Abstract
This study investigates the effectiveness of hydrothermal and alkaline pretreatment methods in enhancing the concentration of fermentable sugars derived from rice husk waste. After the pretreatments, enzymatic hydrolysis and fermentation processes were executed to evaluate the ethanol production from each pretreatment. Rice husk powder measuring ≤250 µm was used. For the alkaline pretreatment, sodium hydroxide (NaOH) was used at concentrations ranging from 0.5, 1 to 1.5% w/v. The efficacy of the hydrothermal pretreatment method was evaluated after 15, 30 and 45 min at 120 °C. The enzymatic hydrolysis process was performed over 144 h at 50 °C, pH 4.8 with an enzyme loading of 30 FPU (filter paper units). Fermentation was carried out at 37 °C using a strain of Saccharomyces cerevisiae Hansen 1883 (NCYC 366). Results indicated that the optimal conditions for alkaline pretreatment were observed at a 1.5% NaOH, while the best hydrothermal procedure was achieved at 120 °C and 45 min. The impact of these pretreatments was assessed based on the efficiency of enzymatic hydrolysis. The alkaline pretreatment resulted in 81.70% conversion of cellulose to glucose and 96.30% conversion of hemicellulose to xylose. In contrast, the hydrothermal pretreatment achieved 93% cellulose-to-glucose conversion and 83.35% hemicellulose-to-xylose conversion. The ethanol production registered ranged from 13 to 13.23 g·L−1, corresponding to a conversion factor of 0.43 for ethanol from fermentable sugars.
1. Introduction
The rapid growth of the population has led to an increase in food production, especially for products such as rice, and, therefore, an increase in the generation of waste [1]. World rice production has reached 754.6 million tons/year, which generates approximately 150.92 million tons of husks [2], representing approximately 20% of the total weight of the grain. This residue is rich in cellulose, hemicellulose and lignin [3,4]. The main component of rice husk is cellulose, which is made up of linear chains of β-(1-4)-D-glucopyranosyl units of approximately 10,000 glycoside units, and hemicellulose, which is a heteropolysaccharide composed of monomers such as xylose, glucose, arabinose and gluconic acid [5], which can be enzymatically hydrolyzed to their monomers for subsequent use in the production of food, chemicals and biofuels [6]. Other components found in rice hulls include lignin and silicon dioxide, at concentrations of approximately 15% and 20%, respectively, which are inhibitors that block cellulase access to cellulose and hemicellulose irreversibly [7,8].
The application of physicochemical pretreatments is effective when the lignin and silicon dioxide content are high, and these pretreatments are intended to promote the separation of cellulose and hemicellulose from lignin and silicon dioxide and avoid steric hindrance, thus improving the formation of fermentable sugars [9]. One of the advantages of the enzymatic hydrolysis of cellulose and hemicellulose is its specificity; they do not produce undesirable by-products that may limit the use of cellobiose, glucose and xylose, and recent advances in genetic engineering have made it possible to obtain genetically modified microorganisms, which produce enzymes that use shorter reaction times and act at a slightly acidic pH without the need for expensive pretreatments [10]. The β-glucosidase (cellulase), in its free state, has the ability to hydrolyze cellulose and hemicellulose, and its activity is affected when it is adsorbed by solid compounds such as silica dioxide, which is present in rice hulls. Lignin is an aromatic compound with a complex structure containing functional groups, such as aliphatic hydroxyl, phenolic hydroxyl and methoxyl, that provide structural support, impermeability and resistance against microbial attack and oxidative stress [11]. Among biofuels, second-generation bioethanol (2 EG) is the most widely used and can be produced from residual biomass, for example bagasse and straw collected directly from agricultural fields or agro-industrial or agroforestry residues; it is a more viable and alternative renewable option [12]. Its production is carried out in the following three stages: pretreatment, enzymatic hydrolysis and fermentation. The pretreatment aims to expose the cellulose and hemicellulose molecules existing in the lignocellulosic biomass, which are originally physically protected by an outer layer of lignin. Enzymatic hydrolysis is a selective conversion process used to transform the biomass components into simple and fermentable sugars (C5 and C6). Finally, the fermentable sugars can be converted into ethanol and carbon dioxide using yeast in the fermentation stage.
Several researchers have studied hydrothermal or alkaline pretreatments to increase the concentration of fermentable sugars before the enzymatic hydrolysis stage. For instance, Alrumman applied an alkaline pretreatment to date palm cellulose to increase the concentration of sugars before the fermentation stage [13]. Phaiboonsilpa et al. studied the fermentability of xylose, arabinose and glucose, but did not study pretreatments to enhance the fermentable sugar concentration [14]. Sun et al. evaluated the combination of thermo-mechanical and alkali pretreatments to improve the enzymatic hydrolysis of Eucalyptus urophylla [15]. The present research provides a comparison between alkali and hydrothermal pretreatment methods to determine which is the best in terms of the depolymerization of cellulose and hemicellulose to obtain cellobiose, glucose and xylose during enzymatic hydrolysis. After that, the produced sugars are converted to ethanol through fermentation with Saccharomyces cerevisiae.
2. Materials and Methods
Rice husk of the INIAP-14 Filipino variety, with seeds improved by the National Institute of Agricultural Research, which has approximately 66.43% of carbohydrates (cellulose and hemicellulose) and whose monomers are hexoses and pentoses was used as a matrix in this study. The samples were collected in the rice sector called “La Cuca”, belonging to the Arenillas, El Oro province, Ecuador. Coordinates: 3°33′ S 80°04″O 3°33′ S 80°04″ W.
2.1. Rice Husk Characterization
Structural and non-structural carbohydrates and lignin were determined. For the cellulose content, 300 mg of rice was mixed with 3 mL of 72% v/v solution of H2SO4 under constant stirring at 200 rpm for 2 h at 55 °C. Then, 10 mL of cold water was added to the mixture until final dilution for 10 min. The cellulose was pooled through three repeated cycles of centrifugation at 3200 rpm for 15 min. The remaining solid residue was resuspended in distilled water and mixed for 5 min. The supernatant obtained was dialyzed with deionized water for 1 week, until reaching a final pH of 5–6. The cellulose yield was measured after drying the suspension at 100 °C until constant weight [16].
The hemicellulose content was determined following the method from Qiu [17]. In short, 3 g of sample was mixed in 10 mL of deionized water at 3000 rpm, heating at 85 °C for 1 h. A solution of NaOH 50% (w/v) was added until pH 11.5. Once the mixture was cooled down, it was centrifuged at 6000 rpm for 20 min and the supernatant was separated from the residue by decantation. Concentrated hydrochloric acid was added to the alkaline extract until pH of 4.5 and centrifuged three times at 10,000 rpm for 10 min. Hemicellulose A (insoluble in acid) was collected by centrifugation at 10,000 rpm for 30 min. Two volumes of ethanol (100%) were gradually added to the supernatant, allowing the larger rice husk fraction, called hemicellulose B, to precipitate. This was left overnight until a white flocculated precipitate formed at the bottom of the beaker. The clear alcohol/water mixture above the precipitate was removed by decantation. The white flocculent precipitate was transferred to another beaker and filtered. The solid fraction obtained was washed with ethanol and dried in a vacuum oven at 50 °C overnight. The remaining insoluble residue after the alkaline extraction was suspended in water and the pH was adjusted to 5.5–6.0. The undissolved residue was collected by vacuum filtration using Whatman No. 1 filter paper. The pellet was rinsed with 100% ethanol and dried in a vacuum oven [18].
The lignin was determined using a modified Klason lignin method. In short, 1 g of grounded and dried rice husk (≤250 µm) was mixed with 15 mL of H2SO4 (72% v/v) at room temperature, with constant stirring at 120 rpm for 2 h. The solution was diluted with deionized water to a final concentration of 3% and heated at 120 °C and 2 atm for 1 h in an autoclave (Sterilclav-75). Once cold, the solution was filtered, and the acid-insoluble lignin was determined gravimetrically, while the soluble lignin was precipitated by acidification. The combined filtrate was acidified with 5N H2SO4 to a final pH 2–3. The precipitate was centrifuged, washed to neutral pH, and then dried at 100 °C to constant weight [19]. The total lignin was calculated using Equation (1).
2.2. Physicochemical Pretreatments Applied to Rice Husk
To decrease the degree of crystallinity, increase the contact surface area and reduce the buoyancy of the cellulose, hemicellulose and lignin, the rice husk was ground in a jaw mill (Fritsch—Pulverisette 19) until obtaining a particle size of ≤ 250 µm. Solutions of 5% (m/v) of rice husk/water were used for the pretreatments [20]. The experiments were performed in triplicate.
2.2.1. Alkaline Pretreatment
For the alkaline delignification process, three different concentrations of NaOH of 0.5, 1 and 1.5% (v/v) were added to the rice husk solutions of 5% (m/v) and stirred at 120 rpm for 1 h [21,22]. The pH of solutions was measured using a GLP-22 pH meter (CRISON Instruments, Barcelona, Spain).
2.2.2. Hydrothermal Pretreatment
For the hydrothermal pretreatment, the 5% (m/v) rice husk solutions were heated in an autoclave at 120 °C for three different periods of time of 15, 30 and 45 min [19]. Then the pH of the solution was adjusted to 4.8 to continue with the enzymatic hydrolysis process.
2.3. Enzymatic Hydrolysis of Cellulose and Hemicellulose
After the alkaline and hydrothermal pretreatments, the enzymatic hydrolysis of cellulose and hemicellulose present in the rice husk waste was carried out in 500 mL of water. Prior to enzyme application, the pH was adjusted to 4.8 by adding 20% hydrochloric acid (v/v). Celluclast 1.5L (β-glucosidase) enzyme was added at a concentration of 30 FPU per gram of carbohydrates, and placed on an orbital shaker at 50 °C and 200 rpm for 144 h [23]. Samples were taken every 24 h, centrifuged for 5 min at 10,000 rpm using an ELMI-Sky Line mini centrifuge and filtered through a 0.22 µm syringe filter (Millipore, Bedford, MA, USA). The production of sugars (cellobiose, glucose and xylose) was quantified by high-performance liquid chromatography (HPLC).
2.4. Alcoholic Fermentation
Prior to alcoholic fermentation, a lyophilized strain of Saccharomyces cerevisiae Hansen 1883 (NCYC 366) from the Spanish Type Culture Collection (CECT) of the University of Valencia was activated. The activation of the lyophilized culture of S. cerevisiae consisted of adding 0.3 mL of the sterile liquid medium into a opened glass ampoule. The resulting suspension was placed into a flask containing 100 mL yeast extract peptone dextrose (10 g·L−1 yeast extract, 20 g·L−1 peptone and 20 g·L−1 of dextrose). The mixture was incubated at 37 °C with constant shaking in an orbital incubator at 130 rpm for 24 h [22]. After the incubation, the S. cerevisiae cells were harvested through centrifugation at 5000 rpm for 5 min in sterile water.
For alcoholic fermentation, 1 g·L−1 of (NH4)2SO4, folic acid (0.4 mg), and biotin (0.3 mg) was added to the hydrolysates resulting from the hydrolysis experiments and sterilized at 121 °C for 15 min. After reaching room temperature, 1.2 mL of activated yeast was added into the hydrolysates. The fermentations were carried out at 37 °C under anaerobic conditions and constant stirring at 120 rpm [23].
2.5. Analytical Methods
2.5.1. IR Spectroscopy (FTIR)
To measure the effect of the alkaline and hydrothermal pretreatments applied to the rice husk, Fourier Transform Infrared Spectroscopy (FTIR) analysis was performed using a 380 FTIR Spectrophotometer (Thermo Scientific, Waltham, MA, USA). Samples were pelletized using potassium bromide (KBr) by pressing a mixture of 2 mg sample with 400 mg KBr. To eliminate interference due to the presence of moisture, the samples and KBr were separately dehydrated at 105 °C to constant weight before tableting. The spectra were observed at a frequency range of 400–4000 cm−1 with a resolution of 4 cm−1 and 32 scans. The baseline was corrected and normalized in transmittance.
2.5.2. Quantification of Fermentable Sugars and Ethanol
The sugars obtained from enzymatic hydrolysis, particullarly cellobiose, glucose, xylose, and the ethanol resulting from alcoholic fermentation were determined using HPLC (Agilent 1100 Technologies Inc., Santa Clara, CA, USA) with a refractive index detector (G-1362A XR RI) and a SUPERCOGEL C-610H column. The temperature of the column was set at 50 °C, and H2SO4 5 mM was used as the mobile phase at a flow rate of 0.6 mL·min−1.
3. Results and Discussion
3.1. Chemical Characterization of Rice Husk
The rice husk contains 64.4% holocellulose (cellulose and hemicellulose), 8.21% lignin, and 17.41% ash. The composition of the studied rice husk is comparable with those reported by Oliveira et al. in Brazil [24] and Bhattacharjee [25] in India, with 60.45% of holocellulose and 20.48% of lignin. The rice husk composition is essential to obtain second-generation ethanol, hence the amount of cellulose and hemicellulose available to be hydrolyzed to fermentable sugars was determined.
3.2. Rice Husk Proximal Analysis
Elemental analysis (CHNS) of rice husk reveals a composition of 39.67% carbon, 5.10% hydrogen, 0.39% nitrogen and traces of sulfur. Similar elemental concentrations were reported in studies conducted in Brazil [24].
3.3. Determination of the Crystallinity of Rice
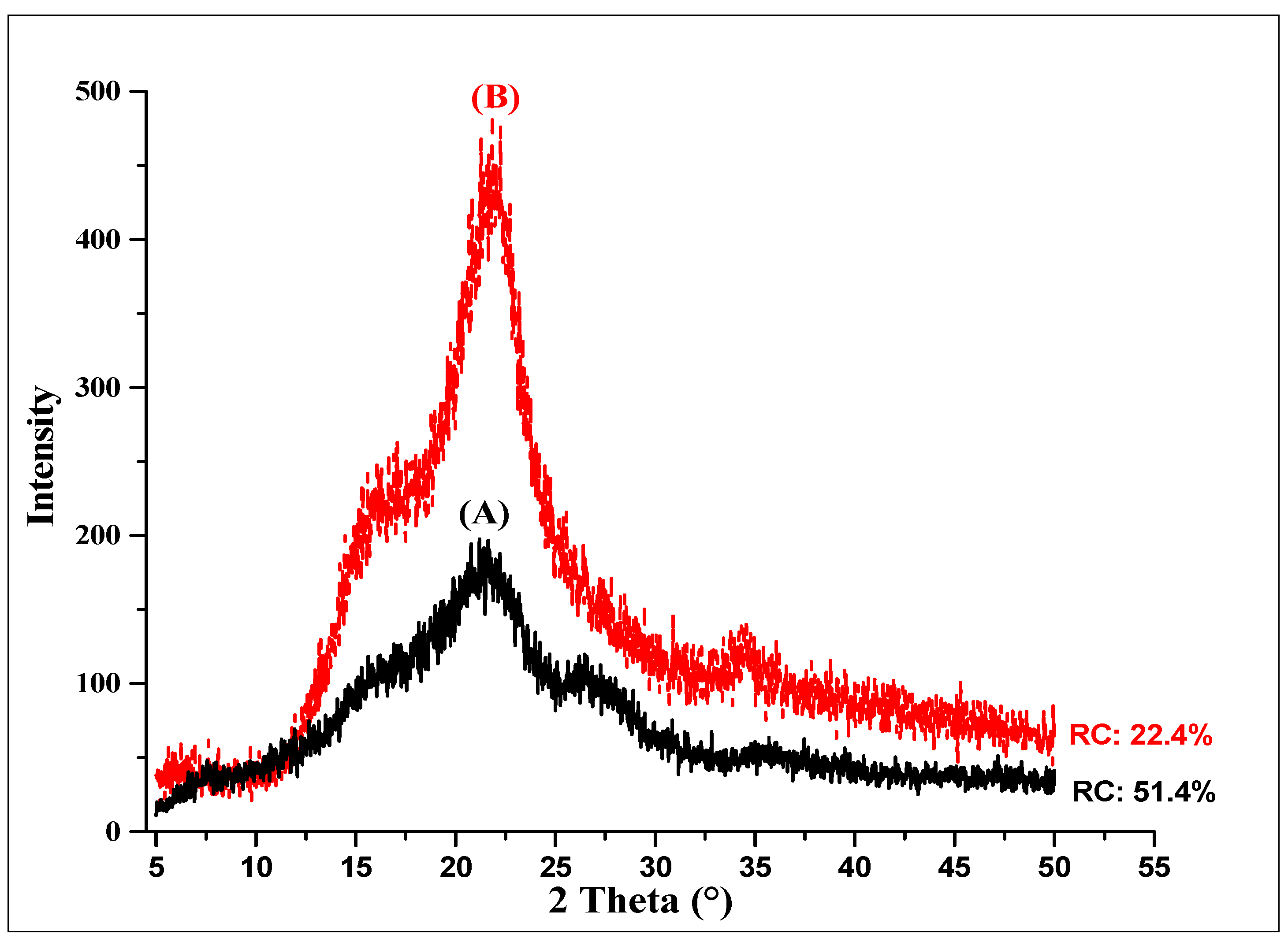
X-ray diffraction analysis was performed to establish the reduction in the crystallinity of the rice husk when it was subjected to mechanical fractionation (≤250 µm). Figure 1 shows the XRD profile of the rice husk with and without grinding, presenting the three characteristic peaks of lignocellulosic biomasses [26].
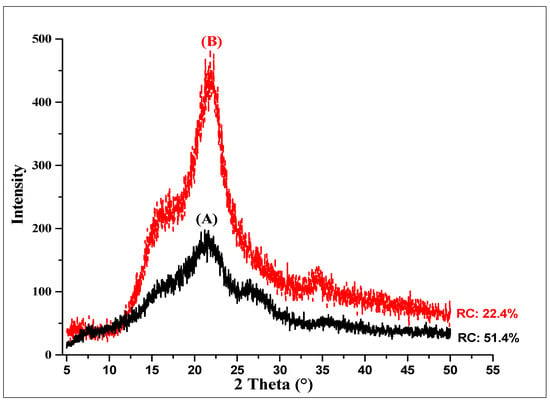
Figure 1.
Relative crystallinity of raw and milled rice husk (≤250 µm). (A) Raw rice husk. (B) Milled rice husk.
The raw rice husk (A) presented higher relative crystallinity (CR) than the milled husk (≤250 µm) (B), which is attributed to the reduction in particle size. The relative crystallinity of the milled rice husks decreased 45.34% in terms of the raw rice husk, thereby enhancing the enzymatic hydrolysis capacity of this residue. The reduction in particle size facilitates the accessibility of enzymes into the structured cell wall, contributing to enzymatic hydrolysis [27].
3.4. Chemical Characterization of Rice Husk Ashes
The analysis of oxides present in the rice husk revealed that the rice husk ash contains a high concentration of silicon dioxide (88%), calcium oxide (9.3%) and K2O, P2O5, and CaO (3%). Studies on this type of ash report similar values in the silicon dioxide concentration [28]. Several reports indicate that these compounds inhibit the enzymatic hydrolysis and/or the fermentation process [29,30]. Thus, its removal is crucial.
3.5. FTIR Analysis of Residue after Alkaline and Hydrothermal Pretreatments
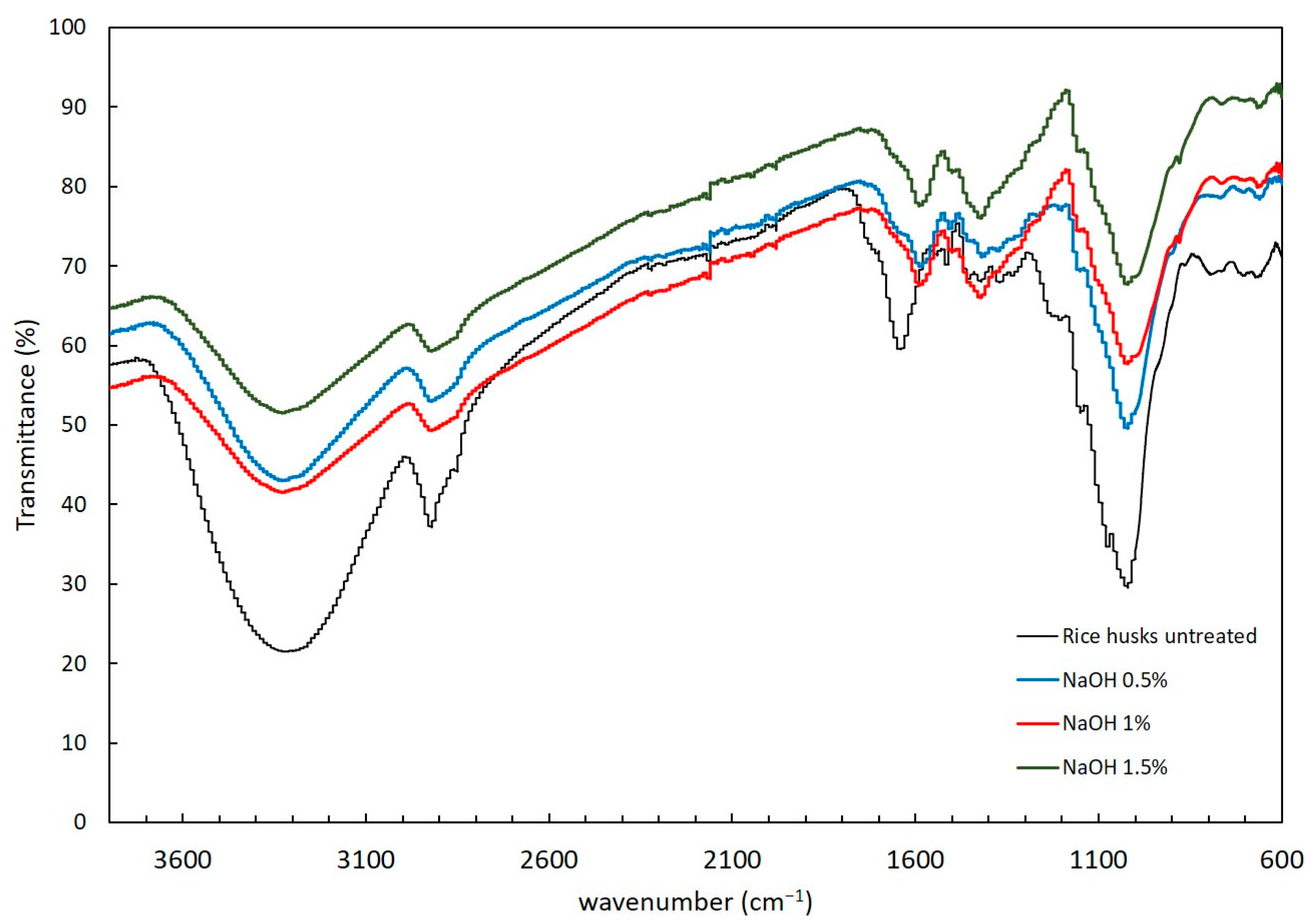
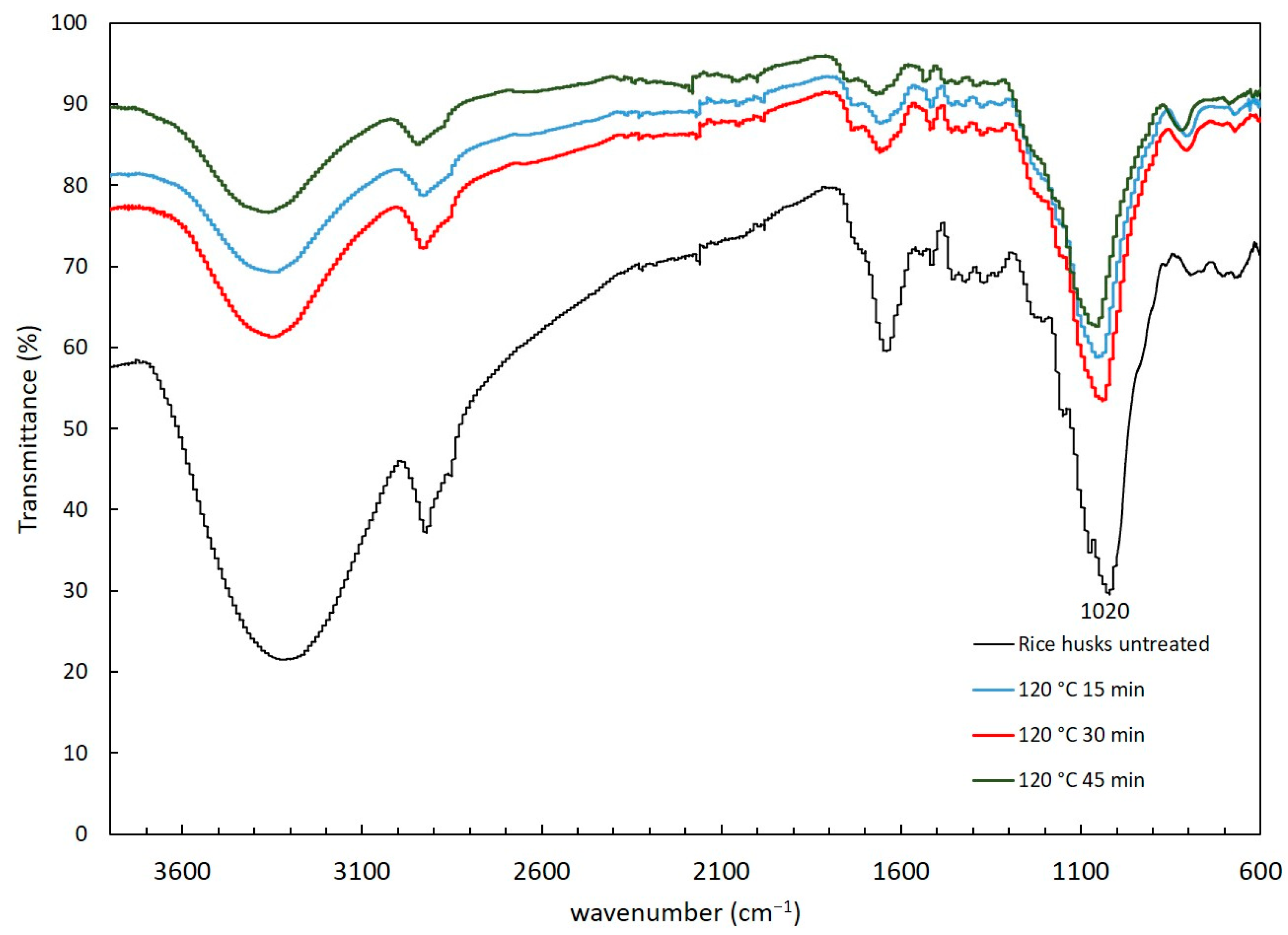
The functional groups present in the solid residue after alkaline and hydrothermal pretreatments were determined to understand the structural changes caused by the pretreatments. Figure 2 and Figure 3 show the FTIR spectra after alkaline and hydrothermal pretreatments, respectively. The rice husk without any pretreatment was evaluated as control. Peaks corresponding to cellulose, hemicellulose and lignin were identified. The presence of a wide band at 3600 to 3040 cm−1 likely corresponds to the presence of alcoholic, hydroxyl and phenolic bonds in the carbohydrate and lignin content of the biomass [31]. Acetyl groups in hemicellulose were detected from 1728 to 1736 cm−1 indicative of the C=O moiety. The stretching vibrations present in the range of 1100–1000 cm−1 were attributed to Si-O-Si present in rice husk [32]. The aromatic ring vibrations attributed to lignin were detected at 1632 cm−1, 1510 cm−1 and 1236 cm−1 [33].
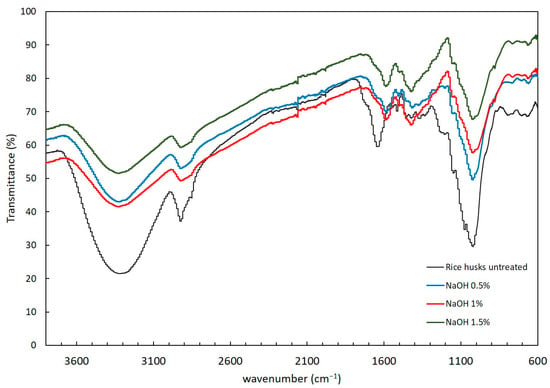
Figure 2.
FTIR spectra of the rice husks treated with NaOH (0.5, 1 and 1.5%).
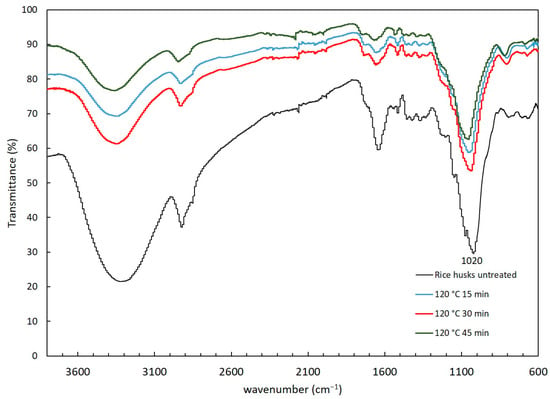
Figure 3.
FTIR spectra of rice husks with hydrothermal pretreatment at 120 °C (15, 30 and 45 min of exposition).
The FTIR spectrum revealed changes in rice husk after pretreatment with NaOH (Figure 2). After each NaOH pretreatment, the stretching vibration at 1640 cm−1 related to the C=O of acetyl groups from hemicelluloses shifts to 1584 cm−1, indicative of the decomposition of initial hemicelluloses. The peak observed at 1216 cm−1 attributed to the C-O stretching vibration in lignin [34] disappeared in the treatments at all the NaOH concentrations applied. Shoda et al. indicates that peak values closer to 1510 cm−1 are associated with lignin [34] and tend to shift to ~1505 cm−1. Similarly, other investigations report that the reduction in peak intensity is caused by the reduction in the lignin content, which is directly related to the reduction in the calorific value [35]. Overall, the NaOH treatment leads to the decomposition of hemicelluloses and contributes to delignification of the substrate.
Following hydrothermal treatment, distinct peaks corresponding to hemicellulose and lignin were observed, indicating significant structural modifications in the rice husk components. The stretching vibration at 1640 cm−1, originally assigned to hemicellulose shit to 1659 cm−1, suggests alterations in the hemicellulose structure. Additionally, the band at 1216 cm−1 associated with lignin exhibited a decrease in intensity as the duration of the hydrothermal pretreatment increased, implying a reduction in lignin content.
A notable change, not observed in the alkaline pretreatment, was identified from 1020 cm−1 to 1040 cm−1. The band at 1020 cm−1 corresponds to the stretching bonds C-O/C-H in cellulose and the stretching vibration of β-(1-4) linkages [36]. This shift suggests that hydrothermal pretreatment induced modifications in the structural composition of hemicellulose, cellulose and lignin of the rice husk.
Overall, these findings indicate that hydrothermal pretreatment effectively altered the structural composition of holocellulose, cellulose and lignin in the rice husk. These structural modifications are crucial as they contribute to the softening and swelling of holocellulose and reduce lignin concentration, thereby increasing the surface area available for enzymatic attack. This enhanced accessibility is vital for subsequent processes aimed at biomass conversion or utilization.
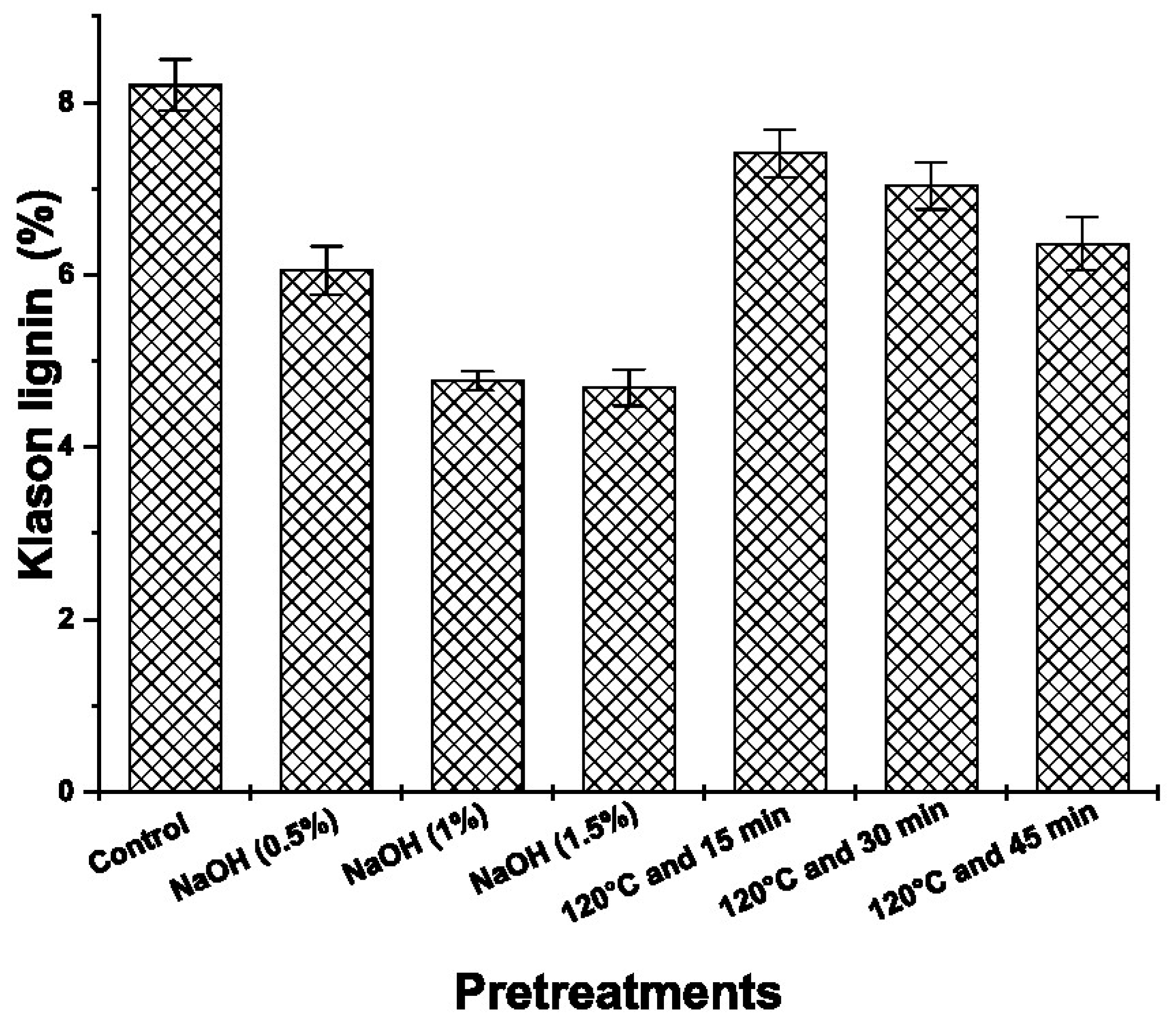
3.6. Klason Lignin Quantification
Figure 4 shows the effects of adding NaOH in three different concentrations (0.5, 1 and 1.5%) and applying hydrothermal pretreatment at 120 °C for three different periods of time (15, 30 and 45 min).
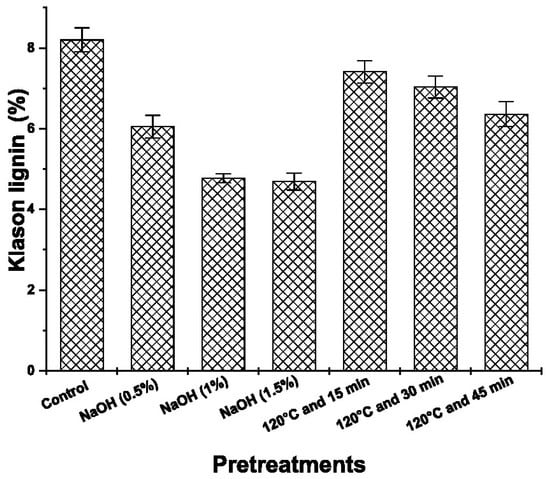
Figure 4.
Klason lignin content after hydrothermal and alkaline pretreatments.
A reduction of 36.4% of lignin was observed in the pretreatment with 1.5% NaOH, compared to the hydrothermal treatment. This indicates that the lignin of the rice husk contains various methoxylated phenolic compounds with ether bonds that NaOH can reduce phenolic hydroxyl. The literature reports that alkaline pretreatment can reduce lignin due to the cleavage of the α-aryl ether bond caused by the saponification of the ether bond [20].
The hydrothermal treatment at 120 °C for 45 min showed the highest lignin reduction of 12.66% compared to the control rice husk sample. The results agreed with studies conducted in chestnut shell, where a hydrothermal-alkaline pretreatment (1–5% NaOH and 100 °C), significantly affected the delignification and polymerization of hemicellulose in more than 2.4 times [7].
3.7. Enzymatic Hydrolysis of Rice Husk with Hydrothermal and Alkaline Pretreatments
3.7.1. Partial Hydrolysis of Cellulose to Cellobiose
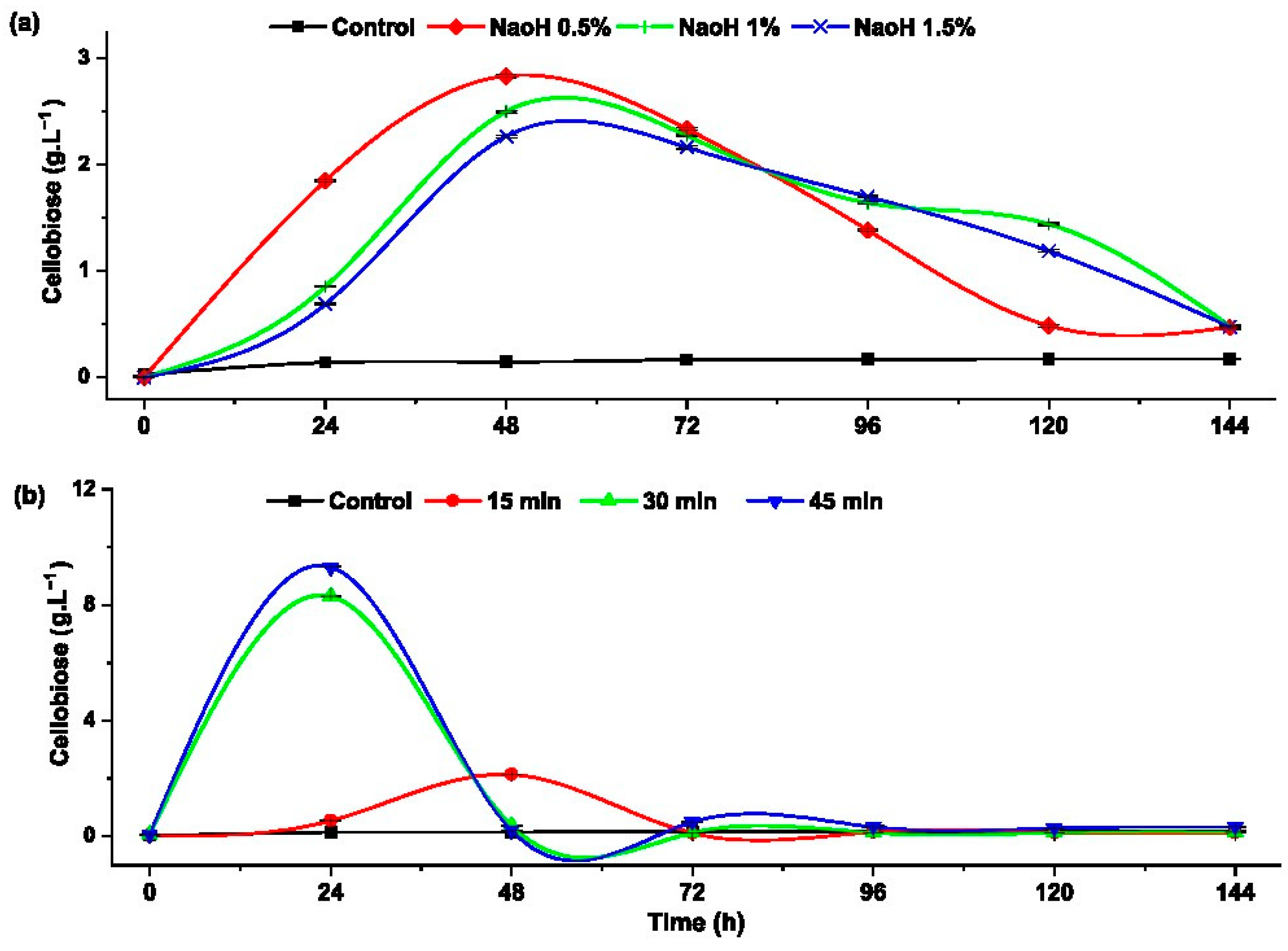
Cellobiose is a disaccharide with β-glucosidics bonds and prebiotic characteristics., which can be obtained from the partial hydrolysis of cellulose using β-glucosidase enzymes. Understanding this reaction provides insights into the conversion from cellobiose to glucose and further conversion to ethanol in the fermentation process [37]. Figure 5 shows how the pretreatment affects the formation of cellobiose during the enzymatic hydrolysis.
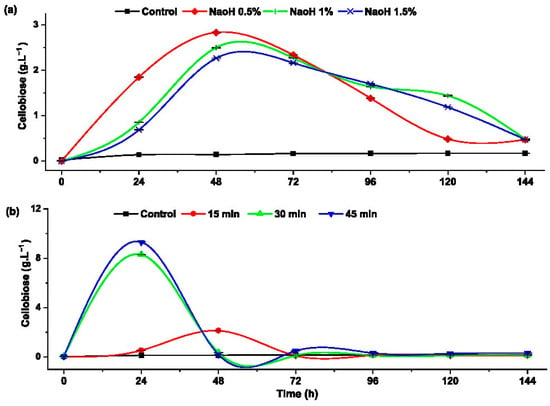
Figure 5.
Cellobiose formation during the progress of the enzymatic hydrolysis of rice husk with: (a) alkaline pretreatment and (b) hydrothermal pretreatment.
Figure 5a illustrates the rice husk pretreated with 0.5% NaOH, which yielded the highest cellobiose concentration of 2.83 ± 0.012 g·L−1. This value exceeded concentrations obtained with 1% NaOH (0.3 g·L−1) and 1.5% NaOH (0.57 g·L−1). It can be observed that formation of cellobiose is inversely proportional to the NaOH concentration.
Regarding thermal treatment (Figure 5b), the highest concentration of cellobiose (9 g·L−1) was reached during the first 24 h of enzymatic hydrolysis in the sample pretreated at 120 °C for 45 min. This was followed by the sample thermally pretreated at 120 °C for 30 min. In contrast, the sample pretreated at 120 °C for 15 min showed no significant difference in cellobiose concentration compared to the control. When comparing these results with the existing literature, there is no specific information on cellobiose production from rice husk. However, several studies have reported cellobiose production using different substrates and microorganisms. For instance, Cao et al. reported the production of approximately 2 g·L−1 of cellobiose from Icelandicmoss lichenan using news glucanases and pure barley β-glucan [8]. Additionally, Wu et al. produced 7.7 g·L−1 of cellobiose from Avicel using a genetically modified fungus-Neurospora crassa [37].
3.7.2. Total Enzymatic Hydrolysis of Cellulose
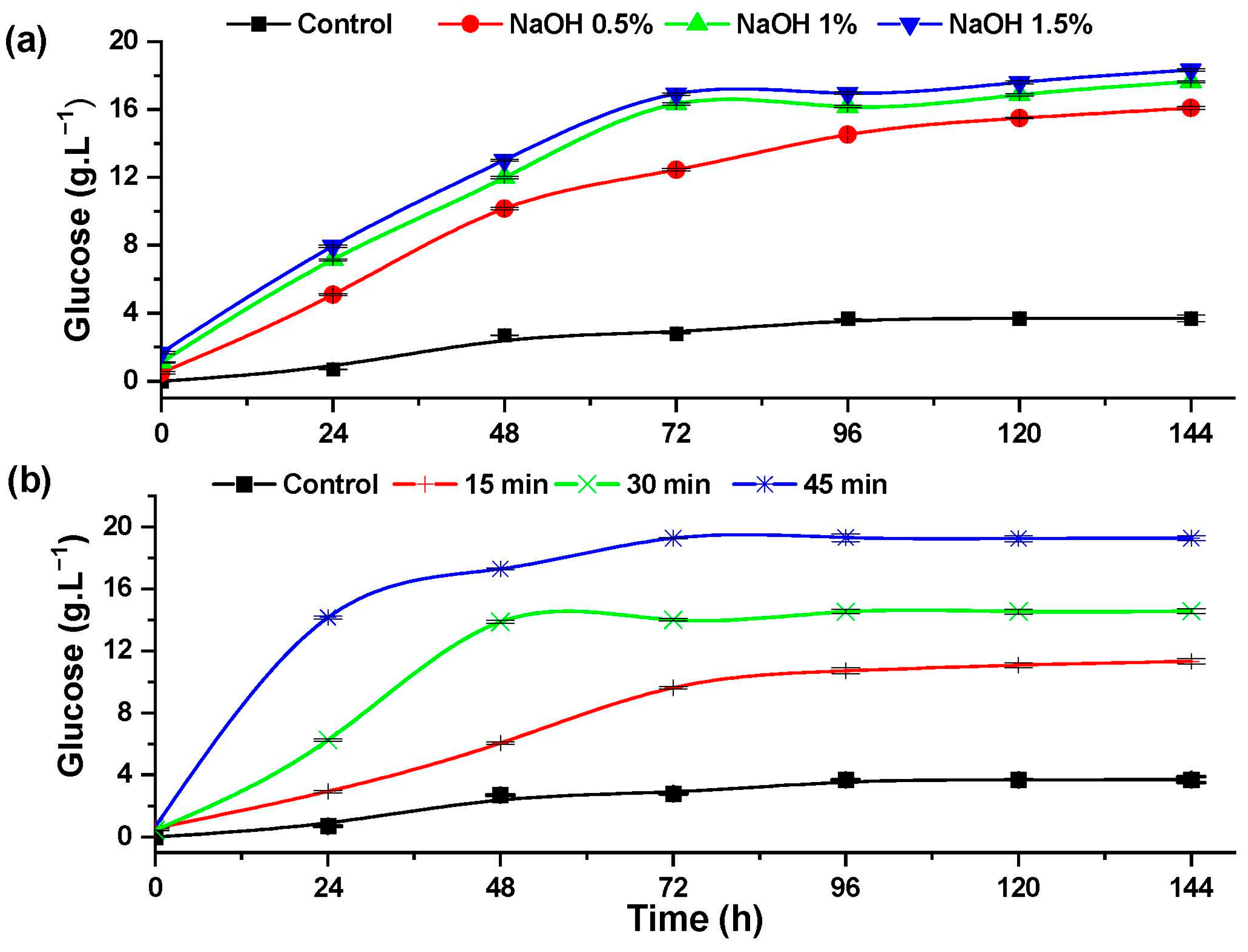
An increase in the concentration of glucose during the enzymatic hydrolysis of rice husk indicates the effect of the pretreatments in the degradation of cellulose. Figure 6 shows the effect of the alkaline and hydrothermal pretreatments, which increased the glucose content compared to the control experiment.
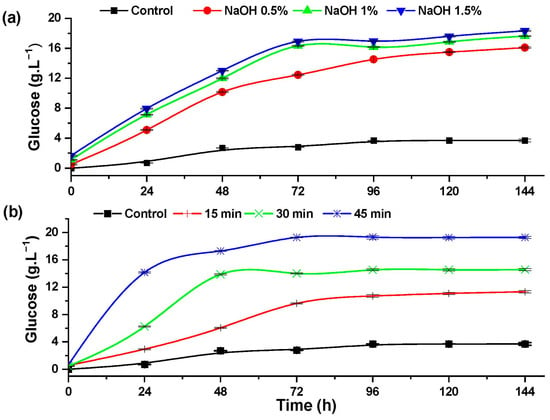
Figure 6.
Glucose formation during the progress of the enzymatic hydrolysis of rice husk with: (a) alkaline pretreatment and (b) hydrothermal pretreatment.
The rice husk pretreated with 1.5% NaOH (Figure 6a) reached a glucose concentration of 18.33 ± 0.1 g·L−1, representing 81.70% of cellulose hydrolysis efficiency. Conversely, during the hydrothermal pretreatment (Figure 6b), a glucose concentration of 19.28 ± 0.17 g·L−1 was achieved after 72 h of enzymatic hydrolysis in the experiment pretreated at 120 °C for 45 min, representing 85.93% of cellulose hydrolysis.
Studies of alkaline extraction of cellulose from Eucalyptus and other hardwoods reported that alkaline pretreatment helps to increase the enzymatic hydrolysis of cellulose and also contributes the softening of lignin [38]. Hydrolysis of cotton stalks pretreated with 3% NaOH at 130 KPa of pressure and a temperature of 125 °C for 40 min achieves 0.293 g of reducing sugar per gram of biomass [39].
3.7.3. Hydrolysis of Hemicellulose to Xylose
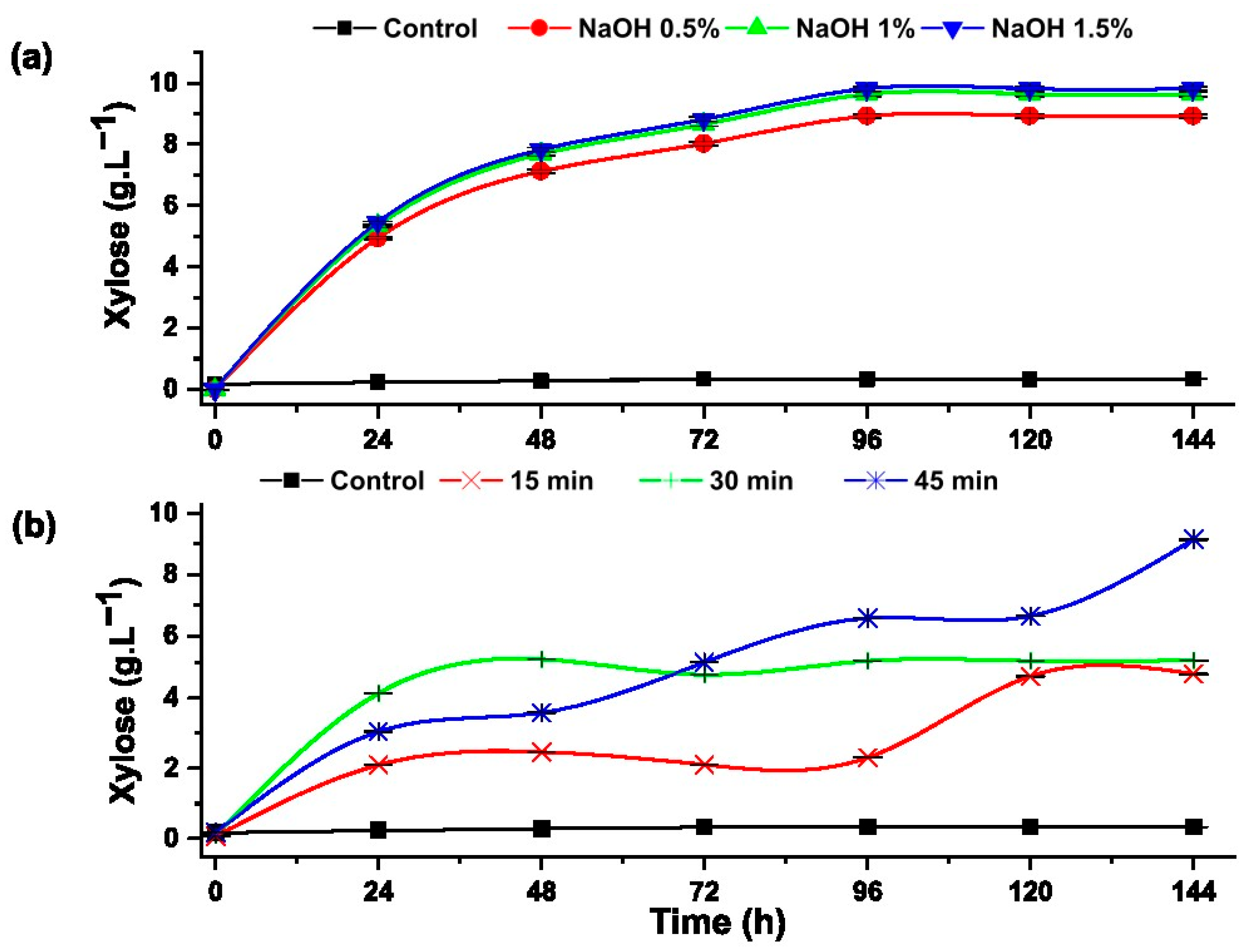
The presence of xylose indicates the degradation of hemicellulose and the effectiveness of pretreatments. Figure 7 shows the effect of alkali and hydrothermal pretreatments on the production of xylose from rice husk.
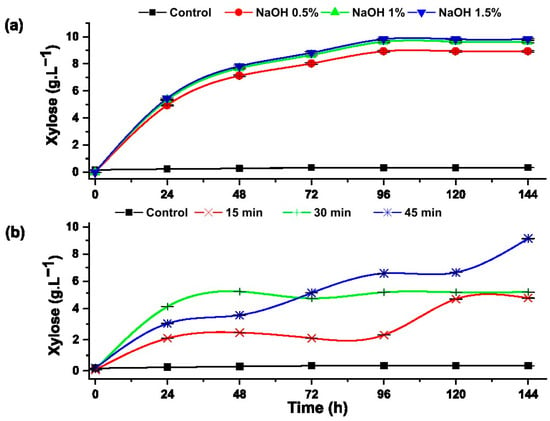
Figure 7.
Formation of xylose during the progress of the enzymatic hydrolysis of rice husk with: (a) alkaline pretreatment and (b) hydrothermal pretreatment.
Overall, the alkaline pretreatment using NaOH in three different concentrations (0.5, 1 and 1.5%) showed better synergy with enzymatic hydrolysis compared to hydrothermal treatment.
In the pretreatment with NaOH (Figure 7a), the maximum efficiency was reached at 1.5% NaOH, obtaining a xylose concentration of 9.82 ± 0.06 g·L−1 after 96 h of enzymatic hydrolysis, representing 96.30% conversion of hemicellulose. Hutterer et al. reported an improvement in the enzymatic hydrolysis of hemicellulose present in Eucalyptus pretreated with 2% NaOH and 90 °C for 2 h, because this alkali softens the lignin and thus leaves the hemicellulose free for enzymatic attack [40]. Zhong et al. applied alkaline-hydrothermal pretreatment to the rice husk, applying 2% NaOH hydroxide and 130 °C for 30 min, obtaining similar concentrations of xylose and the formation of hydroxymethylfurfural [41].
The experiments hydrothermally pretreated at 120 °C for 45 min reached the highest xylose concentration of 8.50±0.01 g·L−1, which represents 83.35% of the hemicellulose present in the rice husk, while the samples pretreated at 120° C for 15 and 30 min reached 5.07 ± 0.01 g·L−1 and 4.67±0.02 g·L−1, respectively. Studies carried out on enzymatic hydrolysis of the corn cob indicate that after convective heating, a fraction of hemicellulose is hydrolyzed [39].
3.8. Fermentation Alcoholic of Rice Husk Hydrolyzate with Alkaline and Hydrothermal Pretreatments
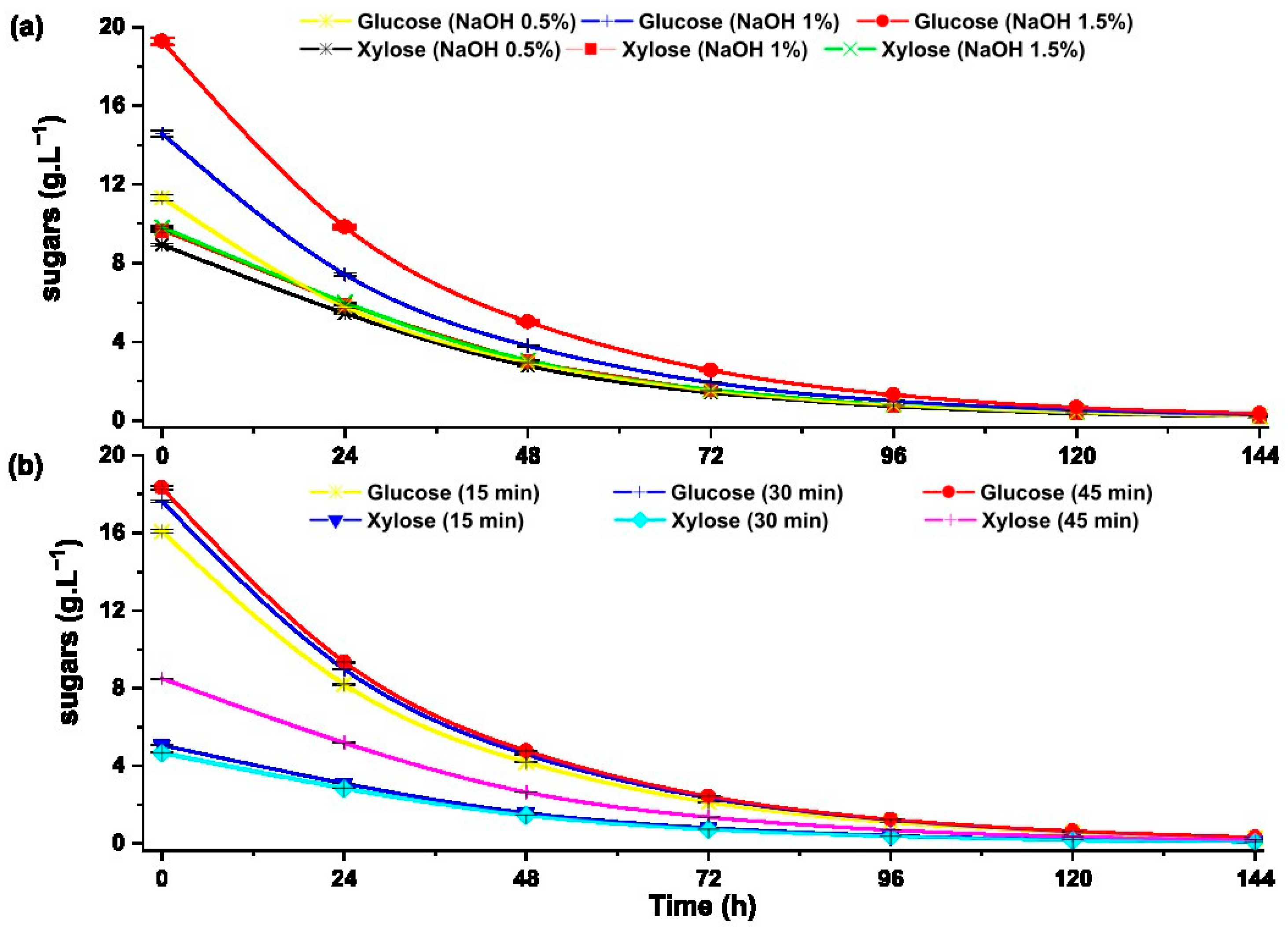
Alcoholic fermentation is a simple conversion of fermentable sugars into ethanol by the action of the yeast S. cerevisiae under anaerobic conditions. The fermentation efficiency depends on the type of biomass, nutrients available in the substrate, and optimal temperature [6]. The ability of S. cerevisiae Hansen 1883 (NCYC 366) to ferment fermentable sugars and xylose obtained by enzymatic hydrolysis pretreated by alkaline and hydrothermal procedures was evaluated (Figure 8).
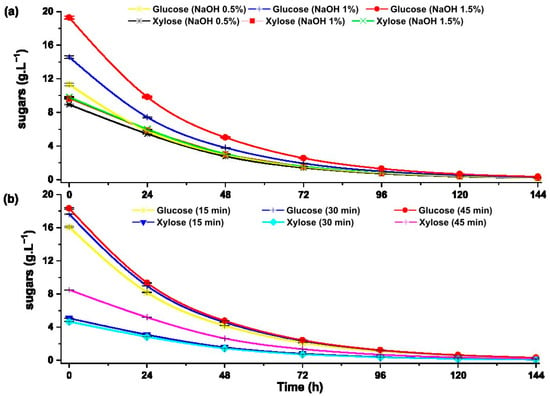
Figure 8.
Substrate uptake by S. cerevisiae Hansen 1883 (NCYC 366) during fermentation from the hydrolysates pretreated with: (a) alkaline pretreatment and (b) hydrothermal pretreatment.
The concentration of glucose and xylose decreases significantly during the first 72 h of alcoholic fermentation due to the action of S. cerevisiae Hansen 1883 (NCYC 366) yeast. Phaiboonsilpa et al. reports greater digestibility of glucose, xylose and arabinose in the hydrolyzed sugarcane bagasse during the first 72 h of alcoholic fermentation using a genetically modified Pichia stipitis (ATC. 58376) [14].
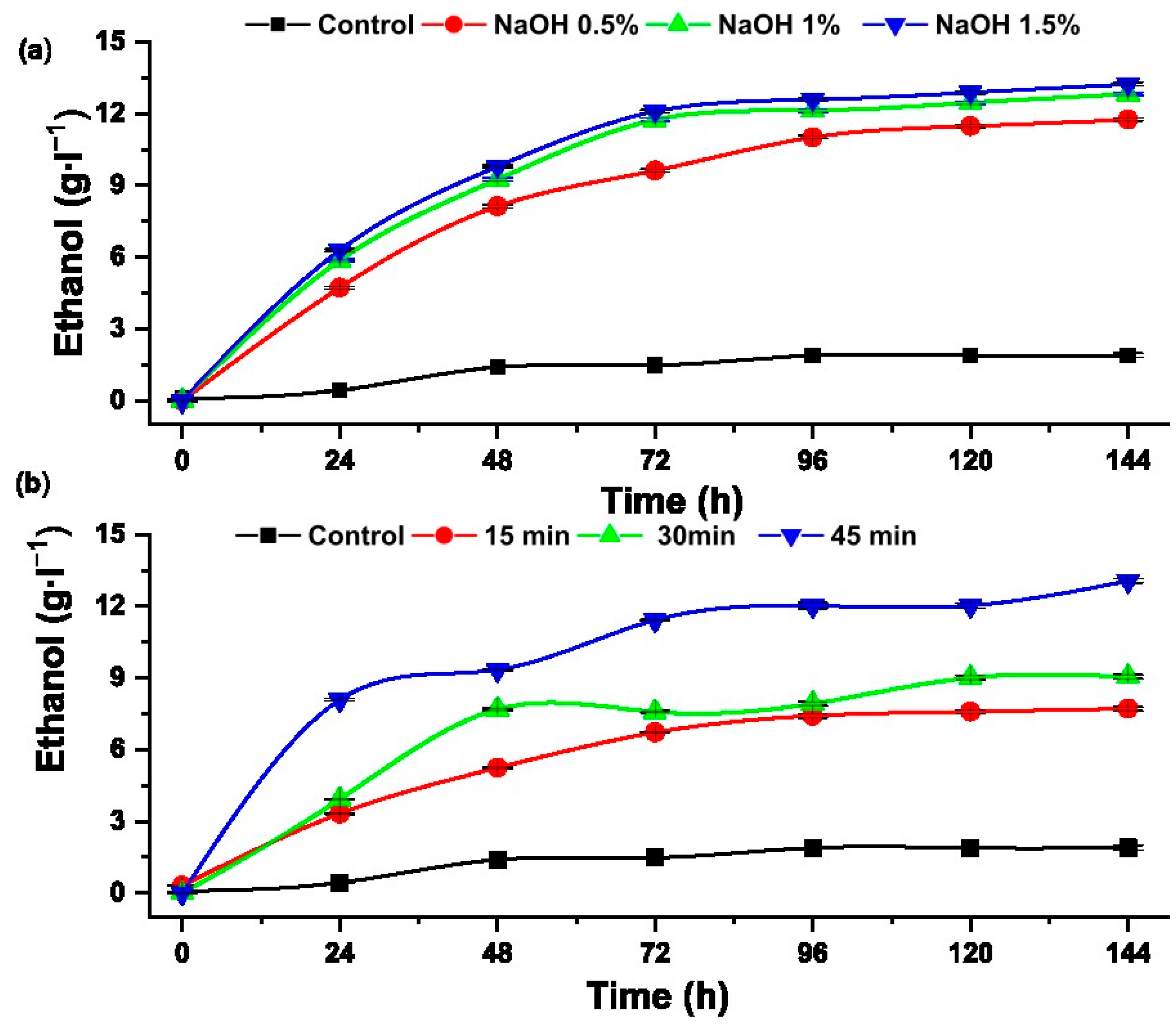
Figure 9 shows the ethanol concentrations obtained from the alcoholic fermentation of glucose and xylose of the samples with hydrothermal and alkaline pretreatment.
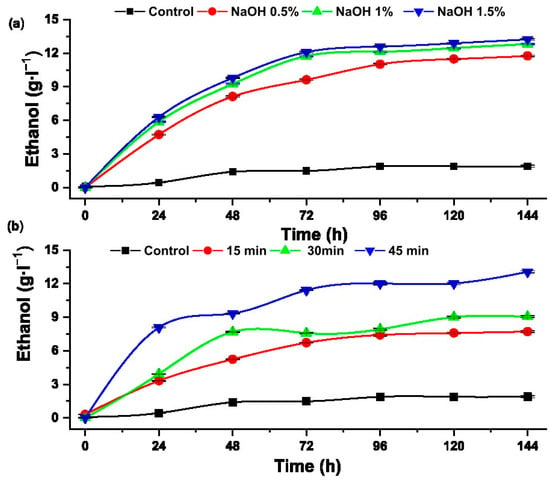
Figure 9.
Ethanol concentration produced from hydrolysates pretreated with (a) alkaline pretreatment and (b) hydrothermal.
The hydrolysate pretreated with sodium hydroxide (1.5%) showed a greater reduction in total sugars, from 28.15 to 3.94 g·L−1, and produced 13.23 ± 0.08 g·L−1 of ethanol, equivalent to a yield of 86%. Research on bioethanol production from pentose and hexose obtained from the hydrolysis of sugarcane bagasse, reported a maximum ethanol production of 93.97% [42]. Sugar consumption depends on the nutrients, such as nitrogen and phosphorus, that are added to the substrate and on the ability to metabolize sugars present in the hydrolysates [43].
In the other case, the hydrolysate hydrothermally pretreated at 120 °C for 45 min showed a greater reduction in total sugars and higher formation of ethanol, equivalent to a yield of 84.83%. This result is in concordance with that reported by Ko et al., who reached > 80% efficiency applying alcoholic fermentation to hardwood hydrolysates, stating that the fraction rich in xylose is efficiently converted into ethanol in 46 h and the fraction rich in glucose takes longer, from 56 to 80 h [44].
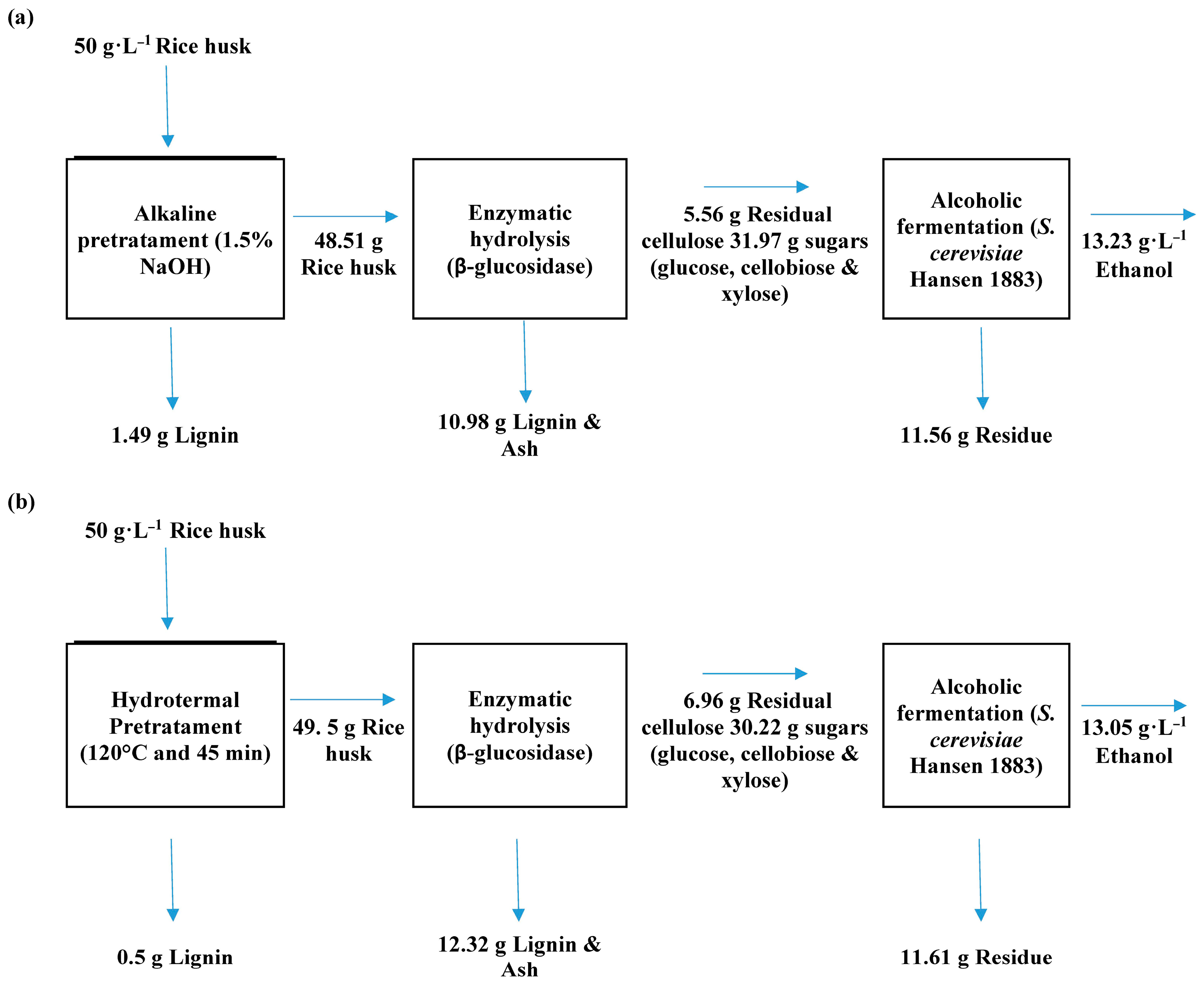
Figure 10 shows the mass balances of the two processes applied. The effect of the pretreatment in the overall process can be observed. The mass balances were calculated in the base of the best conditions during the pretreatments (i.e., 1.5% of NaOH for alkaline and 120 °C and 45 min for hydrothermal).
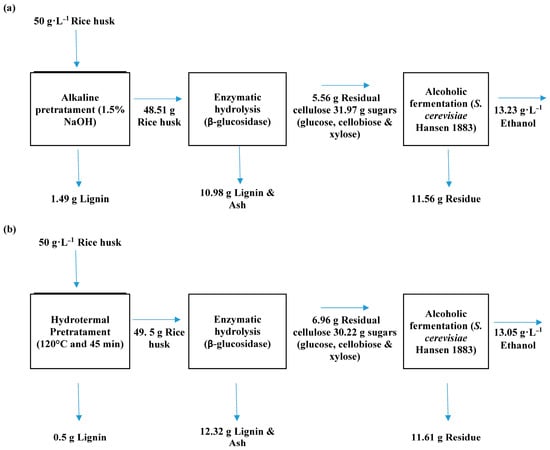
Figure 10.
Mass balance of the two process applied: (a) with alkaline pretreatment and (b) with hydrothermal pretreatment.
The alkaline pretreatment was better than the hydrothermal pretreatment in terms of lignin removal (1.49 g vs. 0.5 g) and had a better impact on the production of fermentable sugars during enzymatic hydrolysis (31.97 g for alkali vs. 30.22 g for hydrothermal).
4. Conclusions
The pretreatments evaluated in the present research improve the enzymatic hydrolysis and fermentation process from rice husk waste. The alkaline pretreatment achieved better results in terms of sugar production (glucose, cellobiose and xylose) than hydrothermal pretreatment. The concentration of fermentable sugars and xylose fermented by Saccharomyces cerevisiae in the hydrolysates obtained with 1.5% alkaline pretreatment decreased by 86% during the first 72 h of alcoholic fermentation, producing 13.23 ± 0.08 g·L−1 of ethanol; and 72 h later, 100% conversion of fermentable sugars to ethanol was reached. The fermentable sugars obtained from the samples hydrothermally pretreated at 120 °C for 45 min fermented faster than the other pretreatments applied, achieving greater reductions in xylose after 48 h and glucose in 72 h of alcoholic fermentation, and greater production of ethanol, reaching a yield of 84.83%.
Author Contributions
Conceptualization, J.A.-A.; methodology, B.L., E.C. and J.S.; validation, B.M.; formal analysis, J.A.-A., C.B. and J.S.; investigation, B.M.; resources, J.A.-A. and D.S.; data curation, J.A.-A.; writing—original draft preparation, J.A.-A. and C.B.; writing—review and editing, B.L. and C.B.; visualization, E.C.; supervision, V.B.; project administration, D.S.; funding acquisition, V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by Universidad Técnica de Machala.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
There is no conflict of interests.
References
- Ahmed, B.; Aboudi, K.; Tyagi, V.K.; álvarez-Gallego, C.J.; Fernández-Güelfo, L.A.; Romero-García, L.I.; Kazmi, A.A. Improvement of Anaerobic Digestion of Lignocellulosic Biomass by Hydrothermal Pretreatment. Appl. Sci. 2019, 9, 3853. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Rajan, K.; Djioleu, A.; Rocha, T.M.; Brumano, L.P.; de Souza Melo, Y.C.; dos Santos, J.C.; Rosa, C.A.; Carrier, D.J.; da Silva, S.S. Sustainable Second-Generation Ethanol Production from Switchgrass Biomass via Co-Fermentation of Pentoses and Hexoses Using Novel Wild Yeasts. Bioenergy Res. 2022, 15, 1157–1168. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery Process for the Simultaneous Recovery of Lignin, Hemicelluloses, Cellulose Nanocrystals and Silica from Rice Husk and Arundo Donax. Ind. Crops Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Bárcena, A.; Samaniego, J.L.; Péres, W.; Alatorre., J.E. The Climate Emergency in Latin America and the Caribbean. The Path Ahead—Resignation or Action? ECLAC: Santiago, Chile, 2020; ISBN 9789211220322. [Google Scholar]
- Beidaghy Dizaji, H.; Zeng, T.; Hölzig, H.; Bauer, J.; Klöß, G.; Enke, D. Ash Transformation Mechanism during Combustion of Rice Husk and Rice Straw. Fuel 2022, 307, 121768. [Google Scholar] [CrossRef]
- Beukes, N.; Pletschke, B.I. Effect of Alkaline Pre-Treatment on Enzyme Synergy for Efficient Hemicellulose Hydrolysis in Sugarcane Bagasse. Bioresour. Technol. 2011, 102, 5207–5213. [Google Scholar] [CrossRef]
- Bianco, F.; Şenol, H.; Papirio, S. Enhanced Lignocellulosic Component Removal and Biomethane Potential from Chestnut Shell by a Combined Hydrothermal–Alkaline Pretreatment. Sci. Total Environ. 2021, 762, 144178. [Google Scholar] [CrossRef]
- Cao, J.W.; Deng, Q.; Gao, D.Y.; He, B.; Yin, S.J.; Qian, L.C.; Wang, J.K.; Wang, Q. A Novel Bifunctional Glucanase Exhibiting High Production of Glucose and Cellobiose from Rumen Bacterium. Int. J. Biol. Macromol. 2021, 173, 136–145. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin Utilization: A Review of Lignin Depolymerization from Various Aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Khare, S.K.; Pandey, A.; Larroche, C. Current Perspectives in Enzymatic Saccharification of Lignocellulosic Biomass. Biochem. Eng. J. 2015, 102, 38–44. [Google Scholar] [CrossRef]
- Dutta, S.K.; Chakraborty, S. Mixing Effects on the Kinetics and the Dynamics of Two-Phase Enzymatic Hydrolysis of Hemicellulose for Biofuel Production. Bioresour. Technol. 2018, 259, 276–285. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Villaflores, O.B.; Ordono, E.E.; Caparanga, A.R. Effects of Acidified Aqueous Glycerol and Glycerol Carbonate Pretreatment of Rice Husk on the Enzymatic Digestibility, Structural Characteristics, and Bioethanol Production. Bioresour. Technol. 2017, 228, 264–271. [Google Scholar] [CrossRef]
- Alrumman, S.A. Enzymatic Saccharification and Fermentation of Cellulosic Date Palm Wastes to Glucose and Lactic Acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef]
- Phaiboonsilpa, N.; Chysirichote, T.; Champreda, V.; Laosiripojana, N. Fermentation of Xylose, Arabinose, Glucose, Their Mixtures and Sugarcane Bagasse Hydrolyzate by Yeast Pichia Stipitis for Ethanol Production. Energy Rep. 2020, 6, 710–713. [Google Scholar] [CrossRef]
- Sun, S.; Cao, X.; Sun, S.; Xu, F.; Song, X.; Sun, R.C.; Jones, G.L. Improving the Enzymatic Hydrolysis of Thermo-Mechanical Fiber from Eucalyptus urophylla by a Combination of Hydrothermal Pretreatment and Alkali Fractionation. Biotechnol. Biofuels 2014, 7, 116. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP). Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 13 September 2024).
- Qiu, S.; Yadav, M.P.; Yin, L. Characterization and Functionalities Study of Hemicellulose and Cellulose Components Isolated from Sorghum Bran, Bagasse and Biomass. Food Chem 2017, 230, 225–233. [Google Scholar] [CrossRef]
- Iftikhar, M.; Asghar, A.; Ramzan, N.; Sajjadi, B.; Chen, W.Y. Biomass Densification: Effect of Cow Dung on the Physicochemical Properties of Wheat Straw and Rice Husk Based Biomass Pellets. Biomass Bioenergy 2019, 122, 1–16. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Shi, C.; Li, M.; Guan, Y.; Yu, C.Y.; Yamada, T.; Sacks, E.J.; Peng, J. Determination of Hemicellulose, Cellulose and Lignin Content Using Visible and near Infrared Spectroscopy in Miscanthus Sinensis. Bioresour. Technol. 2017, 241, 603–609. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Song, F.; Xia, F.; Huang, X.; Zhang, Z.; Cheng, Y.; Wang, H. Combining Hydrothermal-Alkaline/Oxygen Pretreatment of Reed with PEG 6,000-Assisted Enzyme Hydrolysis Promote Bioethanol Fermentation and Reduce Enzyme Loading. Ind. Crops Prod. 2020, 153, 112615. [Google Scholar] [CrossRef]
- Mishra, A.; Ghosh, S. Bioethanol Production from Various Lignocellulosic Feedstocks by a Novel “Fractional Hydrolysis” Technique with Di Ff Erent Inorganic Acids and Co-Culture Fermentation. Fuel 2019, 236, 544–553. [Google Scholar] [CrossRef]
- Ayala, H.; Kaiser, D.; Pavón, S.; Molina, E.; Siguenza, J.; Bertau, M.; Lapo, B. Valorization of Cocoa’s Mucilage Waste to Ethanol and Subsequent Direct Catalytic Conversion into Ethylene. J. Chem. Technol. Biotechnol. 2022, 97, 2171–2178. [Google Scholar] [CrossRef]
- Wang, L.; York, S.W.; Ingram, L.O.; Shanmugam, K.T. Simultaneous Fermentation of Biomass-Derived Sugars to Ethanol by a Co-Culture of an Engineered Escherichia Coli and Saccharomyces Cerevisiae. Bioresour. Technol. 2019, 273, 269–276. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose Fibers Extracted from Rice and Oat Husks and Their Application in Hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Catalytic Pyrolysis of Rice Husk with SnCl2, Al2O3.4SiO2.H2O, and MoS2 for Improving the Chemical Composition of Pyrolysis Oil and Gas. J. Indian Chem. Soc. 2022, 99, 100728. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Characterization of Nanocellulose Extracted from Short, Medium and Long Grain Rice Husks. Ind. Crops Prod. 2020, 154, 112627. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Zhu, J.Y.; Ralph, S.A. Enzymatic Hydrolysis of Biomass: Effect of Crystallinity, Particle Size, and Lignin Removal. In Proceedings of the 16th ISWFPC, Tianjin, China, 8–10 June 2011. [Google Scholar]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for Energy and Bio-Silica Production from Rice Husk: A Comprehensive Review and Emerging Prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Khaleghian, H.; Molaverdi, M.; Karimi, K. Silica Removal from Rice Straw to Improve Its Hydrolysis and Ethanol Production. Ind. Eng. Chem. Res. 2017, 56, 9793–9798. [Google Scholar] [CrossRef]
- Bazargan, A.; Wang, Z.; Barford, J.P.; Saleem, J.; McKay, G. Optimization of the Removal of Lignin and Silica from Rice Husks with Alkaline Peroxide. J. Clean. Prod. 2020, 260, 120848. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Y.; Yang, F.; Tang, C.; Huang, Q.; Zhang, J. Impact of Delignification on Morphological, Optical and Mechanical Properties of Transparent Wood. Compos. Part. A Appl. Sci. Manuf. 2019, 117, 324–331. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M.I. Synthesis and Characterization of Rice Husk Biochar via Hydrothermal Carbonization for Wastewater Treatment and Biofuel Production. Sci. Rep. 2020, 10, 18851. [Google Scholar] [CrossRef]
- Monte, L.S.; Escócio, V.A.; de Sousa, A.M.F.; Furtado, C.R.G.; Leite, M.C.A.M.; Visconte, L.L.Y.; Pacheco, E.B.A.V. Study of Time Reaction on Alkaline Pretreatment Applied to Rice Husk on Biomass Component Extraction. Biomass Convers. Biorefin. 2018, 8, 189–197. [Google Scholar] [CrossRef]
- Uju; Shoda, Y.; Nakamoto, A.; Goto, M.; Tokuhara, W.; Noritake, Y.; Katahira, S.; Ishida, N.; Nakashima, K.; Ogino, C.; et al. Short Time Ionic Liquids Pretreatment on Lignocellulosic Biomass to Enhance Enzymatic Saccharification. Bioresour. Technol. 2012, 103, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Collins, S.R.A.; Elliston, A.; Wellner, N.; Dicks, J.; Roberts, I.N.; Waldron, K.W. Release of Cell Wall Phenolic Esters during Hydrothermal Pretreatment of Rice Husk and Rice Straw. Biotechnol. Biofuels 2018, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Pertile, G.; Panek, J.; Oszust, K.; Siczek, A.; Oleszek, M.; Gryta, A.; Frąc, M. Effect of Different Organic Waste on Cellulose-Degrading Enzymes Secreted by Petriella Setifera in the Presence of Cellobiose and Glucose. Cellulose 2019, 26, 7905–7922. [Google Scholar] [CrossRef]
- Wu, W.; Hildebrand, A.; Kasuga, T.; Xiong, X.; Fan, Z. Direct Cellobiose Production from Cellulose Using Sextuple Beta-Glucosidase Gene Deletion Neurospora Crassa Mutants. Enzym. Microb. Technol. 2013, 52, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, J.; Wen, J.; Bian, J.; Li, M.; Peng, F.; Sun, R. Structure and Distribution Changes of Eucalyptus Hemicelluloses during Hydrothermal and Alkaline Pretreatments. Int. J. Biol. Macromol. 2019, 133, 514–521. [Google Scholar] [CrossRef]
- Du, S.K.; Zhu, X.; Wang, H.; Zhou, D.; Yang, W.; Xu, H. High Pressure Assist-Alkali Pretreatment of Cotton Stalk and Physiochemical Characterization of Biomass. Bioresour. Technol. 2013, 148, 494–500. [Google Scholar] [CrossRef]
- Hutterer, C.; Schild, G.; Potthast, A. A Precise Study on Effects That Trigger Alkaline Hemicellulose Extraction Efficiency. Bioresour. Technol. 2016, 214, 460–467. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, P.; Zhang, Y.H.P. A Kinetic Model of One-Pot Rapid Biotransformation of Cellobiose from Sucrose Catalyzed by Three Thermophilic Enzymes. Chem. Eng. Sci. 2017, 161, 159–166. [Google Scholar] [CrossRef]
- Moodley, P.; Gueguim Kana, E.B. Bioethanol Production from Sugarcane Leaf Waste: Effect of Various Optimized Pretreatments and Fermentation Conditions on Process Kinetics. Biotechnol. Rep. 2019, 22, e00329. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Jiang, B.; Mu, W. Enzymatic Approaches to Rare Sugar Production. Biotechnol. Adv. 2017, 35, 267–274. [Google Scholar] [CrossRef]
- Ko, J.K.; Um, Y.; Woo, H.M.; Kim, K.H.; Lee, S.M. Ethanol Production from Lignocellulosic Hydrolysates Using Engineered Saccharomyces Cerevisiae Harboring Xylose Isomerase-Based Pathway. Bioresour. Technol. 2016, 209, 290–296. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).