevičienė, I. The Effect of Digestate from Liquid Cow Manure on Spring Wheat Chlorophyll Content, Soil Properties, and Risk of Leaching. Agronomy 2023, 13, 626. [Google Scholar] [CrossRef]
- Foereid, B.; Szőcs, J.; Patinvoh, R.; Horváth, I. Effect of anaerobic digestion of manure before application to soil—Benefits for nitrogen utilisation? Int. J. Recycl. Org. Waste Agric. 2020, 10, 89. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Wang, S.; Wang, Z.; Liu, Y.; Hu, Z.; Zhan, X. Environmental sustainability assessment of pig manure mono- and co-digestion and dynamic land application of the digestate. Renew. Sustain. Energy Rev. 2020, 137, 110476. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Han, N.; Li, X.; Bai, R.; Magaña, J.; Shimizu, N. By-Product from Livestock Waste Recovery System Used as Fertilizer: Bioactive Compounds and Antioxidant Activity of Tomato Fruit as Affected by Fertilization under Field and Greenhouse Conditions. Fermentation 2023, 9, 714. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Shimizu, N.; Magaña, J.; Gong, P.; Na, R. Impact of organic fertilization by the digestate from by-product on growth, yield and fruit quality of tomato (Solanum lycopersicon) and soil properties under greenhouse and field conditions. Chem. Biol. Technol. Agric. 2023, 10, 70. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The effectiveness of digestate use for fertilization in an agricultural cropping system. Plants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Nasmia; Binangkari, K.L.; Rusaini; Rosyida, E.; Nurdin, M.S.; Yala, Z.R. The addition of seaweed extract (Sargassum sp.) as fertilizer on culture media on the total density of Skeletonema costatum cells. IOP Conf. Ser. Earth Environ. Sci. 2022, 1075, 012012. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Dumitru, M. Studies Concerning the Utilisation of Digestate in Biogas Plants; University of Agricultural Sciences and Veterinary Medicine of Bucharest/Ceres Publishing House: Bucharest, Romania, 2014. [Google Scholar]
- Panuccio, M.R.; Papalia, T.; Attinà, E.; Giuffrè, A.; Muscolo, A. Use of digestate as an alternative to mineral fertilizer: Effects on growth and crop quality. Arch. Agron. Soil Sci. 2018, 65, 700–711. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Parisi, M.; Bezzi, G.; Parisi, B.; Allesina, G.; Pedrazzi, S.; Francia, E. Using digestate and biochar as fertilizers to improve processing tomato production sustainability. Agronomy 2020, 10, 138. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Wu, S.B.; Qu, Y.H.; Dong, R.J.; Pang, C.L. Use of solid digestate as a growing medium for tomato seedlings. Adv. Mater. Res. 2013, 726, 3001–3006. [Google Scholar] [CrossRef]
- Morra, L.; Cozzolino, E.; Salluzzo, A.; Modestia, F.; Bilotto, M.; Baiano, S.; del Piano, L. Plant growth, yields and fruit quality of processing tomato (Solanum lycopersicon L.) as affected by the combination of biodegradable mulching and digestate. Agronomy 2021, 11, 100. [Google Scholar] [CrossRef]
- Barzee, T.; Edalati, A.; El-Mashad, H.; Wang, D.; Scow, K.; Zhang, R. Digestate Biofertilizers Support Similar or Higher Tomato Yields and Quality Than Mineral Fertilizer in a Subsurface Drip Fertigation System. Front. Sustain. Food Syst. 2019, 3, 58. [Google Scholar] [CrossRef]
- Erraji, H.; Tallou, A.; Laiche, H.; Asehraou, A. Anaerobic digestates from cow dung and food waste as fertilizers: Effect on tomato growth and yield. BIO Web Conf. 2024, 115, 07001. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Torres-Conde, E.G.; Rosellón-Druker, J.; Cabanillas-Terán, N.; Jáuregui-Haza, U. The Great Atlantic Sargassum Belt: Impacts on the Central and Western Caribbean-A Review. Harmful Algae 2025, 144, 102838. [Google Scholar] [CrossRef]
- López-Aguilar, H.; Kennedy-Puentes, G.; Gómez, J.; Huerta-Reynoso, E.; Peralta-Pérez, M.D.R.; Zavala-Díaz de la Serna, F.; Pérez-Hernández, A. Practical and Theoretical Modeling of Anaerobic Digestion of Sargassum spp. in the Mexican Caribbean. Pol. J. Environ. Stud. 2021, 30, 3151–3161. [Google Scholar] [CrossRef]
- López-Aguilar, H.A.; Morales-Durán, B.; Quiroz-Cardoza, D.; Pérez-Hernández, A. Lag phase in the anaerobic Co-digestion of Sargassum spp. and organic domestic waste. Energies 2023, 16, 5462. [Google Scholar] [CrossRef]
- Paletta, R.; Candamano, S.; Bruno, M.D.L.; Desiderio, G.; Castro, Y.A. Effect of unconventional pretreatments on the morphology and biochemical methane potential of Sargassum spp. Algal Res. 2025, 86, 103915. [Google Scholar] [CrossRef]
- Paletta, R.; Filippelli, P.; Candamano, S.; Galluccio, L.; Macilletti, A.; Castro, Y.; Tursi, A.; Amaro, E.; Frias, J. Efficient reuse of Sargassum spp. biomass and organic fraction of municipal solid waste by anaerobic co-digestion in the Dominican Republic: Evaluation of biochemical methanogenic potential and reaction rates. Appl. Chem. Eng. 2024, 7, 2081. [Google Scholar] [CrossRef]
- Czekała, W.; Lewicki, A.; Pochwatka, P.; Czekała, A.; Wojcieszak, D.; Jóźwiakowski, K.; Waliszewska, H. Digestate management in polish farms as an element of the nutrient cycle. J. Clean. Prod. 2020, 242, 118454. [Google Scholar] [CrossRef]
- Stoumpou, V.; Novakovic, J.; Kontogianni, N.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Assessing straw digestate as feedstock for bioethanol production. Renew. Energy 2020, 153, 261–269. [Google Scholar] [CrossRef]
- Bernardo, M.; Correa, C.R.; Ringelspacher, Y.; Becker, G.C.; Lapa, N.; Fonseca, I.; Esteves, I.A.; Kruse, A. Porous carbons derived from hydrothermally treated biogas digestate. Waste Manag. 2020, 105, 170–179. [Google Scholar] [CrossRef] [PubMed]
- ASTM C702/C702M-18; Standard Practice for Reducing Samples of Aggregate to Testing Size. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM E1131-08(2014); Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM International: West Conshohocken, PA, USA, 2014.
- Al Seadi, T. Biogas Handbook; University of Southern Denmark: Esbjerg, Denmark, 2008. [Google Scholar]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Enhancing biogas production from caribbean pelagic Sargassum utilising hydrothermal pretreatment and anaerobic co-digestion with food waste. Chemosphere 2021, 275, 130035. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; AP, Y.; Mohamed, I.M.; Iwasaki, M.; Tangtaweewipat, S.; Ihara, I.; Sakai, R.; Umetsu, K. Thermophilic anaerobic digestion of Sargassum fulvellum macroalgae: Biomass valorization and biogas optimization under different pre-treatment conditions. J. Environ. Chem. Eng. 2021, 9, 106405. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- NTEA-006-SMA-RS-2006; Que establece los requisitos para la producción de los mejoradores de suelos elaborados a partir de residuos orgánicos. Secretaría de Medio Ambiente: México City, Mexico, 2012.
- NOM-004-SEMARNAT-2002; Protección ambiental—Lodos y biosólidos—Especificaciones y límites máximos permisibles de contaminantes para su aprovechamiento y disposición final. Diario Oficial de la Federación: Ciudad de México, Mexico, 2002.
- Océanne-Amaya, C.; Gigault, J.; Dassié, É.P.; Baudrimont, M.; Gourves, P.; Amaral-Zettler, L.; Pascal, P. Metals and metalloids concentrations in three genotypes of pelagic Sargassum from the Atlantic Ocean Basin-scale. Mar. Pollut. Bull. 2022, 178, 113564. [Google Scholar] [CrossRef]
- Chávez-Vergara, B.; Solleiro-Rebolledo, E.; López-Martínez, R.; Beltrán-Paz, O.; Ceniceros-Gómez, Á.E.; Yañez-Mendoza, G. The release of arsenic is a hidden risk during the in-situ decomposition of landed sargassum litter. Aquat. Bot. 2025, 199, 103884. [Google Scholar] [CrossRef]
- Moro Flores, J.P.; Aquino Alves, L.; de Oliveira Denardin, L.G.; Posselt Martins, A.; Campanhola, B.E.; Vasconcellos, I.A.; de Faccio Carvalho, P.C.; Tiecher, T. Soil K forms and K budget in integrated crop-livestock systems in subtropical paddy fields. Soil Tillage Res. 2021, 213, 105070. [Google Scholar] [CrossRef]
- Slopiecka, K.; Liberti, F.; Massoli, S.; Bartocci, P.; Fantozzi, F. Chemical and physical characterization of food waste to improve its use in anaerobic digestion plants. Energy Nexus 2022, 5, 100049. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Harvey, P.J.; Nielsen, B.V. Methane production from Sargassum muticum: Effects of seasonality and of freshwater washes. Energy Built Environ. 2021, 2, 235–242. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Murto, M. Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J. Environ. Manag. 2010, 91, 1573–1579. [Google Scholar] [CrossRef]
- Saratale, R.G.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Saratale, G.D. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).