Carbon Recovery from Wastewater Feedstocks: Synthesis of Polyhydroxyalkanoates for Target Applications
Abstract
1. Introduction
2. Basis of PHA and EPS Synthesis in Prokaryotes
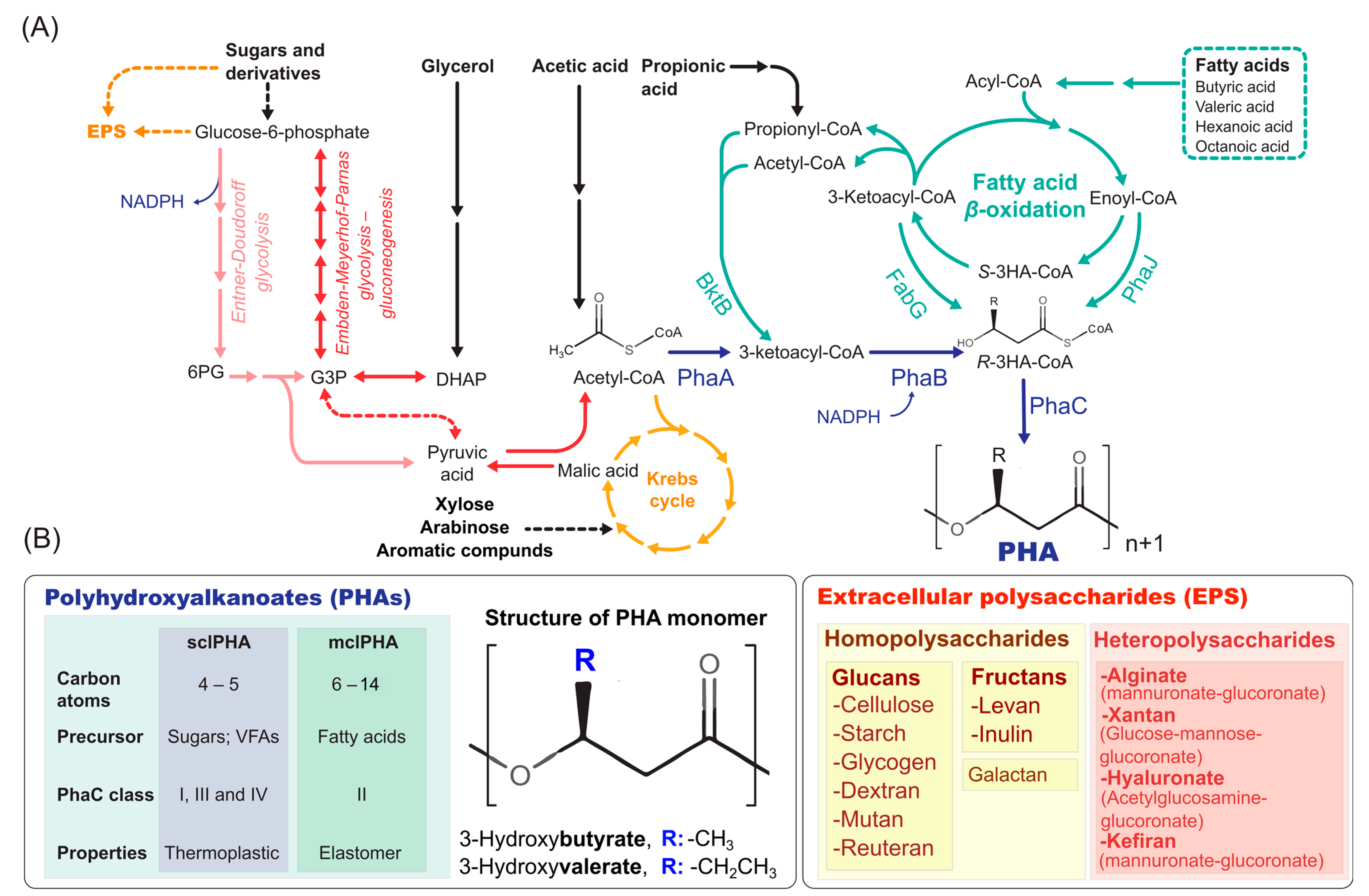
2.1. Types of PHA
2.2. Metabolic Pathways of PHA Synthesis
2.3. Microbial Synthesis of EPS
3. Wastewater Feedstocks
| Source | Feedstock | Pre- Treatment | Main C Sources a | Bioprocess b | MMC | Products | Ref. |
|---|---|---|---|---|---|---|---|
| Municipal WWTPs | WAS | AF | Acetic acid: 0.9 g L−1; | Pilot SBR | Enriched AS | P(3HB-co-15 mol%-3HV) | [62] |
| propionic acid: 0.2 g L−1; | |||||||
| butyric acid: 0.1 g L−1 | |||||||
| WAS | AF | Acetic acid: 0.8 g L−1; | Pilot SBR | Enriched AS | P(3HB-co-3 mol%-3HV) | [63] | |
| propionic acid: 0.1 g L−1; | |||||||
| butyric acid: 0.2 g L−1 | |||||||
| WAS | TH and AF | Acetic acid: 3.9 g L−1; | Pilot fed-batch | Enriched AS | P(3HB-co-10 mol%-3HV) | [64] | |
| propionic acid: 1.4 g L−1; | |||||||
| butyric acid: 2.6 g L−1; | |||||||
| valeric acid: 0.5 g L−1 | |||||||
| Primary sludge | AF | Acetic acid: 1.5 g L−1; | Pilot fed-batch | Non- enriched AS | P(3HB-co-30–40 mol%-3HV) | [49] | |
| propionic acid: 2.4 g L−1; | |||||||
| butyric acid: 1.2 g L−1; | |||||||
| valeric acid: 0.4 g L−1 | |||||||
| WAS or primary sludge | AF | Acetic acid: 2.3–2.7 g L−1; | Pilot fed-batch | Enriched AS | P(3HB-co-25–30 mol%-3HV) | [65,66] | |
| propionic acid: 1.0–1.2 g L−1; | |||||||
| butyric acid: 1.3–1.4 g L−1; | |||||||
| valeric acid: 0.5–0.8 g L−1 | |||||||
| Agro-industry | Olive mill effluent | AF | Acetic acid: 3.6–7.1 g L−1; | Pilot batch, pilot SBR | Enriched and non-enriched AS | P(3HB-co-11 mol%-3HV) | [47,67] |
| propionic acid: 1.0–1.3 g L−1; | |||||||
| butyric acid: 2.1–6.0 g L−1; | |||||||
| valeric acid: 0.6–1.5 g L −1; | |||||||
| oleanolic acid | |||||||
| Fruit processing effluent | AF | Acetic acid: 1 g L−1; | Pilot SBR | Enriched AS | P(3HB-co-3HV); P(3HB-co-1 mol%-3HV-co-41 mol%-3HHx) | [68,69] | |
| butyric acid: 1.6 g L−1; | |||||||
| valeric acid: 0.02 g L−1; | |||||||
| hexanoic acid: 11.8 g L−1 | |||||||
| Tomato processing effluent | AF | Acetic acid: 3.3 g L−1; | Pilot SBR | Enriched AS | P(3HB-co-45 mol%-3HV) | [52] | |
| propionic acid: 2.1 g L−1; | |||||||
| butyric acid: 2.4 g L−1; | |||||||
| valeric acid: 1.2 g L−1 | |||||||
| Food processing industry | Whey permeate | AF | Acetic acid: 0.69 g L−1; | Pilot SBR | Non- enriched MMC | P(3HB-co-1 mol%-3HV) | [70] |
| propionic acid: 0.03 g L−1; | |||||||
| butyric acid: 0.8 g L−1; | |||||||
| valeric acid: 0.02 g L−1 | |||||||
| Ice cream factory effluent | AF | VFAs: 3.1 g L−1 | Pilot aerobic continuous | Non-enriched AS | PHA | [71] | |
| Citrus processing effluent | Non | Acetic acid, | Lab fed-batch | Enriched AS | P(3HB) and EPS | [72] | |
| sucrose, | |||||||
| fructose, | |||||||
| glucose | |||||||
| Potato processing effluent | Non | Acetic acid: 6.3 g L−1 | Pilot SBR | Enriched AS | P(3HB) | [73] | |
| Mussel processing effluent | AF | Acetic acid: 0.6–1.2 g L−1; | Lab fed-batch | Non- enriched AS | P(3HB-co-30 mol%-3HV) | [37] | |
| propionic acid: 0.2–0.4 g L−1; | |||||||
| butyric acid: 0.10–0.15 g L−1; | |||||||
| valeric acid: 0.08–0.1 g L−1 | |||||||
| carbohydrates: 0.17–0.41 g L−1; | |||||||
| Protein: 0.1–0.2 g L−1 | |||||||
| Candy industry effluent | AF | VFAs | Pilot fed-batch | Enriched AS | P(3HB-co-3HV) | [74] | |
| Paper mill and Kraft mill effluents | Paper mill effluent | AF | Acetic acid: 1.8 g L−1; | Pilot SBR | Enriched AS | [75] | |
| propionic acid: 1 g L−1; | |||||||
| butyric acid: 1.5 g L−1; | |||||||
| valeric acid: 0.8 g L−1 | |||||||
| Kraft mill effluent | Non | Total phenolic compounds: 0.25 g L−1; | Lab batch MBBR | Non- enriched AS from kraft-mill WWTP | PHAs | [76] | |
| lignin derivates | |||||||
| Kraft mill effluent | Non | Total phenolic compounds: 0.25 g L−1; | Lab aerobic batch | Non- enriched AS from (i) kraft mill; (ii) paper mill; (iii) municipal WWTPs | PHAs | [14] | |
| lignin derivates | |||||||
| Paper mill effluent | Non | acetic acid: 0.3 g L−1; | Lab MBBR | Non- enriched AS | PHAs | [77] | |
| propionic acid: 0.4 g L−1; | |||||||
| butyric acid: 0.2 g L−1 | |||||||
| Paper mill effluent | AF | Acetic acid: 1.4–2.1 g L−1; | Lab batch | Enriched AS | P(3HB-co-53–69 mol%-3HV) | [78] | |
| propionic acid: 1.6–2.4 g L−1; | |||||||
| butyric acid: 0.8–1.2 g L−1; | |||||||
| valeric acid: 0.2–0.3 g L−1 | |||||||
| Biodiesel production | Crude glycerol | Non | 0.8w w−1 glycerol | Lab SBR | Enriched AS | P(3HB) | [79] |
3.1. Municipal Wastewater
3.2. Agro-Industrial Wastewater
3.3. Food Processing Wastewater
3.4. Lignocellulosic Biomass Processing Wastewater
3.5. Crude Glycerol
3.6. Challenges and Opportunities of PHA Production with Wastewater Feedstocks
4. Recovery of EPS from Wastewater Streams
5. Applications of Microbial Biopolymers
6. Quality Control
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Acidogenic fermentation |
| AGS | Activated granular sludge |
| ALE | Alginate-like exopolysaccharide |
| AS | Activated sludge |
| BktB | 3-Ketovalerate producing 3-ketothiolase |
| COD | Chemical oxygen demand |
| EPS | Extracellular polysaccharide |
| GHG | Greenhouse gas |
| MBBR | Moving-bed biofilm bioreactor |
| MMC | Mixed microbial culture |
| PHA | Polyhydroxyalkanoate |
| PhaC | Polyhydroxyalkanoate synthase enzyme |
| PhaA | 3-Ketothiolase |
| PhaB | Acetoacetyl-CoA reductase |
| PhaJ | R-specific enoyl-CoA hydratase |
| PhaG | (R)-3-Hydroxyacyl-ACP-CoA transacylase |
| PhaZ | Polyhydroxyalkanoate depolymerase |
| mclPHA | Medium-chain-length polyhydroxyalkanoate |
| P(3HB) | Poly(3-hydroxybutyrate) |
| P(3HB-co-3HV) | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| P(3HB-co-3HHx) | Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| P(3HB-co-4HB) | Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) |
| R-3HB-CoA | (R)-3-hydroxybutyryl-CoA |
| SBR | Sequence batch bioreactor |
| sclPHA | Short-chain-length polyhydroxyalkanoate |
| TH | Thermic hydrolysis |
| VSS | Volatile suspended solids |
| WAS | Waste-activated sludge |
| WWTP | Wastewater treatment plant |
| WRRF | Wastewater resource recovery facility |
| 3HB | 3-Hydroxybutyrate monomer |
| 3HV | 3-Hydroxyvalerate monomer |
| 4HB | 4-Hydroxybutyrate monomer |
References
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- EIB. Wastewater as a Resource; European Investment Bank: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; López-Maldonado, E.A. Biosolids management and utilizations: A review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Cardoso, B.J.; Rodrigues, E.; Gaspar, A.R.; Gomes, Á. Energy performance factors in wastewater treatment plants: A review. J. Clean. Prod. 2021, 322, 129107. [Google Scholar] [CrossRef]
- Leiva, A.M.; Monsalves, N.; Gómez, G.; Vidal, G. Fate of antibiotic resistance genes in organic wastes from sewage treatment plants in the framework of circular economy. In Occurrence and Behavior of Emerging Contaminants in Organic Wastes and Their Control Strategies, 1st ed.; Kumar, S., Huang, K., Ahmad, S., Eds.; Elsevier Inc. Press: Amsterdam, The Netherlands, 2024; pp. 3–20. ISBN 978-0-443-13585-9. [Google Scholar]
- Ghimire, U.; Sarpong, G.; Gude, V.G. Transitioning wastewater treatment plants toward circular economy and energy sustainability. ACS Omega 2021, 6, 11794–11803. [Google Scholar] [CrossRef]
- Leiva, A.M.; Gómez, G.; González-Rocha, G.; Piña, B.; Vidal, G. Performance of full-scale rural wastewater treatment plants in the reduction of antibiotic resistance bacteria and antibiotic resistance genes from small-city effluents. J. Environ. Chem. Eng. 2024, 12, 112322. [Google Scholar] [CrossRef]
- Nielsen, P.H. Microbial biotechnology and circular economy in wastewater treatment. Microb. Biotechnol. 2017, 10, 1102–1105. [Google Scholar] [CrossRef]
- Seeger, M.; Macaya, C.; Vílchez, A.; Castillo-Novales, D.; Sepúlveda-Mardones, M.; Bravo, G.; Vega-Celedón, P.; Durán, R.E.; Rivera, E.G.; Cámara, B.; et al. Biotechnology and microbial genomics for circular bioeconomy. In The Handbook of Circular Bioeconomy, 1st ed.; Zilberman, D., Zhuang, J., Wesseler, J., Khanna, M., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Da Cruz Pradella, J.G. Economics and industrial aspects of PHA production. In The Handbook of Polyhydroxyalkanoates, 1st ed.; Koller, M., Ed.; CRC Press: Boca Ratón, FL, USA, 2021; Volume 2, pp. 389–402. ISBN 9780367275594. [Google Scholar]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) Production: A Feasible Economic Option for the Treatment of Sewage Sludge in Municipal Wastewater Treatment Plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Reis, A.; Silva, C.; Pintado, M. Simulation Tool for the Techno-Economic Assessment of PHA Production in Wastewater Treatment Plants. Processes 2022, 13, 295. [Google Scholar] [CrossRef]
- Soo, A.; Kim, J.; Shon, H.K. Technologies for the wastewater circular economy—A review. Desalin. Water Treat. 2024, 317, 100205. [Google Scholar] [CrossRef]
- Pozo, G.; Villamar, C.A.; Martínez, M.; Vidal, G. Effect of organic load and nutrient ratio on the operation stability of the moving bed bioreactor for kraft mill wastewater treatment and the incidence of polyhydroxyalkanoate biosynthesis. Water Sci. Technol. 2012, 66, 370–376. [Google Scholar] [CrossRef]
- Novelli, L.D.D.; Sayavedra, S.M.; Rene, E.R. Polyhydroxyalkanoate (PHA) production via resource recovery from industrial waste streams: A review of techniques and perspectives. Bioresour. Technol. 2021, 331, 124985. [Google Scholar] [CrossRef]
- Sepúlveda-Mardones, M.; Campos, J.L.; Magrí, A.; Vidal, G. Moving forward in the use of aerobic granular sludge for municipal wastewater treatment: An overview. Rev. Environ. Sci. Biotechnol. 2019, 18, 741–769. [Google Scholar] [CrossRef]
- Karahan, O.; Orhon, D.; van Loosdrecht, M.C. Simultaneous storage and utilization of polyhydroxyalkanoates and glycogen under aerobic conditions. Water Sci. Technol. 2008, 58, 945–951. [Google Scholar] [CrossRef]
- Kumar, V.; Darnal, S.; Kumar, S.; Kumar, S.; Singh, D. Bioprocess for co-production of polyhydroxybutyrate and violacein using Himalayan bacterium Iodobacter sp. PCH194. Bioresour. Technol. 2021, 319, 124235. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhao, Y.; Huang, W.; Zhang, L.; Wu, F.; Ye, J.; Chen, G.Q. Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine. Nat. Commun. 2020, 11, 3313. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, M.A.; Escalante-Avilés, M.; Rodríguez-Hernández, A.I.; López-Cuellar, M.R.; Aguirre-Loredo, R.Y.; Martínez-Juárez, V.M.; Chavarría-Hernández, N. Co-production of polyhydroxyalkanoates (PHAs) and exopolysaccharides (EPSs) by halophilic archaeon Haloferax mucosum. New J. Chem. 2024, 48, 20188–20200. [Google Scholar] [CrossRef]
- European Commission. 2020. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 7 July 2025).
- Obruca, S.; Sedlacek, P.; Koller, M. The underexplored role of diverse stress factors in microbial biopolymer synthesis. Bioresour. Technol. 2021, 326, 124767. [Google Scholar] [CrossRef]
- Méndez, V.; Sepúlveda, M.; Izquierdo-Fiallo, K.; Macaya, C.C.; Esparza, T.; Báez-Matus, X.; Seeger, M. Surfing in the storm: How Paraburkholderia xenovorans thrives under stress during biodegradation of toxic aromatic compounds and other stressors. FEMS Microbiol. Rev. 2025, 49, fuaf021. [Google Scholar] [CrossRef]
- Vilchez, A.; Guajardo, G.; Sepúlveda, M.; Seeger, M.; Acevedo, F.; Navia, R. Analyses of substrates and bacterial genera in biological polyhydroxyalkanoates production performance: A review. Biores. Technol. Rep. 2025, 31, 102224. [Google Scholar] [CrossRef]
- Nomura, C.T.; Taguchi, S. PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. Appl. Microbiol. Biotechnol. 2007, 73, 969–979. [Google Scholar] [CrossRef]
- Steinbüchel, A. Perspectives for biotechnological production and utilization of biopolymers: Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 2001, 1, 1–24. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegradation 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Santullano, N.; Villegas, P.; Mardones, M.S.; Durán, R.E.; Donoso, R.; González, A.; Seeger, M. Genome-wide metabolic reconstruction of the synthesis of polyhydroxyalkanoates from sugars and fatty acids by Burkholderia sensu lato species. Microorganisms 2021, 9, 1290. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 376, 15–33. [Google Scholar] [CrossRef]
- Vital-Vilchis, I.; Karunakaran, E. Make it or break it: A review on PHA synthase and depolymerase proteins. J. Polym. Environ. 2024, 33, 1267–1291. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Marsch, R.; Pérez-Guevara, F. Investigation on the evolutionary relation of diverse polyhydroxyalkanoate gene clusters in Betaproteobacteria. J. Mol. Evol. 2018, 86, 470–483. [Google Scholar] [CrossRef]
- Huang, S.; Wang, M.; Yuan, J.; Chen, Y.; Tang, L. Enhancing medium-chain-length polyhydroxyalkanoate (mcl-PHA) synthesis in mixed microbial cultures via targeted substrate composition: A meta-omics guided approach. Bioresour. Technol. 2025, 432, 132700. [Google Scholar] [CrossRef]
- Argiz, L.; Fra-Vázquez, A.; Del Río, Á.V.; Mosquera-Corral, A. Optimization of an enriched mixed culture to increase PHA accumulation using industrial saline complex wastewater as a substrate. Chemosphere 2020, 247, 125873. [Google Scholar] [CrossRef]
- Pinto-Ibieta, F.; Serrano, A.; Cea, M.; Ciudad, G.; Fermoso, F.G. Beyond PHA: Stimulating intracellular accumulation of added-value compounds in mixed microbial cultures. Bioresour. Technol. 2021, 337, 125381. [Google Scholar] [CrossRef] [PubMed]
- Zahra, S.A.; Purba, L.D.A.; Abdullah, N.; Yuzir, A.; Iwamoto, K.; Lei, Z.; Hermana, J. Characteristics of algal-bacterial aerobic granular sludge treating real wastewater: Effects of algal inoculation and alginate-like exopolymers recovery. Chemosphere 2023, 329, 138595. [Google Scholar] [CrossRef]
- Sen, S.; Tiwari, O.N.; Arya, R.K.; Bhowmick, T.K.; Gayen, K. New insights on microbial extracellular polysaccharides: Production, biological activity, and applications. Biomass Convers. Biorefin. 2025, 1–30. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Priyanka, P.; Arun, A.B.; Rekha, P.D. Sulfated exopolysaccharide produced by Labrenzia sp. PRIM-30: Characterization and prospective applications. Int. J. Biol. Macromol. 2014, 69, 290–295. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Ibrahim, M.I.A. Production and characterization of exopolysaccharide from newly isolated marine probiotic Lactiplantibacillus plantarum EI6 with in vitro wound healing activity. Front. Microbiol. 2022, 13, 903363. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, J.; Chen, G.Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Pérez, D.; Castro, M.; Urtuvia, V.; Castillo, T.; Díaz-Barrera, A.; Peña, C. Production and recovery of poly-3-hydroxybutyrate P(3HB). of ultra-high molecular weight using fed-batch cultures of Azotobacter vinelandii OPNA strain. J. Chem. Technol. Biotechnol. 2019, 94, 1853–1860. [Google Scholar] [CrossRef]
- Mouzakitis, Y.; Adamides, E.D. Techno-economic assessment of an olive mill wastewater (OMWW) biorefinery in the context of circular bioeconomy. Engineering 2022, 3, 488–503. [Google Scholar] [CrossRef]
- Ahuja, V.; Singh, P.K.; Mahata, C.; Jeon, J.M.; Kumar, G.; Yang, Y.H.; Bhatia, S.K. A review on microbes mediated resource recovery and bioplastic (polyhydroxyalkanoates) production from wastewater. Microb. Cell Factories 2024, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Werker, A.; Bengtsson, S.; Johansson, P.; Mugnusson, P.; Gustafsson, E.; Hjort, M.; Anterrieu, S.; Karabegovic, L.; Alexandersson, T.; Karsson, A.; et al. Production quality control of mixed culture poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blends using full-scale municipal activated sludge and non-chlorinated solvent extraction. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 330–378. ISBN 9780367275594. [Google Scholar]
- Neumann, P.; Pesante, S.; Venegas, M.; Vidal, G. Developments in pre-treatment methods to improve anaerobic digestion of sewage sludge. Rev. Environ. Sci. Biotechnol. 2016, 15, 173–211. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Bengtsson, S.; Karlsson, A.; Alexandersson, T.; Quadri, L.; Hjort, M.; Johansson, P.; Werker, A. A process for polyhydroxyalkanoate (PHA) production from municipal wastewater treatment with biological carbon and nitrogen removal demonstrated at pilot-scale. New Biotechnol. 2017, 35, 42–53. [Google Scholar] [CrossRef]
- Limbergen, H.V.; Top, E.M.; Verstraete, W. Bioaugmentation in activated sludge: Current features and future perspectives. Appl. Microbiol. Biotechnol. 1998, 50, 16–23. [Google Scholar] [CrossRef]
- Boon, N.; Top, E.M.; Verstraete, W.; Siciliano, S.D. Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl. Environ. Microbiol. 2003, 69, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Geng, N.; Wu, Z.; Xiong, W.; Su, H. Artificially constructing mixed bacteria system for bioaugmentation of nitrogen removal from saline wastewater at low temperature. J. Environ. Manag. 2022, 324, 116351. [Google Scholar] [CrossRef]
- Han, X.; Jin, Y.; Yu, J. Rapid formation of aerobic granular sludge by bioaugmentation technology: A review. Chem. Eng. J. 2022, 437, 134971. [Google Scholar] [CrossRef]
- Pimenov, N.V.; Nikolaev, Y.A.; Dorofeev, A.G.; Grachev, V.A.; Kallistova, A.Y.; Mironov, V.V.; Vanteeva, A.V.; Grigor’Eva, N.V.; Berestovskaya, Y.Y.; Gruzdev, E.V.; et al. Bioaugmentation of anammox activated sludge with a nitrifying bacterial community as a way to increase the nitrogen removal efficiency. Microbiology 2022, 91, 133–142. [Google Scholar] [CrossRef]
- Krainara, S.; Suksong, W.; Suraraksa, B.; Prommeenate, P.; Thayanukul, P.; Luepromchai, E. Bioaugmentation of activated sludge with the immobilized 2-mercaptobenzothiazole-degrading bacterial consortium for rubber industrial wastewater treatment. J. Water Process Eng. 2023, 55, 104129. [Google Scholar] [CrossRef]
- Muter, O. Current trends in bioaugmentation tools for bioremediation: A critical review of advances and knowledge gaps. Microorganisms 2023, 11, 710. [Google Scholar] [CrossRef]
- Feng, X.; Deng, M.; Yu, J.; Wang, J.; Jin, W. Highly efficient removing polycyclic aromatic hydrocarbons in cooking wastewater by bio-augmentation activated sludge with Nocardioides sp. JWJ-L0 through sodium acetate co-metabolism. J. Water Process Eng. 2024, 58, 104844. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, L.; Huang, L.; Qin, Y.; Zhang, J.; Zhang, J.; Yan, Q. Enhanced nitrogen removal through bioaugmentation with Stutzerimonas stutzeri SW22: From denitrification mechanism to optimized sequencing batch reactor. J. Water Process Eng. 2025, 71, 107252. [Google Scholar] [CrossRef]
- Mineo, A.; van Loosdrecht, M.M.; Mannina, G. From waste activated sludge to polyhydroxyalkanoate: Insights from a membrane-based enrichment process. Chem. Eng. J. 2025, 475, 160089. [Google Scholar] [CrossRef]
- Mannina, G.; Mineo, A. Polyhydroxyalkanoate production from fermentation of domestic sewage sludge monitoring greenhouse gas emissions: A pilot plant case study at the WRRF of Palermo University (Italy). J. Environ. Manag. 2023, 348, 119423. [Google Scholar] [CrossRef]
- Lorini, L.; Munarin, G.; Salvatori, G.; Alfano, S.; Pavan, P.; Majone, M.; Valentino, F. Sewage sludge as carbon source for polyhydroxyalkanoates: A holistic approach at pilot scale level. J. Clean. Prod. 2022, 354, 131728. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Valentino, F.; Hjort, M.; Cirne, D.; Karabegovic, L.; Gerardin, F.; Werker, A. Polyhydroxyalkanoate (PHA) production from sludge and municipal wastewater treatment. Water Sci. Technol. 2014, 69, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Werker, A. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Carucci, G.; Papini, M.P.; Riccardi, C.; Majone, M.; Carrasco, F. Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res. 2005, 39, 2076–2084. [Google Scholar] [CrossRef]
- Matos, M.; Cruz, R.A.; Cardoso, P.; Silva, F.; Freitas, E.B.; Carvalho, G.; Reis, M.A. Combined strategies to boost polyhydroxyalkanoate production from fruit waste in a three-stage pilot plant. ACS Sustain. Chem. Eng. 2021, 9, 8270–8279. [Google Scholar] [CrossRef]
- Silva, F.; Matos, M.; Pereira, B.; Ralo, C.; Pequito, D.; Marques, N.; Reis, M.A. An integrated process for mixed culture production of 3-hydroxyhexanoate-rich polyhydroxyalkanoates from fruit waste. Chem. Eng. J. 2022, 427, 131908. [Google Scholar] [CrossRef]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef]
- Chakravarty, P.; Mhaisalkar, V.; Chakrabarti, T. Study on poly-hydroxyalkanoate (PHA) production in pilot scale continuous mode wastewater treatment system. Bioresour. Technol. 2010, 101, 2896–2899. [Google Scholar] [CrossRef]
- Traina, F.; Corsino, S.F.; Capodici, M.; Licitra, E.; Di Bella, G.; Torregrossa, M.; Viviani, G. Combined recovery of polyhydroxyalkanoates and reclaimed water in the mainstream of a WWTP for agro-food industrial wastewater valorisation by membrane bioreactor technology. J. Environ. Manag. 2024, 351, 119836. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Bengtsson, S.; De Grazia, G.; Alexandersson, T.; Quadri, L.; Johansson, P.; Werker, A. Mixed-culture polyhydroxyalkanoate (PHA) production integrated into a food-industry effluent biological treatment: A pilot-scale evaluation. J. Environ. Chem. Eng. 2020, 8, 104469. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Visser, C.; Korving, L. PHARIO: Stepping Stone to a Sustainable Value Chain for PHA Bioplastic Using Municipal Activated Sludge; Stichting Toegepast Onderzoek Waterbeheer: Amersfoort, The Netherlands, 2017; pp. 1–93. [Google Scholar]
- Tamis, J.; Mulders, M.; Dijkman, H.; Rozendal, R.; Van Loosdrecht, M.C.; Kleerebezem, R. Pilot-scale polyhydroxyalkanoate production from paper mill wastewater: Process characteristics and identification of bottlenecks for full-scale implementation. J. Environ. Eng. 2018, 144, 04018107. [Google Scholar] [CrossRef]
- Pozo, G.; Villamar, A.C.; Martínez, M.; Vidal, G. Polyhydroxyalkanoates (PHA) biosynthesis from kraft mill wastewaters: Biomass origin and C:N relationship influence. Water Sci. Technol. 2011, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Jarpa, M.; Pozo, G.; Baeza, R.; Martínez, M.; Vidal, G. Polyhydroxyalkanoate biosynthesis from paper mill wastewater treated by a moving bed biofilm reactor. J. Environ. Sci. Health A 2012, 47, 2052–2059. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, B.; Li, F.; Chen, Z. Substrate strategy optimization for polyhydroxyalkanoates producing culture enrichment from crude glycerol. Bioresour. Technol. 2020, 311, 123516. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Alonso, Á.; Pei, R.; van Loosdrecht, M.C.; Kleerebezem, R.; Werker, A. Scaling-up microbial community-based polyhydroxyalkanoate production: Status and challenges. Bioresour. Technol. 2021, 327, 124790. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.; Carvalho, G.; Kragelund, C.; Silva, A.F.; Barreto Crespo, M.T.; Reis, M.A.; Nielsen, P.H. Link between microbial composition and carbon substrate-uptake preferences in a PHA-storing community. ISME J. 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Mamo, Z.; Abera, S.; Tafesse, M. Shotgun metagenomic analysis reveals the diversity of PHA producer bacterial community and PHA synthase gene in Addis Ababa municipal solid waste disposal area ‘Qoshe’. Ann. Microbiol. 2024, 74, 33. [Google Scholar] [CrossRef]
- Argiz, L.; Gonzalez-Cabaleiro, R.; Correa-Galeote, D.; del Rio, A.V.; Mosquera-Corral, A. Open-culture biotechnological process for triacylglycerides and polyhydroxyalkanoates recovery from industrial waste fish oil under saline conditions. Sep. Purif. Technol. 2021, 270, 118805. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhu, L.; Liu, B.; Liu, S.; Huang, H.; Wen, Q. Effects of salinity regulation strategies on the enrichment of polyhydroxyalkanoate (PHA) producing mixed cultures: Microbial community succession and metabolic mechanisms. Chem. Eng. J. 2025, 506, 160001. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Marreiros, B.C.; Reis, M.A. Polyhydroxyalkanoates production by mixed microbial culture under high salinity. Sustainability 2022, 14, 1346. [Google Scholar] [CrossRef]
- Koller, M.; Obruča, S. Biotechnological production of polyhydroxyalkanoates from glycerol: A review. Biocat. Agric. Biotechnol. 2022, 42, 102333. [Google Scholar] [CrossRef]
- Baruah, N.; Haajanen, R.; Rahman, M.T.; Pirttilä, A.M.; Koskimäki, J.J. Biosynthesis of polyhydroxybutyrate by Methylorubrum extorquens DSM 13060 is essential for intracellular colonization in plant endosymbiosis. Front. Plant Sci. 2024, 15, 1302705. [Google Scholar] [CrossRef]
- Kumar, P.; Jun, H.B.; Kim, B.S. Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain LL1. Int. J. Biol. Macromol. 2018, 107, 2552–2558. [Google Scholar] [CrossRef]
- Sruamsiri, D.; Thayanukul, P.; Suwannasilp, B.B. In situ identification of polyhydroxyalkanoate (PHA)-accumulating microorganisms in mixed microbial cultures under feast/famine conditions. Sci. Rep. 2020, 10, 3752. [Google Scholar] [CrossRef]
- Yasuda, S.; Suenaga, T.; Orschler, L.; Agrawal, S.; Lackner, S.; Terada, A. Metagenomic insights into functional and taxonomic compositions of an activated sludge microbial community treating leachate of a completed landfill: A pathway-based analysis. Front. Microbiol. 2021, 12, 640848. [Google Scholar] [CrossRef]
- Pavan, F.A.; Junqueira, T.L.; Watanabe, M.D.; Bonomi, A.; Quines, L.K.; Schmidell, W.; de Aragão, G.M. Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem. Eng. J. 2019, 146, 97–104. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Biopolymers production by mixed culture and their applications in water and wastewater treatment. Water Environ. Res. 2015, 87, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Sarvajith, M.; Nancharaiah, Y.V. Properties of alginate-like exopolymers recovered from flocculent and granular microbial sludges of different biological treatment systems treating real municipal wastewater. Sep. Purif. Technol. 2023, 313, 123460. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Z.; Wen, Q.; Liu, S.; Wang, Y.; Wang, Z. Sequential recovery of extracellular alginate and intracellular polyhydroxyalkanoate (PHA) from mixed microbial culture PHA production system. J. Clean. Prod. 2024, 448, 141668. [Google Scholar] [CrossRef]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.; Lin, Y. Flame retardant property of flax fabrics coated by extracellular polymeric substances recovered from both activated sludge and aerobic granular sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef]
- Lin, Y.M.; Nierop, K.G.J.; Girbal-Neuhauser, E.; Adriaanse, M.; van Loosdrecht, M.C.M. Sustainable polysaccharide-based biomaterial recovered from waste aerobic granular sludge as a surface coating material. Sustain. Mater. Technol. 2015, 4, 24–29. [Google Scholar] [CrossRef]
- Pandey, A.; Adama, N.; Adjallé, K.; Blais, J.F. Sustainable applications of polyhydroxyalkanoates in various fields: A critical review. Int. J. Biol. Macromol. 2022, 221, 1184–1201. [Google Scholar] [CrossRef]
- Giubilini, A.; Messori, M.; Bondioli, F. 3D-printed poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)-cellulose-based scaffolds for biomedical applications. Biomacromolecules 2023, 24, 3961–3971. [Google Scholar] [CrossRef]
- Rambo, C.R.; Costa, C.M.; Carminatti, C.A.; Recouvreux, D.O.S.; d’Acampora, A.J.; Porto, L.M. Osteointegration of poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds incorporated with violacein. Mater. Sci. Eng. C 2012, 32, 385–389. [Google Scholar] [CrossRef]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Development and thermochemical characterization of an antioxidant material based on polyhydroxybutyrate electrospun microfibers. Int. J. Biol. Macromol. 2021, 183, 772–780. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millan, R.; Sanchez, A.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2021, 119, 111602. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Banerjee, S. Valorization of biowaste for sustainable 3D printing in the pharmaceutical and biomedical fields: Advances, challenges, and future perspectives. ACS Sustain. Resour. Manag. 2025, 3, e9515. [Google Scholar] [CrossRef]
- Perez-Torrero, E.; Luna-Rodriguez, L.E.; Gomez-Herrera, M.L.; Rivera-Muñ, E.M. Pattern of x-ray diffraction test for quality control of the components that resembling the bone tissue constituents. J. Biosci. Med. 2025, 13, 152–161. [Google Scholar] [CrossRef]
- Amores-Monge, V.; Goyanes, S.; Ribba, L.; Lopretti, M.; Sandoval-Barrantes, M.; Camacho, M.; Corrales-Ureña, Y.; Vega-Baudrit, J.R. Pineapple agro-industrial biomass to produce biomedical applications in a circular economy context in Costa Rica. Polymers 2022, 14, 4864. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Cerri, M.; Wille, F.; Arn, S.; Bucheli, T.D.; Widmer, F.; Werz, R.; McNeill, K.; Manfrin, A.; Sander, M. An analytical workflow to quantify biodegradable polyesters in soils and its application to incubation experiments. Environ. Sci. Technol. 2025, 59, 8108–8118. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Navia, R. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- Murugan, P.; Ong, S.Y.; Hashim, R.; Kosugi, A.; Arai, T.; Sudesh, K. Development and evaluation of controlled release fertilizer using P(3HB-co-3HHx) on oil palm plants (nursery stage) and soil microbes. Biocatal. Agric. Biotechnol. 2020, 28, 101710. [Google Scholar] [CrossRef]
- Tsekova, P.; Nachev, N.; Valcheva, I.; Draganova, D.; Naydenov, M.; Spasova, M.; Stoilova, O. Encapsulation of Bacillus subtilis in electrospun poly(3-hydroxybutyrate) fibers coated with cellulose derivatives for sustainable agricultural applications. Polymers 2024, 16, 2749. [Google Scholar] [CrossRef]
- Sfez, S.; Meester, S.; Vlaeminck, S.; Dewulf, J. Improving the resource footprint evaluation of products recovered from wastewater: A discussion on appropriate allocation in the context of circular economy. Resour. Conserv. Recycl. 2019, 148, 132–144. [Google Scholar] [CrossRef]
- Keith, M.; Koller, M.; Lackner, M. Carbon recycling of high value bioplastics: A route to a zero-waste future. Polymers 2024, 16, 1621. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, R.; Wu, Y.; Xue, P. Additive manufacturing of wood flour/polyhydroxyalkanoates (PHA) fully bio-based composites based on micro-screw extrusion system. Mater. Des. 2021, 199, 109418. [Google Scholar] [CrossRef]
- Plouzeau, M.; Belyamani, I.; Fatyeyeva, K.; Marais, S.; Kobzar, Y.; Cauret, L. Recyclability of poly(hydroxybutyrate-co-hydroxyhexanoate) (PHBH) for food packaging applications. Food Packag. Shelf Life 2023, 40, 101170. [Google Scholar] [CrossRef]
- Ding, K.; Xu, L.; Chen, Y.; Li, W.; Chai, X.; Dai, X.; Wu, B. Mechanistic insights into polyhydroxyalkanoate-enhanced denitrification capacity of microbial community: Evolution of community structure and intracellular electron transfer of nitrogen metabolism. Sci. Total Environ. 2023, 856, 159147. [Google Scholar] [CrossRef]
- Tomietto, P.; Loulergue, P.; Paugam, L.; Audic, J.L. Biobased polyhydroxyalkanoate (PHA) membranes: Structure/performances relationship. Sep. Purif. Technol. 2020, 252, 117419. [Google Scholar] [CrossRef]
- Vermeer, C.M.; Rossi, E.; Tamis, J.; Jonkers, H.M.; Kleerebezem, R. From waste to self-healing concrete: A proof-of-concept of a new application for polyhydroxyalkanoate. Res. Conser. Recycl. 2021, 164, 105206. [Google Scholar] [CrossRef]
- Singh, A.K.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef]
- Food and Drug Administration. Use of International Standard ISO 10993-1: “Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process”; Guidance Document; Guidance for Industry and FDA Staff, Dockets Management: Rockville, MD, USA, 8 September 2023. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 15 September 2025).
- Walsh, M.; Schenk, G.; Schmidt, S. Realising the circular phosphorus economy delivers for sustainable development goals. npj Sustain. Agric. 2023, 1, 2. [Google Scholar] [CrossRef]
- European Sustainable Phosphorus Platform. Circular Economy Action—CAP 2025: Summary of Research & Development Policy Recommendations, Version 2.2.24; ESPP: Brussels, Belgium, January 2025; Available online: https://www.phosphorusplatform.eu/images/Events/CEA-CAP%2021-22%20Jan%202025/Frank%20ESPP%20summary%20RD%20policy%20recommendations%20v2_2_24.pdf (accessed on 15 September 2025).
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulation (EC) Nos 1069/2009 and 1107/2009, and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, L170, 1–114. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 15 September 2025).
- European Union. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union 2004, L338, 4–17. Available online: https://eur-lex.europa.eu/eli/reg/2004/1935/2021-03-27 (accessed on 15 September 2025).


| Wastewater/Resource | MMC | Bioprocess | EPS Type | Application | Ref. |
|---|---|---|---|---|---|
| Municipal wastewater | Enriched AS | Fed-batch | ALE | N.D. | [93] |
| Municipal wastewater | Non-enriched AGS and AS | SBR | ALE | Wastewater and surplus biosolid treatment | [94] |
| Municipal wastewater | AGS bioaugmented with microalgae | SBR | ALE | N.D. | [39] |
| Municipal wastewater | Non-enriched AGS | SBR | N.D. | Flame retardant | [95] |
| Municipal wastewater | Non-enriched AS | Aerobic continuous reactor | Flame retardant | ||
| Municipal/Slaughterhouse wastewater | Non-enriched AGS | SBR | Methyl furaldehyde and levoglucosenone | Tissue coating enhancing. Hydrophobicity | [96] |
| WAS a | Synthetic MMC | Aerobic batch | N.D. | Flocculation and metal chelator agent | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda, M.I.; Seeger, M.; Vidal, G. Carbon Recovery from Wastewater Feedstocks: Synthesis of Polyhydroxyalkanoates for Target Applications. Resources 2025, 14, 156. https://doi.org/10.3390/resources14100156
Sepúlveda MI, Seeger M, Vidal G. Carbon Recovery from Wastewater Feedstocks: Synthesis of Polyhydroxyalkanoates for Target Applications. Resources. 2025; 14(10):156. https://doi.org/10.3390/resources14100156
Chicago/Turabian StyleSepúlveda, Mario I., Michael Seeger, and Gladys Vidal. 2025. "Carbon Recovery from Wastewater Feedstocks: Synthesis of Polyhydroxyalkanoates for Target Applications" Resources 14, no. 10: 156. https://doi.org/10.3390/resources14100156
APA StyleSepúlveda, M. I., Seeger, M., & Vidal, G. (2025). Carbon Recovery from Wastewater Feedstocks: Synthesis of Polyhydroxyalkanoates for Target Applications. Resources, 14(10), 156. https://doi.org/10.3390/resources14100156











