Abstract
Several researchers recognize the importance of plants as effective tools for environmental biomonitoring. The black poplar (Populus nigra L.) often emerges as a useful bioindicator of air quality in urban environments, where this tree species is widely employed for urban areas. Here, we used ICP-MS analysis to assess the presence and concentration of trace elements, with a special focus on heavy metals (HMs), in black poplar leaves and soil samples from three urbanized sites showing varying degrees of environmental quality. Specifically, the foliar concentrations of Zn (173.3 ppm), Cd (0.7 ppm), Co (1.1 ppm) and As (0.2 ppm) exceeded reference values for unpolluted sites, indicating potential environmental hazards. Additionally, we correlated the foliar concentrations of HMs with those quantified in soil and with air quality data provided by the regional air quality-monitoring network. Subsequently, we estimated the values of foliar fluctuating asymmetry, and evaluated their relationships with HM concentrations in both leaves and soil. Our results suggest that element concentrations in black poplar leaves are related to soil contamination and atmospheric quality, and the extent depends on the proximity to relevant pollution sources. Furthermore, the study species showed a pronounced accumulation capacity for some HMs (i.e., Zn, Cd) commonly found in particulate matter. The extent of foliar fluctuating asymmetry is related to atmospheric quality and HM soil concentration, possibly because of the growth anomalies induced by this kind of environmental contamination. Overall, our data indicate the study species can supply an effective biomonitoring service in urbanized contexts, offering valuable insights into the occurrence and biological implications of heavy metal contamination.
1. Introduction
Current estimates suggest that approximately 4 million people succumb to air pollution annually, as about 91% of the global population resides in areas where minimum air quality thresholds are surpassed [1]. Particulate matter (PM) (i.e., PM10 and PM2.5 having aerodynamic diameters < 10 μm and <2.5 μm, respectively) is the prevalent source of air pollution in urban areas due to adjacent industrial zones, vehicular traffic, and resuspended dust [2]. Although PM settles on surfaces, wind and runoff can disperse or relocate it, leading to soil accumulation [1]. The hazardousness of PM-related pollutants is underscored by their mobility toward deeper soil layers, which can easily lead to groundwater contamination, and by their bioavailability, which enables their penetration into food chains after being absorbed by vegetation [3,4].
Most of the concerns regarding the impact of PM contamination on human health and other aspects of environmental quality originate from their richness in metals, with a major emphasis on heavy metals (HMs). Indeed, while some metals serve as essential cofactors for numerous enzymes (e.g., Mg, Fe, Zn, Mn, Cu, and Se), others (e.g., Cd, Hg, Pb, Cr), inhaled or absorbed through food [5], contribute to the generation of Reactive Oxygen Species (ROS). The presence of ROS in humans leads to various diseases, including vascular disorders, neurodegeneration, and cancer [6]. Metals have been integral to human daily life, but in recent decades, their concentration in soil and air has witnessed a significant increase [7]. Accordingly, large-scale surveys, such as those provided by the European Environment Agency, indicate that HMs represent the prevalent kind of pollutant in contaminated sites; in particular, high concentrations of Cd, Cu, Hg, Pb and Zn appear linked to large-scale human activities, i.e., industrialization and intensive agriculture. HM contamination poses serious hazards on ecosystem biological components and human health, due to the pronounced mobility, low degradation rate, and great bioaccumulation potential that characterize such highly toxic contaminants [2].
The harm posed by HMs to both people and ecosystems can be mitigated through the establishment of rigorous limit values and extensive monitoring networks operating in agreement with reliable guidelines. Monitoring strategies can include both active and passive techniques. The former involves the analysis of drawing air over filters at control stations, as is commonly carried out by the national and regional environmental agencies. In contrast, passive monitoring techniques can involve biomonitors, i.e., the use of model organisms to determine the presence of pollutants through detectable metabolic or biochemical damage [8], and bioaccumulators, i.e., the use of model organisms to determine the occurrence and extent of pollutants based on their ability to accumulate one or more pollutants in their tissues [9]. For the measurement of metal bioavailability, vascular plants are preferable to traditional chemical tests, because they provide a direct measure of metal bioaccumulation without the need for interpolations of extraction concentration for an effect [10,11]. Therefore, urban green areas can represent a relevant option for implementing extensive and low-cost networks aiming to monitor the levels of HM contamination. Urban tree species, especially, can easily absorb airborne pollutants through leaves, or uptake soil pollutants through their root system. In particular, leaves can be more sensitive to pollution than other plant organs (roots, bark, or flowers), as they often represent the main sink for the accumulated metals [12,13]. However, discerning the pathways of metal absorption and determining whether the accumulated metals originate from air absorption or soil uptake remains challenging. Indeed, the uptake of metals by trees depends on various factors, including the pollutant concentration in environmental matrices, metal bioavailability, and the tree species [14].
The black poplar (Populus nigra L.) is one of the tree species most commonly employed in urban phytomanagement interventions [15]. Such a dioecious tree, belonging to the Salicaceae family, is characterized by a highly developed root system, a rapid biomass production, and an enhanced potential for metal accumulation [16]. These species absorb metals through both the root system and the leaves; subsequently, metals are translocated and stored in the leaves [17].
In Southern Italy, the black poplar is widespread in public green areas as well in natural riparian habitats, providing an optimal chance for testing its potential in monitoring the levels of contamination across sites with different environmental quality. Accordingly, in this paper, we used this species as the model organism, to perform trace-element analyses (with a major emphasis on HMs leaf concentration) and phenotypic measures of plant stress (i.e., leaf fluctuating asymmetry) to be compared to the air and soil quality of three urbanized sites with different environmental quality rates. The determination of trace element concentration in leaf and soil samples was carried out using inductively coupled plasma mass spectrometry (ICP-MS), an analytical approach that is effective in determining multiple elements at trace levels due to its high sensitivity, precision, and wide linear dynamic range [18,19]. Then, trace-element analyses were integrated with morphological measures of fluctuating asymmetry (FA). In plant species, FA is frequently used as a proxy of developmental instability that can result from various environmental drivers, including pollution and other kinds of stress [20,21,22]. The founding assumption for FA is that individuals growing in polluted environments lack the ability to rectify random developmental errors, resulting in greater deviations from the axis of bilateral symmetry [22]. Nonetheless, generalizing the informative value of FA is often challenged by its variability across species and traits [23].
The obtained data were used to address the following questions: (1) do trace elements soil concentrations reflect the levels of air quality of the three study sites?; (2) do trace element concentrations in black poplar leaves fit the between-site patterns of air quality and soil contamination?; (3) does the FA in black poplar leaves match the between-site patterns of HM contamination and give an indication of its biological effects?
2. Materials and Methods
2.1. Study Area and Sampling Sites
The Calabria Region occupies the southernmost portion of the Italian Peninsula. In recent years, the territory of this region has experienced a significant increase in industrial activities, vehicular traffic, and the construction of an international airport. The rise of such anthropogenic activities significantly contributed in lowering the environmental quality in both the urban and surrounding areas. This is particularly evident for the area known as Lamezia Plain. Indeed, this area hosts the most important airport of the region (SUF international airport), one of the main regional railway hubs, and the largest industrial hub of the Southern Italy, which covers approximately 1100 hectares [24].
In September 2021, a comparable amount of black poplar leaves and soil samples were collected from three different sites that can represent different levels of air quality (Figure 1). The first sampling site (NIC: 38°57′52.2″ N, 16°17′44.0″ E, altitude 147 m a.s.l.) is a small green area located in an urbanized area (the Nicastro city) of the Lamezia Plain; here, the vehicular traffic represents the greatest anthropogenic pressure; in addition, this site is just 6.77 km away from the SUF airport. The second site (LAM: 38°55′11.5″ N, 16°14′04.4″ E, altitude 18 m a.s.l.) lies in an agricultural area of the Lamezia Plain, very close (~1.5 km) to SUF airport. The third site (REN: 39°21′03.8″ N, 16°14′16.9″ E, altitude 190 m a.s.l.) is located northwards, within a large riparian park in the Rende city, and shows a higher environmental quality compared the other sites. Moreover, the NIC and REN coincided with two sampling stations (Nicastro 1—European code IT2087A, and Rende 2—European code IT2086A, respectively) belonging to the regional air quality monitoring network (RAQMN), established by the Regional Agency for the Environmental Protection of Calabria (ARPACAL). The RAQMN sampling stations provide data relative to the local air quality in the form of a synthetic air quality index (AQI). The AQI is calculated by combining the contribution that each monitored pollutant makes to the air quality under investigation through a specific sub-index. Specifically, the AQI is derived from the semi-addition of the sub-index for particulate matter (PM10) and the highest of the sub-indices calculated for each monitored pollutant.
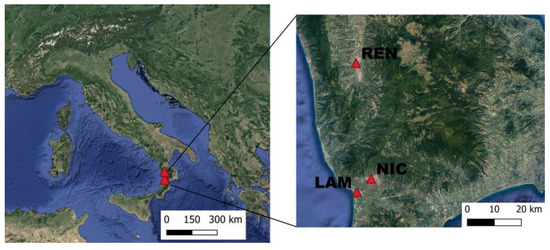
Figure 1.
Geographical location of the three study sites in the context of the Italian Peninsula. Background image from Google satellite.
The PM10 sub-index is chosen as the main reference, because it is the parameter that statistically makes the greatest contribution to the air quality assessment; moreover, PM10 is the parameter with the highest frequency of annual exceedances. Unfortunately, although the calculation of AQI closely depends on PM10, it does not provide information about the composition of the particulate matter. In contrast, Imax is the highest of the sub-indices relative to the further pollutants monitored (e.g., NO2, CO, SO2, O3). The sub-index for individual pollutants is a dimensionless quantity obtained by referring the recorded value to the most restrictive of the limit or target values indicated by the Italian legislation (Legislative Decree 155/2010). Therefore, values of AQI < 1.0 indicate that non pollutants exceed the thresholds set by law; while AQI > 1.0 indicate that at least one pollutant exceeds the limit value. Then, air quality at each sampling station is expressed over a range of 7 levels (from excellent to very unhealthy) that follow an increasing gradient of AQI. For our study, we used daily data recorded over one month (22 June 2021–22 July 2021) by the sampling stations Nicastro 1 and Rende 2. Although no data of air quality were available for SUF, we assumed it possesses an intermediate level of air quality compared to the other two sites; indeed, in spite of this site lying within a sparsely urbanized area, it is still in proximity to the industrial hub of the Lamezia Plain.
2.2. Plant and Soil Sampling
All details regarding the design of the plant and soil sampling are summarized in Table 1. A sample of 40 leaves was collected on 5 trees in each site; the leaves were picked up at a height of 4 m by sampling the tree canopy in all directions. Subsequently, from the 40 leaves taken from each tree, 10 leaves were randomly selected and used to perform measurements of fluctuating asymmetry, while 5 well-preserved leaves per plant, representing different regions of the tree canopy, were selected for ICP-MS procedures. Additionally, two soil samples were taken in each site at a depth of 0–20 cm in the area hosting the sampled trees; both the soil samples were processed by ICP-MS to determine the level of heavy metal soil contamination in each site.

Table 1.
Sampling plane adopted for collecting plant and soil samples.
2.3. ICP-MS Analysis on Plant and Soil Samples
Sample pretreatment and ICP analyses were carried out at the DiSTeM department laboratories, according to the preparation protocol and analytical procedures adopted in Calabrese et al. [25]. Hence, leaf and soil samples were firstly oven dried at a constant temperature of 40 °C for 24 h and were completely powdered in agate jars by planetary ball mills. A total of 250 mg of the powdered sample was mineralized for 60 min by Closed Vessel Acid Digestion by using a microwave oven (MARS Xpress 5, CEM), using Teflon vessels with 3 mL concentrated HNO3 (ultrapure grade 65%), 2 mL H2O2, (ultrapure 39%) and 5 mL milliQ-water. Control samples were added to each batch of samples as follows: six certified reference materials (CRM), three replicates of one random sample and one method blank (acid solution without any powder). The certified reference materials included leaves (NIST 1515, NCS DC 07603, NCS DC 07604, NCS DC 07605) and soils (NCS DC 77302 and NCS DC 87104). Digested solutions were filtered with syringe filters (25 mm diameter, 0.45 µm pore size) and were further diluted with ultrapure water up to a total volume of 20 mL. Thirty-five elements (Al, As, B, Ba, Be, Bi, Br, Ca, Cd, Cu, Co, Cr, Cs, Fe, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Rb, S, Sb, Se, Si, Sn, Sr, Te, Tl, Ti, V, U, Zn) were quantified in the laboratories of INGV of Palermo by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), using an Agilent 7800 and an Agilent 5900, respectively. The certified reference materials, method blanks, and replicates were used to assess the precision and accuracy of the results.
2.4. Estimating Fluctuating Asymmetry (FA)
Foliar FA was determined on fresh leaves. The measurements were carried out by digital caliper with a 20 mm gauge and 0.01 mm of resolution, by considering the central vein as the latitudinal axis and measuring the width of the right and left sides of the lamina in correspondence with the point of maximum foliar amplitude along the longitudinal foliar axis (Figure 2).

Figure 2.
Measurements carried out on the leaf lamina for estimating fluctuating asymmetry.
Then, our FA estimations relied on the concept of mean absolute difference, as this way of measuring FA is appropriate for comparing two or more populations through analysis of variance [20]. In more detail, we quantified FA by computing two indices (FA1 and FA2) as formulated by Palmer [26], and Palmer and Strobeck [27].
Therefore:
While:
where R and L, respectively, are the width of the right and left side of a lamina, while n is the total number of measured leaves; and S accounted for the average lamina size as it was calculated for each leaf as follows:
Hence, FA1 and FA2 differed because the latter included the average lamina size as a correction factor for FA measurements, thereby limiting the potential inflation due to the leaf size variations on final FA estimates [28].
2.5. Data Analyses
The ICP-MS analyses allowed us to determine the soil and leaf concentration of 35 trace elements, and the overall relationships between patterns of soil and leaf concentration were checked by carrying out a Mantel test (no. permutations: 9999) between the soil and leaf matrix (both accounting for 35 elements × 3 sites). To reduce possible distortions in the multivariate test, due to large differences in number dimension among concentrations of different elements, the concentration values were log-transformed before performing the Mantel test. The normality of the log-transformed data was then checked by the Shapiro–Wilk normality test.
Although our analysis permitted a comprehensive assessment of soil and leaf trace elements, we paid more attention to a selected array of HMs (i.e., Pb, As, Cd, Co, Ni, Zn), whose results were crucial to monitor as they are particularly toxic to human health and the environment. Indeed, the selected HMs are often included as particles in PM10 [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], and the Italian Environmental Regulations establishes rigorous limits to their release into the environment. Therefore, to establish the occurrence of contamination in the study sites based on the biomonitoring capacity of black poplar, the foliar concentration of the target HMs was compared to the relative “Reference Plant” values [30], which represent the expected leaf concentration in unpolluted areas [30,31].
Furthermore, the actual bioavailability of the target HMs in the study sites, and their biological hazardousness, was estimated by computing the bioaccumulation factor (BAF) from the leaf and soil concentration of each selected HM. According to Luoma and Bryan [32], the BAF can be calculated as the ratio between the concentration of heavy metals in the plant tissues and their relative concentration in the soil.
Therefore, in our case, for each heavy metal, the equation is as follows:
where C leaves is the average concentration of a given HM in the foliar tissue, and C Soils the average concentration of the same HM in the soil.
BAF = (C leaves/C soil)
The BAF represents the ability of a given element to migrate through the soil–plant system; accordingly, values > 1 indicate that the plant has a strong ability in absorbing the element from the soil and accumulating it in its tissues [33].
The between-site variations of FA were evaluated by analysis of variance (ANOVA) with a Bonferroni post hoc test. Before carrying out ANOVA, the 10 within-individuals replicates available for each plant were averaged to a single individual value of FA. The possible influence of environmental contamination in destabilizing the normal developmental processes of the studied biological systems was evaluated by performing a Pearson correlation test between the individual levels of FA and soil, and the leaf concentration of trace elements.
Statistical analyses were performed by using the free software package PAST3 [34].
3. Results
3.1. Patterns of Soil Trace Elements and Air Quality among Study Sites
Overall, the ICP-MS analyses allowed us to determine the concentration of 35 trace elements in the soil samples collected in the three study sites; the complete element list, with their relative concentration in each soil sample from each study site, is reported in Table S1. Compared to the other sites, NIC showed a higher concentration of 28 out of 35 elements, including many heavy metals (Table 2). In contrast, LAM and REN exhibited, respectively, the highest concentration of 3 and 4 trace elements (Table 2).

Table 2.
Average concentration (ppm) of soil (sx) and leaf (dx) trace elements recorded in the three study sites. Elements marking the highest concentration in a given site are in bold.
Regarding the limits set by the national environmental policies, the soil concentration of As exceeded the PTCs set by the D.L. 156/2006 in NIC and LAM (Table 3). In contrast, the concentration of other target elements (i.e., Zn, Ni, Co, Cd, and Pb) remained below the threshold value in all sites (Table 3); nonetheless, the soil samples from NIC exhibited a higher average concentration of Cd, Co, Pb, and Zn than other sites.

Table 3.
Comparison between leaf heavy metal concentrations detected in the three study sites and the ‘Reference Plant’ values (RP) as set by Markert Bernd [30]. Values exceeding the RP threshold are in bold.
The 1-month records of air quality obtained by the ARPACAL sampling stations agreed with the above-described patterns of soil trace elements, by indicating a worse air quality at NIC (sampling station Nicastro1: average AQI = 0.817—Acceptable) than at REN (sampling station Rende 2: average AQI = 0.380—Good).
3.2. Patterns of Foliar Trace Elements among Study Sites and Relationships with Soil Contamination
The complete list and concentration of trace elements identified in all leaf samples are reported in Table S2. The between-site patterns of average concentrations (Table 2) were similar to those observed for soil trace elements. Specifically, 21 out of 35 elements showed the highest concentration in NIC (Table 2), while LAM and REN revealed the highest concentration of 11 and 2 elements, respectively (Table 2).
The between-site patterns of leaf trace elements agreed with the soil ones for 20 elements (Table 2). Accordingly, the highest leaf concentration of many HMs (e.g., Mn, Ni, Zn, Rb, Mo, Sb, Pb) was found in sites where the soil samples indicated a more pronounced contamination by those elements (Table 2). With regard to the target HMs, the soil from LAM and NIC revealed a similar concentration of As that was quite higher than that found in REN; such a pattern was mirrored by the leaf values. The normality test on log-transformed data indicated that soil and leaf concentrations met normality assumption in all the study sites (NIC: p = 0.45; LAM = 0.25; REN = 0.22). The Mantel test confirmed a significant overall correlation between soil and leaf concentration patterns (r = 0.634; p < 0.001).
With reference to the selected HMs, the foliar concentration of As in samples from LAM and NIC exceeded the ‘Reference Plant’ values for unpolluted sites (Table 3). In contrast, the leaf concentrations of Zn, Cd, and Co exceeded such a reference value in all the study sites, although for the latter two elements the foliar concentration did not match the between-site soil patterns (Table 3). Finally, for the remnant heavy metals (i.e., Pb, and Ni) the detected foliar concentration remained below the Reference Plant threshold (Table 3).
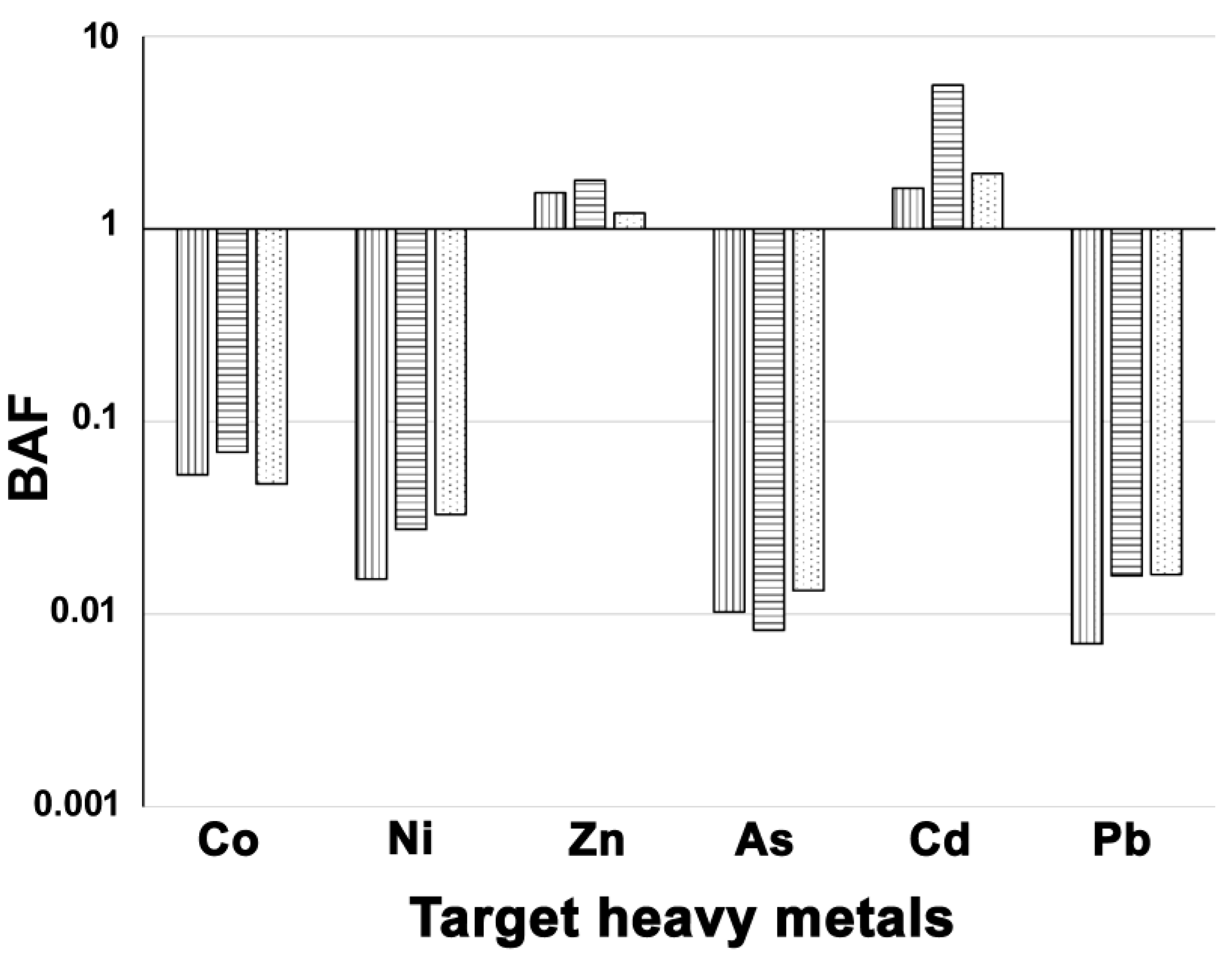
The calculation of the bioaccumulation factor for the selected HMs indicated a BAF > 1 for Zn and Cd in all study sites, while for the other metals BAF < 1 (Figure 3).
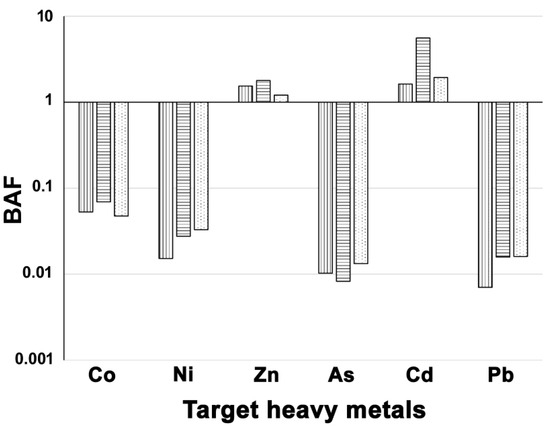
Figure 3.
Scores of bioaccumulation factor (BAF) for the PM10 components recorded in the three study sites. Legend for sites: NIC, vertically dashed bars; LAM, horizontally dashed bars; REN, dotted bars.
3.3. Between-Site Patterns of Fluctuating Asymmetry and Relationships with HMs in Soil and Leaves
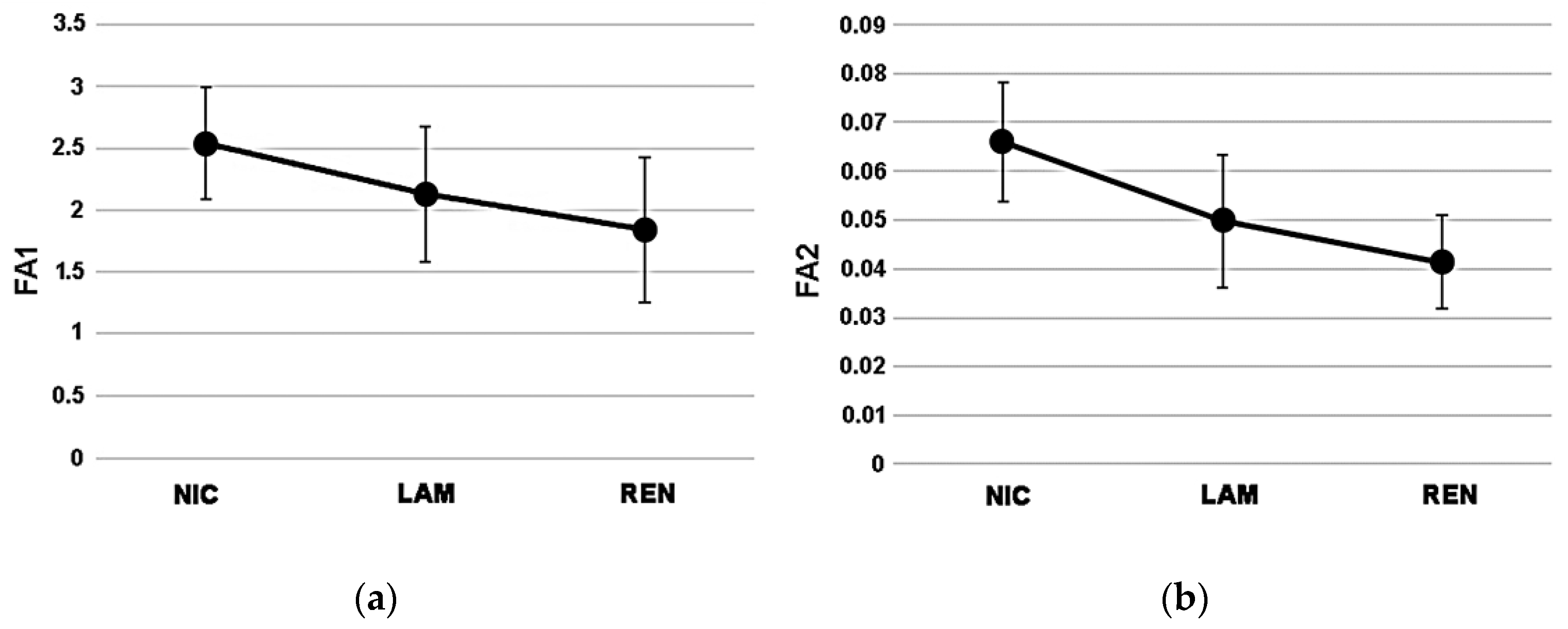
The individual values for the fluctuating asymmetry values of each site are provided in Table S3. According to the average values of FA1 (Figure 4a) and FA2 (Figure 4b), NIC exhibited the highest extent of fluctuating asymmetry (FA1 = 2.54 ± 0.550; FA2 = 0.07 ± 0.012), followed by LAM with intermediate values (FA1 = 2.13 ± 0.454; FA2 = 0.05 ± 0.014), and REN with the lowest scores (FA1 = 1.84 ± 0.584; FA2 = 0.04 ± 0.010).
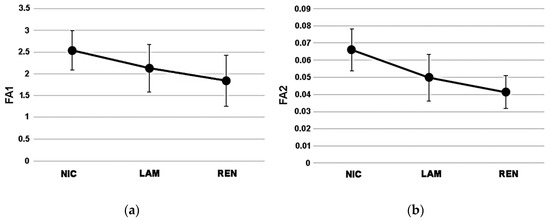
Figure 4.
Variation patterns (average ± sd) of FA1 (a) and FA2 (b) index among study sites.
ANOVA did not show significant differences of FA1 among study sites (F = 2.191; p = 0.15); in contrast, the FA2 index marked a significant variation among the three sites (F = 5.506; p = 0.02). In more detail, the Bonferroni post hoc test indicated a significant difference between NIC and REN (p = 0.008), while no differences occurred between NIC and LAM (p = 0.08) and between LAM and REN (p = 0.29).
The outcomes of the correlation tests between the estimators of foliar fluctuating asymmetry and the soil and leaf concentrations of trace elements are shown in Table 4. Both FA1 and FA2 were positively correlated with the concentration of various soil trace elements, including many HMs like Mn, Rb, Te, Tl, and Bi; with regard to common PM10 components, the two FA estimators showed positive relationships with the soil concentrations of Pb, and Zn (Table 4). In contrast, the relationships between FA measures and leaf trace elements appeared to be weaker. In this case, the FA2 index seemed more sensitive, and this resulted in a higher amount of trace elements. Additionally, the foliar concentration of elements like Zn, Rb, Cu, Mn, Be were positively related to at least one FA estimator, by agreeing with the relationships observed between soil trace elements and foliar fluctuating asymmetry (Table 4).

Table 4.
Correlation between estimators of fluctuating asymmetry (FA1 and FA2) and concentration of soil (sx) and leaf (dx) trace elements. Significant correlations are in bold.
4. Discussion
Our data on the soil concentration of trace elements aligned substantially with the air quality data provided by the monitoring network of the Regional Environmental Agency, indicating a lower environmental quality in NIC compared to REN study site. In addition, the data reported in Table 2 fit the assumption of intermediate contamination levels in LAM. Especially, the soil samples from NIC and LAM indicated that such sites experience a higher contamination of heavy metals (e.g., As, Pb, Ni, Co, Zn, Cd). Such findings confirm the expectation of close relationships between patterns of air and soil quality, as widely testified in the literature [35,36]. In addition, in the absence of data inherent in the PM10 composition, the recorded relationship between HM soil contamination and a PM10—based air quality index can furnish an indirect clue about the role played by HMs in affecting the air quality of the study sites.
Noteworthy, the As soil concentration at NIC and LAM was much higher than REN. Identifying the causes for the presence of such a toxic element is a challenging task, as its presence in urban soils can stem from a large combination of anthropogenic (e.g., industrial settlements, agricultural practices, waste disposal) and natural factors [37]. Additionally, as already described for groundwater contamination [24], the closeness to a large industrial hub may have substantially contributed to the increase in soil contamination in NIC and LAM.
The uptake of soil elements by plants depends on a complex interplay of numerous factors (including species identity, kind of element, pH and other soil features), and it is difficult to discriminate whether the absorption comes from air, water, or soil [11]. Nonetheless, the leaf trace element patterns relative to the three study sites (Table 2), and the outcome of the multivariate correlation test evidence a great congruence between leaf and soil concentration of trace elements, including various HMs. Such a finding further emphasizes the hazardousness of these pollutants, due to their pronounced mobility across abiotic and biotic components of environmental systems [2,3,4], and evidence the role of plant leaves as an accumulation sink for accumulated metals and their utility in biomonitoring tasks [12]. Accordingly, compared to the thresholds proposed by Markert [30] Markert et al. [31] for discriminating polluted sites from unpolluted ones, the foliar accumulation of potential PM10–related HMs detected in the leaves of black poplar suggested the occurrence of a pronounced contamination by Zn, Co, Cd in all the study sites, and by As at NIC and LAM (Table 3). It is worth noting that, based on the permitted concentrations set by current national legislation (i.e., Legislative Decree 156/2006), the As soil concentrations found at NIC and LAM are the unique values exceeding the threshold proposed for areas suitable to public greens, private and residential use (Table S4). Establishing more rigorous policies would probably allow us to better incorporate the actual biological hazard connected to the presence of HMs in urban contexts.
Accordingly, in spite of larger samples than those collected in this work generally being required to detect FA signals [26,27], the patterns of foliar fluctuating asymmetry found in our study could be seen as an expression of the contamination level of the sampled sites. Indeed, the between-site patterns of FA estimators and their relationships with soil and leaf trace elements indicated an overall agreement with the contamination profile of the study sites that emerged from ICP-MS analyses on soil and leaf samples, and with the available air quality data. Such a finding suggests that the presence of pollutants in the environment, and the relative bioaccumulated fraction, may have interfered with the normal developmental processes of the study species. Indeed, along with a high foliar concentration of elements, that many authors [38,39] recognized to be essential for plant metabolism (e.g., Mg, Ca, K, Si, and S), our analyses also revealed the foliar accumulation of elements with high potential toxicity. (e.g., Zn, Cd, Ni, As, Pb). For instance, the leaf concentration of Zn ranged between 101.5 ppm (REN) and 173.3 ppm (NIC); according to Lidon [40], at low concentrations (10~160 ppm), Zn is an important constituent of proteins and enzymes involved in plant growth [37], while it becomes toxic at concentrations ranging between 70 and 400 ppm [41,42]. Moreover, the ability of black poplar to accumulate HMs [12] was further evidenced by the high bioaccumulation factor we detected for Cd (at all sites) and Zn (at NIC and LAM), which emphasizes the usefulness of such a tree species for biomonitoring and, possibly, reducing the contamination of common pollutants in urbanized areas. In contrast, despite the positive correlation between FA2 and soil Pb, the foliar concentration of this relevant PM10 component remained quite low and similar across study sites. Such a low foliar content of Pb could be due to the fact that its absorption occurs primarily through the root system rather than through particle deposition. The presence of Pb in the soil is commonly associated with abnormal morphologies, interference with normal enzymatic activities, and reduced chlorophyll production [43,44]. Therefore, plants tend to accumulate Pb within their root system, by limiting translocation to leaves [38]. Hence, determining Pb concentration in roots would facilitate further assessment of the actual capacity of black poplar for accumulating such a pollutant.
5. Conclusions
Our work evidenced close interrelationships among patterns of foliar accumulation and soil contamination by HMs, which in turn agreed with the levels of atmospheric quality possessed by the investigated sites. According to the available atmospheric data, the sites with lower air quality (i.e., NIC and LAM), had a higher amount of contaminants in soils and plants. As expected, the study sites located near relevant transport and industrial hubs underwent a higher degree of contamination. Comparing the levels of HM accumulation within our biological samples with those expected in pristine environments evidenced a relevant contamination by Zn (173.3 ppm), Cd (0.70 ppm), and Co (1.10 ppm), in all sites, and by As (0.20 ppm) at the NIC site. Such a finding suggested the contamination of heavy metals might be more relevant than that derived from current environmental policies in all the study sites. Future work on the analysis of PM10 composition in the considered sites could provide further relevant information on the sources of heavy metals found in the leaves. Additionally, the poplar’s leaves were an effective mean for indicating the extent of environmental pollution and its possible biological effect. Moreover, the high bioaccumulation factor (BF > 1) detected for some elements (i.e., Zn and Cd) in all study sites indicated the studied plant could contribute to reducing the extent of the contamination by highly toxic pollutants that are very frequent in urban areas. In addition, the patterns of foliar FA in this species appeared to be related to the contamination levels, although additional data from more intensive sampling tasks would be useful to gain a better understanding of the bioindication potential of FA measures taken on black poplar’s leaves. In the future, the study could continue by determining the metals that can certainly be produced by motor vehicle traffic, such as platinum, palladium and rhodium, which are present in catalytic converters [44] Overall, the black poplar was confirmed to be an effective candidate for improving the healthiness of urban areas, where it can provide valuable nature-based options in environmental monitoring and restoration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/resources13060085/s1, Table S1: Trace element concentrations (ppm) in soil samples from each study site; Table S2: Total concentration of elements in LAM, NIC and REN leaves (ppm); Table S3: Individual FA1 and FA2 values in LAM, NIC and REN; Table S4: Comparison between soil heavy metal concentrations detected in the three study sites and permitted threshold concentration (PTC) set by L.D. 156/2006 for soils used as public green, private and residential sites. Values exceeding thresholds are in bold.
Author Contributions
Conceptualization, G.P., D.G. and M.F.L.R.; methodology, L.R., S.C., L.B. and I.F.; oversight of laboratory procedures and formal analysis, G.P., D.G. and C.A.; writing—original draft preparation, G.P., D.G. and M.F.L.R.; writing—review and editing, L.R., S.C., L.B., I.F. and C.A.; overall supervision, D.G., C.A. and M.F.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data the present manuscript refers to are freely available as Supplementary Materials.
Acknowledgments
The authors are grateful to Luigi Cipresso for practical support in field and laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sorrentino, M.C.; Capozzi, F.; Wuyts, K.; Joosen, S.; Mubiana, V.K.; Giordano, S.; Samson, R.; Spagnuolo, V. Mobile biomonitoring of atmospheric pollution: A new perspective for the moss-bag approach. Plants 2021, 10, 2384. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J. Declining urban and community tree cover in the United States. Urban For. Urban Green. 2018, 32, 32–55. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.; Pigna, M. Mobility and biovailability of Heavy Metals and metalloids in soil environments. J. Soil. Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Luo, X.; Yu, S.; Li, X. The mobility, bioavailability, and human bioaccessibility of trace metals in urban soils of Hong Kong. Appl. Geochem. 2012, 27, 995–1004. [Google Scholar] [CrossRef]
- Barreca, S.; Orecchio, S.; Orecchio, S.; Abbate, I.; Pellerito, C. Macro and micro elements in traditional meals of Mediterranean diet: Determination, estimated intake by population, risk assessment and chemometric analysis. J. Food Comp. Anal. 2023, 123, 1–16. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Remon, E.; Bouchardon, J.L.; Le Guédard, M.; Bessoule, J.J.; Conord, C.; Faure, O. Are plants useful as accumulation indicators of metal bioavailability? Environ. Pollut. 2013, 175, 1–7. [Google Scholar] [CrossRef]
- Harmsen, J. Measuring Bioavailability: From a Scientific Approach to Standard Methods. J. Environ. Qual. 2007, 36, 1420–1428. [Google Scholar] [CrossRef]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Ugolini, F.; Tognetti, R.; Raschi, A.; Bacci, L. Quercus ilex L. as bioaccumulator for heavy metals in urban areas: Effectiveness of leaf washing with distilled water and considerations on the trees distance from traffic. Urban For. Urban Green. 2013, 12, 576–584. [Google Scholar] [CrossRef]
- Isinkaralar, K. The large-scale period of atmospheric trace metal deposition to urban landscape trees as a biomonitor. Biomass Convers. Biorefinery 2022, 5, 6455–6464. [Google Scholar] [CrossRef]
- Levei, L.; Cadar, O.; Babalau-Fuss, V.; Kovacs, E.; Torok, A.I.; Levei, E.A.; Ozunu, A. Use of black poplar leaves for the biomonitoring of air pollution in an urban agglomeration. Plants 2021, 10, 548. [Google Scholar] [CrossRef]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef]
- He, J.; Ma, C.; Ma, Y.; Li, H.; Kang, J.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Cadmium tolerance in six poplar species. Environ. Sci. Pollut. Res. 2013, 20, 163–174. [Google Scholar] [CrossRef]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustoš, P.; Zupan, M.; Wenzel, W.W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef]
- Masson, P.; Dalix, T.; Bussière, S. Determination of major and trace elements in plant samples by inductively coupled plasma-mass spectrometry. Commun. Soil Sci. Plant Anal. 2010, 41, 231–243. [Google Scholar] [CrossRef]
- Yener, I.; Temel, H.; Tokul-Olmez, O.; Firat, M.; Oral, E.V.; Akdeniz, M.; Senturk, K.; Kaplaner, E.; Ozturk, M.; Ertaş, A. Trace element analysis by icp-ms and chemometric approach in some euphorbia species: Potential to become a biomonitor. Iran. J. Pharm. Res. 2019, 18, 1704–1724. [Google Scholar]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating Asymmetry: Methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Abeli, T.; Zubani, L.; Bonomi, C.; Parolo, G.; Gargano, D. Is phenotypic canalization involved in the decline of the endemic Aquilegia thalictrifolia? Rethinking relationships between fluctuating asymmetry and species conservation status. Plant Species Biol. 2016, 31, 247–255. [Google Scholar] [CrossRef]
- Mendes, G.; Boaventura, M.G.; Cornelissen, T. Fluctuating Asymmetry as a Bioindicator of Environmental Stress Caused by Pollution in a Pioneer Plant Species. Environ. Entomol. 2018, 47, 1479–1484. [Google Scholar] [CrossRef]
- Mabrouk, L.; Mabrouk, W.; Mansour, H.B. High leaf fluctuating asymmetry in two native plants growing in heavy metal-contaminated soil: The case of Metlaoui phosphate mining basin (Gafsa, Tunisia). Environ. Monit. Assess. 2020, 192, 406. [Google Scholar] [CrossRef]
- Vespasiano, G.; Cianflone, G.; Cannata, C.B.; Apollaro, C.; Dominici, R.; De Rosa, R. Analysis of groundwater pollution in the Sant’Eufemia plain (Calabria-South Italy). Ital. J. Eng. Geol. Environ. 2016, 16, 5–15. [Google Scholar]
- Calabrese, S.; D’alessandro, W.; Bellomo, S.; Brusca, L.; Martin, R.S.; Saiano, F.; Parello, F. Characterization of the Etna volcanic emissions through an active biomonitoring technique (moss-bags): Part 1–Major and trace element composition. Chemosphere 2015, 119, 1447–1455. [Google Scholar] [CrossRef]
- Palmer, A.R. Fluctuating asymmetry analyses: A primer. In Developmental Instability: Its Origins and Evolutionary Implications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 335–364. [Google Scholar]
- Palmer, A.R.; Strobeck, C. Fluctuating Asymmetry: Measurement, Analysis, Patterns. Ann. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Graham, J.H. Fluctuating asymmetry and developmental instability, a guide to best practice. Symmetry 2021, 13, 9. [Google Scholar] [CrossRef]
- Di Vaio, P.; Magli, E.; Caliendo, G.; Corvino, A.; Fiorino, F.; Frencetese, F.; Saccone, I.; Santagada, V.; Severino, B.; Onorati, G.; et al. Heavy Metals Size Distribution in PM10 and Environmental-Sanitary Risk Analysis in Acerra (Italy). Atmosphere 2018, 9, 58. [Google Scholar] [CrossRef]
- Markert Bernd, A. Establishing of “reference plant” for inorganic characterization of different plant species by chemical fingerprinting. Water Air Soil Pollut. 1992, 64, 533–538. [Google Scholar] [CrossRef]
- Markert Bernd, A.; Breure, A.M.; Zechmeister, H. Bioindicators and biomonitors: Chapter 1 Definitions, strategies and principles for bioindication/biomonitoring of the environment. Trace Met. Other Contam. Environ. 2003, 6, 3–39. [Google Scholar]
- Luoma, S.N.; Bryan, G.W. Trace metal bioavailability: Modeling chemical and biological interactions of sediment bound zinc. In Chemical Modeling—Speciation, Sorption, Solubility, and Kinetics in Aqueous Systems; Jenne, E.A., Ed.; American Chemical Society: Washington, DC, USA, 1979; pp. 577–611. [Google Scholar]
- Rong, G.; Chu, Y.; Liu, S.; Kataweteetham, L.; Zhu, J. Cd uptake in upright/leaning trees. BioResources 2021, 16, 3422–3436. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Mantese, S.; Yayintas, Ö.T.; Bas, B.; Irkin, L.C.; Yilmaz, S. Heavy Metal and Composition of Soil, Atmospheric Deposition, and Moss with Regard to Integrated Pollution Assessment approach. Environ. Manag. 2021, 67, 833–851. [Google Scholar] [CrossRef]
- Alahabadi, A.; Ehrampoush, M.H.; Miri, M.; Aval, H.E.; Yousefzadeh, S.; Ghaffari, H.R.; Ahmadi, E.; Talebi, P.; Fathabadi, Z.A.; Babai, F.; et al. A comparative study on capability of different tree species iin accumulating heavy metals from soil and ambient air. Chemosphere 2017, 172, 459–467. [Google Scholar] [CrossRef]
- Shrivastava, A.; Ghosh, D.; Dash, A.; Bose, S. Arsenic Contamination in Soil and Sediment in India: Sources, Effects, and Remediation. Curr. Pollut. Rep. 2015, 1, 35–46. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC: Boca Raton, FL, USA, 2010; pp. 143–149. [Google Scholar]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Lidon, F.C. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Hajar, E.W.I.; Bin Sulaiman, A.Z.; Sakinah, A.M.M. Assessment of Heavy Metals Tolerance in Leaves, Stems and Flowers of Stevia Rebaudiana Plant. Procedia Environ. Sci. 2014, 20, 386–393. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanov, V.B. Physiological aspects of cadmium and lead toxic effects on higher plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Amorello, D.; Barreca, S.; Orecchio, S. Voltammetry for Monitoring Platinum, Palladium and Rhodium in Environmental and Food Matrices. Chem. Sel. 2023, 8, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).