Geostatistical and Multivariate Assessment of Radon Distribution in Groundwater from the Mexican Altiplano
Abstract
1. Introduction
2. Materials and Methods
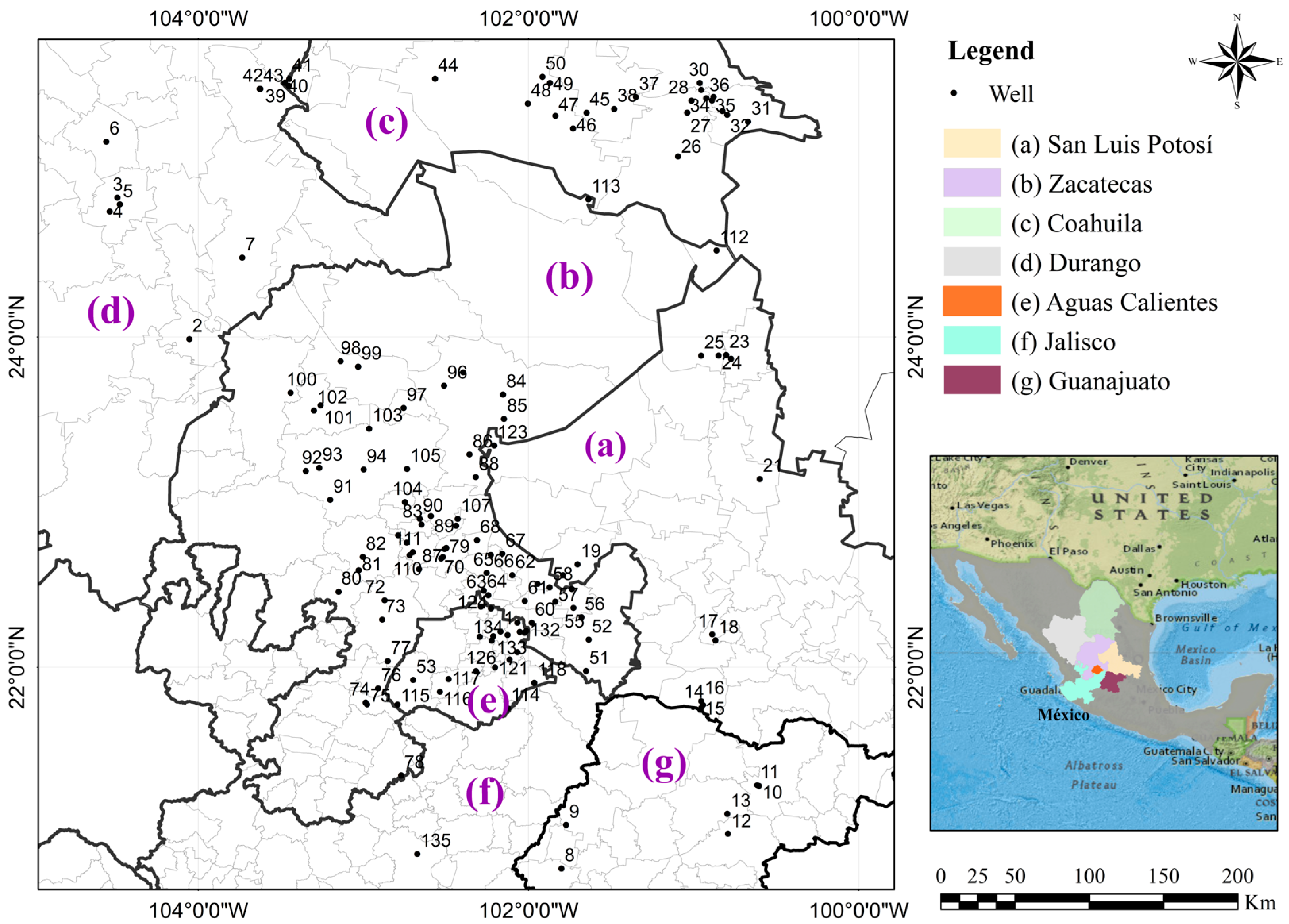
2.1. Study Site
2.2. Data and Experiment
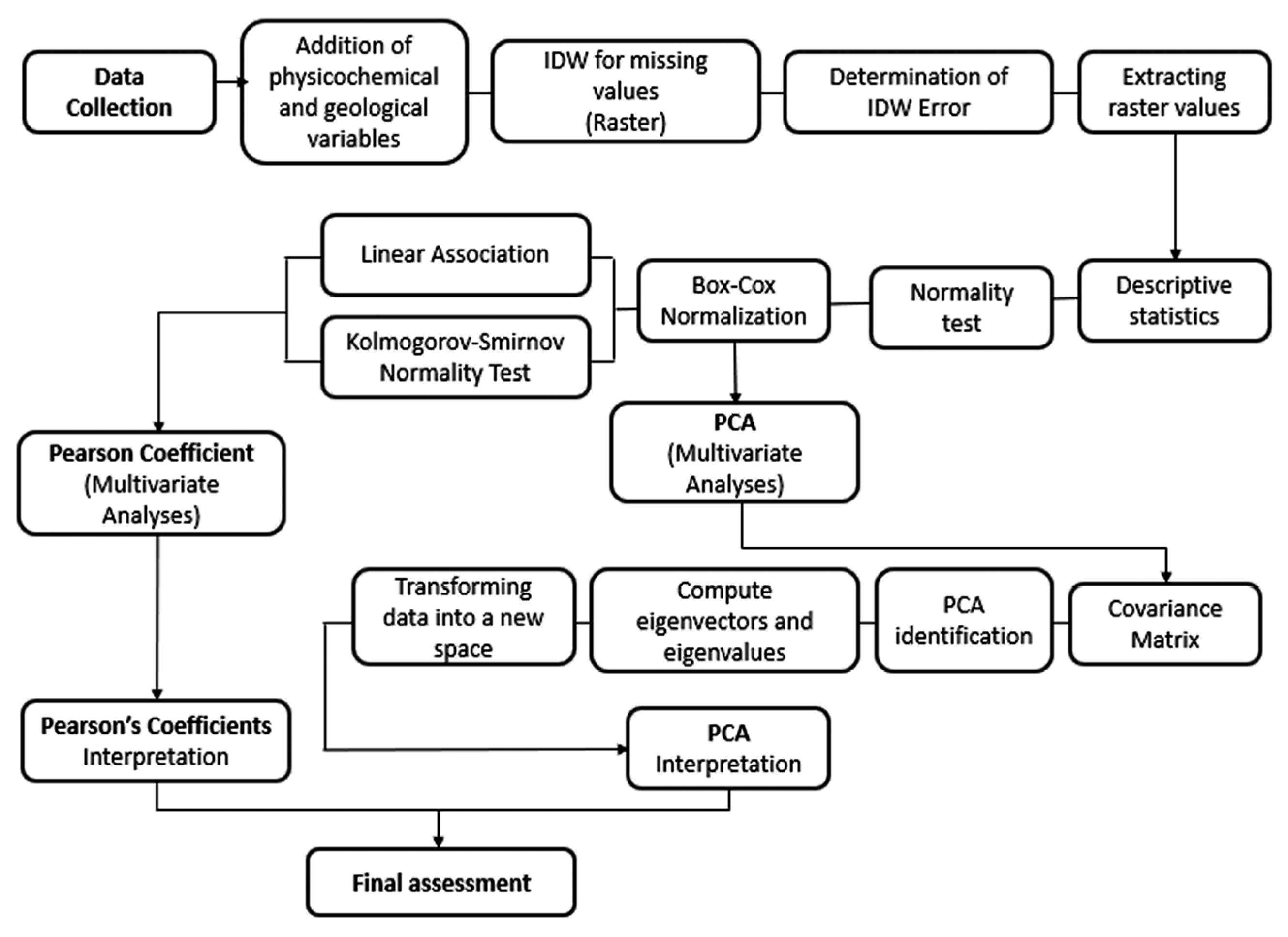
2.3. Data Processing
2.3.1. Spatial Distribution Analysis
2.3.2. Statistical Analysis
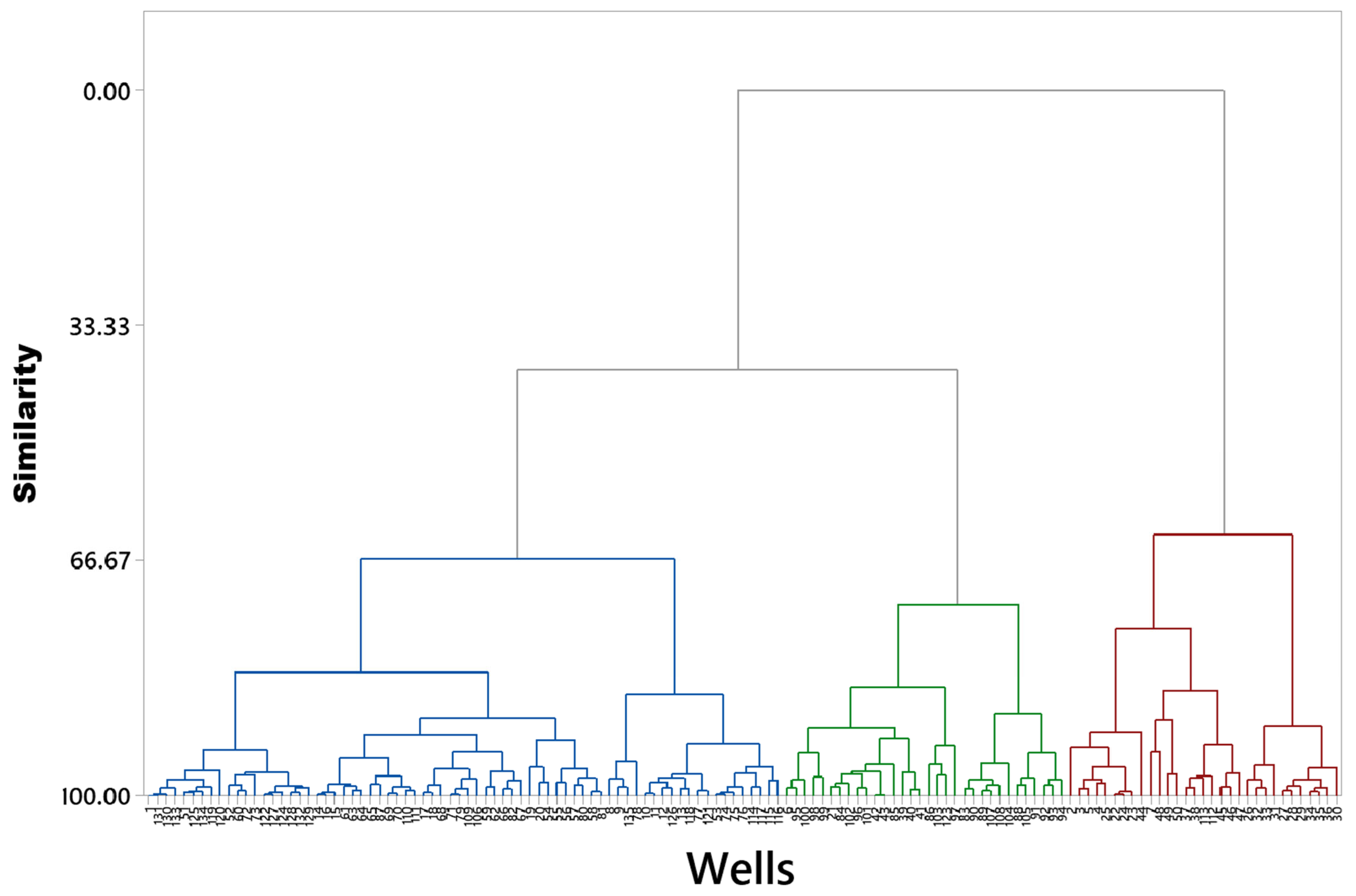
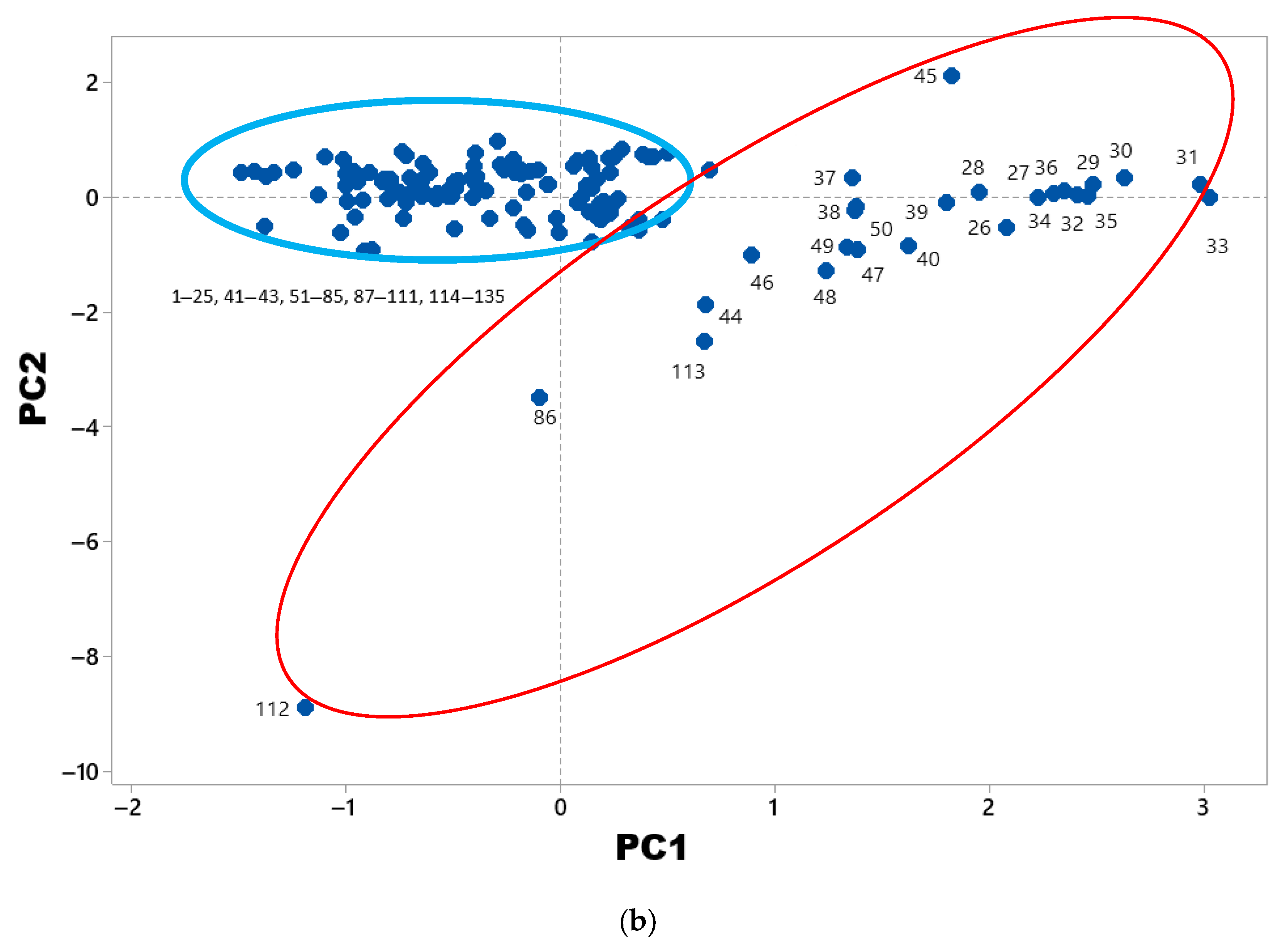
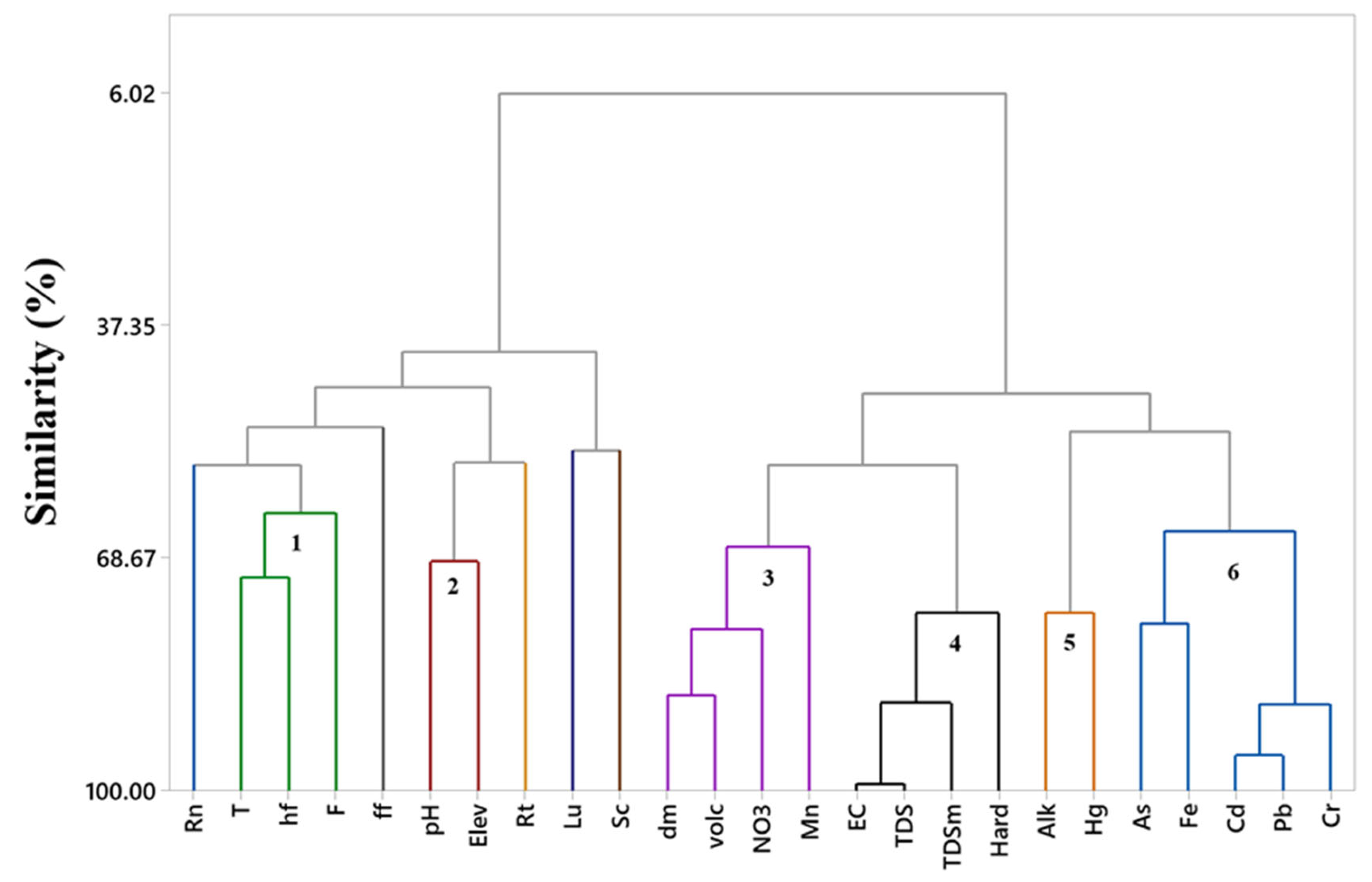
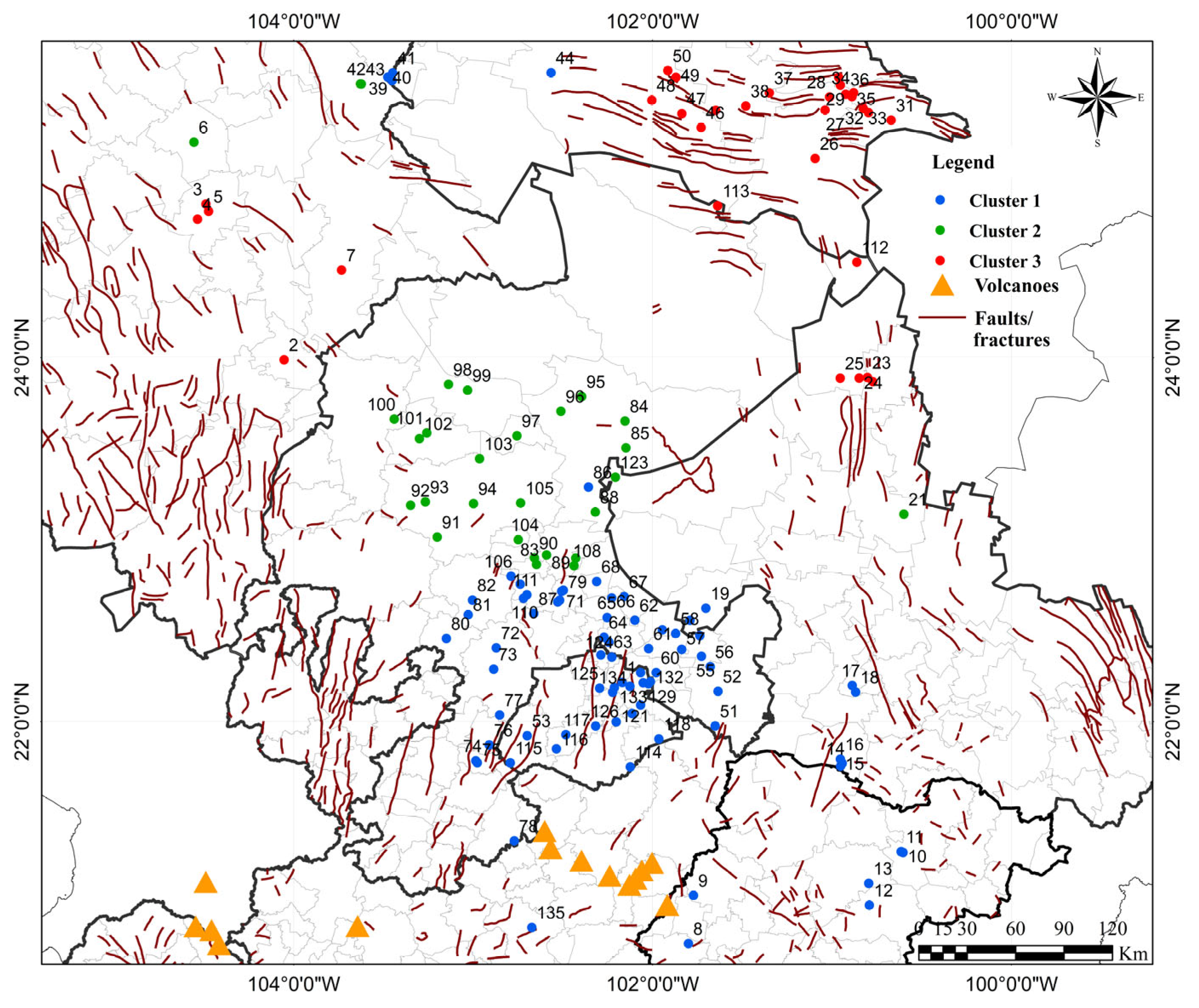
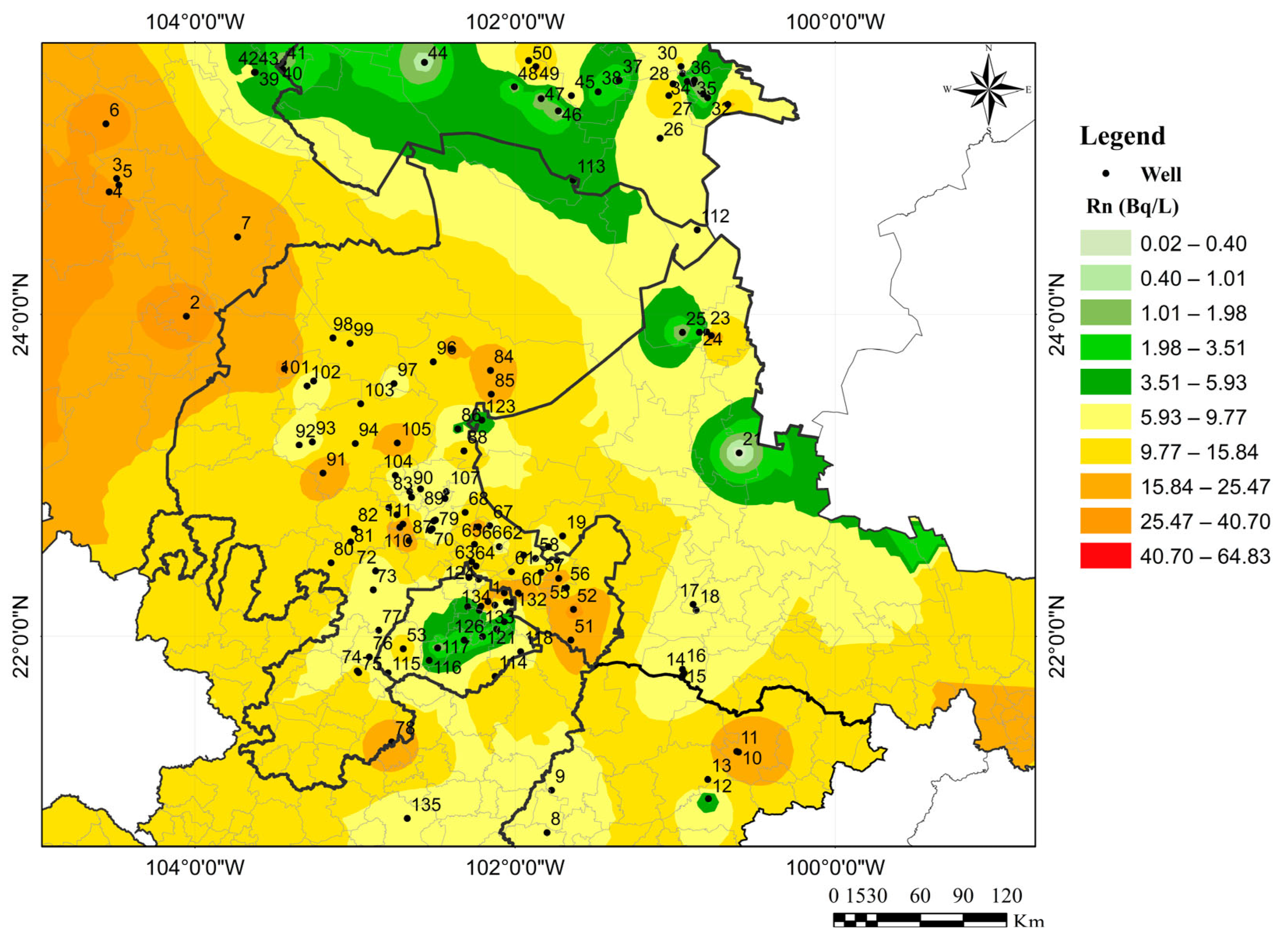
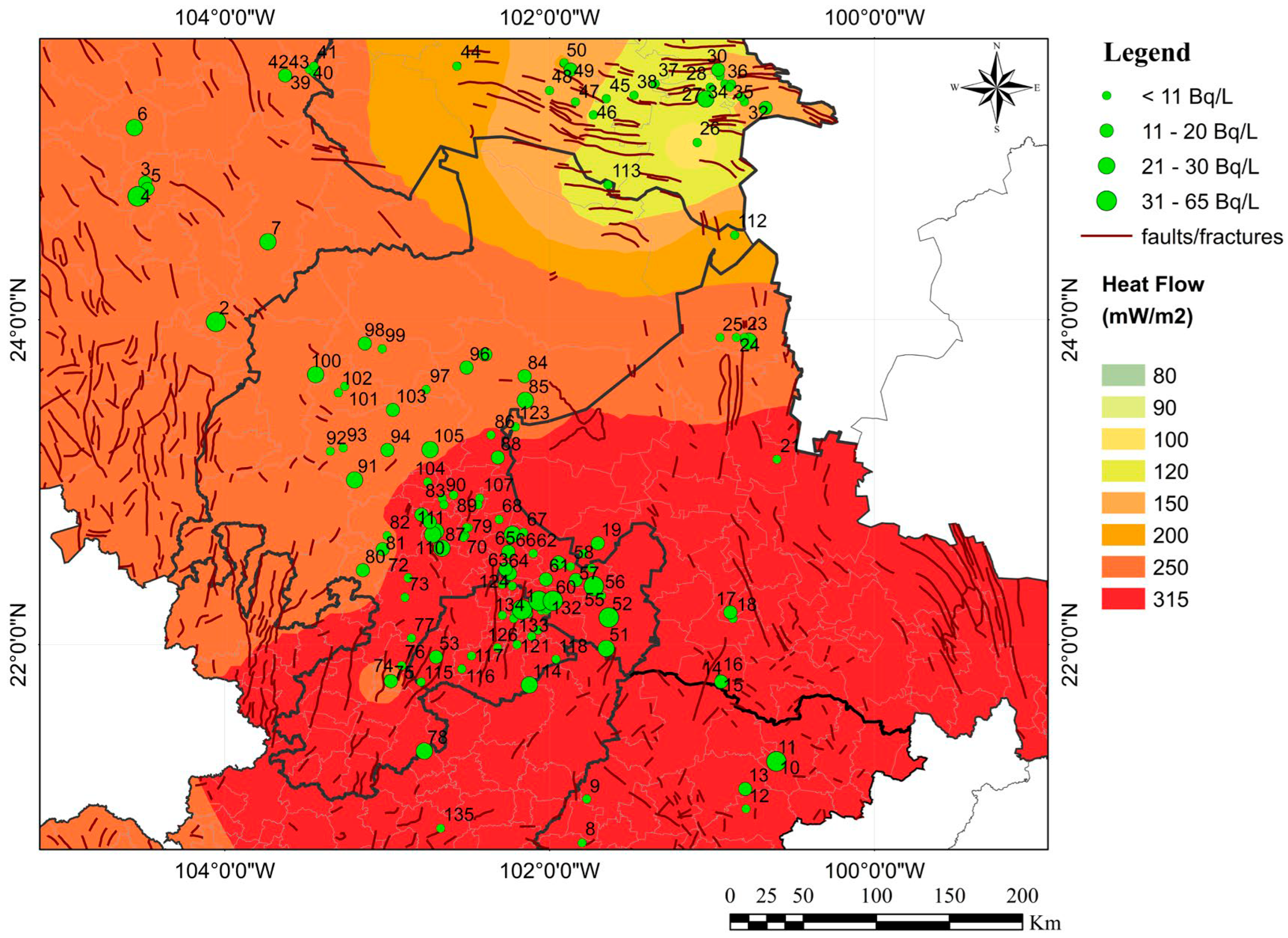
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| State | Aquifers |
| Aguascalientes | Valle de Aguas Calientes, Valle de Calvillo, El Llano, Venadero, and Valle de Chicalote |
| Coahuila | Saltillo Sur, Saltillo-Ramos Arizpe, Región Mazanera Zapaliname, General Cepeda-Sauceda, Principal-Región Lagunera, and La Paila |
| Durango | Vicente Guerreo-Poanas, San Juan del Río, Nazas, Peñón Blanco, and Villa Juárez |
| Jalisco | Tepatitlán |
| Guanajuato | La Muralla, Valle de León, Laguna Seca, and Cuenca Alta del Rio Laja |
| San Luis Potosí | Jaral de Berrios-Villa de Reyes, San Luis Potosí, Salinas de Hidalgo, Matehuala-Huizache, Cedral-Matehuala, Vanegas-Catorce, and El Barril |
| Zacatecas | Pinos, Villa Hidalgo, Loreto, La Blanca, Ojo Caliente, Chupaderos, Guadalupe Bañuelos, Villa Nueva, Jalpa-Juchipila, Nochistlán, Jerez, Calera, Guadalupe de las Corrientes, Puerto Madero, Benito Juárez, Agua Naval, Abrego, El Palmar, Sain Alto, El Salvador, and Cedros |
Appendix B
References
- World Health Organization. Guías Para la Calidad del Agua Potable Incluye el Primer Apéndice. Recomendaciones. Available online: https://sswm.info/sites/default/files/reference_attachments/OMS%202006.%20Gu%C3%ADa%20para%20la%20calidad%20dl%20agua%20potable.pdf (accessed on 10 March 2024).
- Prasad, Y.; Prasad, G.; Choubey, V.M.; Ramola, R.C. Geohydrological control on radon availability in groundwater. Radiat. Meas. 2009, 44, 122–126. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Anil, K.M.; Divya, L.; Jessen, G.; Magesh, S.B.; Rashmi, R. Determination of Gross Alpha Activity and Physic-Chemical Parameters of Borewell Samples of Mysore District, India. Int. Res. J. Environ. Sci. 2015, 4, 6–11. [Google Scholar]
- Chok, N.S. Pearson’s Versus Spearman’s and Kendall’s Correlation Coefficients for Continuous Data. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2008. [Google Scholar]
- Segovia, N.; Bulbulian, S. Radon determination in ground water. In Desarrollo; IAEA: Mexico City, Mexico, 1991. [Google Scholar]
- Olguin, M.T.; Segovia, N.; Tamez, E.; Alcántara, M.; Bulbulian, S. Radon concentration levels in ground water from Toluca, Mexico. Sci. Total Environ. 1993, 130–131, 43–50. [Google Scholar] [CrossRef] [PubMed]
- López, R.N.; Segovia, N.; Cisniega, M.G.; López, M.B.E.; Armienta, M.A.; Seidel, J.L.; Peña, P.; Godínez, L.; Tamez, E. Determination of radon, major and trace elements in water samples from springs and wells of northern Mexico State, Mexico. Geofísica Int. 2002, 41, 407–414. [Google Scholar]
- Segovia, N.; Tamez, E.; Peña, P.; Carrillo, J.; Acosta, E.; Armienta, M.A.; Iturbe, J.L. Groundwater flow system in the valley of Toluca, Mexico: An assay of natural radionuclide specific activities. Appl. Radiat. Isot. 1999, 50, 589–598. [Google Scholar] [CrossRef]
- De Iaco, S.; Maggio, S.; Palma, M. Radon Predictions with Geographical Information System Covariates: From Spatial Sampling to Modeling. Geogr. Anal. 2017, 49, 215–235. [Google Scholar] [CrossRef]
- Knutsson, G.; Olofsson, B. Radon content in groundwater from drilled wells in the Stockholm region of Sweden. Nor. Geol. Unders. Bull. 2002, 439, 79–85. [Google Scholar]
- Zafar, W.A.; Ahmed, J.; Barkat, A.; Nabi, A.; Mahmood, R.; Manzoor, S.; Iqbal, T. Spatial mapping of radon: Implication for fault delineation. Geochem. J. 2018, 52, 359–371. [Google Scholar] [CrossRef]
- Kasić, A.; Kasumović, A.; Adrović, F.; Hodžić, M. Radon measurements in well and spring water of the Tuzla area, Bosnia and Herzegovina. Arch. Ind. Hyg. Toxicol. 2016, 67, 332–339. [Google Scholar] [CrossRef]
- Skeppström, K.; Olofsson, B. Uranium and radon in groundwater. Eur. Water 2007, 17, 51–62. [Google Scholar]
- Villalba, L.; Colmenero Sujo, L.; Montero Cabrera, M.E.; Cano Jimenez, A.; Renteria Villalobos, M.; Delgado Mendoza, C.J.; Jurado Tenorio, L.A.; Davila Rangel, I.; Herrera Peraza, E.F. Radon concentrations in ground and drinking water in the state of Chihuahua, Mexico. J. Environ. Radioact. 2005, 80, 139–151. [Google Scholar] [CrossRef]
- Colmenero Sujo, L.; Montero Cabrera, M.E.; Villalba, L.; Renteria Villalobos, M.; Torres Moye, E.; Garcia Leon, M.; Garcia-Tenorio, R.; Mireles Garcia, F.; Herrera Peraza, E.F.; Sanchez Aroche, D. Uranium-238 and thorium-232 series concentrations in soil, radon-222 indoor and drinking water concentrations and dose assessment in the city of Aldama, Chihuahua, Mexico. J. Environ. Radioact. 2004, 77, 205–219. [Google Scholar] [CrossRef]
- Lopez, F.O.; Canoba, A.C. 222 Rn gas diffusion and determination of its adsorption coefficient on activated charcoal. J. Radioanal. Nucl. Chem. 2002, 252, 515–521. [Google Scholar] [CrossRef]
- Hopke, P.K.; Borak, T.B.; Doull, J.; Cleaver, J.E.; Eckerman, K.F.; Gundersen, L.C.S.; Harley, N.H.; Hess, C.T.; Kinner, N.E.; Kopecky, K.J.; et al. Health Risks Due to Radon in Drinking Water. Environ. Sci. Technol. 2000, 34, 921–926. [Google Scholar] [CrossRef]
- Grande, E.; Moran, J.E. Patterns in Radon Activity in California Groundwater. ACS EST Water 2021, 1, 2390–2402. [Google Scholar] [CrossRef]
- Pásztor, L.; Szabó, K.Z.; Szatmári, G.; Laborczi, A.; Horváth, Á. Mapping geogenic radon potential by regression kriging. Sci. Total Environ. 2016, 544, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Chaminé, H.I.; Espinha Marques, J.; Carvalho, J.M.; Pereira, A.J.S.C.; Carvalho, M.R.; Fonseca, P.E.; Pérez-Alberti, A.; Rocha, F. A comprehensive analysis of groundwater resources using GIS and multicriteria tools (Caldas da Cavaca, Central Portugal): Environmental issues. Environ. Earth Sci. 2015, 73, 2699–2715. [Google Scholar] [CrossRef]
- Guida, D.; Guida, M.; Cuomo, A.; Guadagnuolo, D.; Siervo, V. Assessment and Mapping of Radon-prone Areas on a regional scale as application of a Hierarchical Adaptive and Multi-scale Approach for the Environmental Planning. Case Study of Campania Region, Southern Italy. WSEAS Trans. Syst. 2013, 12, 105–120. [Google Scholar]
- Skeppström, K.; Olofsson, B. A prediction method for radon in groundwater using GIS and multivariate statistics. Sci. Total Environ. 2006, 367, 666–680. [Google Scholar] [CrossRef]
- Gong, G.; Mattevada, S.; O’Bryant, S.E. Comparison of the accuracy of kriging and IDW interpolations in estimating groundwater arsenic concentrations in Texas. Environ. Res. 2014, 130, 59–69. [Google Scholar] [CrossRef]
- Custodio, E. Groundwater in volcanic hard rocks. In Groundwater in Fractured Rocks, Selected Papers from the Groundwater in Fractured Rocks; Krásný, J., Sharp, J.M., Eds.; Taylor and Francis: Prague, Czech Republic, 2003; pp. 95–108. [Google Scholar]
- CONAGUA. Aguas Subterráneas. Disponibilidad por Acuíferos. 2023. Available online: https://sigagis.conagua.gob.mx/gas1/sections/Disponibilidad_Acuiferos.html (accessed on 28 July 2025).
- CONABIO: Explorador de Cambio Climático y Biodiversidad. 2023. Available online: https://www.biodiversidad.gob.mx/pais/cambio-climatico (accessed on 16 July 2025).
- García Amaro de Miranda, E. Distribución de la Precipitación en la República Mexicana. Investig. Geográficas 2003, 50, 67–76. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-46112003000100009&lng=es&tlng=es (accessed on 9 September 2025).
- Ferrari, L.; Pasquaré, G.; Venegas-Salgado, S.; Romero-Rios, F. Geology of the western Mexican Volcanic Belt and adjacent Sierra Madre Occidental and Jalisco Block. In Cenozoic Tectonics and Volcanism of Mexico; Delgado-Granados, H., Aguirre-Díaz, G.J., Stock, J.M., Eds.; Geological Society of America: Boulder, CO, USA, 2000; pp. 65–83. [Google Scholar]
- Valenzuela-Vásquez, L.; Ramírez-Hernández, J.; Reyes-López, J.; Sol-Uribe, A.; Lázaro-Mancilla, O. The origin of fluoride in groundwater supply to Hermosillo City, Sonora, México. Environ. Geol. 2006, 51, 17–27. [Google Scholar] [CrossRef]
- Shah, T.; Bruke, J.; Vullholth, K.; Angelica, M.; Custodio, E.; Daibes, F. Groundwater: A global assessment of scale and significance. In Water for Food Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Molden, D., Ed.; Earthscan: London, UK, 2007; pp. 395–423. [Google Scholar]
- Tositti, L.; Cinelli, G.; Brattich, E.; Galgaro, A.; Mostacci, D.; Mazzoli, C. Assessment of lithogenic radioactivity in the Euganean Hills magmatic district (NE Italy). J. Environ. Radioact. 2017, 166, 259–269. [Google Scholar] [CrossRef]
- CONAGUA-SINA. Calidad del Agua en México. Gobierno de México. Available online: https://www.gob.mx/conagua/articulos/calidad-del-agua (accessed on 17 May 2023).
- Schubert, M.; Buerkin, W.; Peña, P.; Lopez, A.E.; Balcázar, M. On-site determination of the radon concentration in water samples: Methodical background and results from laboratory studies and a field-scale test. Radiat. Meas. 2006, 41, 492–497. [Google Scholar] [CrossRef]
- Hernández Lalinde, J.D.; Espinosa Castro, J.F.; Peñaloza Tarazona, M.E.; Rodriguez, J.E.; Chacón Rangel, J.G.; Toloza Sierra, C.A.; Arenas Torrado, M.K.; Toloza Sierra, C.A.; Arenas Torrado, M.K.; Carrillo Sierra, S.M.; et al. Sobre el uso adecuado del coeficiente de correlación de Pearson: Definición, propiedades y suposiciones. AVFT–Arch. Venez. Farmacol. Ter. 2019, 37, 587–595. [Google Scholar]
- Marimuthu, S.; Mani, T.; Sudarsanam, T.D.; George, S.; Jeyaseelan, L. Preferring Box-Cox transformation, instead of log transformation to convert skewed distribution of outcomes to normal in medical research. Clin. Epidemiol. Glob. Health 2022, 15, 101043. [Google Scholar] [CrossRef]
- Berger, V.W.; Zhou, Y. Kolmogorov–Smirnov Test: Overview. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Matiatos, I.; Alexopoulos, A.; Godelitsas, A. Multivariate statistical analysis of the hydrogeochemical and isotopic composition of the groundwater resources in northeastern Peloponnesus (Greece). Sci. Total Environ. 2014, 476–477, 577–590. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; D’Enza, A.I.; Markos, A.; Tuzhilina, E. Publisher Correction: Principal component analysis. Nat. Rev. Methods Primers 2023, 3, 22. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Federal Register 40 Parts 141 and 142: National Primary Drinking Water Regulations; Radionuclides: Proposed Rule. 1991. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/10003I8N.PDF?Dockey=10003I8N.PDF (accessed on 9 September 2025).
- Zhao, R.; Yi, J. Geochemistry of thermal waters in the Changbaishan volcanic geothermal system, Northeast China—Implications for vapor-liquid separation controls on geothermal fluid composition. Arab. J. Geosci. 2021, 14, 419. [Google Scholar] [CrossRef]
- Liu, S.; Tang, X.; Han, X.; Zhang, D.; Wang, G. Hydrochemistry of the Geothermal in Gonghe Basin, Northeastern Tibetan Plateau: Implications for Hydro-Circulation and the Geothermal System. Water 2023, 15, 1971. [Google Scholar] [CrossRef]
- Gárfias, J.; Arroyo, N.; Aravena, R. Hydrochemistry and origins of mineralized waters in the Puebla aquifer system, Mexico. Environ. Earth Sci. 2010, 59, 1789–1805. [Google Scholar] [CrossRef]
- REDPA-CONAGUA: Consulta a la Base de Datos del REPDA; Registro Publico de Derechos de Agua. Available online: https://app.conagua.gob.mx/consultarepda.aspx (accessed on 3 July 2025).
- CONAGUA S. Estadísticas del Agua en México. México, DF: CONAGUA (2011) Estadísticas del Agua en México Comisión Nacional del Agua Reporte. 2011. Available online: https://www.gob.mx/cms/uploads/attachment/file/259373/_2011_EAM2011.pdf (accessed on 9 September 2025).
- CONAGUA. Disponibilidad Media Anual de Agua Subterránea del Acuífero Saltillo–Ramos Arizpe (0510); Comisión Nacional del Agua: Mexico City, Mexico, 2024; Available online: https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/coahuila/DR_0510.pdf (accessed on 9 September 2025).
- CONAGUA. Actualización de la Disponibilidad Media Anual de Agua Subterránea del Acuífero Región Manzanera–Zapalinamé (0511), Estado de Coahuila; Comisión Nacional del Agua: Mexico City, Mexico, 2024; Available online: https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/coahuila/DR_0511.pdf (accessed on 9 September 2025).
- CONAGUA. Disponibilidad Media Anual de Agua Subterránea del Acuífero Cedral-Matehuala (2407), Estado de San Luis Potosí; Comisión Nacional del Agua: Mexico City, Mexico, 2024; Available online: https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/sanluispotosi/DR_2407.pdf (accessed on 9 September 2025).
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef]
- Reyes-Gómez, V.M.; Alarcón-Herrera, M.T.; Gutiérrez, M.; López, D.N. Fluoride and Arsenic in an Alluvial Aquifer System in Chihuahua, Mexico: Contaminant Levels, Potential Sources, and Co-occurrence. Water Air Soil Pollut. 2013, 224, 1433. [Google Scholar] [CrossRef]
- Nickson, R.; McArthur, J.; Burgess, W.; Ahmed, K.M.; Ravenscroft, P.; Rahmanñ, M. Arsenic poisoning of Bangladesh groundwater. Nature 1998, 395, 338. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Postma, D.; Larsen, F.; Minh Hue, N.T.; Duc, M.T.; Viet, P.H.; Nhan, P.Q.; Jessen, S. Arsenic in groundwater of the Red River floodplain, Vietnam: Controlling geochemical processes and reactive transport modeling. Geochim. Cosmochim. Acta 2007, 71, 5054–5071. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F. The Ecology of Arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef]
- Zheng, Y.; Stute, M.; van Geen, A.; Gavrieli, I.; Dhar, R.; Simpson, H.J.; Schlosser, P.; Ahmed, K.M. Redox control of arsenic mobilization in Bangladesh groundwater. Appl. Geochem. 2004, 19, 201–214. [Google Scholar] [CrossRef]
- NOM-127-SSA1-2021; Official Mexican Standard. Environmental Health. Water for Human Use and Consumption. Permissible limits for Water Quality (Agua Para Uso y Consumo Humano. Límites Permisibles de la Calidad del Agua). Deparment, H., Ed.; Official Gazette of the Federation: Mexico City, Mexico, 2022.
- CONAGUA. Actualización de la Disponibilidad Media Anual de Agua Subterránea del Acuífero Principal–Región Lagunera (0523), Estado de Coahuila; Comisión Nacional del Agua: Mexico City, Mexico, 2024; Available online: https://www.gob.mx/cms/uploads/attachment/file/102876/DR_0523.pdf (accessed on 9 September 2025).
- López, J.A.; Rodríguez, G.; Chavarría, I.; Ornelas, O.D.; Sajo-Bohus, L. Correlation between underground radon gas and dormant geological faults. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 2016, 4, 265–275. [Google Scholar] [CrossRef]
- Santiago Carrasco, B.; Martínez Ramos, C.J.; Sánchez Bermeo, G.; Chiapa García, R.; Palacios García, R. Informe Final de la Cartografía Geológico-Minera Escala 1:250000, Carta Monterrey Clave G14–7, Estados de Coahuila, Nuevo León y Zacatecas; Consejo de Recursos Minerales: Coahuila, Mexico, 2000. [Google Scholar]
- Espinosa Aramburu, E.; Meneses Garibay, F.; Ignacio Jiménez, D. Carta Geológico-Minera General Cepeda, Clave G14–C32, Escala 1:50000; Mexicano, S.G., Ed.; Servicio Geológico Mexicano: Mexico City, Mexico, 2004. [Google Scholar]
- Rodríguez Rodríguez, J.S. Carta Geológico-Minera Sierra El Laurel Clave G14–C42, Escala 1:50000, Estado de Coahuila; Servicio Geológico Mexicano: Mexico City, Mexico, 2006. [Google Scholar]
- Carrasco Brígido, S.; Escamilla de la Rosa, J.E. Carta Geológico-Minera Agua Nueva Clave G14–C43, Escala 1:50000, Estado de Coahuila; Servicio Geológico Mexicano: Mexico City, Mexico, 2007. [Google Scholar]
- Carrillo-Rivera, J.J.; Varsányi, I.; Kovács, L.Ó.; Cardona, A. Tracing Groundwater Flow Systems with Hydrogeochemistry in Contrasting Geological Environments. Water Air Soil Pollut. 2007, 184, 77–103. [Google Scholar] [CrossRef]
- Mendoza-Amézquita, E.; Armienta-Hernández, M.A.; Ayora, C.; Soler, A.; Ramos-Ramírez, E. Potential lixiviation of trace elements in tailings from the mines La Asunción and Las Torres in the Guanajuato Mining District, Mexico. Rev. Mex. Cienc. Geológicas 2018, 23, 75–83. [Google Scholar]
- Valencia-Moreno, M.; Ochoa-Landín, L.; Noguez-Alcántara, B.; Ruiz, J.; Pérez-Segura, E. Características metalogenéticas de los depósitos de tipo pórfido cuprífero en México y su situación en el contexto mundial. Boletín Soc. Geológica Mex. 2006, 58, 1–26. [Google Scholar] [CrossRef]
- Roy, P.D.; García-Arriola, O.A.; Selvam, S.; Vargas-Martínez, I.G.; Sánchez-Zavala, J.L. Geochemistry of some fluoride and nitrate enriched water resources from the Oriental Basin: A prospective health risk hotspot from eastern-central Mexico. Environ. Geochem. Health 2025, 47, 114. [Google Scholar] [CrossRef]
- Zhu, G.; Cui, G.; Zhang, L.; Feng, Q.; Zhang, E.; Niu, Z.; Xu, W.; Li, X.; Deng, Z. Hydrogeochemical evidence for the geothermal origin of sedimentary hot dry rock in Gonghe Basin, Northwest China. Environ. Earth Sci. 2023, 82, 549. [Google Scholar] [CrossRef]
- Zielinski, R.A. Experimental Leaching of Volcanic Glass: Implications For Evaluation of Glassy Volcanic Rocks as Sources of Uranium. In Uranium in Volcanic and Volcaniclastic Rocks; Goodell, P.C., Waters, A.C., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1981; Volume 13, pp. 1–11. [Google Scholar]
- Maithani, P.B.; Srinivasan, S. Felsic Volcanic Rocks, a Potential Source of Uranium—An Indian Overview. Energy Procedia 2011, 7, 163–168. [Google Scholar] [CrossRef]
- Nieto-Samaniego, A.F.; Del Pilar-Martínez, A.; Suárez-Arias, A.M.; Angeles-Moreno, E.; Alaniz-Álvarez, S.A.; Levresse, G.; Xu, S.; Olmos-Moya, M.d.J.P.; Báez-López, J.A. The geology and tectonic evolution of the Mesa Central of Mexico during the Cenozoic era: A review: Geology and tectonics of the Mesa Central. Rev. Mex. Cienc. Geológicas 2023, 40, 187–213. [Google Scholar] [CrossRef]
- Del Pilar-Martínez, A.; Nieto-Samaniego, A.F.; Alaniz-Alvarez, S.A.; Angeles-Moreno, E. Geology of the southern Mesa Central of Mexico: Recording the beginning of a polymodal fault system. J. Maps 2020, 16, 199–211. [Google Scholar] [CrossRef]
- Schubert, M.; Paschke, A.; Lieberman, E.; Burnett, W.C. Air–Water Partitioning of 222Rn and its Dependence on Water Temperature and Salinity. Environ. Sci. Technol. 2012, 46, 3905–3911. [Google Scholar] [CrossRef]
- Reimann, C.; Hall, G.E.; Siewers, U.; Bjorvatn, K.; Morland, G.; Skarphagen, H.; Strand, T. Radon, fluoride and 62 elements as determined by ICP-MS in 145 Norwegian hard rock groundwater samples. Sci Total Env. 1996, 192, 1–19. [Google Scholar] [CrossRef]
- Salih, I.; Bäckström, M.; Karlsson, S.; Lund, E.; Pettersson, H.B.L. Impact of fluoride and other aquatic parameters on radon concentration in natural waters. Appl. Radiat. Isot. 2004, 60, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cinti, D.; Vaselli, O.; Poncia, P.P.; Brusca, L.; Grassa, F.; Procesi, M.; Tassi, F. Anomalous concentrations of arsenic, fluoride and radon in volcanic-sedimentary aquifers from central Italy: Quality indexes for management of the water resource. Environ. Pollut. 2019, 253, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, M.; Raymond, J.; Martel, R.; Drolet, J.-P.; Dugdale, S.J.; Bergeron, N. Analysis of Large-Scale Groundwater-Driven Cooling Zones in Rivers Using Thermal Infrared Imagery and Radon Measurements. Water 2023, 15, 873. [Google Scholar] [CrossRef]
- Zhou, H.; Wan, Y.; Su, H.; Li, C. Spatial–temporal evolution of soil gas Rn before two Ms ≥ 5.0 earthquakes in the mid-eastern of the Qilian fault zone (QLF). Sci. Rep. 2023, 13, 21491. [Google Scholar] [CrossRef]
- Aranda-Gómez, J.J.; Luhr, J.F.; Housh, T.B.; Valdez-Moreno, G.; Chávez-Cabello, G. Late Cenozoic intraplate-type volcanism in central and northern México: A review. In Geology of México: Celebrating the Centenary of the Geological Society of México; Alaniz-Álvarez, S.A., Nieto-Samaniego, Á.F., Eds.; Geological Society of America: Boulder, CO, USA, 2007; pp. 187–2250. [Google Scholar]
- Fite, J.; Tracol, R.; Gran-Aymerich, L.; Grandguillot, G.; Guillotin, L.; Davezac, H.; Loyen, J. La Qualité Radiologique de L’eau Mise en Distribution en France: 2005–2007; Autorité du Súreté Nucléaire MdlSedS, Institut de Radioprotection et de Sûreté Nucléaire, Eds.; Francia, 2009; pp. 1–37. Available online: https://bretagne-environnement.fr/notice-documentaire/qualite-radiologique-leau-mise-distribution-france-2005-2007 (accessed on 9 September 2025).
- Prol-Ledesma, R.M.; Morán-Zenteno, D.J. Heat flow and geothermal provinces in Mexico. Geothermics 2019, 78, 183–200. [Google Scholar] [CrossRef]
- Macedo Serrano, E.; Prol Ledesma, R.M. GIS-based analysis for regional exploration in the geothermal province of intraplate volcanism in the Center of Mexico. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 23–28 April 2023. [Google Scholar]
- Sukanya, S.; Noble, J.; Joseph, S. Application of radon (222Rn) as an environmental tracer in hydrogeological and geological investigations: An overview. Chemosphere 2022, 303, 135141. [Google Scholar] [CrossRef]
- Mair, J.; Petermann, E.; Lehné, R.; Henk, A. Can neotectonic faults influence soil air radon levels in the Upper Rhine Graben? An exploratory machine learning assessment. Sci. Total Env. 2024, 956, 177179. [Google Scholar] [CrossRef]
- Stanley, J.; Reading, L. Determination of radon source in basaltic groundwater, using geochemical tracers and chemometric statistical analysis. Heliyon 2025, 11, e41859. [Google Scholar] [CrossRef]
- Morales Arredondo, I.; Flores Ocampo, I.Z.; Armienta, M.A.; Morán Ramírez, J.; Hernández Hernández, M.A.; Landa Arreguin, J.F. Identificación de las fuentes de nitratos mediante métodos hidrogeoquímicos e isotópicos en el agua subterránea del Bajío Guanajuatense. Geofísica Int. 2020, 59, 169–194. [Google Scholar] [CrossRef]
- Bonotto, D.M.; Bueno, T.O. The natural radioactivity in Guarani aquifer groundwater, Brazil. Appl. Radiat. Isot. 2008, 66, 1507–1522. [Google Scholar] [CrossRef]
- Cho, B.W.; Choo, C.O. Geochemical Behavior of Uranium and Radon in Groundwater of Jurassic Granite Area, Icheon, Middle Korea. Water 2019, 11, 1278. [Google Scholar] [CrossRef]
| Variable | Data Source | Parameter |
|---|---|---|
| Physicochemical | Measured | Radon (Rn), pH, measured total dissolved solids (TDSm) *, electrical conductivity (EC), and temperature (T). |
| Physicochemical | From SINA-CONAGUA | Alkalinity (Alk), total dissolved solids (TDS) *, fluorides (F), water hardness (Hard), nitrates (NO3), arsenic (As), cadmium (Cd), chromium (Cr), mercury (Hg), lead (Pb), manganese (Mn), and iron (Fe) |
| Geological | From SINA-CONAGUA | Heat flow (hf), proximity to mineral deposits (dm), proximity to volcanic centers (volc), proximity to faults and fractures (ff), rock type (Rt), land use (Lu), soil classification (Sc), and elevation (elev) |
| Variables | Mean | Median | Min | Max | σ | CV * | |
|---|---|---|---|---|---|---|---|
| Physical and chemical (measured) | Rn (Bq/L) | 10.5 | 7.2 | 0.02 | 64.8 | 11.1 | 105.2 |
| mTDS (mg/L) | 351 | 288 | 0.4 | 2764 | 391 | 111.3 | |
| pH | 7.6 | 7.8 | 6.1 | 9.5 | 0.7 | 9.6 | |
| EC (μS/cm) | 807.9 | 585 | 98.3 | 4920 | 587.8 | 72.8 | |
| T (°C) | 27.3 | 27.1 | 18.9 | 37.3 | 3.4 | 12.6 | |
| Geological | hf (mW/m2) | 237 | 253 | 75 | 315 | 55.4 | 23.4 |
| dm (m) | 10917 | 9120 | 133 | 47136 | 8126 | 74.4 | |
| volc (m) | 195,707 | 154,853 | 16,341 | 450,446 | 114,931 | 58.7 | |
| ff(m) | 13,451 | 10,315 | 1176 | 45,654 | 10,599 | 78.8 | |
| Elev (m) | 1926 | 1996 | 185 | 2398 | 333 | 17.3 | |
| Physical and chemical | Alk (mg/L) | 163 | 149 | 1.5 | 628 | 80 | 49.1 |
| TDS (mg/L) | 527 | 423 | 60.7 | 3149 | 369 | 70 | |
| Hard (mg/L) | 177 | 130 | 2.2 | 1004 | 153 | 86.1 | |
| NO3 (mg/L) | 3.5 | 1.7 | 0.01 | 37 | 5.02 | 143.2 | |
| F (mg/L) | 1.5 | 1.1 | 0.01 | 7.2 | 1.1 | 76.2 | |
| As (μg/L) | 17.8 | 15.8 | 0.3 | 110 | 15.6 | 87.6 | |
| Cd (μg/L) | 1.8 | 2 | 0.02 | 5 | 1.04 | 57.9 | |
| Cr (μg/L) | 3.7 | 3.9 | 0.03 | 16.2 | 2.3 | 61.1 | |
| Hg (μg/L) | 0.3 | 0.3 | 0 | 5 | 0.44 | 129 | |
| Pb (μg/L) | 3.1 | 3.4 | 0.03 | 8.4 | 1.9 | 59.5 | |
| Mn (μg/L) | 30.6 | 7.3 | 0.2 | 425 | 67.9 | 222 | |
| Fe (μg/L) | 39.1 | 25 | 0.3 | 497 | 51.9 | 133 | |
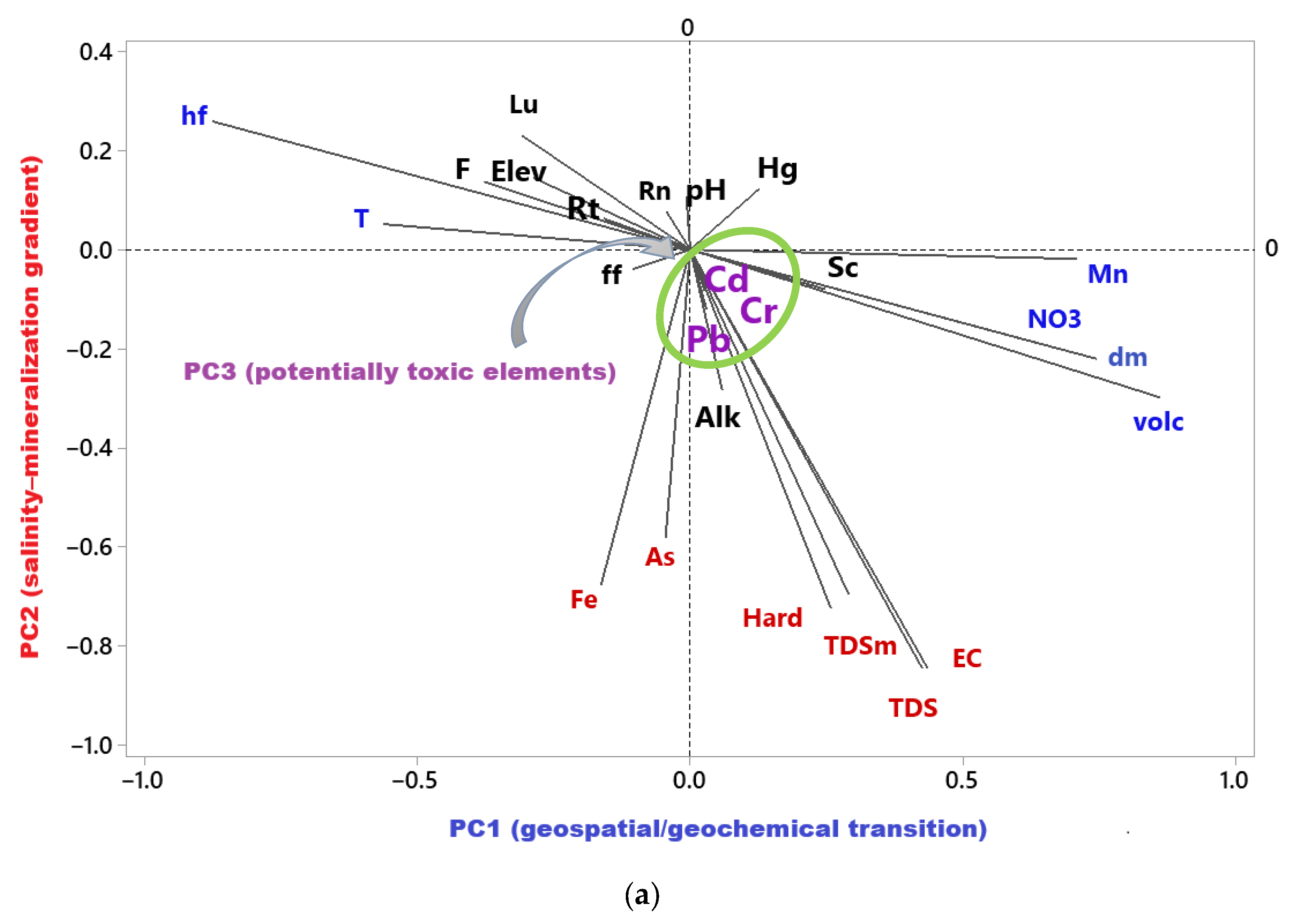
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 |
|---|---|---|---|---|---|---|---|---|
| Rn | −0.04 | 0.08 | −0.12 | −0.06 | 0.04 | −0.02 | 0.05 | 0.87 |
| pH | −0.01 | 0.11 | −0.26 | 0.05 | 0.80 | −0.16 | −0.05 | −0.06 |
| T | −0.56 | 0.05 | 0.11 | −0.04 | −0.08 | −0.12 | −0.28 | 0.36 |
| hf | −0.88 | 0.26 | −0.07 | −0.19 | 0.01 | −0.09 | 0.01 | 0.02 |
| Volc | 0.75 | −0.22 | −0.14 | 0.20 | −0.27 | −0.23 | 0.03 | 0.00 |
| Dm | 0.86 | −0.30 | 0.01 | 0.09 | −0.13 | −0.02 | 0.10 | 0.06 |
| Ff | −0.11 | −0.04 | −0.13 | −0.10 | 0.07 | −0.81 | 0.10 | 0.05 |
| Rt | −0.16 | 0.06 | −0.06 | −0.11 | 0.08 | 0.26 | −0.61 | 0.05 |
| Lu | −0.31 | 0.23 | 0.04 | −0.10 | −0.12 | 0.18 | 0.70 | −0.10 |
| Sc | 0.25 | −0.08 | −0.11 | −0.01 | 0.10 | −0.03 | 0.55 | 0.19 |
| Elev | −0.29 | 0.14 | −0.08 | −0.32 | 0.67 | 0.22 | 0.01 | 0.10 |
| Alk | 0.06 | −0.28 | −0.04 | 0.86 | −0.08 | 0.04 | 0.10 | −0.08 |
| EC | 0.44 | −0.84 | 0.08 | 0.00 | −0.14 | −0.08 | 0.00 | −0.07 |
| TDS | 0.43 | −0.85 | 0.06 | 0.01 | −0.13 | −0.06 | 0.01 | −0.08 |
| TDSm | 0.29 | −0.70 | 0.51 | −0.06 | −0.04 | −0.10 | −0.02 | −0.13 |
| F | −0.38 | 0.14 | −0.02 | 0.29 | 0.42 | −0.16 | −0.06 | 0.24 |
| Hard | 0.26 | −0.72 | −0.19 | 0.16 | −0.19 | −0.11 | 0.00 | −0.18 |
| NO3 | 0.64 | −0.19 | 0.41 | −0.14 | −0.24 | −0.32 | −0.07 | −0.09 |
| As | −0.05 | −0.58 | 0.39 | 0.13 | 0.07 | 0.04 | 0.08 | 0.35 |
| Cd | 0.03 | −0.08 | 0.91 | 0.34 | −0.07 | 0.05 | −0.04 | −0.01 |
| Cr | 0.05 | −0.11 | 0.85 | −0.07 | −0.13 | 0.10 | 0.03 | −0.10 |
| Hg | 0.13 | 0.12 | 0.39 | 0.75 | 0.02 | 0.11 | −0.06 | 0.01 |
| Pb | 0.03 | −0.12 | 0.93 | 0.05 | −0.14 | 0.07 | −0.02 | −0.02 |
| Mn | 0.71 | −0.02 | 0.23 | −0.15 | −0.02 | 0.37 | 0.06 | −0.01 |
| Fe | −0.16 | −0.68 | 0.35 | 0.07 | −0.01 | 0.38 | −0.10 | 0.12 |
| Eigenvalue | 4.29 | 3.73 | 3.54 | 1.83 | 1.59 | 1.37 | 1.31 | 1.22 |
| Proportion of Variance (%) | 17.1 | 14.9 | 14.2 | 7.3 | 6.4 | 5.5 | 5.2 | 4.9 |
| Cumulative Proportion (%) | 17.1 | 32 | 46.2 | 53.5 | 59.9 | 65.4 | 70.6 | 75.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizarro Sánchez, A.; Renteria-Villalobos, M.; Cabadas Báez, H.V.; Villarreal Vega, A.; Balcázar, M.; Zepeda Mondragón, F. Geostatistical and Multivariate Assessment of Radon Distribution in Groundwater from the Mexican Altiplano. Resources 2025, 14, 154. https://doi.org/10.3390/resources14100154
Bizarro Sánchez A, Renteria-Villalobos M, Cabadas Báez HV, Villarreal Vega A, Balcázar M, Zepeda Mondragón F. Geostatistical and Multivariate Assessment of Radon Distribution in Groundwater from the Mexican Altiplano. Resources. 2025; 14(10):154. https://doi.org/10.3390/resources14100154
Chicago/Turabian StyleBizarro Sánchez, Alfredo, Marusia Renteria-Villalobos, Héctor V. Cabadas Báez, Alondra Villarreal Vega, Miguel Balcázar, and Francisco Zepeda Mondragón. 2025. "Geostatistical and Multivariate Assessment of Radon Distribution in Groundwater from the Mexican Altiplano" Resources 14, no. 10: 154. https://doi.org/10.3390/resources14100154
APA StyleBizarro Sánchez, A., Renteria-Villalobos, M., Cabadas Báez, H. V., Villarreal Vega, A., Balcázar, M., & Zepeda Mondragón, F. (2025). Geostatistical and Multivariate Assessment of Radon Distribution in Groundwater from the Mexican Altiplano. Resources, 14(10), 154. https://doi.org/10.3390/resources14100154









