Abstract
Functional ingredients rich in bioactive compounds can be added to conventional ingredients for the formulation of food to increase its nutraceutical potential. Three prickly pear parts, namely flowers, seeds, and seed cake were utilized in the current work as natural antioxidant resources. The flower extract gave the best amounts of antioxidants as estimated by spectrophotometric methods, which were 68.08 mg GAE/100 g DM for total phenolics; 6.91 mg QE/100 g DM for total flavonoids; 13.05 mg QE/100 g of DM for flavonols; and 0.22 mg/100 g of DM for condensed tannins. The three extracts showed a high proportion of antioxidant effect as determined by DPPH, ABTS, FRAP, and TAA in vitro assays. Chemical analysis and sensory testing were performed on biscuits that were made by adding powdered flowers, seeds, or seed cake. The biscuits made from the seed cake gave a higher protein content (83.97 mg/100 g) and the biscuits made from the seeds had the best ash concentration (3.21%), while the biscuits containing flower powder had the highest anti-radical activity (81.04%). Sensory analysis disclosed the preference for biscuits formulated with flowers or seeds (80%) by the experts. These findings demonstrated that the nutritional value of the biscuits was improved by their enrichment with cactus parts powders, and they were appreciated by tasters.
1. Introduction
The Opuntia genus (Cactaceae family) is estimated to have >300 species, including cactus pear (Opuntia ficus-indica L.). This plant is native to Mexico and extensively scattered throughout the American hemisphere, while it was brought to Europe and North Africa by the Spanish conquerors during the 15th century [1,2]. The cactus pear is found in all of Algeria except the south of the country and is abundant, particularly in the east. Recently, 52,000 hectares of O. ficus-indica were planted in the four eastern departments, namely Oum El Bouaghi, Tebessa, Khenchela, and Souk-Ahras [3]. However, cactus fruits do not attract much attention from Algerian people since their use is still seasonal and they have little commercial value [4]. However, cactus fruits are processed to make juice, juice concentrates, jams, syrups, alcoholic beverages, natural liquid sweeteners, or fruit jellies, and this results in an enormous amount of waste [5], including flowers, seeds, and seed cakes, which are thrown away during industrial processing [6]. These by-products still have value since they contain antioxidants (flavonoids, phenolic acids, and ascorbate) and pigments (carotenoids and betalains), which are recognized as bioactive compounds [7].
It is now believed that the great diversity of the chemical structures and the antioxidant qualities of cactus parts are what give them their nutritional and health benefits and enable them to find applications in various fields such as medicine, pharmacy, cosmetics, agri-food, and biological control [8]. O. ficus indica flowers and seeds show a strong ability to scavenge free radicals [9,10] and can be a valuable source for functional food formulation with their contribution of antioxidants, dietary fiber, and minerals [11].
Cereal-based supplies have been extensively researched for functionalization due to their widespread use worldwide [12]. Amongst these products, biscuits are highly sought-after and can be found on the market in a variety of forms and recipes. Today, the food industry, particularly the bakery food sector, is searching for innovative recipes to create healthier foods that satisfy the demands of consumers [13]. The different parts of the O. ficus-indica (OFI) plant have the potential for use in bakery products as high dietary fiber and antioxidant content can improve their nutritional profile [11]. In this direction, OFI cladodes powder has been used at 5% added to wheat flour, increasing the bread’s antioxidant capacity and overall phenolic content without degrading its sensory appeal [14]. According to Dick et al. [15], crackers containing 5% cladode flour and 2% cactus mucilage scored the highest attributes in sensory assessments, indicating that they could provide tasty and wholesome substitutes for gluten-free crackers. The roasted cactus pear seed flour added to bread dough impacted the nutritional and sensory aspects of bread [15]. The dietary fiber, fat, and ash contents, as well as the phenolic concentration and antioxidant activity, significantly increased, while the bread’s sensory qualities remained unaffected at supplementation levels of up to 6% [16]. Cactus pear peels were used as of partial sugar and gluten substitute in biscuits and they have improved their acceptability and textural properties [17]. In recent papers [18,19], cladode flour from OFI was reported to be added up to 25% and 15% in preparing cookies.
Nonetheless, as far as the authors are aware, there are no studies in the literature on the fortification of biscuits with cactus by-products selected herein (flowers, seeds, and seed cake) nor a comparison between them. In this study, an evaluation of OFI by-products was conducted by analyzing the proximate composition, the phenolic contents, and the antioxidant activities of flowers, seeds, and seed cakes of OFI, native from Algeria. The biscuits were prepared by substituting partially wheat flour with cactus pear flower, seeds, and cake powders. Physico-chemical parameters and anti-oxidative capacity of biscuits were also assessed. To guarantee customer acceptability, the effect on sensory qualities was also evaluated.
2. Materials and Methods
2.1. OFI By-Product Extract
The OFI by-products, namely flowers (F), seeds (S), and seed cake (SC), were offered by a private company that processes and valorizes cactus products (Taghzouith locality, Bejaia department, Algeria). The plant samples were dried in an air oven at 40 °C until a residual moisture content <7% was reached (about 48 h), milled by an IKA grinder (A11 Basic grinder, IKA Works, Petaling Jaya, Malaysia) with a mesh of ≤500 µm. For extraction, 10 g of each powder sample was mixed with 100 mL of 36% aqueous ethanol, then immersed in an ultrasonic bath (300 W) and irradiated with 50 Hz power at 53 °C for 60 min, as already reported [10].
After extraction, the mixtures were filtered to recover the extracts, which were stored at 4 °C.
2.2. Phenolics Quantification and Antioxidant Activity Assessment
The total phenolic content (TPC) was assessed by the Folin–Ciocalteu method. The total flavonoids (TFC) and flavonol (FL) contents were determined by the aluminum trichloride technique. The condensed tannins (CT) amount was evaluated by the vanillin method. The procedures for each dosage were already reported in our previous study [20]. TPC was expressed as mg gallic acid equivalent, GAE/100 g DM, TFC as a mg quercetin equivalent, QE/100 g DM, FLC as a mg QE/100 g DM, and CTC as a mg catechin equivalent (CE)/100 g DM.
All the results were expressed in milligrams of standard equivalent per 100 g of dry matter (mg/100 g DM).
The antioxidant capacity of the extracts was measured spectrophotometrically using the DPPH, ABTS, FRAP, and phosphomolybdate methods (for total antioxidant activity, TAA), as already reported [10], and the results were expressed as IC50 (mg/mL).
2.3. Analysis of Flowers, Seeds, and Seed Cake Powders
Physicochemical characterization and proximal analysis of the samples were carried out by the Association of Official Analytical Chemists’ methods [21] by evaluating water content, ash content, pH, and soluble dry residue. For the protein contents and total sugars, the procedures described by Bradford et al. and DuBois et al. [22,23] were adopted, respectively. The functional properties of the samples were evaluated by determining the apparent density (AD), water holding capacity (WHC), oil holding capacity (OHC), swelling capacity, and foaming capacity by the methods reported by Bouazizi et al. [17].
2.4. Biscuit Production and Physicochemical Characterization
The following biscuit recipe was employed: 920 g whole soft wheat flour, 200 g white sugar, 400 g margarine, 216 g eggs, and 40 g baking powder. Every ingredient was bought locally. A portion of the wheat flour (30%) in the enriched biscuits was replaced with the F, S, and SC powders. Using a home kneading machine, the dough was rolled and shaped into round biscuits (5 cm in diameter and 0.5 cm in thickness), which were then baked for 11 min at 180 °C in an electric static oven. To evaluate the physicochemical properties of the products, the biscuits were milled and sifted to obtain a powder <500 µm. The extraction was carried out by mixing 1 g of biscuit powder with 9 mL of ethanol 50% (v/v) and stirring for 10 min. The specimens underwent a 10-min centrifugation at 5000 rpm and 25 °C and the upper phase was recovered [24].
The pH, protein, ashes contents, radical scavenging activity, and dry matter content (drying at 105 ± 2 °C for 24 h) were evaluated.
2.5. Sensory Evaluation
At the University of Bejaia’s Sensory Laboratory, sensory analyses were performed one day after manufacture to determine the differences between the sensory characteristics of enriched and non-enriched biscuits and to identify those that were most appreciated by a panel of 10 expert judges. The judges who participated in the research were staff of the University aged between 30 and 52 years.
Each biscuit was arranged in a clear plastic container, sealed tightly, and labeled with a code consisting of three digits. Samples were presented to subjects in a randomized and balanced order at room temperature (25 ± 1 °C). Before starting the test and in between samples, individuals were instructed to rinse their mouths.
To characterize the different biscuits prepared, 9 descriptors were selected: color, smell, sweet taste, aftertaste, texture to the touch, thickness, texture, consistency, and reliability.
Then, an assessment of overall liking was made on a 9-point hedonic scale, from “dislike extremely” (1) to “like extremely” (9).
2.6. Statistical Analysis
Results were reported as the mean values of three replicates along with the related standard deviations. One-way analysis of variance (ANOVA) was performed on the data using STATISTICA 10.0. Tukey’s post hoc test was used to differentiate means with significant differences (p < 0.05). Correlations between different classes of phenolic amounts and antioxidant capacities were also determined by achieving a Spearman’s correlation analysis. An Internal Preference Map (IPM) was produced for exploratory purposes using Principal Component Analysis (PCA) on the overall liking data of all individuals. The XLSTAT statistical software was used for all analyses of the sensory data.
3. Results
3.1. OFI By-Products Powders Analysis
All the samples were acidic but they differed significantly in this respect (p ≤ 0.05), with F being the most acidic, followed by SC then S.
With regard to ash, OFI flowers had a highly significant content (p ≤ 0.05) of 6.54% compared to seeds (2.20%) and seedcake (1.03%).
Seeds showed higher sugar content with respect to the flowers and the cake.
The trend for protein contents is the same as that of sugar since the seeds are richer than the flowers and notably the cake.
In terms of technological characteristics, the cakes and the seeds were denser than the flowers, which have the highest capacity to absorb both water and oil.
None of the three samples have the ability to swell. When compared to OFI flower powder and cakes, the seed powder had a higher capacity for foaming (Table 1). The low protein and starch composition may be the cause of the low foaming capacities.

Table 1.
Physico-chemical analyses of the OFI by-products powders.
3.2. Phenolic Contents and Antioxidant Effects of OFI By-Product Extracts
The major antioxidant compound classes that exhibit antioxidant ability are general phenolics, flavonoids (which comprise also flavonols), and tannins.
For flower extract, the total phenolic content (TPC) was 68.080 mg GAE/100 g DM. As compared to the TPC value reported by other authors under other circumstances, this one is deficient.
The samples’ flavonoid and flavonol concentrations were not exceptionally considerable. However, it is still the flowers that had significantly (p < 0.05) the best contents, which were 13.050 and 6.910 mg QE/100 g DM, respectively.
The condensed tannins content was very low in all examined samples and comprised between 0.120 and 0.220 mg CE/100 g DM.
According to the antioxidant capacity results (Table 2), all OFI by-products exert an active role in scavenging free radicals, especially DPPH• and reducing metals (iron and molybdenum in FRAP and TAA, respectively). In nearly every test, the OFI flower extract with higher phenolic content was the most effective, showing the lowest IC50 values.

Table 2.
Total phenolic content (TPC) (expressed as mg gallic acid equivalent, GAE/100 g DM), total flavonoids (TFC) (as mg quercetin equivalent, QE/100 g DM), flavonols content (FLC) (mg QE/100 g DM), condensed tannins contents (CTC) (as mg catechin equivalent, CE/100 g DM), and antioxidant activity (IC50 in mg/mL) of OFI by-product extracts.
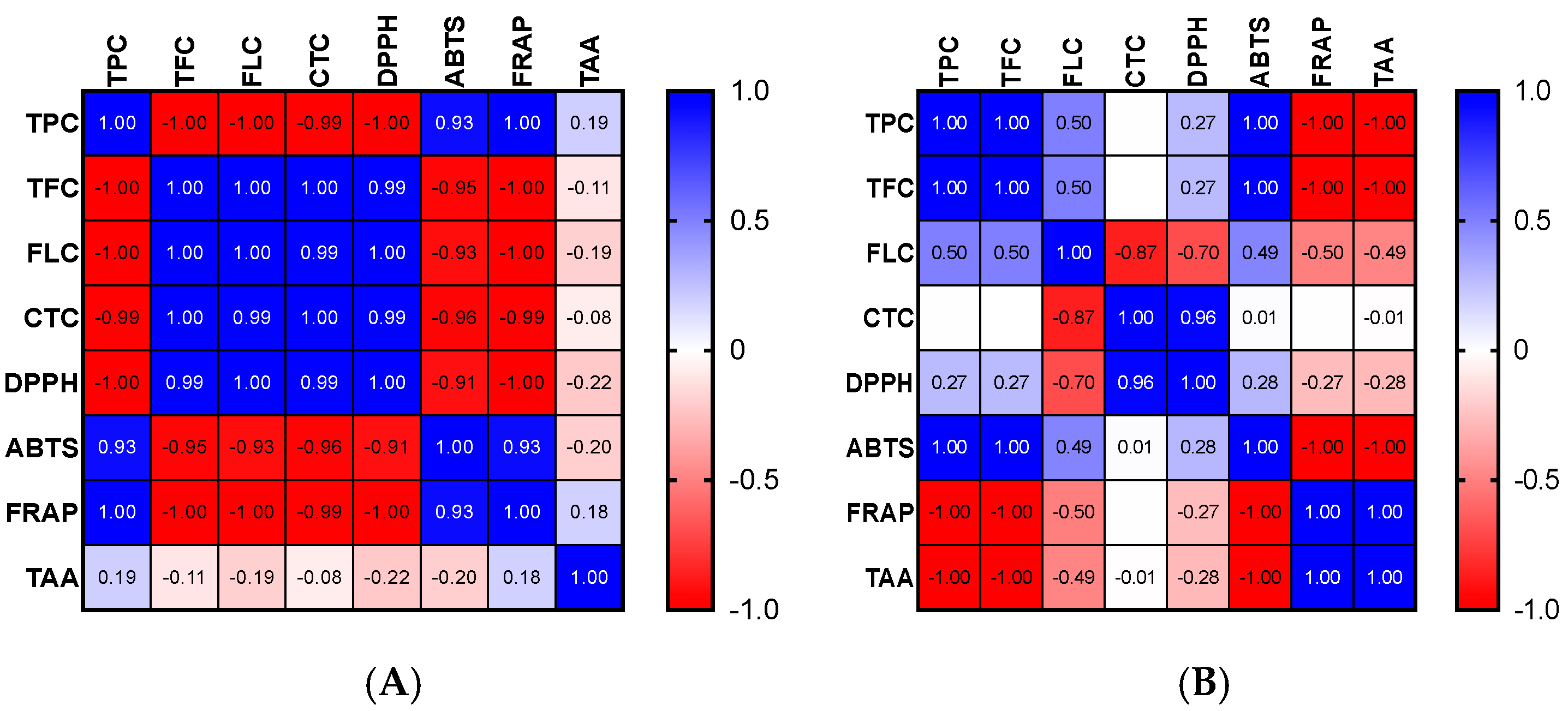
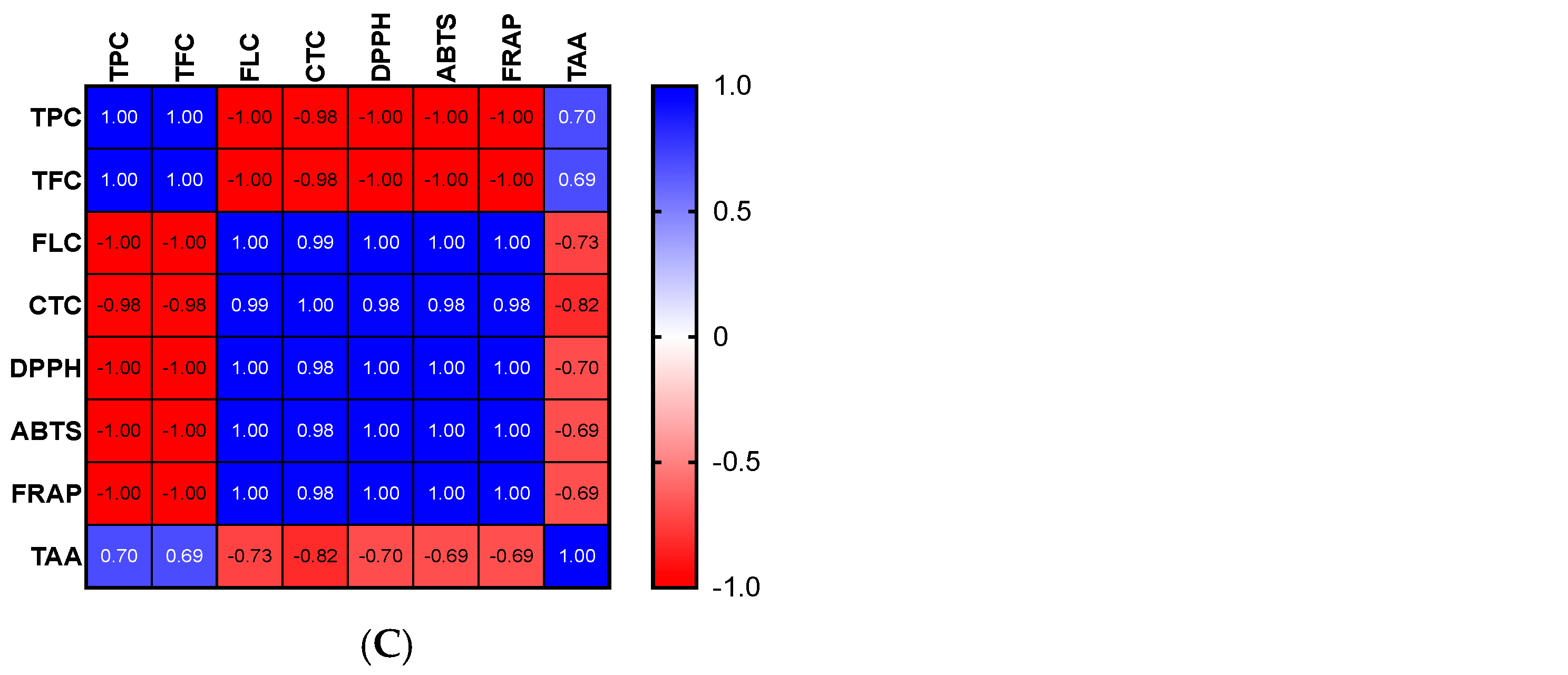
The high phenolic content of the extracts may be the cause of the apparent antioxidant capacity of the various parts of OFI. Indeed, it is possible to establish a link between the content of phenolics and the free radicals−scavenging effect. For the OFI flower extract, significant positive correlations were found between the TPC, ABTS, and FRAP tests as well as between TFC, FLC, CTC, and DPPH (Figure 1A). Significant positive correlations among TPC, TFC, and ABTS as well as between CTC and DPPH were discovered for seeds (Figure 1B). With regard to seed cake extract, more significant positive correlations were observed between FLC, CTC, DPPH, ABTS, and FRAP and to a lesser extent between TPC, TFC, and TAA (Figure 1C).
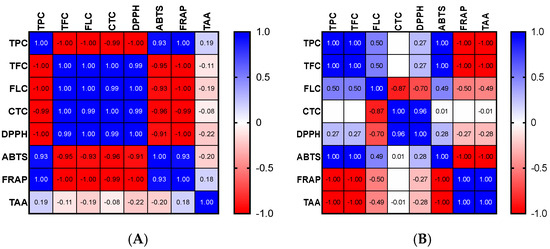
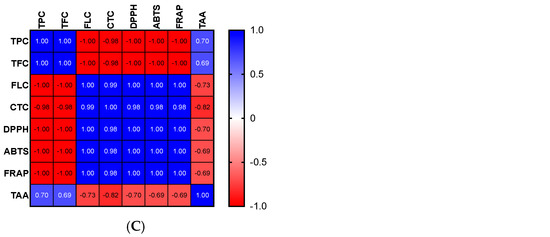
Figure 1.
Correlograms of phenolic contents and antioxidant activities of OFI flowers (A), seeds (B), and seed cake (C). The Spearman’s “r” coefficients were calculated: 0 < r < +1 showed direct correlations, while −1 < r < 0 inverse correlations. The red color indicates negative correlations, the blue color positive correlations, and the white color the absence of correlations. TPC: total phenolic content, TFC: total flavonoid content, FLC: flavonols content, CTC: condensed tannins content, DPPH: scavenging of DPPH radical, ABTS: scavenging of ABTS radical, FRAP: antioxidant power, and TAA: total antioxidant activity (TAA) determined by phosphomolybdate assay.
3.3. Biscuit Formulation with OFI By-Products
3.3.1. Physico-Chemical Parameters and Antioxidant Effect of the Biscuits
In comparison to the enriched biscuits, the control biscuit has a higher moisture content (8.200%).
The ash content was significantly increased in the enriched biscuits; this could be clarified through the enrichment powders’ high ash content (Table 3).

Table 3.
Physico-chemical and antioxidant properties of control and enriched biscuits.
Meanwhile, the biscuits enriched with seed powder had the highest protein content, followed by those enriched with seed cake powder and flower powder. The biscuits made with wheat flour had the lowest protein content of only 13.111 mg/100 g of biscuit.
When comparing the enriched biscuits to the control, the pH dropped noticeably.
As for the antioxidant activity, enriched biscuits showed much higher scavenging activity than that of the control biscuits. Interestingly, the F biscuits exhibited the highest activity against the DPPH radical followed by S and SC biscuits, while the C biscuit had the least impact.
3.3.2. Sensorial Analysis
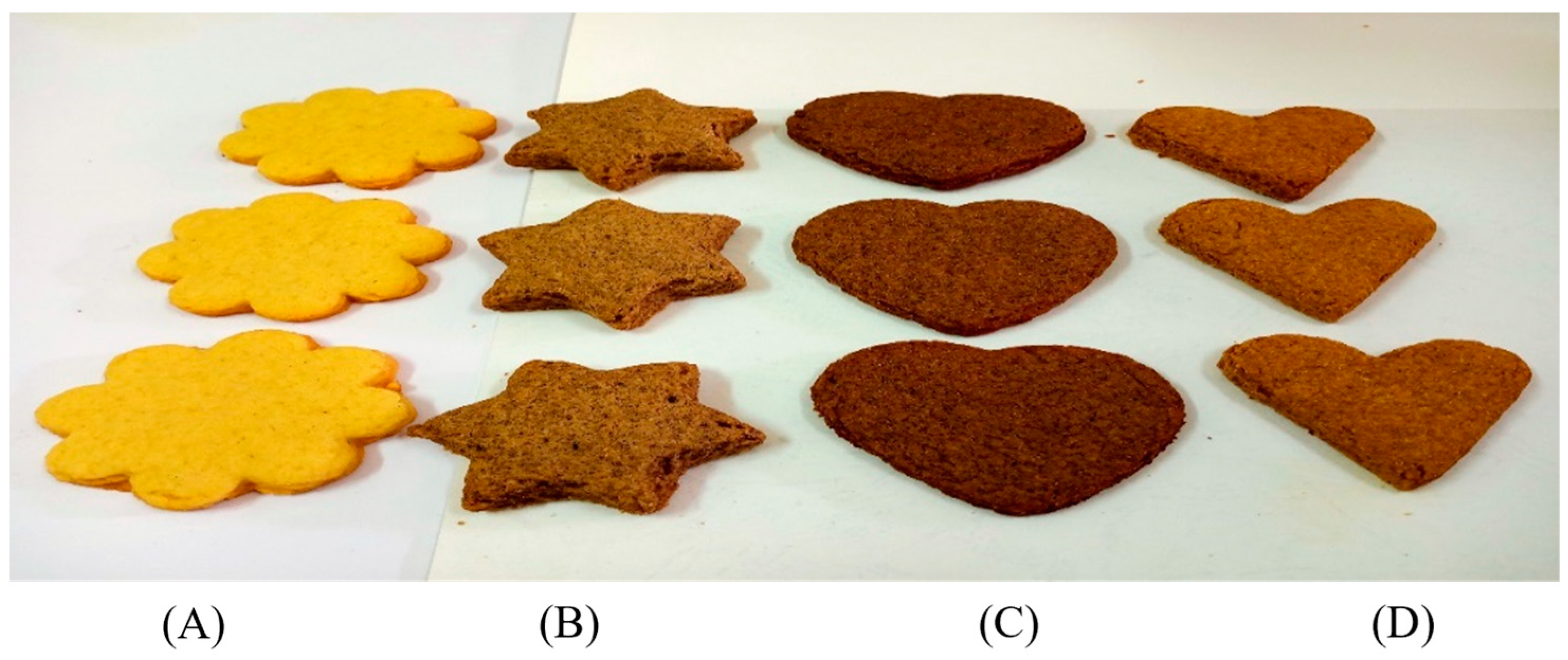
Samples of the biscuits prepared with the addition of OFI by-products are shown in Figure 2.
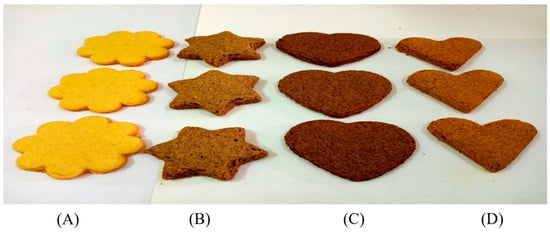
Figure 2.
Control biscuits (A) and biscuits prepared with 30 g/100 g of OFI seed cake (B), seeds (C), and flowers (D).
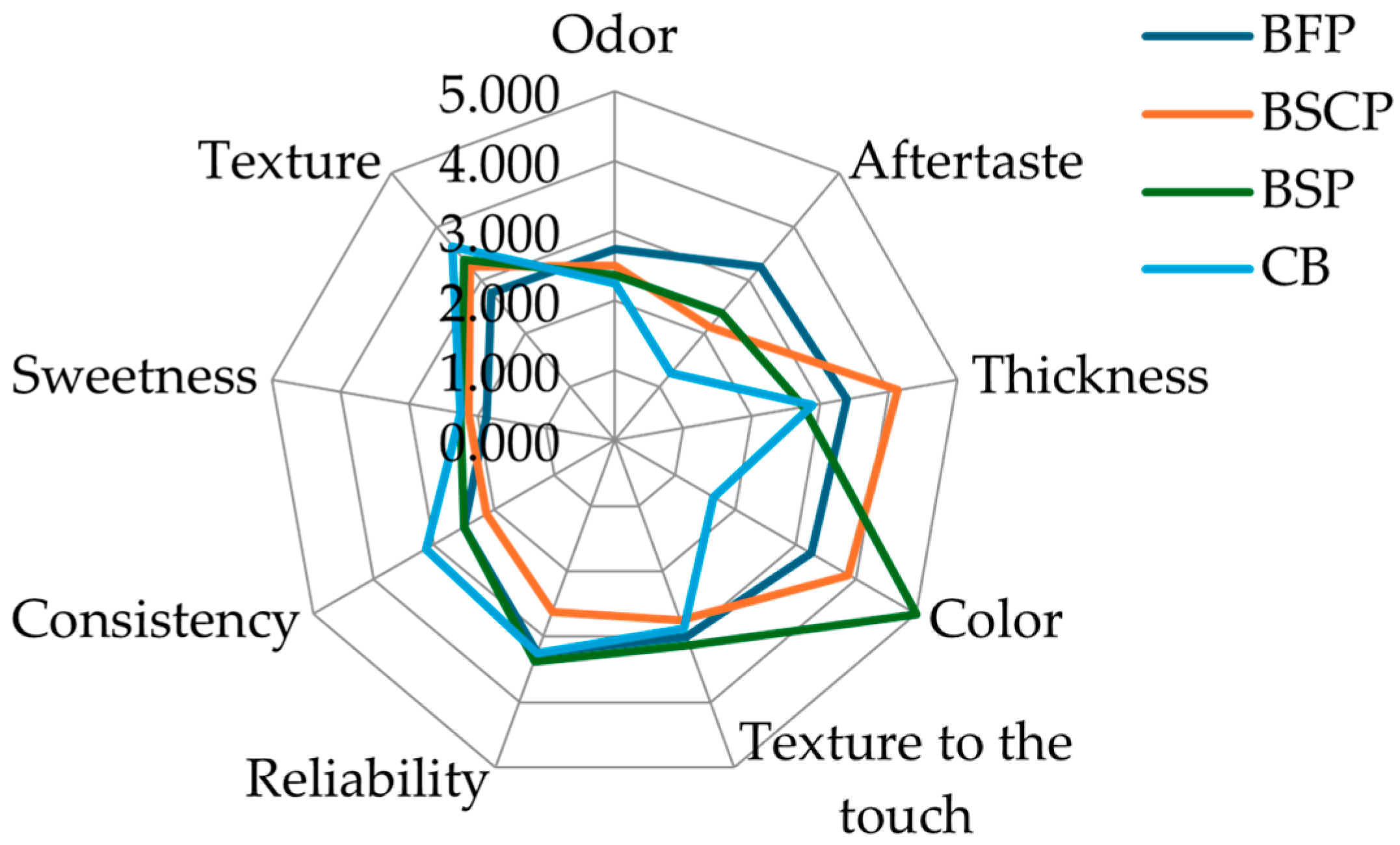
There were notable variations in the biscuits’ sensory profile across some characteristics (Figure 3).
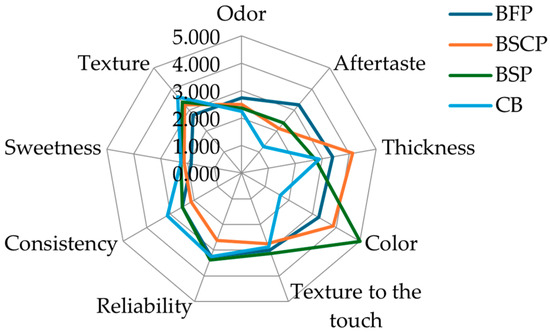
Figure 3.
Radar graph of sensory features of the biscuits obtained with wheat flour (control, CB) and various parts of OFI powder (BFP: Biscuit with flowers powder, BSP: Biscuit with seeds powder, BSCP: Biscuit with seed cakes powder).
In comparison to the control biscuits, the enriched biscuits had a stronger color, aftertaste, and less consistency and texture. The resulting coloration of the OFI samples was reflected in the sensory assessment of color; biscuits enriched with seed and seed cake powders have a deeper color and hence displayed the highest scores of 5 and 3.8, respectively. The odor was powerful in the enriched biscuits and nearly missing in the control.
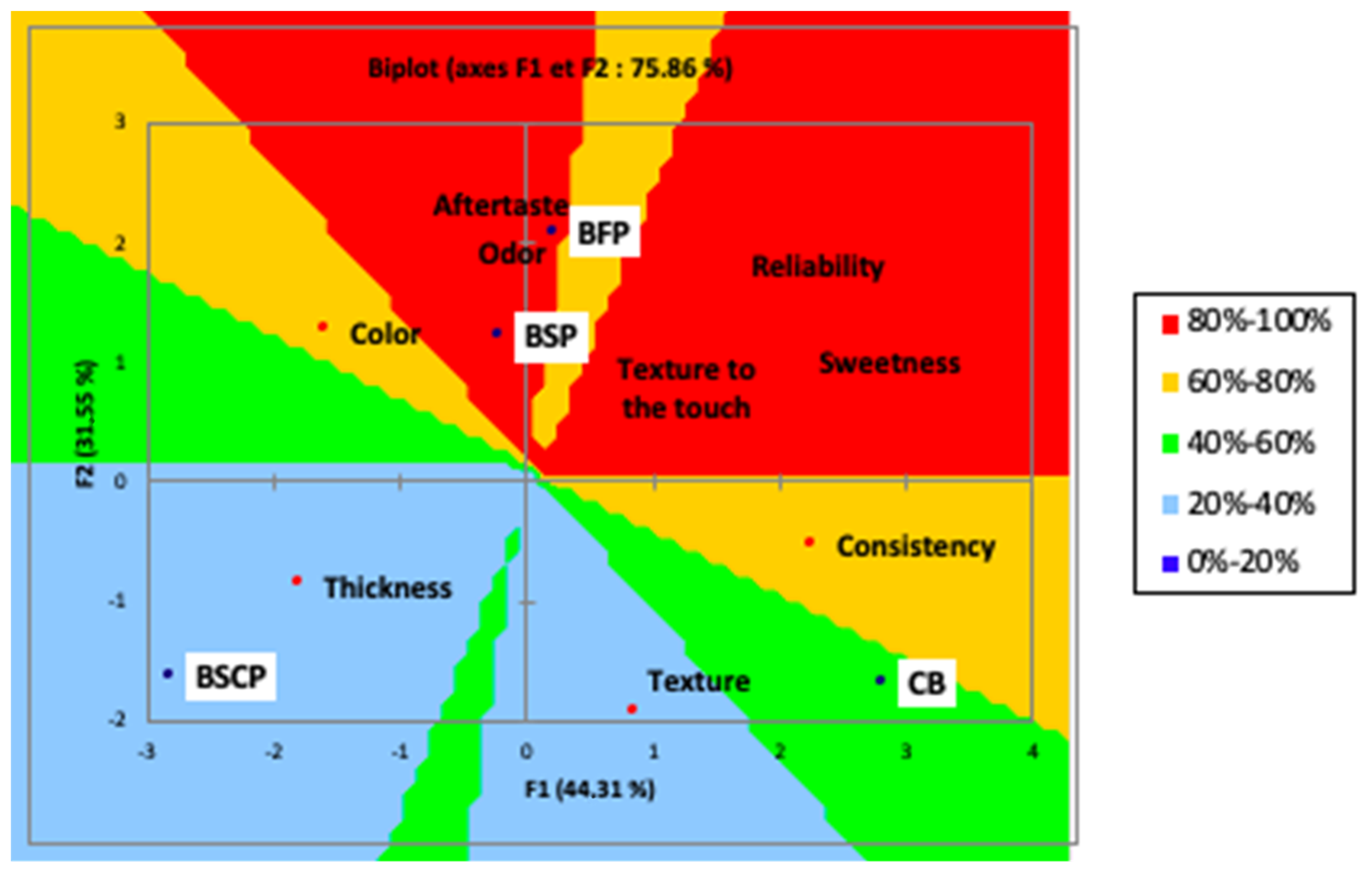
With respect to the overall acceptability, as shown in PREEFMAP (Figure 4), the biscuits formulated with seed cake had a percentage between 20% and 40%, while the biscuits enriched with flowers and seeds had a percentage between 80% and 100%.
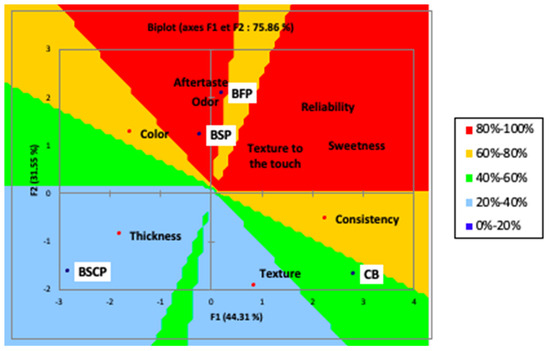
Figure 4.
Preference mapping (PREEFMAP) of the prepared biscuits. (control, CB) and OFI by-products powder (BFP: Biscuit with flowers powder, BSP: Biscuit with seeds powder, BSCP: Biscuit with seed cakes powder).
The control biscuit had a percentage between 40 and 60%. When compared to other biscuits, the flower-based biscuit is distinguished more by its aftertaste and odor. The color of the seed-based biscuit, the thickness and color of the cake-based biscuit, and the texture and consistency of the control biscuit differentiated them.
4. Discussion
Before mixing OFI by-product powders with wheat flour to make biscuits, samples were subjected to physicochemical and functional properties with phytochemical analysis. A general correlation could exist between the studied by-product characteristics and those of formulated biscuits.
Based on their proximate composition, the by-products under investigation are rich in sugars, proteins, and minerals, which might be advantageous to biscuit formulation. Nonetheless, the results obtained herein for the flower ash content are not in line with those of Ennouri et al. [25]. There is an array of explanations for the disparities in ash content that have been noted. The primary determinants of the ash content are soil type, pH, accessibility to water, and location [26]. It is also greatly impacted by crop year and genotype [27]. The dry ashing method to measure ash content in biomass is also crucial. The correct combination of ashing temperature, ashing time, and sample size must be chosen when utilizing dry ashing for ash analysis. For some samples, ashing at 550 °C for 6 h gave higher ash values than ashing at 550 °C overnight or ashing at 600 °C for 6 h [28].
Also, for seed, our values are different from those reported by Habibi [29] (0.072%). The seed cakes’ ash content is comparable to that of the studies conducted by Borchani et al. [30] and Borchani [31], which showed ash contents of 1.29% and 1.58%, respectively. The extraction of edible oil or the application of high pressure may have contributed to the cakes’ low ash content [32]. It is crucial to note that the mineral-rich sample is advantageous for the formulation of baked products since some cations such as calcium and magnesium enhance gluten’s mechanical properties to facilitate interactions with side groups of amino acids [33].
Concerning the content of soluble solids (°Brix), which is one of the indicators of sugar content, it can be inferred that biscuits with the addition of flower powder were sweeter than biscuits prepared with seed and seed cake powders. Ennouri et al. [25] report that OFI flowers at the full-flowering stage have a high soluble sugar content of 0.60 ± 0.03 g/kg on a dry weight basis.
For the sugar contents, the findings of other authors contrast with our results; hence, in a study conducted in 2019, Berrabah et al. [34] established that low total sugar values of 31, 44, 53, 36, 40, and 54 mg/100 g were acquired from Opuntia ficus indica flowers belonging to six distinct areas of Algeria (Tizi Ouzou, Relizane, Ain Defla, Mascara, Tiaret, and Msila). Reversely, higher levels, 1290 and 480 mg/100 g for the two red varieties of Opuntia (Opuntia microdasys and Opuntia macrorhiza), were detected in the seeds [35].
The outcomes attained by other researchers for the protein amounts were better than we estimated. Ennouri et al. [25] reported that the protein content of OFI flowers is about 80 mg/100 g DM. Additionally, Borchani et al. [31] found that the seed cakes of many cactus pear cultivars have exceptionally high protein levels, ranging from 6500 to 7650 mg/100 g DM. Nebbache et al. [36] discovered that the seeds have a protein concentration of 4480 mg/100 g DM.
The variances in climate, cultivar, genetics, harvesting period, and soil characteristics of the region where cactus pears grow could be the cause of the discrepancies in results between this study and the other literature as reported by estimating the approximate composition of Ethiopian cactus seeds [37].
Intrinsic physicochemical features known as functional properties are typically associated with the interaction of oil and water. These consist of swelling capacity (SWC), water holding capacity (WHC), oil absorption capacity (OAC), and oil holding capacity (OHC). It is possible to predict the technological effect of a component on a food product by looking at its functional features. For example, higher OHC is better for stabilizing emulsions and high-fat food items, while high WHC prevents syneresis and modifies the viscosity and texture of particular food products [38].
In this current investigation, flower flour demonstrated the highest capacity to absorb both water and oil. WHC of flour is significant in the food formulation process since it influences the final product’s physicochemical and sensory qualities (tenderness, friability, etc.). This property is dependent on the structure of polysaccharides and proteins. In addition, the use of flour in a food formulation is linked to its interaction with water and water absorption is a very essential quality factor in bakery products [39]. The kind and concentration of proteins, fibers, and starch, as well as the technological processes used in the preparation of food powders, are all related to the capacity to hold onto oil [40]. Food formulation relies heavily on an ingredient’s capacity to hold onto oil or fat to enhance mouthfeel and preserve flavor. For baked products needing a high oil retention capacity, flour with a high oil absorption capacity can be helpful [41].
On the other side, the phenolics known as potential antioxidants of cactus by-products were estimated. As compared to the TPC value reported by other authors under other circumstances, this one is deficient. Benayad et al. [42], who studied flowers and used maceration and accelerated solvent extraction (ASE) techniques, found that the acetone extracts produced the highest quantities (285 and 318 mg GAE/100 g DM for both techniques, respectively) in comparison to the methanolic extracts (234 and 240 mg GAE/100 g DM, respectively). There were notable TPC levels in aqueous extracts of flowers from several Algerian locations ranging from 752 to 1089 mg GAE/100 g [34].
Bousbia et al. [9], who used aqueous methanol (80%) for maceration, achieved a high level of 737.76 mg GAE/100 g DM. Likewise, the TPC of the seed cakes we found is much lower than the results found by Borchani et al. [31] who carried out a study on the seed cakes of different varieties of cactus pear. Nonetheless, the TPC content of the seeds we found (56.80 ± 0.45 mg GAE) was similar to that of the seeds of different varieties of OFI, which can be identified by their color (green, yellow, orange, and red) [4,43]. Other researchers found higher quantities of flavonoids either in the flowers [4,8,27,30,37,38] or seed cakes [31]. Nevertheless, the levels of flavonols were not reported in the literature to the best author’s knowledge. With regard to condensed tannins results, they are comparable to the findings of some research on the seeds of various OFI varieties from Bejaia locality (Algeria) [43] but are lower than those of another region either from Algeria (Souk Ahras) [4] and flowers from many Algerian areas [34].
Following the measurement of phenolic substances, various in vitro experiments were used to assess the antioxidant impact. The exploitation of the OFI by-product antioxidant activity has been the subject of several studies, with variable results throughout the literature. Better findings were reported by several authors who employed the DPPH test for both flowers from various regions [9,34] and seeds of different varieties [9,35,43]. In our earlier study, OFI flowers were also more effective in scavenging the ABTS radical [10]. Likewise, the iron reduction was more significant in flowers [9,34], seeds [4,9,35], and cake [31] assessed in other works.
The quantity of phenolics and antioxidant activity ranges significantly amongst plant species as it varies depending on the season, the degree of maturity, and the stage of vegetative development for a given species, with the added variation in the environmental conditions [44]. Moreover, differences in the reported values of phenolic compounds in plant extracts are influenced by the extraction solvents and methodologies applied.
Correlations are required to verify the implication of the various phenolics in the various antioxidant assays. This enables us to differentiate between the antioxidant impact of each by-product based on its phenolic composition. Our findings are not supported by those of Bouaouich et al. [4]. The difference in the correlation rate between phenolics and antioxidant activities may be due to the different compositions of flowers, seeds, and cake hydroalcoholic extract. Platzer et al. [45] stated that the substituents of the phenolic compounds play a major role in their antioxidant action, whereas their backbone has a small effect. This could be clarified also through the fact that the actions are carried out by other components. For instance, orange cactus fruit scavenging DPPH radical was associated with phenolics, ascorbic acid, and carotene while fresh purple fruit betalains were associated with chelating activity [46]. Adding to that, the majority of phenolics have been reported in the literature to have a high capacity to scavenge free radicals; however, when examining a complex extract, synergic and antagonist effects must be taken into account, as they rely on the combined action of all the constituents.
OFI by-products are a potentially health-promoting functional ingredient for use in bakery products. The selection of biscuits as a food matrix was based on their widespread availability, extended shelf life, and ease of export. Meanwhile, the mix between wheat flour and OFI by-products was considered due to their intriguing nutrients and phytochemicals components, which makes it suitable to meet the demand for healthier foods. The choice of 30% was based on the subsequent work of Bouazizi et al. [17] who formulated biscuits with 10, 20, and 30% cactus peel powder and discovered that the fragrance, taste, color, and general acceptability of biscuits made with 30 g/100 g of prickly pear flour were higher. In addition, a higher percentage (up to 30%) of F, S, and SC in the formula increased the antioxidant potential of the proposed biscuits.
It is crucial to note that the selection of a single proportion is related to the fact that we aimed to compare the relative efficacy of the cactus main by-products (F, S, and SC). Consequently, in subsequent research, the most effective functional ingredient will undergo more thorough testing.
After the formulated biscuit analysis, it turned out that the findings of Bouazizi et al. [17] using various proportions of OFI peel flour corroborated the results obtained herein. The higher protein content we found in OFI by-products added-biscuits is probably what caused the moisture content to decrease. Saadoudi [47] asserts that a negative association exists between baked products’ protein content and their water content. Similar findings were also reported by Pourmohammadi et al. [48], who developed innovative dietary fiber-enriched biscuits using resistant starch from corn and wheat and found a high level of moisture in control samples. The low water-holding capacity of the OFI by-product powder made the dough drier and harder and enhanced the textural properties (crispiness included) of the enriched biscuits [49]. However, one of the key elements that adversely impacts the solubility and water absorption of cereal products is their degree of crystallinity. The degree of crystallinity of wheat is about 27.7; it was most likely impacted by the powdered OFI by-product added. Therefore, that difference could explain the higher moisture content observed in the control sample with respect to enriched biscuits [50]. Diminished moisture content is advantageous to the shelf life of biscuits since most unwanted microbes might struggle to survive [51].
The ash content increased in our added biscuits, like in a previous work undertaken by Bouazizi et al. [17] in biscuits enriched with OFI fruit peel flour. In their study, Ali et al. [16] examined the effects of roasted cactus pear seed flour on the nutritional and sensory aspects of bread. They discovered that the dietary fiber, fat, and ash contents significantly increased. With regard to the pH value, our results agree with Menasra [39], who suggests that its decrease may result from the enrichment of biscuit powders, changing their composition in the process.
The incorporation of cactus by-products into biscuits could increase health benefits by increasing antioxidant properties. From our results, it can be hypothesized that the occurrence of appropriate phytocompounds in OFI by-products enhanced biscuits’ in vitro antioxidant activity. A similar increase in radical scavenging effect was shown in biscuits formulated with OFI fruit peel flour [17]. In addition to the richness of OFI fruit peel in active and functional biomolecules, the presence of fibers and polyphenols gives biscuits technological properties such as kneading ability, flavor retention, and antioxidant capacity [17]. Msaddak et al. [14] observed that replacing wheat flour with cladode powder at 5% improved the total phenolics content and the bread’s antioxidant potential without negatively affecting its sensory acceptability. The phenolic concentration and antioxidant activity of the bread enriched with roasted OFI seed flour have also been considerably improved [16]. Refined wheat flour commonly used to make biscuits is high in starch but poor in protein and fiber. The enrichment of biscuits with natural products significantly changes their chemical composition and antioxidant capacity. In the recipe for biscuits, a variety of food waste or by-products along with those of OFI, including peel, germs, and biomass waste such as leaves, have been partially substituted for cereal flour. These products can reduce calorie content while maintaining the functionalities of sucrose and fat and enhancing nutritional values (dietary fiber, proteins, minerals, and bioactive compounds) [52]. For instance, the use of carob by-products (germ and seed peel) improved, particularly the amount of protein, fiber, minerals, essential fatty acids, and antioxidant potential of biscuits [53]. The biscuits incorporated with 6% mandarin peel powder enhanced the DPPH antioxidant activity from 23.72% in the control to 73% in the functionalized biscuits [54]. The antioxidant activity of muffin doughs containing pomegranate peel at 5%, 10%, and 15% was increased 10-, 21-, and 27-fold, respectively [55]. The enhancement of the biscuit’s antioxidant effect is principally related to the phenolic compounds of the by-products. They have a possible action in protecting lipids against the oxidation process [56]. Foods enriched with polyphenols proved their ability to combat obesity by influencing adipocyte differentiation, lipid metabolism, and energy and food needs [57].
By using sensory analysis, we may emphasize the judges’ appreciation. Interestingly, the enriched biscuits were the ones that the judges preferred. According to Dick et al. [15], crackers containing 5% cladode flour and 2% cactus mucilage scored the highest attributes in sensory assessments, indicating that they could provide tasty and wholesome substitutes for gluten-free crackers. The fortification with OFI by-products was associated with the most increases in color, smell, and taste acceptance scores. This is likely because they contain certain reducing sugars that can undergo non-enzymatic browning reactions and have a better propensity for retaining smell.
Also, the prickly pear flour addition in cookie preparation showed a significant difference (in positive) for acceptance descriptors, particularly at a higher percentage of addition (20 and 30 g/100 g) [17].
5. Conclusions
Overall, the findings showed that it is feasible to produce durum wheat biscuits enhanced with OFI waste, notably flowers and seeds. We found that these by-products contained a sustainable amount of nutritional (sugars, proteins, and ashes) and bioactive compounds (polyphenolic compounds) and it was the flowers that exhibited the highest contents. There was a correlation between phenolic class content, namely total phenolics, total flavonoids, flavonols, and condensed tannins, with the outcomes of DPPH, ABTS, FRAP, and TAA assays, pointing out that they are considered as a main contributor to the antioxidant effect of the samples. After formulating biscuits, it was found that by substituting wheat flour with powdered OFI flowers, seeds, and seed cakes, the nutritional value of the biscuits was improved. So, we were able to produce biscuits that were higher in sugar, protein, minerals, and greater antioxidant activity. The sensory analysis showed the acceptability and appreciation of the judges of the biscuits with a particular preference for the biscuit made from flowers or seeds, which had a percentage between 80% and 100%. Their execution could provide a new use for a widespread by-product of the cactus manufacturing sector. To validate the tendencies found, a bigger sample size and consideration of participant ethnicity must be included in a subsequent investigation. Further studies should also focus on confirming that adding cactus by-products does not affect the stability of the antioxidant compounds or the biscuits’ sensory attributes over time.
Author Contributions
Conceptualization: F.B.(Fatiha Brahmi); Writing—original draft: F.B.(Fatiha Brahmi); Writing—review and editing: F.B. (Federica Blando); Formal analysis: A.O. and L.S.-B.; Software: A.O. and L.S.-B.; Methodology: N.H. and L.H.; Supervision: L.B.-M. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding for the Algerian part. For the Italian part, the work has been partially funded by the Project “ON Foods—Research and Innovation Network on food and nutrition Sustainability, Safety and Security—Working ON Foods”, a project funded by the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3—Call for proposals No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors gratefully acknowledge Mahmoud Fahmi Elsebai from the Faculty of Pharmacy, Mansoura University, Egypt, for linguistic revision. The authors would like also to thank everybody who helped to accomplish this research study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Louppis, A.P.; Constantinou, M.S.; Kontominas, M.G.; Blando, F.; Stamatakos, G. Geographical and Botanical Differentiation of Mediterranean Prickly Pear Using Specific Chemical Markers. J. Food Compos. Anal. 2023, 119, 105219. [Google Scholar] [CrossRef]
- Griffith, M.P. The Origins of an Important Cactus Crop, Opuntia ficus-indica (Cactaceae): New Molecular Evidence. Am. J. Botany 2004, 91, 1915–1921. [Google Scholar] [CrossRef]
- Mazari, A.; Mahdeb, A. Importance nutritionnelle et agro-économique des produits issus. Rech. Agron. 2021, 19, 43–63. [Google Scholar]
- Bouaouich, A.; Bouguerche, F.; Mahiaoui, H.; Peron, G.; Bendif, H. Phytochemical Elucidation and Antioxidant Activity of Seeds from Three Prickly Pear (Opuntia ficus-indica L.) Cultivars from Algeria. Appl. Sci. 2023, 13, 1444. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Ayoub, T.E.M.; Rohn, S. (Eds.) Opuntia Spp.: Chemistry, Bioactivity and Industrial Applications; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-78443-0. [Google Scholar]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Lo Turco, V. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Komla, M.G.; Mariod, A.A. (Eds.) Nopal Cactus (Opuntia ficus-indica (L.) Mill) as a Source of Bioactive Compounds; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-31884-0. [Google Scholar]
- Blando, F.; Albano, C.; Jiménez-Martínez, C.; Cardador-Martínez, A. Opuntia, ficus-indica [L.] Mill. and Other Species: Source of Bioactives and Their Molecular Mechanisms of Action to Promote Human Health. In Molecular Mechanisms of Functional Food; Wiley: Hoboken, NJ, USA, 2023; pp. 193–237. [Google Scholar]
- Bousbia, N.; Mazari, A.; Lamoudi, L.; Akretche-Kelfat, S.; Dif, M.E. Evaluation of the phytochemical composition and the antioxidant activity of cactus pear flowers and fruit derivatives. Rev. Abrobiologia 2022, 12, 3235. [Google Scholar]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; De Bellis, L.; Negro, C.; Haddadi-Guemghar, H.; Madani, K.; Makhlouf-Boulekbache, L. Optimization of the Conditions for Ultrasound-Assisted Extraction of Phenolic Compounds from Opuntia ficus-indica [L.] Mill. Flowers and Comparison with Conventional Procedures. Ind. Crops Prod. 2022, 184, 114977. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.D.B. New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods 2023, 12, 2494. [Google Scholar] [CrossRef]
- Otles, S.; Nakilcioglu-Tas, E. Cereal-Based Functional Foods. In Functional Foods; Chhikara, N., Panghal, A., Chaudhary, G., Eds.; Scrivener Publishing LLC.: Austin, TX, USA, 2022. [Google Scholar] [CrossRef]
- Pinto, D.; Castro, I.; Vicente, A.; Bourbon, A.I.; Cerqueira, M.Â. Functional Bakery Products: An Overview and Future Perspectives. In Bakery Products Science and Technology; Zhou, W., Hui, Y.H., De Leyn, I., Pagani, M.A., Rosell, C.M., Selman, J.D., Therdthai, N., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 431–452. ISBN 978-1-119-96715-6. [Google Scholar]
- Msaddak, L.; Abdelhedi, O.; Kridene, A.; Rateb, M.; Belbahri, L.; Ammar, E.; Nasri, M.; Zouari, N. Opuntia ficus-indica Cladodes as a Functional Ingredient: Bioactive Compounds Profile and Their Effect on Antioxidant Quality of Bread. Lipids Health Dis. 2017, 16, 32. [Google Scholar] [CrossRef]
- Dick, M.; Limberger, C.; Cruz Silveira Thys, R.; De Oliveira Rios, A.; Hickmann Flôres, S. Mucilage and Cladode Flour from Cactus (Opuntia monacantha) as Alternative Ingredients in Gluten-Free Crackers. Food Chem. 2020, 314, 126178. [Google Scholar] [CrossRef]
- Ali, R.F.; El-Anany, A.M.; Mousa, H.M.; Hamad, E.M. Nutritional and Sensory Characteristics of Bread Enriched with Roasted Prickly Pears (Opuntia ficus-indica) Seed Flour. Food Funct. 2020, 11, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of Prickly Pear (Opuntia ficus-indica L.) Peel Flour as an Innovative Ingredient in Biscuits Formulation. LWT-Food Sci. Technol. 2020, 124, 109155. [Google Scholar] [CrossRef]
- Nabil, B.; Ouaabou, R.; Ouhammou, M.; Essaadouni, L.; Mahrouz, M. Functional Properties, Antioxidant Activity, and Organoleptic Quality of Novel Biscuit Produced by Moroccan Cladode Flour “Opuntia ficus-indica”. J. Food Qual. 2020, 2020, 3542398. [Google Scholar] [CrossRef]
- Aparicio-Ortuño, R.; Jiménez-González, O.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Cladodes of Opuntia Ficus Indica as a Functional Ingredient in the Production of Cookies: Physical, Antioxidant and Sensory Properties. Sustain. Food Technol. 2024, 2, 816–825. [Google Scholar] [CrossRef]
- Mindjou, S.; Brahmi, F.; Belkhiri, W.; Bouanane, F.; Bouchalal, N.; Madani, K. Quantification of the Antioxidants and Assessment of the Antioxidant Activity of Two Cucurbita Species Harvested in Bejaia (Algeria). Curr. Nutr. Food Sci. 2020, 16, 190–197. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists’ Methods. Official Methods of Analysis; Association of Official Analytical Chemistry: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Parsaei, M.; Goli, M.; Abbasi, H. Oak Flour as a Replacement of Wheat and Corn Flour to Improve Biscuit Antioxidant Activity. Food Sci. Nutr. 2018, 6, 253–258. [Google Scholar] [CrossRef]
- Ennouri, M.; Ammar, I.; Khemakhem, B.; Attia, H. Chemical Composition and Antibacterial Activity of Opuntia ficus-indica F. Inermis (Cactus Pear) Flowers. J. Med. Food 2014, 17, 908–914. [Google Scholar] [CrossRef]
- Finell, M.; Nilsson, C. Variations in Ash Content, Pulp Yield, and Fibre Properties of Reed Canary-Grass. Ind. Crops Prod. 2005, 22, 157–167. [Google Scholar] [CrossRef]
- Morris, C.F.; Li, S.; King, G.E.; Engle, D.A.; Burns, J.W.; Ross, A.S. A Comprehensive Genotype and Environment Assessment of Wheat Grain Ash Content in Oregon and Washington: Analysis of Variation. Cereal Chem. 2009, 86, 307–312. [Google Scholar] [CrossRef]
- Liu, K. Effects of Sample Size, Dry Ashing Temperature and Duration on Determination of Ash Content in Algae and Other Biomass. Algal Res. 2019, 40, 101486. [Google Scholar] [CrossRef]
- Habibi, Y. Contribution à l’étude Morphologique, Ultrastructurale et Chimique de la Figue de Barbarie. Les Polysaccharides Pariétaux: Charactérisation et Modification Chimique. Ph.D. Dissertation, Université Joseph-Fourier-Grenoble I, Grenoble, France, 2004. [Google Scholar]
- Borchani, M.; Yaich, H.; Abbès, F.; Blecker, C.; Besbes, S.; Attia, H.; Masmoudi, M. Physicochemical, Functional and Antioxidant Properties of the Major Protein Fractions Extracted from Prickly Pear (Opuntia ficus indica L.) Seed Cake. Waste Biomass Valor. 2021, 12, 1749–1760. [Google Scholar] [CrossRef]
- Borchani, M. Contribution à la Valorisation de la Pulpe, de la peau et du Tourteau de Graines de Figue de Barbarie (Opuntia spp.). Ph.D. Dissertation, Université de Sfax, Sfax, Tunisia, 2022. [Google Scholar]
- Ait Benhamou, A.; Boussetta, A.; Kassab, Z.; Nadifiyine, M.; Hamid Salim, M.; Grimi, N.; El Achaby, M.; Moubarik, A. Investigating the Characteristics of Cactus Seeds By-Product and Their Use as a New Filler in Phenol Formaldehyde Wood Adhesive. Int. J. Adhes. Adhes. 2021, 110, 102940. [Google Scholar] [CrossRef]
- Sehn, G.A.R.; Nogueira, A.D.C.; Almeida, E.L.; Chang, Y.K.; Steel, C.J. Fortification of Wheat Dough with Calcium and Magnesium Ions Affects Empirical Rheological Properties. Cereal Chem. 2015, 92, 405–410. [Google Scholar] [CrossRef]
- Berrabah, H.; Taïbi, K.; Ait Abderrahim, L.; Boussaid, M. Phytochemical Composition and Antioxidant Properties of Prickly Pear (Opuntia ficus-indica L.) Flowers from the Algerian Germplasm. Food Meas. 2019, 13, 1166–1174. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Seeds of Opuntia spp. as a Novel High Potential by-Product: Phytochemical Characterization and Antioxidant Activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef]
- Nebbache, S.; Chibani, A.; Chadli, R.; Bouznad, A. Chemical composition of Opuntia ficus-indica (L.) fruit. Afr. J. Biotechnol. 2009, 8, 1623–1624. [Google Scholar]
- Reda, T.H.; Atsbha, M.K. Nutritional Composition, Antinutritional Factors, Antioxidant Activities, Functional Properties, and Sensory Evaluation of Cactus Pear (Opuntia ficus-indica) Seeds Grown in Tigray Region, Ethiopia. Int. J. Food Sci. 2019, 2019, 5697052. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food Industry By-Products Used as Functional Ingredients of Bakery Products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Menasra, A. Etude de la Formulation et des Traitements Technologiques des Biscuits Enrichis. Ph.D. Dissertation, University of Batna 1, Batna, Algeria, 2020. [Google Scholar]
- Vioque, J.; Alaiz, M.; Girón-Calle, J. Nutritional and Functional Properties of Vicia Faba Protein Isolates and Related Fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef] [PubMed]
- David, O.; Arthur, E.; Kwadwo, S.O.; Badu, E.; Sakyi, P.; Student, P.G. Proximate Composition and Some Functional Properties of Soft Wheat Flour. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 753. [Google Scholar]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic Composition, Antioxidant and Anti-Inflammatory Activities of Extracts from Moroccan Opuntia ficus-indica Flowers Obtained by Different Extraction Methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil Composition and Characterisation of Phenolic Compounds of Opuntia ficus-indica Seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Brahmi, F.; Lounis, N.; Mebarakou, S.; Guendouze, N.; Yalaoui-Guellal, D.; Madani, K.; Boulekbache-Makhlouf, L.; Duez, P. Impact of Growth Sites on the Phenolic Contents and Antioxidant Activities of Three Algerian Mentha Species (M. pulegium L., M. rotundifolia (L.) Huds., and M. spicata L.). Front. Pharmacol. 2022, 13, 886337. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M.; Osthoff, G.; Hugo, A. Relationship and Correlation between Antioxidant Content and Capacity, Processing Method and Fruit Colour of Cactus Pear Fruit. Food Bioprocess Technol. 2018, 11, 1527–1535. [Google Scholar] [CrossRef]
- Saadoudi, M. Qualité et Sécurité Alimentaire. Ph. D. Dissertation, Universite of Batna 1, Batna, Algeria, 2019. [Google Scholar]
- Pourmohammadi, K.; Abedi, E.; Farahmandi, S.; Mahmoudi, M.R.; Hashemi, S.M.B.; Torri, L. Modeling the Effects of Corn and Wheat Resistant Starch on Texture Properties and Quality of Resistant Starch-enrichment Dough and Biscuit. J. Food Process Eng. 2019, 42, e12962. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant Starch as Functional Ingredient: A Review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Ramashia, S.E.; Mamadisa, F.M.; Mashau, M.E. Effect of Parinari Curatellifolia Peel Flour on the Nutritional, Physical and Antioxidant Properties of Biscuits. Processes 2021, 9, 1262. [Google Scholar] [CrossRef]
- Goubgou, M.; Songré-Ouattara, L.T.; Bationo, F.; Lingani-Sawadogo, H.; Traoré, Y.; Savadogo, A. Biscuits: A Systematic Review and Meta-Analysis of Improving the Nutritional Quality and Health Benefits. Food Prod. Process Nutr. 2021, 3, 26. [Google Scholar] [CrossRef]
- Martin-Diana, A.B.; Izquierdo, N.; Albertos, I.; Sanchez, M.S.; Herrero, A.; Sanz, M.A.; Rico, D. Valorization of Carob’s Germ and Seed Peel as Natural Antioxidant Ingredients in Gluten-Free Crackers: Carob Antioxidant By-Products in Gluten-Free Snack. J. Food Process. Preserv. 2017, 41, e12770. [Google Scholar] [CrossRef]
- Ojha, P.; Thapa, S. Quality Evaluation of Biscuit Incorporated with Mandarin Peel Powder. Sci. Study Res. Chem. Chem. Eng. 2017, 18, 19–30. [Google Scholar]
- Topkaya, C.; Isik, F. Effects of Pomegranate Peel Supplementation on Chemical, Physical, and Nutritional Properties of Muffin Cakes. J. Food Process Preserv. 2019, 43, e13868. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized Biscuits with Bioactive Ingredients Obtained by Citrus Lemon Pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing Obesity through Natural Polyphenols: A Review. Future Foods 2020, 1–2, 100002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).