Abstract
The synthesis of inorganic nanoparticles for diverse applications is an active research area that involves physical and chemical methods, which typically are expensive, involve hazardous chemical reagents, use complex equipment and synthesis conditions, and consume large amounts of time and energy. Thus, green synthesis methods have emerged as eco-friendly and easy alternatives for inorganic nanoparticle synthesis, particularly the use of plant-based extracts from fruit juice, leaves, seeds, peel, stem, barks, and roots, which act as reducing, capping, and stabilizing agents, contributing to the Sustainable Development Goals and circular economy principles. Therefore, diverse inorganic nanoparticles have been synthesized using plant-based extracts, including gold, silver, titanium dioxide, zinc, copper, platinum, zirconium, iron, selenium, magnesium, nickel, sulfur, cobalt, palladium, and indium nanoparticles, which exhibit different biological activities such as antioxidant, antimicrobial, dye degradation, cytotoxic, analgesic, sedative, wound-healing, skin protection, sensor development, and plant-growth-promoting effects. Therefore, this review summarizes the advantages and limitations of plant-based extracts as reducing, capping, and stabilizing agents for inorganic nanoparticle green synthesis.
1. Introduction
In the current scientific era, nanotechnology has emerged as an active research area with a wide range of applications, including physics, biology, chemistry, medicine, electronics, engineering, and environmental sciences [1], attributed to the superior physical, chemical, thermal, mechanical, and biological properties (surface area, stability, adsorption, optical, mechanical strength, lower, melting points, catalytic, antimicrobial, antioxidant, and biocompatibility) that nanomaterials exhibit in comparison with their bulk materials [2,3]. Various physical and chemical methods have been exploited to synthesize inorganic nanoparticles; however, these methods are expensive, involve hazardous chemical reagents, use complex equipment and synthesis conditions, and require huge energy and time consumption [4]. To overcome these drawbacks, green synthesis methods are a viable, technological, and eco-friendly alternative for the synthesis of NPs [4,5].
In the green synthesis approach, biological resources such as bacteria, fungi, yeast, viruses, algae, and plants have been investigated as reducing agents for synthesizing inorganic nanoparticles [4,5]. Particularly, the extract of different plant parts can be used, such as the aerial parts, flowers, leaves, roots, stem bark, and seeds, which contain bioactive compounds able to act as reducing agents during the synthesis of inorganic nanoparticles [2]. The main components typically required to reduce the oxidation state of a metal (often in a salt form) are polyphenols, flavonoids, and stilbenes, among others [4]. These compounds act as antioxidants; thus, they can interact with metallic salts to reduce the oxidation value of the metal [5]. In green synthesis using plant extracts, the concentration of these compounds varies depending on the method of extraction applied and the part of the plant used [4,6].
Regarding the plant-based sources of bioactive compounds, it has been reported that approximately 40–50% of global food waste globally comes from plant-based sources, including fruits, vegetables, roots, tubers, edible flowers, leaves, peel, and seeds. These sources can be used to obtain natural antioxidant compounds for synthesizing inorganic nanoparticles. This strategy of using plant-based extracts to synthesize inorganic nanoparticles directly contributes to the United Nations’ Sustainable Development Goals for the 2030 Agenda and aligns with the principles of the circular economy, which focuses on recycling, recovery, and reutilization [7,8]. Therefore, this review aims to summarize the advantages and limitations of using plant-based extracts as reducing, capping, and stabilizing agents for the green synthesis of inorganic nanoparticles.
2. Synthesis of Inorganic Nanoparticles
The general methods for producing nanomaterials can be divided into physical and chemical approaches [9]. In physical methods, experimental conditions are controlled to create nanomaterials from bulk material or desired components. These methods start from bulk materials and are called “top-down” approaches. They encompass techniques like high-energy ball milling, inert gas condensation, laser ablation, wire explosion, arc discharge, and ion sputtering [10,11].
In chemical methods, nanomaterials are manufactured from atoms produced from ions in solution, which assemble to form the nanomaterials [11]. Since the synthesis begins with atoms, these methods are known as “bottom-up” approaches. Methods belonging to this category include chemical reduction, photochemical synthesis, sonochemical routes, electrochemical, solvothermal, micelles and nanoemulsions, interfacial synthesis, biological methods, thermolysis strategies (e.g., pyrolysis, spray pyrolysis), precipitation (mainly for semiconductors and oxides), solvated metal atom dispersion, and hybrid methods that systematically combine several of the previously mentioned methods to create intricate structures [12,13,14].
Due to the nature of the physical and chemical processes, their main disadvantages include high energy and solvent consumption to synthesize nanomaterials, which can increase costs and harm environmental and human health [12]. In this context, the green synthesis of nanoparticles has emerged as an active research area to reduce the negative impact of nanoparticles synthesized by chemical and physical methods.
3. Green Synthesis of Inorganic Nanoparticles
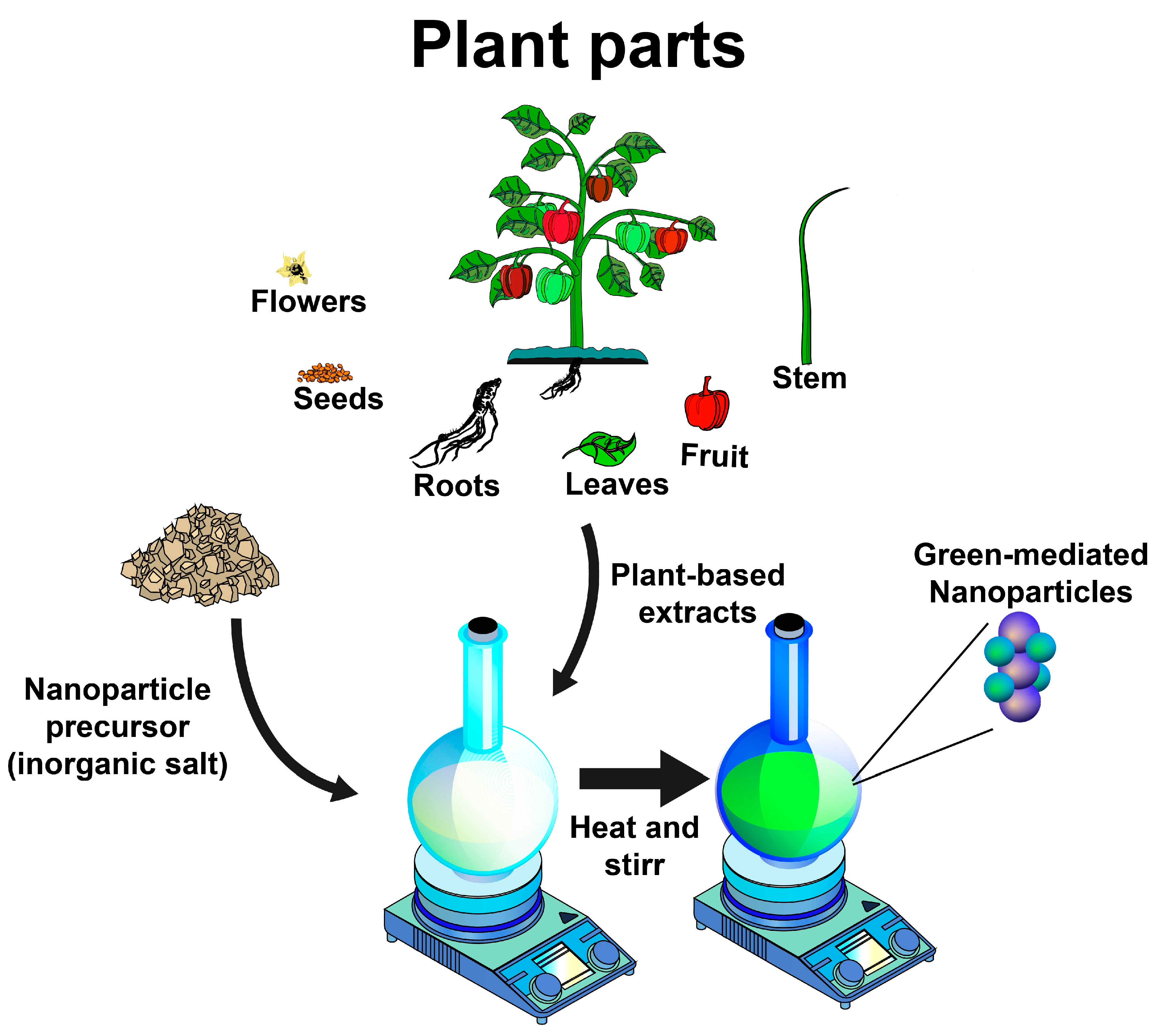
In the green synthesis methods for producing inorganic nanoparticles, bacteria, fungi, algae, and plants are used to reduce the oxidized state of metals, favoring the formation of nanoparticles. Plant-mediated nanoparticle (NP) synthesis can be performed by extracellular and intracellular methods. The extracellular methods involve using plant extracts or isolated phytochemicals as raw materials for the synthesis of NPs, while in the intracellular methods, the NP synthesis takes place inside the cells of plant tissues [2]. The most preferred route for the green synthesis of NPs is the use of plant extracts because this process typically requires ambient pressure and temperature, as well as neutral pH values, usually completed within a few minutes [5]. For the process, plant-extract-mediated bio-reduction involves mixing an aqueous extract with an aqueous solution of the relevant metal salts [14] (Figure 1).
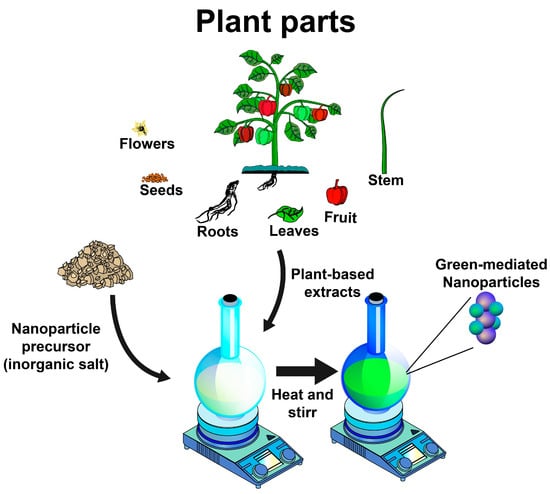
Figure 1.
Schematic representation of typical green synthesis of inorganic nanoparticles using plant-based extracts.
Plants are excellent sources of biomolecules such as phytochemicals (polyphenols, flavonoids, terpenoids, alkaloids, and saponins), polysaccharides, and proteins, which act as reducing, capping, and stabilizing agents for the green synthesis of NPs [3,5]. The roots, seeds, flowers, leaves, peels, fruits, and stem barks of various plant species have been investigated as potential sources for synthesizing NPs [3]. Nonetheless, the source of a plant extract and type and the concentration of phytochemicals, as well as its extraction method, are known to influence the characteristics of a nanostructure due to their hydroxyl and ketone groups, which are capable of binding to metals and showing chelation [4].
4. Inorganic Nanoparticles Synthesized Using Plant-Based Extracts
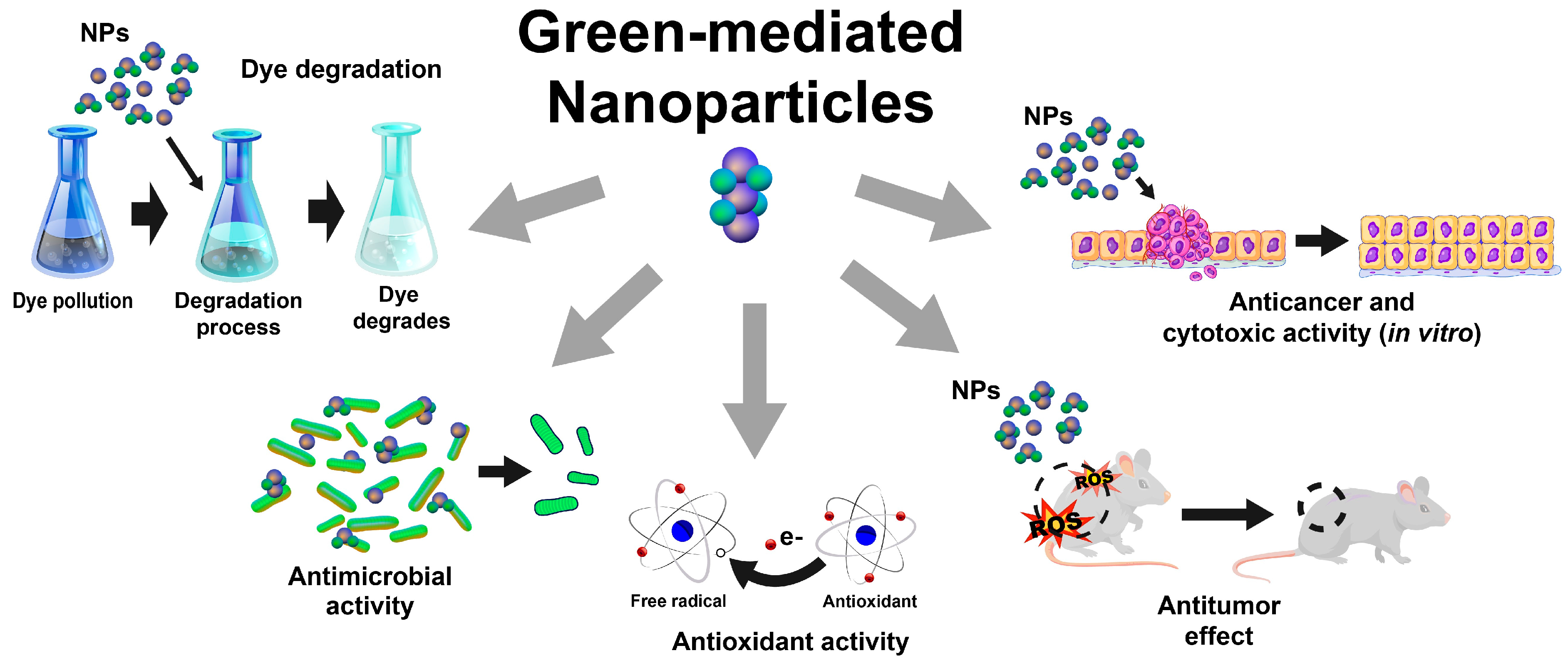
As discussed in the preceding sections, using plant-based extracts as reducing and stabilizing agents for synthesizing inorganic nanoparticles is an opportunity for developing greener synthesis approaches. Therefore, diverse inorganic nanoparticles have been synthesized using plant-based extracts, including gold, silver, titanium dioxide, zinc, copper, platinum, zirconium, iron, selenium, magnesium, nickel, sulfur, cobalt, palladium, and indium nanoparticles, which exhibit different biological activities for diverse applications (Figure 2), as described below.
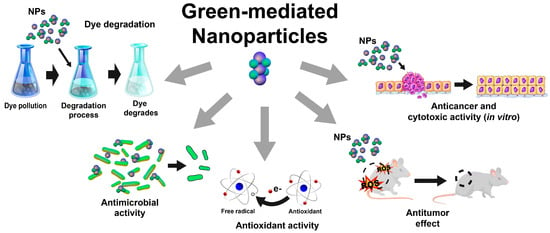
Figure 2.
Schematic representation of most-reported applications of green-mediated inorganic nanoparticles.
4.1. Gold Nanoparticles
Gold nanoparticles (AuNPs) have proved to be a versatile material for diverse applications [15,16,17,18]. Therefore, many routes for its synthesis have been developed involving the reduction of gold cations (Au1+ or Au3+) to gold-zerovalent (Au0) [15,19,20], highlighting the green synthesis route, where diverse rich bioactive compounds from plant-based extracts reduce gold precursors. Then, Au+ ions are bound and capped by phytochemicals to form stable AuNPs [19,21,22] (Table 1). In most cases, the reduction of gold is monitored by a color change from pale pink to ruby red, or to dark violet from yellow for AuCl3 and HAuCl4, respectively, as AuNPs precursors [16,20,21]. The green-mediated AuNPs exhibit XRD patterns of a face center cubic structure [(111), (200), (220), and (311)] [16,18] and UV–visible absorption peaks between 530 and 550 nm [23], with an Eg value of 1.9 eV [20].

Table 1.
Gold nanoparticles synthesized by green chemistry using plant-based extracts.
In general, the methanolic and aqueous extracts from flowers, roots, seeds, fruits, and leaves of different plants have been used for the green synthesis of AuNPs with spherical shapes and sizes from 4 to 100 nm for diverse applications [15,18,20,23]. Khan et al. [25] reported that AuNPs (5.8 nm) can be synthesized using Clerodendrum inerme aqueous leaf extract, which exhibited antimicrobial, antioxidant, and cytotoxic activities. Similar trends were reported in AuNPs (53 nm) synthesized using licorice root extract, which exhibited antioxidant, antimicrobial, and anticancer activities in a concentration-dependent manner. These activities are associated with the bioactive compounds of the licorice root extract that includes phenolics, glycosides, organic acids, terpenes, and fatty acids [21]. Furthermore, it has been reported that the green synthesis of AuNPs is influenced by the concentration of gold precursor [20,21].
Boruah et al. [19] used Moringa oleifera methanolic leaf extract to synthesize spherical-shaped AuNPs with size of 4 nm. They exhibited diverse biological properties, such as antimicrobial, antioxidant, and cytotoxic activities in red blood cells; moreover, they also showed photocatalytic properties against methylene blue dye. The authors suggested that the bioactive compounds of the extract contributed to the enhanced biological and technological properties of the AuNPs. Other studies have found similar trends when using green-mediated AuNPs from Zingiber officinale root (antimicrobial, antioxidant, and cytotoxic properties) [24], Ricinus communis seeds [15], Jatropha integerrima flower [20], and Vitis vinifera fruit (antimicrobial activity) [18]. It was also reported that AuNP-mediated Pistacia chinensis exhibited analgesic and sedative properties in an acetic-acid-induced writhing model in a dose-dependent manner [23]. Furthermore, extracts from plants such as Phoenix dactylifera [18], Simarouba glauca [22], Salvia officinalis, Lippia citriodora, Pelargonium graveolens, Punica granatum [17], and Centella asiatica [16] have been investigated as reducing, capping, and stabilizing agents for the green synthesis of AuNPs.
4.2. Silver Nanoparticles
Among the metal-based nanoparticles, silver nanoparticles (AgNPs) have become very popular in the research community due to their wide range of applications, including for antimicrobial, antioxidant, anticancer, and photocatalytic dye degradation applications [26,27,28,29]. In this context, the green synthesis of AgNPs using plant-based extracts as reducing agents (Ag+ to Ag0) has gained significant attention in recent years [30,31,32], with silver nitrate (AgNO3) being the most used precursor for their synthesis [29,31,32,33]. After mixing AgNO3 aqueous solution and plant extracts, the color of the reaction medium changes from yellowish green to brownish [34,35], suggesting the conversion of Ag ions (Ag+) to metallic silver (Ag0) [31]. The green-mediated AgNPs exhibited surface plasmon resonance around 400–450 nm [31] with a crystalline nature and a face-centered cubic structure XRD pattern of (111), (200), (220), and (331) [35]. On the other hand, some green-synthesized AgNPs exhibited good stability (zeta potential of −15.8 to 3.31 mV) [32]. Various plant-based extracts have been investigated to prepare spherical AgNPs with sizes < 100 nm (Table 2).

Table 2.
Green synthesis of silver nanoparticles using plant-based extracts.
Various plants such as Zataria multiflora, Brillantaisia patula, Crossopteryx febrifuga, Senna siamea, Gymnema sylvestre, Lysiloma acapulcensis, and Achillea millefolium have been used to synthesize silver AgNPs, using extracts from different parts of the plant, such as the leaves, stems, and roots [29,31,33,34]. It was reported that factors such as the plant extract, pH, and temperature significantly influenced the green synthesis of AgNPs [39]. These green-mediated AgNPs have exhibited antimicrobial properties against various pathogenic bacteria and fungi [27,28,31,35,36,37]. According to the authors, the antimicrobial activity of AgNPs is related to their ability to cause cell breakdown, which promotes changes in the cell membrane’s permeability.
It has been found that green-synthesized AgNPs prepared from aqueous extracts derived from Annona muricata peel, Teucrium polium leaves, and Cynara scolymus leaf extracts exhibit anticancer properties at very low concentrations, attributed to their apoptotic properties [30,34,38,39,40]. Additionally, green-synthesized AgNPs using Achillea millefolium and Annona muricata extracts exert anti-inflammatory and antioxidant activities [28,30]. Chand et al. [38] reported that AgNPs synthesized using onion and tomato extracts exhibited photocatalytic properties against cationic dyes in aqueous solutions, with the effect attributed to the capability of AgNPs to produce reactive oxygen species under the UV region [38].
In general, the plant-based extracts used for AgNPs green synthesis proceed through reducing silver ions, primarily by the capability to donate electrons. Tannins were found to play a key role in reducing and capping AgNPs in most cases [39]. Furthermore, some plant-based extracts, such as Centella Asiatica and Tridax, were used to synthesize silver oxide (Ag2O) nanoparticles, which exhibited photocatalytic properties against acid orange dye [27].
4.3. Titanium Dioxide Nanoparticles
Titanium dioxide nanoparticles (TiO2NPs) are one of the most investigated materials due to their photocatalytic properties and chemical and thermal stability in diverse industrial uses [41]. They have been typically synthesized by chemical routes [42]; however, TiO2NPs have been synthesized using green approaches using different plant-based extracts and titanium-isopropoxide or titanium dioxide solution as chemical precursors (Table 3). In most cases, a light-green formation after mixing TiO2 precursor and plant extracts indicates the reduction of Ti ions [43]. The green-mediated TiO2NPs exhibited a strong UV absorption peak around 380 to 400 nm and a crystalline structure in their anatase and rutile forms [Miller index = (101), (110), (103), (004), (112), (200), (105), and (211)] [41] with negative zeta potential (−18.7 to −11.5 mV) and large surface area (105 m2g−1) [44,45,46].

Table 3.
Green synthesis of titanium dioxide nanoparticles using plant-based extracts.
Thakur et al. [41] synthesized TiO2NPs using Azadirachta indica aqueous leaf extract as a stabilizing and reducing agent, with anatase and rutile structures, spherical shapes, and sizes from 15 to 50 nm, which exhibited antibacterial activity against different Gram-negative bacteria. Similarly, TiO2NPs (spherical and 20–70 nm in size) prepared by green synthesis using Mentha arvensis leaf extracts showed antimicrobial activity in a dose-dependent manner [45]. According to the authors, the phytochemicals such as alkaloids, terpenoids, and phenolics in Azadirachta indica and Mentha arvensis acted as stabilizing and reducing agents during TiO2 synthesis. The antimicrobial effect of TiO2 is due to its capability to interact with the cell wall of microorganisms, promoting cell death [41,45].
Sethy et al. [46] reported that TiO2NPs (anatase phase) synthesized using an aqueous leaf extract of Syzygium cumini exhibited photocatalytic properties for removing Pb from wastewater, associated with its ability to generate OH radicals in the presence of light. On the other hand, recent research also showed that TiO2NPs (anatase form, spherical and polygonal shape, 130 nm in size), prepared by a green synthesis approach using Ocimum sanctum leaf extract, could improve the wound-healing efficacy of chitosan hydrogels in diabetics rats [44]. This effect was attributed to the antimicrobial properties of TiO2 [45]. The authors suggested that the presence of phytochemicals in plant-based materials plays an important role in the reduction, capping, and stabilization of TiO2 [44,46].
Srinivasan et al. [43] synthesized TiO2NPs in the anatase phase using aqueous leaf extracts of Sesbania grandiflora, which exhibited square and spherical shapes with sizes ranging from 43 to 56 nm, completing the reduction at room temperature. Furthermore, the study revealed that TiO2NPs exhibited toxicological effects against zebrafish embryos in a dose-dependent manner. Therefore, further studies are needed to fully evaluate the possible toxic effects of TiO2NPs synthesized by green synthesis methods.
Additionally, the green synthesis of TiO2NPs has been performed using different plant-based extracts, including Moringa oleifera [47], Psidium guajava [48], Arbor tristis [49], Eclipta prostrata [50], and Ageratina altissima leaves [51], Vigna unguiculata seeds [52], Calotropis gigantea flowers [53], Aloe vera [54], Vigna radiata legumes [55], and Curcuma longa plant [56]. These TiO2NPs exhibited potential industrial, environmental, and pharmaceutical applications due to their wound healing, antimicrobial, antioxidant, dye photocatalytic degradation, acaricidal, and cytotoxic properties [47,49,51,52,53,55].
4.4. Zinc Nanoparticles
Zinc nanoparticles (ZnNPs) have unique features like being nontoxic, low-cost, biocompatible, multifunctional, and eco-friendly. They have been investigated for diverse applications due to their antimicrobial, antifungal, nanomedicine, antioxidant, and photocatalytic activities [57,58,59]. ZnNPs have been green-synthesized using extracts from the leaves, flowers, stem bark, and fruit juice from various plant species (Table 4), which are mostly spherical, with sizes smaller than 60 nm. The most common Zn precursors are zinc nitrate and zinc acetate [60,61]. After mixing the plant extracts and Zn precursors, a white or yellowish paste is observed, indicating successful formation [62,63]. The green-mediated ZnNPs exhibited a strong UV absorption peak around 300–400 nm [63,64] and a crystalline structure in their hexagonal wurtzite phase [Miller index = (100), (002), (101), (102), (110), (200), (112), (201), (004), and (202)], where each Zn+2 ion was ordered in a tetragonal coordination with a polar symmetry throughout the hexagonal axis [62,65]. These ZnNPs exhibited Eg values from 2.67 to 3.37 eV and a negative zeta potential (−40 mV) [63,65].

Table 4.
Green synthesis of zinc dioxide nanoparticles using plant-based extracts.
Madhukara et al. [60] used Limonia acidissima juice for zinc ferrite (ZnFe2O4) nanoparticle synthesis with photocatalytic properties against Evans blue and methylene blue dyes under visible light in a concentration-dependent manner, as well as antibacterial activity against in a strain- and dose-dependent manner. It has been reported that green-synthesized ZnNPs using Eriobutria japonica seed [57], Hibiscus sabdariffa [58], or Hydnocarpus alpina [67] extracts are active against methylene blue dye in a dose-dependent manner; moreover, they exhibit antimicrobial activity against various bacterial strains [57,67]. However, ZnNPs exhibit higher antimicrobial activity against Gam-negative bacteria than Gram-positive bacteria due to differences in the thickness of the peptidoglycan layer; thus, ZnNPs can enter cells and inhibit their replication and growth. Additionally, ZnNPs synthesized using Lippia adoensis [61], as well as Euphorbia hirta extracts, showed antibacterial activity in a strain- and dose-dependent manner.
Bitopan et al. [66] studied the biocompatibility of ZnNPs using Xanthium indicum leaf extract as a reducing and stabilizing agent and reported that the ZnNPs did not show hemolytic action at lower concentrations; however, negative effects were observed at higher concentrations (>25 mg/mL). The authors mentioned that the ZnNPs synthesized by the green route demonstrated the weakest cytotoxic effects compared with those obtained by the chemical route. Additionally, Ashwini et al. [68] synthesized ZnNPs using Cayratia pedate as an enzyme glucose oxidase immobilizer for biomedical applications.
4.5. Copper Nanoparticles
Copper nanoparticles (CuNPs) are gaining significant attention due to their electrical, optical, mechanical, catalytic, and antimicrobial properties [69]. They have been green-synthesized using aqueous extracts from the leaves, flowers, and fruits from various plants for environmental and antimicrobial purposes [3,70,71,72]. The most common precursors for CuNPs are copper (II) sulfate pentahydrate [3,69,71,73], cupric nitrate trihydrate [70], copper chloride (II) [71,73,74], and copper (II) acetate [75] (Table 5). The formation of CuNPs is confirmed through a color change of the reaction mixture from yellowish to brownish [71,73], yellow to green [3], or blue to brown [72], depending on the Cu precursor. Green-mediated CuNPs exhibited a UV absorption peak ranging from 269 to 580 nm, which depends on the CuNPs’ energy state [3,69]. In most cases, CuNPs exhibited a monocyclic configuration [Miller index = (110), (111), (220), (800), and (713)] [71]. These CuNPs are mostly spherical with sizes ranging from 2 to 80 nm [3,70,71,72,73,74] and a negative zeta potential (−33.98 mV) [74].

Table 5.
Green synthesis of copper nanoparticles using plant-based extracts.
Mali et al. [3] used Celastrus paniculatus aqueous leaf extract as a reducing agent to synthesize spherical CuNPs (Cu purity of 79.87%, size of 2–10 nm) with photocatalytic and antifungal properties. Similarly, it was reported that CuNPs (spherical and size of 63 nm) prepared using Tinospora cardifolia aqueous leaf extract exhibited antibacterial activity and could be impregnated in cotton fabrics [74]. Additionally, Cissus vitiginea leaf extract was used to synthesize CuNPs (spherical and size of 5–20 nm) active against urinary infection pathogens [72]. The antimicrobial properties of CuNPs are based on changes in the cell structure of microorganisms, leading to cell death [3,72,74]. In general, the biomolecules present in plant extracts act as reducing and stabilizing agents during the formation of CuNPs. Particularly, flavonoids are transformed from the enol form to the keto form by releasing a reactive hydrogen atom that reduces Cu2+, which is facilitated at pH 7 [3].
Ismail et al. [69] reported that zerovalent CuNPs synthesized using Duranta erecta aqueous fruit extract showed photocatalytic activity against anionic dyes in a dose-dependent manner. Similarly, CuNPs with photocatalytic properties against anionic and cationic dyes could be synthesized using Jatropha curcas leaf extract [71].
Additionally, Chowdhury et al. [75] synthesized copper oxide nanoparticles (CuONPs) with Lantana camara flower extract in an alkaline hydrolysis process. The resulting rod-shaped nanoparticles (15–23 nm in size) exhibited catalytic properties against acrylonitrile and aniline. Similarly, CuONPs synthesized using Calotropis procera leaf extract exhibited antimicrobial activity. Furthermore, Cu-based nanoparticles have also been doped with inorganic materials to enhance their physicochemical properties. Green-synthesized Cu-doped MoO3 (Cu-MoO3) nanoparticles prepared using Genus Santalum aqueous leaf extract enhanced the photocatalytic properties in degrading hazardous organic pollutants [70]. Cu-doped silver (Cu-Ag) nanoparticles could remove dyes from aqueous solutions [76], while copper–nickel hybrid nanoparticles synthesized using extracts from Zingiber officinale rhizomes showed photocatalytic activity against crystal violet dye [77].
4.6. Platinum Nanoparticles
Over the past few years, researchers have been exploring the use of aqueous extracts from various plant materials, including Cordyceps militaris, Nymphaea tetragona, Atriplex halimus, olive, Saudi’s dates, and tea polyphenols, along with their parts, to synthesis platinum nanoparticles (PtNPs) (Table 6). For this purpose, hexachloroplatinic acid (H2PtCl6) is commonly used as precursor to produce spherical-shaped PtNPs ranging from 1 to 13 nm [78,79,80]. After mixing plant extracts and H2PtCl6, the color of the reaction mixture gradually changed from pale yellow to brown or black, indicating the reduction to Pt0 [79,80,81,82,83]. The green-mediated PtNPs exhibited surface plasmon resonance around 230 to 295 nm and a cubic structure XRD pattern of (111), (200), (220), and (311) [81,82] with a zeta potential of −17.28 to −0.0536 mV [80,84,85].

Table 6.
Green synthesis of platinum nanoparticles using plant-based extracts.
PtNPs have shown promising antimicrobial activity against pathogenic bacteria [79,80,82], as well as antioxidant capacity [80,81,82]. Additionally, PtNPs have been found to possess anticancer properties [79] and inhibit serum aspartate aminotransferase in serum levels of patients with chronic liver disease [83]. PtNPs have also demonstrated antiaging effects/skin protection by promoting collagen I biosynthesis in HFF-1 cells and inhibiting tyrosinase activity in A375 cells [81]. On the other hand, PtNPs with polycrystalline structures were used to develop sensors for hydrogen peroxide detection [78]. All authors agreed that bioactive compounds in plant-based extracts exerted capping and reducing effects, both associated with metal reduction and contribute as a stabilizing agent to forming PtNPs, avoiding their agglomeration [78,79,82]. Furthermore, green synthesis of PtNPs has been performed using hyacinth plant extracts [85] and seaweed Padina gymnospora [82] with potential biomedical applications.
4.7. Zirconium Nanoparticles
Zirconium (ZrNPs) nanoparticles can be synthesized by green chemistry methods with high purity using plant-based extracts for diverse applications, such as antimicrobial and dye photocatalytic degradation [86]. They can be prepared from various precursors, including zirconium isopropoxide [87], zirconylchloride octahydrate [88], zirconyl nitrate [84], and zirconyl chloride [89]. After mixing a Zr precursor and plant extract, color changes from yellow to brown [89] or milky white formation indicates the formation of ZrNPs [88]. Green-mediated ZrNPs exhibited monocyclic structure of baddeleyite [XRD (111), (002), (022), (031), and (131)] [90], a UV absorption peak around 275 nm, an Eg value of 3.7 eV [91], and a zeta potential of −32.8 mV [88,92]. The ZrNPs had cubic, spherical, triangular, and oval shapes with sizes from 10 to <200 nm [87,88] (Table 7).

Table 7.
Green synthesis of zirconium nanoparticles using plant-based extracts.
Kazi et al. [86] used aqueous leaf extracts from Sphagneticola trilobata to synthesize ZrNPs of various shapes and sizes (20 to 100 nm). These nanomaterials showed antimicrobial properties and antimalarial activity. Similarly, spherical ZrNPs (121 nm) synthesized using Phyllanthus niruri aqueous leaf extracts, cubic ZrNPs obtained from Laurus nobilis (20 to 100 nm), and neem-gum nanoparticles displayed antimicrobial activity. In all cases, the antimicrobial effects of ZrNPs were dose-dependent. According to the authors, the antimicrobial properties of ZrNPs are related to their surface energy and surface-related interactions, leading to cell death by disrupting cell membranes and altering membrane fluidity [87,88,89].
Regarding environmental applications, ZrNPs synthesized by green methods with extracts from the pericarp of Sapindus mukorossias and leaves of Phyllanthus niruri exhibited photocatalytic properties for different dyes. These effects were attributed to the photocatalytic and adsorptive properties of ZrNPs [89,93].
The authors agreed that the presence of biomolecules in the plant-based extracts played a crucial role in reducing, capping, chelating, and stabilizing the conversion to ZrNPs due to their antioxidant properties [86,87,89,93]. These phytochemicals also helped to form and stabilize the octahedral complex of Zr2+ phytochemicals [94]. Moreover, zirconium oxide (ZrO2) nanoparticles can be synthesized using different plant-based extracts, including Euclea natalensis roots [95], Salvia Rosmarinus leaves [96], Helianthus annuus seeds [91], Nephelium lappaceum fruit [90], and Nyctanthes arbortristis flowers [92] for diverse industrial applications.
4.8. Iron Nanoparticles
Iron oxide nanoparticles (Fe2O3NPs) have gained significant attention due to their unique physicochemical and catalytic properties for environmental and biomedical applications [97,98], mainly those synthesized by green routes using plant-based extracts. Fe2O3NPs can be prepared from various precursors, including ferric chloride hexahydrate (FeCl3·6H2O) [97], ferric nitrate (Fe(NO3)3·9H2O) [99], and ferrous sulfate (FeSO4) [100]. The formation of a black precipitate ensures the formation of α-Fe2O3 nanoparticles [101] or Fe3O4 [102], which are corroborated by the XRD patterns [101]. The nanoparticles exhibited spherical, semispherical, and cubic shapes with sizes ranging from 4 to 200 nm [97,100,103] (Table 8).

Table 8.
Green synthesis of iron nanoparticles using plant-based extracts.
Generally, the most common form of green-synthesized iron nanoparticles is iron oxide (α-Fe2O3), which has been investigated for dye degradation, antimicrobial, and antioxidant purposes [103,104,105]. In this context, Lohrasbi et al. [97] used an aqueous extract of Plantago major leaves to prepare spherical Fe2O3NPs with sizes from 4 of 30 nm for environmental applications, while Ficus carica fruit extract was used in core–shell form with an average size of 9 nm. Additionally, Fe2O3 or Fe3O4 iron nanoparticles synthesized using Phoenix dactylifera aqueous leaf extract were found to exhibit antioxidant properties [102]. Nas et al. [98] reported that Fe2O3NPs synthesized using Rhus punjabensis extract can be used for antimicrobial, antioxidant, and anticancer applications. This effect is associated with the functional groups of the phenolics and flavonoids on the surface of the nanoparticles [98].
Devi et al. [99] reported that Platanus orientalis leaf extract can be used for the green synthesis of spherical Fe2O3NPs with an average size of 38 nm. These nanoparticles exhibited antifungal activity in a dose-dependent manner, attributed to the capability of Fe2O3NPs to disrupt microbial cell walls. Similarly, it was reported that Fe2O3NPs with irregular shapes and an average size of 21 nm can be synthesized using Carica papaya leaf extract, exhibiting moderate antimicrobial and photocatalytic properties in a dose-dependent manner [101]. During the green synthesis of Fe2O3NPs, the phytochemicals of plant extracts can reduce metallic salts to nanoparticles and act as stabilizing agents, preventing the aggregation of nanoparticles. The bioactive compounds reacted with the iron ions to give Fe2O3NPs, as the first-row transition metals are oxidation-prone. However, it is possible that the phytochemicals are not able to reduce Fe3+ to Fe0 [99,101].
Furthermore, Withania coagulans extract was used as a reducing agent to prepare α-Fe2O3 nanorods (16 nm) with photocatalytic and antimicrobial activities [105]. Similarly, it was reported that using pomegranate seed extract, semispherical Fe2O3NPs (25–55 nm) exhibited the catalytic degradation of reactive blue dyes under UV light [103]. On the other hand, Fe-based nanoparticles doped with other inorganic materials showed enhanced physicochemical properties. Younas et al. [100] found that green-synthesized Fe–Cu bimetallic nanoparticles using Ixora finlaysonian leaf extract with rectangular and cubic shapes and sizes from 50 to 200 nm exhibited antioxidant and photocatalytic dye-degradation activities.
4.9. Selenium Nanoparticles
Selenium nanoparticles (SeNPs) are biocompatible compounds that exhibit low toxicity and high biological activities, which can be synthesized by green chemistry using plant-based extracts from fruits, leaves, peel, and plants such as Crataegus monogyna [106], Melia azedarach [107], Rosmarinus officinali [108], orange [109], Cleistocalyx operculatus [110], Portulaca oleracea [111], Urtica dioica [112], Abelmoschus esculentus [113], Cordia myxa [114], and Withania somnífera [115], with sodium selenite (Na2SeO3), selenium dioxide (SeO2), and selenious acid (H2SeO3, as SeNPs precursors [106,110,115]. The formation of SeNPs can be identified by a color change from light green to brick red [107], colorless to light pink or red [106,114], or from pale yellow to deep red [106,109]. SeNPs exhibited a UV absorption peak ranging from 200 to 302 nm with monocyclic and trigonal phases [106,107,108,109,110] and a zeta potential of −64 mV [113]. These nanoparticles are spherical with sizes ranging from 2 to 200 nm [110,111] and exhibit antioxidant, antimicrobial, antiviral, anticancer, and photocatalytic activities [106,107,111,115] (Table 9).

Table 9.
Green synthesis of selenium nanoparticles using plant-based extracts.
Barzegarparay et al. [106] reported that green-synthesized SeNPs using Crataegus monogyna methanolic fruit extract exhibited anticancer activity; using an ethanolic leaf extract from Withania somnifera and Cordia myxa aqueous fruit extract, SeNPs showed antiproliferative properties [114,115]. This effect was attributed to the apoptotic properties of SeNPs, which were able to arrest the C2/M cell cycle [106], as well as to their antioxidant activity [106,115].
Additionally, SeNPs prepared using Melia azedarach aqueous leaf extract exhibited antifungal properties [107], while those synthesized using Rosmarinus officinali, Abelmoschus esculentus, Cleistocalys operculants leaves, and orange peel extracts exhibit antimicrobial activity [108,109,110,113]. Portulaca oleracea-based green SeNPs exhibited antimicrobial, antiviral, and mosquitocidal properties [111], while Urtica dioica-mediated SeNPs exerted antifungal activity [112]. Additionally, it was reported that SeNPs synthesized using combinations of plant extracts (Allium cepa, Malpighia emarginata, and Gymnanthemum amygdalinum) exhibited antimicrobial activity [116]. Moreover, green-synthesized SeNPs (Withania somnifera leaf extract) have been explored for the photocatalytic degradation of methylene blue dye [115].
4.10. Magnesium Nanoparticles
Magnesium oxide nanoparticles (MgONPs) are an attractive material for antimicrobial purposes [117]. Moreover, they have been investigated for other potential applications, including dye degradation, antioxidant, cytotoxic, and antiaging applications [118,119,120]. The green synthesis of MgONPs has been performed using aqueous extracts from the leaves, flowers, and barks of different plants [118,121,122], when magnesium nitrate (Mg(NO3)2) [121] and magnesium chloride (MgCl2) [123] have been used as precursors (Table 10). The formation of MgONPs can be identified by a color change from pale green to brown [121], brownish to dark brownish-red [119], and colorless to dark brown [117,123]. Moreover, it has been reported that MgONPs exhibited a UV absorption peak around 280–290 nm and a hexagonal or cubic structure [117,118,119,122,123].

Table 10.
Green synthesis of magnesium nanoparticles using plant-based extracts.
Vergheese and Vishal [119] demonstrated that spherical MgONPs (13 nm) synthesized using Trigonella foenum-graecum, Xanthomonas oryzae pv. Oryzae, and Dalbergia sissoo aqueous leaf extract exhibited antibacterial activity [118]. In general, bioactive compounds such as alkaloids, saponins, flavonoids, phenolics, and terpenoids act as a capping and stabilizing agent during synthesis [120]. However, it must be noted that the pH of the reaction solution may affect the reduction ability of bioactive compounds, mainly owing to the concentration of hydroxyl ions in the medium [118].
Younis et al. [118] found that MgONPs (polyhedral and size of 35–55 nm) synthesized using flower extracts of Rosa floribunda charisma exhibited antioxidant, antiaging, and antibacterial activities in a dose-dependent manner against skin pathogens. Additionally, the bark and leaf extracts of Moringa oleifera have been investigated for the synthesis of MgONPs with antioxidant and antimicrobial activities [117,123]. Recently, it has been reported that MgONPs with irregular shapes and sizes < 100 nm synthesized by a green approach (Abrus precatorius aqueous bark extract) exhibited antioxidant, antibacterial, and cytotoxic activities without toxic effects on zebrafish embryos; moreover, MgONPs exhibited the photocatalytic degradation of methylene blue dye [119].
4.11. Nickel Nanoparticles
Nickel/nickel oxide nanoparticles (NiNPs/NiONPs) have been green-synthesized using aqueous extracts from the leaves, seeds, and flowers of various plants for environmental, antioxidant, antimicrobial, anticancer, and antileishmanial applications [76,124,125,126]. In the process, nickel nitrate (Ni(NO3)2) [127], nickel chloride (NiCl2) [128], and nickel sulfate (NiSO4) [129] are the most commonly used precursors (Table 11). During the green synthesis of NiNPs, the color of the reaction mixture changes from green to dark brown [77]. NiNPs were characterized by XRD [Miller index = (110), (111), (200), (220), and (311)], and UV–Vis (surface resonance plasmon of 341 nm and bandgap energy of 1.57 eV) [126,128].

Table 11.
Green synthesis of nickel nanoparticles using plant-based extracts.
Yuan et al. [129] synthesized NiNPs (spherical, 20–36 nm) using Alhagi maurorum leaf aqueous extract that exhibited cytotoxic and anti-human ovarian cancer activity in a dose-dependent manner [125,128]. Similarly, it was reported that nickel ferrite (NiFe2O4) nanoparticles (spherical shape, size of 19 nm) synthesized using Terminalia catappa aqueous leaf extract exhibited anticancer activity in a dose-dependent manner, possibly through an apoptosis mechanism. The average crystallite size of the Ni nanoparticles was reduced by increasing the volume of the plant extract used [124].
Additionally, Rhamnus virgata leaf aqueous extract was used as stabilizing, reducing, and chelating agent during the formation of NiONPs, showing anticancer, antileishmanial, and antimicrobial activities without toxicological effects in human RBCs and macrophages [125]. Ali et al. [126] reported that green-synthesized spherical Ni/NiONPs using the seed extract of Lactuca serriola exhibited antimicrobial properties in a strain- and dose-dependent manner due to the capability of the nanoparticles to modify the cell membrane and block the transport channels. Moreover, Senna auriculata-mediated and Hammada scoparia-mediated NiONPs have exerted antimicrobial properties [76,130].
Furthermore, the seed extract of Hordeum vulgare was used to synthesize NiNPs and NiONPs (<100 nm) and was able to degrade methylene blue dye, which exhibited first-order kinetics [127]. Similarly, it was reported that NiONPs green-synthesized using Syzygium cumini leaf extract with a spherical shape and size of 10 nm exhibited photocatalytic activity against methylene blue and Congo red dyes [128]. Moreover, other plant-based extracts from the seeds, flowers, and leaves from Lactuca Serriola [126], Senna auriculata [130], and Hammada scoparia [76], respectively, have been used for the green synthesis of Ni and NiO nanoparticles for the photocatalytic degradation of crystal violet, methylene blue, and malachite green [76,126,130].
4.12. Sulfur Nanoparticles
Sulfur nanoparticles (SNPs) are biocompatible compounds with a high surface area and catalytic activity, which have great potential for diverse biomedical and agricultural applications [131]. These SNPs have been green-synthesized using aqueous extracts from various plant materials and their parts, including Rosmarinus officinalis [132], Citrus limon [131], Aloe vera [133], Allium sativum [134], and Cinnamomum zeylanicum [135]. Sodium thiosulfate pentahydrate is commonly used as a precursor for spherical SNPs, ranging from 40 to 69 nm in size [131,132,133,134,135]. After mixing the plant extract and sulfur precursor, a yellow color indicates the formation of SNPs [135]. These nanoparticles agreed with the standard XRD orthorhombic sulfur pattern [133] and exhibited a UV absorption peak of around 245 to 295 nm [132,133] with a zeta potential of −10.4 mV [134].
These SNPs have been used as plant-growth-promoting and nematicidal agents [132,134,135] and are effective antimicrobial agents [131]. Furthermore, SNPs were embedded in a chitosan nanohydrogel for wound-healing applications [131]. Table 12 lists some green-synthesized sulfiur SNPs and their applications.

Table 12.
Green synthesis of sulfur nanoparticles using plant-based extracts.
4.13. Other Nanoparticles Synthesized by Green Methods
The other nanoparticles synthesized by the green approach using plant-based extracts as capping, reducing, and stabilizing agents include cobalt [136,137], palladium [138], and indium [139,140]. Gingasu et al. [137] used an aqueous extract of ginger roots and cardamom seeds to synthesize cobalt ferrite nanoparticles (CoFe2O4) with irregular forms and sizes smaller than 100 nm. The authors reported that the Co2+ cation distribution was higher than that of Fe3+, possibly associated with the nature of the plant extracts. Similarly, cobalt oxide (Co3O4) nanoparticles were green-synthesized using Populus cilata aqueous leaf extracts, which exhibited antimicrobial activity [136].
Vinodhini et al. [138] used Allium fistulous, Basella alba, and Tabernaemontana divaricate aqueous leaf extracts to synthesize palladium nanoparticles with photocatalytic activity for Congo red dye degradation. Moreover, palladium nanoparticles exhibited antioxidant and antimicrobial properties in a concentration-dependent manner. Furthermore, palladium nanoparticles prepared with Tabernaemontana divaricate leaf extract showed antidiabetic activity in vitro inhibiting the α-amylase enzyme. According to the authors, polyphenol-rich plant-based extracts played an important role in reducing the metal ions and stabilizing the inorganic nanoparticle formation, showing diverse potential applications [137,138]. On the other hand, Aloe vera plant extract [140] and Astragalus gummifer gums [139] have been used in green synthesis of indium oxide (In2O3) nanoparticles, which exhibited good optical properties for further applications [139,140].
5. Advantages of and Challenges in the Green Synthesis of Nanoparticles
Green synthesis has many advantages compared with physical and chemical processes. The inorganic nanoparticles obtained from green synthesis with plant extracts offers an eco-friendly approach since the chemicals used are the bioactive compounds present in the plant [4]. The compounds that reduce metals ions can stabilize the nanoparticles obtained, which avoids the use of different solvents to achieve reduction and capping [141]. Also, operational conditions are mainly environmental, so external energy is unnecessary for the nanoparticles to form. The compounds present in different parts of the plant vary; thus, they allow the synthesis of nanoparticles with interesting morphologies, sizes, and distributions, which opens the possibility of producing a wide catalog of nanoparticles according to their use [142].
On the other hand, some of the challenges for the synthesis of inorganic nanoparticles through plant extracts include low production yield. This can be attributed to the amount and type of bioactive compounds in the extract, which vary depending on the plant and part selected, the growth location, and period of collection. Also, achieving high reproducibility implies a controlled growth environment for plants since factors such as light, nutrient availability, and soil pH impact the production of the metabolites required for green synthesis [4,143]. Although some plants are specific to some regions, availability can also expand the uses of some plants.
6. Conclusions
Green methods that use plant-based extracts as reducing, capping, and stabilizing agents are environmentally friendly, cost-effective, and feasible alternatives for synthesizing inorganic nanoparticles. This approach contributes to the Sustainable Development Goals and circular economy principles. In recent years, various studies have been conducted regarding the green-mediated synthesis of inorganic nanoparticles such as gold, silver, titanium dioxide, zinc, copper, platinum, zirconium, iron, selenium, magnesium, nickel, sulfur, cobalt, palladium, and indium, using plant extracts from the fruits, leaves, roots, stems, barks, seeds, and peel from different plant species. These green-synthesized nanoparticles exhibit interesting properties, including antioxidant, antimicrobial, dye degradation, cytotoxic, analgesic, sedative, wound-healing, and skin-protection properties, as well as being plant-growth promoters and showing potential for sensor development for diverse applications. On the other hand, synthesizing inorganic nanoparticles using plant-based extracts has many challenges that should be solved (low yield and extraction procedures) for their practical production and application. Therefore, further studies are needed to standardize synthesis processes, mainly the extraction conditions that permit narrowing the gap between research laboratories and industry scale-up.
Author Contributions
Conceptualization, L.M.A.-E. and C.A.V.-C.; methodology, Z.V., J.M.S.-J., J.M.R.-G., E.F.A.-V., E.R.-L., N.R.-B., F.M.-E. and I.B.-L.; writing—original draft preparation, Z.V., L.M.A.-E., C.A.V.-C., J.M.S.-J., J.M.R.-G., E.F.A.-V., E.R.-L., N.R.-B., F.M.-E. and I.B.-L.; writing—review and editing, Z.V., L.M.A.-E. and C.A.V.-C.; visualization, Z.V., L.M.A.-E. and C.A.V.-C.; supervision, L.M.A.-E. and C.A.V.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank Ernesto Emmanuel Hermosillo Martín for technical support as part of his activities of the “Early incorporation into Research Program” from the Centro Universitario de Los Altos of University of Guadalajara.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Ullah Khan, A. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 28, ISBN 9780128135860. [Google Scholar]
- United Nations. Global Sustainable Development Report 2023. Department of Economic and Social Affairs. Sustainable Development. 2023. Available online: https://sdgs.un.org/gsdr/gsdr2023 (accessed on 1 April 2024).
- Anaya-Esparza, L.M.; de la Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications. Molecules 2021, 26, 5431. [Google Scholar] [CrossRef] [PubMed]
- Hamdallah, S.I.; Zoqlam, R.; Erfle, P.; Blyth, M.; Alkilany, A.M.; Dietzel, A.; Qi, S. Microfluidics for pharmaceutical nanoparticle fabrication: The truth and the myth. Int. J. Pharm. 2020, 584, 119408. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.A.; Lodeiro, C.; Capelo, J.L.; Lorenzo, J.; Oliveira, E. Magnetic, fluorescent and hybrid nanoparticles: From synthesis to application in biosystems. Mater. Sci. Eng. C 2020, 106, 110104. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, characteristics, and applications in analytical and other sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H. Multimetallic nanoparticles as alternative antimicrobial agents: Challenges and perspectives. Molecules 2021, 26, 912. [Google Scholar] [CrossRef]
- Rahman, T.U.; Khan, H.; Liaqat, W.; Zeb, M.A. Phytochemical screening, green synthesis of gold nanoparticles, and antibacterial activity using seeds extract of Ricinus communis L. Microsc. Res. Tech. 2022, 85, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Borthakur, B.B.; Bora, U. Green synthesis of gold nanoparticles using ethanolic leaf extract of Centella asiatica. Mater. Lett. 2010, 64, 1445–1447. [Google Scholar] [CrossRef]
- Elia, P.; Zach, R.; Hazan, S.; Kolusheva, S.; Porat, Z.; Zeiri, Y. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 2014, 9, 4007–4021. [Google Scholar]
- Patil, N.A.; Udgire, S.; Shinde, D.R.; Patil, P.D. Green synthesis of gold nanoparticles using extract of Vitis vinifera, Buchananialanzan, Juglandaceae, Phoenix dactylifera plants, and evaluation of antimicrobial activity. Chem. Methodol. 2023, 7, 15–27. [Google Scholar]
- Boruah, J.S.; Devi, C.; Hazarika, U.; Bhaskar Reddy, P.V.; Chowdhury, D.; Barthakur, M.; Kalita, P. Green synthesis of gold nanoparticles using an antiepileptic plant extract: In vitro biological and photo-catalytic activities. RSC Adv. 2021, 11, 28029–28041. [Google Scholar] [CrossRef] [PubMed]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J. King Saud Univ. Sci. 2022, 34, 101830. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Thangamani, N.; Bhuvaneshwari, N. Green synthesis of gold nanoparticles using Simarouba glauca leaf extract and their biological activity of microorganism. Chem. Phys. Lett. 2019, 732, 136587. [Google Scholar] [CrossRef]
- Alhumaydhi, F.A. Green synthesis of gold nanoparticles using extract of Pistacia chinensis and their in vitro and in vivo biological activities. J. Nanomater. 2022, 2022, 23–27. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Guibal, E.; Hamza, M.F.; Hassan, S.E.D.; Alkhalifah, D.H.M.; El-Hossary, D. Green synthesis of gold nanoparticles by aqueous extract of Zingiber officinale: Characterization and insight into antimicrobial, antioxidant, and in vitro cytotoxic activities. Appl. Sci. 2022, 12, 1287. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Lee, C.S. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, B.N.; Harlapur, S.F.; Avinash, B.; Ravikumar, C.R.; Nagaswarupa, H.P.; Anil Kumar, M.R.; Gurushantha, K.; Santosh, M.S. Facile green synthesis of silver oxide nanoparticles and their electrochemical, photocatalytic and biological studies. Inorg. Chem. Commun. 2020, 111, 107580. [Google Scholar] [CrossRef]
- Yousaf, H.; Mehmood, A.; Ahmad, K.S.; Raffi, M. Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater. Sci. Eng. C 2020, 112, 110901. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green synthesis of silver nanoparticles using Annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef]
- Barabadi, H.; Mojab, F.; Vahidi, H.; Marashi, B.; Talank, N.; Hosseini, O.; Saravanan, M. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg. Chem. Commun. 2021, 129, 108647. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, L.; Tkáčiková, L. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green synthesis of silver nanoparticles using Teucrium polium leaf extract and assessment of their antitumor effects against MNK45 human gastric cancer cell line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.M.I.; Krause, R.W.M.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga, and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, M.; Prakasam, A.; Rajkumar, P.V.; Rajeshkumar, S.; Chandrasekaran, R.; Anbarasan, P.M. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. S. Afr. J. Chem. Eng. 2020, 32, 1–4. [Google Scholar]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef] [PubMed]
- Chand, K.; Cao, D.; Eldin Fouad, D.; Hussain Shah, A.; Qadeer Dayo, A.; Zhu, K.; Nazim Lakhan, M.; Mehdi, G.; Dong, S. Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arab. J. Chem. 2020, 13, 8248–8261. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Mustafa, D.E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef]
- Srinivasan, M.; Venkatesan, M.; Arumugam, V.; Natesan, G.; Saravanan, N.; Murugesan, S.; Ramachandran, S.; Ayyasamy, R.; Pugazhendhi, A. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019, 80, 197–202. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Alasiri, A.S.; Ahmad, J.; Alqahtani, A.A.; Abdullah, M.M.; Abdel-Wahab, B.A.; Pathak, K.; Saikia, R.; Das, A.; Sarma, H.; et al. Green synthesis of titanium dioxide nanoparticles using Ocimum sanctum leaf extract: In vitro characterization and its healing efficacy in diabetic wounds. Molecules 2022, 27, 7712. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Metal Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Sivaranjani, V.; Philominathan, P. Synthesize of titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 2016, 12, 1–5. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Gowri, S. Green synthesis of titanium dioxide nanoparticles by nyctanthes arbor-tristis leaves extract. Chalcogenide Lett. 2011, 8, 447–451. [Google Scholar]
- Rajakumar, G.; Rahuman, A.A.; Priyamvada, B.; Khanna, V.G.; Kumar, D.K.; Sujin, P.J. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012, 68, 115–117. [Google Scholar] [CrossRef]
- Ganesan, S.; Babu, I.G.; Mahendran, D.; Arulselvi, P.I.; Elangovan, N.; Geetha, N.; Venkatachalam, P. Green engineering of titanium dioxide nanoparticles using Ageratina altissima (L.) King & H.E. Robines. medicinal plant aqueous leaf extracts for enhanced photocatalytic activity. Ann. Phytomed. Int. J. 2016, 5, 69–75. [Google Scholar]
- Chatterjee, A.; Ajantha, M.; Talekar, A.; Revathyr, N.; Abraham, J. Biosynthesis, antimicrobial and cytotoxic effects of titanium dioxide nanoparticles using Vigna unguiculata seeds. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 95–99. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M.; et al. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac. J. Trop. Med. 2013, 6, 682–688. [Google Scholar] [CrossRef]
- Rao, K.G.; Ashok, C.; Rao, K.V.; Chakra, C.S.; Tambur, P. Green synthesis of TiO2 nanoparticles using Aloe vera extract. Int. J. Adv. Res. Phys. Sci. 2016, 2, 28–34. [Google Scholar]
- Chatterjee, A.; Nishanthini, D.; Sandhiya, N.; Abraham, J. Biosynthesis of titanium dioxide nanoparticles using Vigna radiata seeds. Asian J. Pharm. Clin. Res. 2016, 9, 85–88. [Google Scholar]
- Abdul Jalill, R.D.; Nuaman, R.S.; Abd, A.N. Biological synthesis of titanium dioxide nanoparticles by Curcuma longa plant extract and study its biological properties. World Sci. News 2016, 49, 204–222. [Google Scholar]
- Shabaani, M.; Rahaiee, S.; Zare, M.; Jafari, S.M. Green synthesis of ZnO nanoparticles using loquat seed extract; Biological functions and photocatalytic degradation properties. LWT-Food Sci. Technol. 2020, 134, 110133. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green synthesis, characterization and anti-microbial activities of ZnO nanoparticles using Euphorbia hirta leaf extract. J. King Saud Univ. Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Madhukara Naik, M.; Bhojya Naik, H.S.; Nagaraju, G.; Vinuth, M.; Raja Naika, H.; Vinu, K. Green synthesis of zinc ferrite nanoparticles in Limonia acidissima juice: Characterization and their application as photocatalytic and antibacterial activities. Microchem. J. 2019, 146, 1227–1235. [Google Scholar] [CrossRef]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A. Synthesis of zinc oxide nanoparticles using leaf extract of Lippia adoensis (Koseret) and evaluation of its antibacterial activity. J. Chem. 2020, 7459042. [Google Scholar] [CrossRef]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Anuf, A.R.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Mirza, S.; Hussaini, A.A.; Öztürk, G.; Turgut, M.; Öztürk, T.; Tugay, O.; Ulukuş, D.; Yıldırım, M. Photocatalytic and antibacterial activities of ZnO nanoparticles synthesized from Lupinus albus and Lupinus pilosus plant extracts via green synthesis approach. Inorg. Chem. Commun. 2023, 155, 111124. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: Characterization and their applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Boro, B.; Boruah, J.S.; Devi, C.; Alemtoshi; Gogoi, B.; Bharali, P.; Reddy, P.V.B.; Chowdhury, D.; Kalita, P. A novel route to fabricate ZnO nanoparticles using Xanthium indicum ethanolic leaf extract: Green nanosynthesis perspective towards photocatalytic and biological applications. J. Mol. Struct. 2024, 1300, 137227. [Google Scholar] [CrossRef]
- Ganesh, M.; Lee, S.G.; Jayaprakash, J.; Mohankumar, M.; Jang, H.T. Hydnocarpus alpina Wt extract mediated green synthesis of ZnO nanoparticle and screening of its anti-microbial, free radical scavenging, and photocatalytic activity. Biocatal. Agric. Biotechnol. 2019, 19, 101129. [Google Scholar] [CrossRef]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Gul, S.; Khan, M.I.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process. Synth. 2019, 8, 135–143. [Google Scholar] [CrossRef]
- kumar, E.V.; Niveditha, B.S.; Sushmitha, L.; Usha, B.K.; swamy, B.E.K.; Anitha; Nagaraju, G. Facile green synthesis of Cu-doped MoO3 nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Nano-Struct. Nano-Objects 2023, 36, 101066. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Sahu, S.; Gupta, I.; Ghorai, T.K. Green synthesis of copper nanoparticles from an extract of Jatropha curcas leaves: Characterization, optical properties, CT-DNA binding and photocatalytic activity. RSC Adv. 2020, 10, 22027–22035. [Google Scholar] [CrossRef]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef]
- Shah, I.H.; Ashraf, M.; Sabir, I.A.; Manzoor, M.A.; Malik, M.S.; Gulzar, S.; Ashraf, F.; Iqbal, J.; Niu, Q.; Zhang, Y. Green synthesis and characterization of copper oxide nanoparticles using Calotropis procera leaf extract and their different biological potentials. J. Mol. Struct. 2022, 1259, 132696. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Dave, V.; Tak, K.; Sadhu, V.; Reddy, K.R. Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J. Microbiol. Methods 2019, 160, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Khan, A.; Rashid, M.H. Green synthesis of CuO nanoparticles using Lantana camara flower extract and their potential catalytic activity towards the aza-Michael reaction. RSC Adv. 2020, 10, 14374–14385. [Google Scholar] [CrossRef] [PubMed]
- Bouzekri, O.; El Gamouz, S.; Ed-Dra, A.; Moussout, H.; Dehmani, Y.; Ziyat, H.; El Idrissi, M.; Choukrad, M.; Abouarnadasse, S. Green Synthesis of nickel and copper nanoparticles doped with silver from Hammada scoparia leaf extract and evaluation of their potential to inhibit microorganisms and to remove dyes from aqueous solutions. Sustainability 2023, 15, 1541. [Google Scholar] [CrossRef]
- Abdullah; Hussain, T.; Faisal, S.; Rizwan, M.; Saira; Zaman, N.; Iqbal, M.; Iqbal, A.; Ali, Z. Green synthesis and characterization of copper and nickel hybrid nanomaterials: Investigation of their biological and photocatalytic potential for the removal of organic crystal violet dye. J. Saudi Chem. Soc. 2022, 26, 101486. [Google Scholar] [CrossRef]
- Hu, H.; Wang, F.; Ding, X.; Imhanria, S.; Wang, W.; Zhang, J. Green fabrication of Pt nanoparticles via tea-polyphenols for hydrogen peroxide detection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128201. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Liu, L.; Jing, Y.; Guo, A.; Li, X.; Li, Q.; Liu, W.; Zhang, X. Biosynthesis of platinum nanoparticles with cordyceps flower extract: Characterization, antioxidant activity and antibacterial activity. Nanomaterials 2022, 12, 1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, S.; Jia, H.; Zhou, J.; Xi, J.; Wang, J.; Chen, X.; Wu, L. Green synthesis of platinum nanoparticles by Nymphaea tetragona flower extract and their skin lightening, antiaging effects. Arab. J. Chem. 2023, 16, 104391. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Fawzy, M.; Hosny, M.; Abd El-Monaem, E.M.; Tamer, T.M.; Omer, A.M. Green synthesis of platinum nanoparticles using Atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications. Arab. J. Chem. 2022, 15, 103517. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Rheima, A.M.; Aljaafari, F.M.D.; Al Marjani, M.F.; Abbas, Z.S. Green-synthesis of Platinum nanoparticles using olive leaves extracts and its effect on aspartate aminotransferase activity. Egypt. J. Chem. 2022, 65, 377–382. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.K.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Rajendran, R.B. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- John Leo, A.; Oluwafemi, O.S. Plant-mediated synthesis of platinum nanoparticles using water hyacinth as an efficient biomatrix source—An eco-friendly development. Mater. Lett. 2017, 196, 141–144. [Google Scholar] [CrossRef]
- Kazi, S.; Nirwan, S.; Kunde, S.; Jadhav, S.; Rai, M.; Kamble, D.; Sayyed, S.; Chavan, P. Green synthesis, characterization and bio-evaluation of zirconium nanoparticles using the dried biomass of Sphagneticola trilobata plant leaf. Bionanoscience 2022, 12, 731–740. [Google Scholar] [CrossRef]
- Korde, S.A.; Thombre, P.B.; Dipake, S.S.; Sangshetti, J.N.; Rajbhoj, A.S.; Gaikwad, S.T. Neem gum (Azadirachta indicia) facilitated green synthesis of TiO2 and ZrO2 nanoparticles as antimicrobial agents. Inorg. Chem. Commun. 2023, 153, 110777. [Google Scholar] [CrossRef]
- Chau, T.P.; Kandasamy, S.; Chinnathambi, A.; Alahmadi, T.A.; Brindhadevi, K. Synthesis of zirconia nanoparticles using Laurus nobilis for use as an antimicrobial agent. Appl. Nanosci. 2021, 13, 1337–1344. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Suganthy, N.; Shanmugam, S.; Brindhadevi, K.; Sabour, A.; Alshiekheid, M.; Lan Chi, N.T.; Pugazhendhi, A.; Shanmuganathan, R. Biosynthesis of zirconium nanoparticles (ZrO2 NPs) by Phyllanthus niruri extract: Characterization and its photocatalytic dye degradation activity. Food Chem. Toxicol. 2022, 168, 113340. [Google Scholar] [CrossRef] [PubMed]
- Isacfranklin, M.; Dawoud, T.; Ameen, F.; Ravi, G.; Yuvakkumar, R.; Kumar, P.; Hong, S.I.; Velauthapillai, D.; Saravanakumar, B. Synthesis of highly active biocompatible ZrO2 nanorods using a bioextract. Ceram. Int. 2020, 46, 25915–25920. [Google Scholar] [CrossRef]
- Goyal, P.; Bhardwaj, A.; Mehta, B.K.; Mehta, D. Research article green synthesis of zirconium oxide nanoparticles (ZrO2NPs) using Helianthus annuus seed and their antimicrobial effects. J. Indian Chem. Soc. 2021, 98, 100089. [Google Scholar] [CrossRef]
- Gowri, S.; Rajiv Gandhi, R.; Senthil, S.; Sundrarajan, M. Effect of calcination temperature on nyctanthes plant mediated zirconia nanoparticles; optical and antibacterial activity for optimized zirconia. J. Bionanosci. 2015, 9, 181–189. [Google Scholar] [CrossRef]
- Alagarsamy, A.; Chandrasekaran, S.; Manikandan, A. Green synthesis and characterization studies of biogenic zirconium oxide (ZrO2) nanoparticles for adsorptive removal of methylene blue dye. J. Mol. Struct. 2022, 1247, 131275. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.V.N. Green synthesis of ZrO2 nanoparticles and nanocomposites for biomedical and environmental applications: A review. Environ. Chem. Lett. 2022, 20, 1309–1331. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.F.V.; Fagundes, A.P.; Macuvele, D.L.P.; de Carvalho, E.F.U.; Durazzo, M.; Padoin, N.; Soares, C.; Riella, H.G. Green synthesis of zirconia nanoparticles based on Euclea natalensis plant extract: Optimization of reaction conditions and evaluation of adsorptive properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123915. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Polyvinyl alcohol thin film reinforced by green synthesized zirconia nanoparticles. Ceram. Int. 2018, 44, 19377–19382. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Ghasemi, Y.; Amani, A.M.; Taghizadeh, S. Green synthesis of iron nanoparticles using Plantago major leaf extract and their application as a catalyst for the decolorization of azo dye. Bionanoscience 2019, 9, 317–322. [Google Scholar] [CrossRef]
- Naz, S.; Islam, M.; Tabassum, S.; Fernandes, N.F.; Carcache de Blanco, E.J.; Zia, M. Green synthesis of hematite (α-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J. Mol. Struct. 2019, 1185, 1–7. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Younas, U.; Hassan, S.T.; Ali, F.; Hassan, F.; Saeed, Z.; Pervaiz, M.; Khan, S.; Jannat, F.T.; Bibi, S.; Sadiqa, A.; et al. Radical scavenging and catalytic activity of Fe-Cu bimetallic nanoparticles synthesized from Ixora finlaysoniana extract. Coatings 2021, 11, 813. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Salah Eddine, L.; Abderrhmane, B.; Alonso-González, M.; Guerrero, A.; Romero, A. Green synthesis and characterization of iron oxide nanoparticles by Pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 2020, 17, 100280. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Yıldız, Y.Ş.; Yılmaz, Ş.; Demirezen Yılmaz, D. Green synthesis and characterization of iron oxide nanoparticles using Ficus carica (common fig) dried fruit extract. J. Biosci. Bioeng. 2019, 127, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef] [PubMed]
- Barzegarparay, F.; Najafzadehvarzi, H.; Pourbagher, R.; Parsian, H.; Ghoreishi, S.M.; Mortazavi-Derazkola, S. Green synthesis of novel selenium nanoparticles using Crataegus monogyna extract (SeNPs@CM) and investigation of its toxicity, antioxidant capacity, and anticancer activity against MCF-7 as a breast cancer cell line. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef] [PubMed]
- Adibian, F.; Ghaderi, R.S.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Ghazvini, K.; Youssefi, M.; Soleimanpour, S.; Darroudi, M. Green synthesis of selenium nanoparticles using Rosmarinus officinalis and investigated their antimicrobial activity. BioMetals 2022, 35, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green biosynthesis of selenium nanoparticles using orange peel waste: Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Nguyen, P.T.M.; Pham, N.H.; Le, T.H.; Nguyen, T.H.; Do, D.T.; La, D.D. Green synthesis of selenium nanoparticles using Cleistocalyx operculatus leaf extract and their acute oral toxicity study. J. Compos. Sci. 2022, 6, 307. [Google Scholar] [CrossRef]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, antiviral, and in-vitro cytotoxicity and mosquitocidal activities of Portulaca oleracea-based green synthesis of selenium nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef]
- Hashem, A.H.; Salem, S.S. Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol. J. 2022, 17, 2100432. [Google Scholar] [CrossRef]
- Ghaderi, R.S.; Adibian, F.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Amel Jamehdar, S.; Meshkat, Z.; Soleimanpour, S.; Daroudi, M. Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity. Mater. Technol. 2022, 37, 1289–1297. [Google Scholar] [CrossRef]
- Hosseinpour, L.; Baharara, J.; Zaker Bostanabad, S.; Darroudi, M. Plant-based synthesis of selenium nanoparticles using Cordia myxa fruit extract and evaluation of their cytotoxicity effects. Inorg. Chem. Commun. 2022, 145, 110030. [Google Scholar] [CrossRef]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. Bionanoscience 2019, 9, 105–116. [Google Scholar] [CrossRef]
- dos Santos Souza, L.M.; Dibo, M.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, I.M.; Kobayashi, R.K.T.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Curr. Res. Green Sustain. Chem. 2022, 5, 100303. [Google Scholar] [CrossRef]
- Fatiqin, A.; Amrulloh, H.; Simanjuntak, W. Green synthesis of MgO nanoparticles using Moringa oleifera leaf extract for antimicrobial activity. Bull. Chem. Soc. Ethiop. 2021, 35, 161–170. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Ali, S.; Sudha, K.G.; Thirumalaivasan, N.; Ahamed, M.; Pandiaraj, S.; Rajeswari, V.D.; Vinayagam, Y.; Thiruvengadam, M.; Govindasamy, R. Green synthesis of magnesium oxide nanoparticles by using Abrus precatorius bark extract and their photocatalytic, antioxidant, antibacterial, and cytotoxicity activities. Bioengineering 2023, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Younis, I.Y.; El-Hawary, S.S.; Eldahshan, O.A.; Abdel-Aziz, M.M.; Ali, Z.Y. Green synthesis of magnesium nanoparticles mediated from Rosa floribunda charisma extract and its antioxidant, antiaging and antibiofilm activities. Sci. Rep. 2021, 11, 16868. [Google Scholar] [CrossRef] [PubMed]
- Vergheese, M.; Vishal, S.K.; Mary Vergheese, C. Green synthesis of magnesium oxide nanoparticles using Trigonella foenum-graecum leaf extract and its antibacterial activity. J. Pharmacogn. Phytochem. 2018, 7, 1193–1200. [Google Scholar]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae. Biomed Res. Int. 2019, 5620989. [Google Scholar] [CrossRef]
- Amrulloh, H.; Fatiqin, A.; Simanjuntak, W.; Afriyani, H.; Annissa, A. Antioxidant and antibacterial activities of magnesium oxide nanoparticles prepared using aqueous extract of Moringa Oleifera bark as green agents. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 44–53. [Google Scholar] [CrossRef]
- Sarala, E.; Vinuth, M.; Naik, M.M.; Reddy, Y.V.R. Green synthesis of nickel ferrite nanoparticles using Terminalia catappa: Structural, magnetic and anticancer studies against MCF-7 cell lines. J. Hazard. Mater. Adv. 2022, 8, 100150. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Hameed, S.; Munir, A.; Kanwal, S. Green synthesis and characterizations of nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem. 2019, 33, e4950. [Google Scholar] [CrossRef]
- Ali, T.; Warsi, M.F.; Zulfiqar, S.; Sami, A.; Ullah, S.; Rasheed, A.; Alsafari, I.A.; Agboola, P.O.; Shakir, I.; Baig, M.M. Green nickel/nickel oxide nanoparticles for prospective antibacterial and environmental remediation applications. Ceram. Int. 2022, 48, 8331–8340. [Google Scholar] [CrossRef]
- Din, M.I.; Tariq, M.; Hussain, Z.; Khalid, R. Single step green synthesis of nickel and nickel oxide nanoparticles from Hordeum vulgare for photocatalytic degradation of methylene blue dye. Inorg. Nano-Metal Chem. 2020, 50, 292–297. [Google Scholar] [CrossRef]
- Riaz, T.; Munnwar, A.; Shahzadi, T.; Zaib, M.; Shahid, S.; Javed, M.; Iqbal, S.; Rizwan, K.; Waqas, M.; Khalid, B.; et al. Phyto-mediated synthesis of nickel oxide (NiO) nanoparticles using leaves’ extract of Syzygium cumini for antioxidant and dyes removal studies from wastewater. Inorg. Chem. Commun. 2022, 142, 109656. [Google Scholar] [CrossRef]
- Yuan, C.; Jiang, B.; Xu, X.; Wan, Y.; Wang, L.; Chen, J. Anti-human ovarian cancer and cytotoxicity effects of nickel nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract. J. Exp. Nanosci. 2022, 17, 113–125. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Umamakeshvari, K.; Mohana, V.; Muthuvel, A.; Boshaala, A. Green synthesis of nickel oxide nanoparticles and its photocatalytic degradation and antibacterial activity. J. Mater. Sci. Mater. Electron. 2022, 33, 11864–11880. [Google Scholar] [CrossRef]
- Baloch, H.; Siddiqua, A.; Nawaz, A.; Latif, M.S.; Zahra, S.Q.; Alomar, S.Y.; Ahmad, N.; Elsayed, T.M. Synthesis and characterization of sulfur nanoparticles of Citrus limon extract embedded in nanohydrogel formulation: In vitro and in vivo studies. Gels 2023, 9, 284. [Google Scholar] [CrossRef]
- Al Banna, L.S.; Salem, N.M.; Jaleel, G.A.; Awwad, A.M. Green synthesis of sulfur nanoparticles using Rosmarinus officinalis leaves extract and nematicidal activity against Meloidogyne javanica. Chem. Int. 2020, 6, 137–143. [Google Scholar]
- Himanshi; Joshi, D.P. Comparative study of green synthesised sulphur nanoparticles in different acidic media. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 025003. [Google Scholar] [CrossRef]
- Ismalita; Khairan, K.; Rahmi; Muhammad, S.; Indra, I. Green synthesis of sulfur nanoparticles using Allium sativum: Its effects on the growth of Pogostemon cablin Benth. and the chemical characterization of the patchouli oil after being harvested. Nanotechnol. Environ. Eng. 2022, 7, 359–375. [Google Scholar] [CrossRef]
- Najafi, S.; Razavi, S.M.; Khoshkam, M.; Asadi, A. green synthesized of sulfur nanoparticles and its application on lettuce plants metabolic profiling. Bionanoscience 2022, 12, 116–127. [Google Scholar] [CrossRef]
- Hafeez, M.; Shaheen, R.; Akram, B.; Zain-Ul-Abdin; Haq, S.; Mahsud, S.; Ali, S.; Khan, R.T. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater. Res. Express 2020, 7, 025019. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Calderon-Moreno, J.M.; Culita, D.C.; Patron, L.; Diamandescu, L. Green synthesis of cobalt ferrite nanoparticles using ginger roots or cadmium seeds extract. Rev. Roum. Chim. 2017, 62, 645–653. [Google Scholar]
- Vinodhini, S.; Vithiya, B.S.M.; Prasad, T.A.A. Green synthesis of palladium nanoparticles using aqueous plant extracts and its biomedical applications. J. King Saud Univ. Sci. 2022, 34, 102017. [Google Scholar] [CrossRef]
- Chitturi, K.L.; Yaramma, A.; Merugu, R.; Dachepalli, R.; Kandhadi, J. Synthesis and characterisation of In2O3 nanoparticles from Astragalus gummifer. Adv. Nanoparticles 2016, 5, 114–122. [Google Scholar] [CrossRef]
- Phokha, S.; Seraphin, S.; Maensiri, S.; Laokul, P.; Klinkaewnarong, J.; Phokha, S.; Seraphin, S. Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. Optoelectron. Adv. Mater. Commun. 2008, 2, 161–165. [Google Scholar]
- Zhang, T.; Dang, M.; Zhang, W.; Lin, X. Gold nanoparticles synthesized from Euphorbia fischeriana root by green route method alleviates the isoprenaline hydrochloride induced myocardial infarction in rats. J. Photochem. Photobiol. B Biol. 2020, 202, 111705. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Al jomaa, A.; kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).