Abstract
The genus Pyrus has a long history in Sardinia (Italy), where two wild pear species (P. spinosa Forssk. and P. pyraster (L.) Burgsd.) and Pyrus communis L. cultivars are extensively distributed. Even if neglected, these taxa represent well-adapted key resources for redesigning sustainable farming systems. This report aims at shedding light on the phenolic fingerprint and antioxidant properties of wild pear fruits and comparing their traits with those of the studied pear cultivar germplasm (PCG). Fruits of wild pear species were collected, and flesh, peel, and core subsamples were analyzed. Moreover, available data from previous research on PCG were analyzed. The contents of total phenolics (TotP), total flavonoids (TotF), and condensed tannins (CT), as well as the antioxidant capacity, were similar in the flesh of the two wild species. However, P. spinosa had significantly higher values of TotP (89 g GAE kg−1 DM) and CT (33 g DE kg−1 DM) in the peel. Eleven individual phenolic compounds were identified and quantified in the fruit flesh, 14 in both peel and core. For both wild species, arbutin and chlorogenic acid were the main phenolic compounds, followed by the quercetin glycosides. Comparing the antioxidant capacity and TotF fruit flesh values of wild pears with those of PCG, the latter resulted up to 15-fold lower. The wild types showed unique metabolite profiles. Results support novel insights on the phytochemicals of wild pear fruits.
Keywords:
Pyrus spinosa; P. pyraster; peel; core; flesh; bioactive compounds; arbutin; chlorogenic acid; isorhamnetin derivative; PCA 1. Introduction
Within the Rosaceae family, the genus Pyrus represents one of the oldest and economically most important fruit crops in the temperate zones after apples (Malus domestica L.) and before peaches (Prunus persica L.) [1]. There are at least 26 primary Pyrus species, widely distributed in Europe, Asia, and North Africa [2]. Major centers of diversity include the Mediterranean basin, Georgia, and Central Asia. Pear crops are thought to have been cultivated in Europe as early as 1000 BC. More than 5000 cultivars have been identified around the world. Among wild pear species, Pyrus pyraster (L.) Burgsd. (=Pyrus communis ssp. pyraster L.) is distributed in southern, central, and western Europe. In southern Europe, this species is sporadically reported from northern Spain, Sicily, Sardinia, and Corsica [3]. In contrast, P. spinosa Forssk. is mainly distributed in the Mediterranean region and Iran [4]. P. spinosa was known as P. amygdaliformis Vill. for the characteristic almond-shaped leaves [5], which are now referred to as synonyms [6]. Phenotypic and morphologic variability, as well as the genetic diversity of wild pears in Europe, is not well studied, as only a few papers cover the genetic diversity of P. pyraster and are usually very localized and conducted on a small number of samples [4,7,8,9]. A recent paper deals with the fruit morphological and chemical characteristics of nine natural populations of P. pyraster; moreover, the population variability and the relationship between fruit characteristics and geographical and environmental conditions were also investigated [10].
Within the Mediterranean basin, the island of Sardinia (Italy) is one of the most important areas for the extension and richness of endemic species and sub-species [11]. In Sardinia, the genus Pyrus has a long history [8], which involves domestication processes of three autochthonous species: Pyrus communis L. with many ancient cultivars and two wild species, P. spinosa and P. pyraster, whose morphological differences are described [6,12]. Wild pears are widespread throughout Sardinia, from coastal to higher elevation (till 1000–1500 m) areas, regardless of soil type [13]; however, P. pyraster is confined to internal areas [14]. In Sardinia, these two wild species are popularly called “Pirastru” [15], and, curiously, Pirastru is also a family surname. Thereafter, in this study, we refer to P. spinosa and P. pyraster as wild pears.
Wild pears have been traditionally used as rootstock to spread, by grafting, germplasm pear cultivars [13]. Additionally, the small fruits of wild pears are an excellent feed resource for farm animals, deer, and wild boars. Its wood is very hard, compact, and resistant, and it is traditionally used for inlay work [14] (Figure 1).

Figure 1.
(A): traditional mask from Central Sardinia made of Pyrus spinosa wood, which is wore at Carnival for evoking ancient farming rites (Courtesy of Leonardo Murgia); (B): scattered plant of wild pear at flowering in a natural pasture context.
The Sardinian pear germplasm represents a very important source of genetic diversity, which has been investigated at the molecular level for the first time by Sau et al. [12]. Up to date, only one paper has reported on the fingerprinting of phenolic compounds and antioxidant capacity in the fruit fractions of representative ancient pear cultivars grown in Central Sardinia [16]. No details regarding the bioactive phenolic compounds contained in the fruit of Sardinian wild pears have been reported so far, to the best of our knowledge. Undoubtedly, wild pears represent underutilized and neglected fruit species and, at the same time, are a well-adapted native resource useful to redesign current farming systems into resilient groves supporting sustainable production and ecosystem services [17,18]. It has also been recognized that wild pears may represent an excellent tree species to improve carbon storage and increase biodiversity in pasturelands and agroforestry systems [19].
The amount of phenolic molecules might vary between pear species, cultivars, and plant organs [20]. Metabolite contents and sensory qualities of leaves and fruits of P. amygdaliformis grown in the Kurdistan region were studied by Saadatian et al. [20], who found gallic, caffeic, coumaric, chlorogenic, rosmaric acids, rutin, quercetin, and apigenin in its fruit. The presence of phenolic compounds in different plant parts of wild species, including P. pyraster, was highlighted by Stoenescu et al. [21].
Undoubtedly, the chemical diversity of wild pears remains unexplored in Sardinia and information on their fruit phenolic composition and antioxidant capacity will be useful to support the exploitation of nutritional, pharmaceutical, and nutraceutical uses.
With this in mind, this research concerns a specific area of central Sardinia, with the aim of: (i) revealing the phenolic composition and antioxidant capacity in three fruit parts (i.e., flesh, peel, and core) from P. pyraster and P. spinosa naturally growing in the selected area; and (ii) evaluating the relationship between the phenolic fingerprint of fruit from wild pears and germplasm cultivars already investigated in the same area. As the previously studied ancient pear cultivars and the wild pears under investigation shared the same environment, growth season, and analytical methods, some already published results [16] were used for comparison.
2. Materials and Methods
2.1. Reagents and Standards
Reagents were supplied as follows: sodium carbonate, HPLC-grade acetonitrile, and methanol by Carlo Erba (Milan, Italy); Trolox, ABTS ((2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt)); DPPH (1,1-diphenyl-2-picrylhydrazyl), Folin Ciocalteau by Merck Life Science S.R.L, (Milan, Italy). Ultrapure water was taken from a Milli-Q system supplied by Merck Life Science S.R.L (Milan, Italy).
Phenolic standard arbutin, gallic acid, chlorogenic acid, catechin, epicatechin, rutin, quercetin 3-galattoside, quercetin 3-glucoside, and isorhamnetin 3-rutinoside of analytical grade were supplied by Merck Life Science S.R.L (Milan, Italy).
2.2. Plant Material
Fruits from a spontaneous flora of P. spinosa and P. pyraster were harvested in Sardinia (Italy), 40° N, 8° E, 200 m a.s.l. The climate of the site is typically Mediterranean; the mean annual air temperature is 16.6 °C with a mild winter, and the long-term average annual precipitation is 580 mm. In the study area, the two abovementioned wild pear species are largely represented as isolated trees within Mediterranean agroforestry systems. Their fruits were harvested in November 2021 from 5 different trees for each species in the same field, under the same environmental, bioclimatic, and agronomic conditions.
Fruits free from defects and mechanical damage were carefully selected for this study. Each subsample consisted of 30 fruits similar in appearance and characteristics. This process was replicated three times to ensure the statistical strength of the results.
Sample preparation and extraction were carried out according to Piluzza et al. [16], and precisely the adoption of the same analytical procedures makes possible the correct comparison of wild pears with already investigated pear cultivar germplasm. Briefly, fruit were manually peeled, divided vertically into three components: flesh, peel, and core (without seeds), and separately frozen at −20 °C. Then, it was freeze-dried at −55 °C for 80 h using a Heto Lyolab 3000. Once lyophilized, the samples were ground into a fine powder using a mill. To maintain sample quality and integrity, the powdered samples were stored in total darkness at −20 °C until further analysis. Concerning sample extraction, 250 mg of the lyophilized powder was extracted with a 3 mL methanol/water (80:20 v/v) mixture and left for 24 h in the dark. After centrifugation (10 min at 3900 rpm) and filtration (0.20 μm polytetrafluoroethylene syringe), the methanolic extracts were stored at −20 °C until further analysis. Antioxidant capacity, total phenolic content (TotP), non-tannic phenolics (NTP), tannic phenolics (TP), condensed tannins (CT), and individual phenolic compounds were evaluated in the methanolic extract of samples.
2.3. Antioxidant Capacity and Total Phenolic Content
Antioxidant capacity was evaluated by means of the ABTS ((2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt)) and DPPH (1,1-diphenyl-2-picrylhydrazyl) assays [22]. The results were expressed in terms of Trolox equivalent antioxidant capacity (TEAC) as mmol Trolox equivalents·100 g−1 dry matter (mmol TEAC·100 g−1 DM). Spectrophotometric analysis with the Folin–Ciocalteau reagent was used to quantify TotP, NTP, and TP of extracts, and the results were expressed as g of gallic acid equivalent (GAE)·kg−1 dry matter (DM) [23]. Total flavonoids (TotF) were quantified by colorimetric assay using the AlCl3 method, and the results were expressed as g of catechin equivalent (CE)·kg−1 DM [24]. The butanol assay was used for quantification of the extractable CT from the sample, expressed as g delphinidin equivalent (DE)·kg−1 DM [25].
2.4. Analysis of Individual Phenolic Compounds by Reverse Phase-High-Performance Liquid Chromatography (RP-HPLC)
The individual phenolic compounds were analyzed using an Agilent 1260 series HPLC instrument (Agilent Technologies, Palo Alto, CA, USA) equipped with a quaternary pump, degasser, column thermostat, autosampler, and diode array detector. Chromatographic separation was performed according to Re et al. [25]. The column was a Zorbax Eclipse plus C18 (250 mm × 4.6 mm, 5 µm; Agilent), and the flow rate was 0.8 mL·min−1. The injection volume was 10 μL, and the detection wavelengths were set to 280, 330, and 350 nm. Data were processed using the Agilent OpenLAB CDS Chem-Station edition 2012. Molecule identifications were achieved as a function of the retention time and spectra of available standards, which were selected from the literature concerning their phenolic composition as well as by adding standard solutions to the sample. Quantification of individual phenolic compounds was performed using the external standard curve method, obtained with five concentration increments for each standard in duplicate and expressed in mg g−1 DM. The calibration curves for each standard solution, the limit of detection (LOD), and the limit of quantification (LOQ) are reported in Table S4.
2.5. Data Analyses
Data analyses were subjected to a one-way analysis of variance to test the effect of different wild pear species on the following variables of fruit components: concentrations of total phenolics, total flavonoids, antioxidant capacity, and individual phenolic compounds. Differences between means were assessed using the Fisher’s least significant difference (LSD) procedure for mean separation. The significance level was fixed at p ≤ 0.05 for all the statistical analyses. On values of flesh, peel, and core from wild pear species, a linear association between the variables was assessed using the Pearson correlation coefficient. The analysis was conducted at significance levels of p ≤ 0.05, 0.01, and 0.001. To summarize the information obtained from analyzing phenolic compounds, the original data set, which also included PCG plus Coscia cultivar values [16], was analyzed using Principal Component Analysis (PCA). Univariate analysis and correlation analysis were performed with Statgraphics Centurion XVI [26]. Pearson linear correlation analysis was performed with Jamovi software. Additionally, PAST 4.16c software [27] and Jamovi software [28] were used for principal component analysis (PCA).
3. Results
3.1. Antioxidant Capacity and Total Phenolic Content
The average values and standard deviations of total antioxidant capacity (TEAC), TotP, NTP, TP, TotF, and CT contents of flesh, peel, and core extracts of wild pears are reported in Table 1. Overall, the phytochemical contents varied between wild pears. The composition of the wild pear fruit flesh showed quite similar values, except for the TotF and CT contents, which exhibited statistically significant variations. P. spinosa had the highest CT content, whereas P. pyraster was characterized by the highest level of TotF content.

Table 1.
Total antioxidant capacity (TEAC) by ABTS and DPPH methods, total phenolics (TotP), non-tannic phenolics (NTP), tannic phenolics (TP), total flavonoids (TotF), and condensed tannins (CT) in the fruit flesh, peel, and core of the investigated wild pear species.
Instead, peel composition showed more statistically significant differences within species (Table 1). P. spinosa was characterized by higher values of TEAC (DPPH), TotP, TP, and CT than P. pyraster. The largest differences between species occurred for the CT content, with P. spinosa having a 2-fold higher content than P. pyraster.
Statistically significant differences were also detected in the composition of the fruit core (Table 1). The core of P. spinosa showed the highest antioxidant capacity and TotP and TotF contents. As already found in the flesh and peel, the core CT content of P. spinosa was again higher than that of P. pyraster. The differences in phytochemical contents between species were more evident in the core than in other fruit parts: in P. spinosa, the contents of TotP, NTP, and TotF were 1.7, 4.6, and 1.9-fold higher than in P. pyraster, respectively.
In P. spinosa, the TotP content in the core reached 107.2 g GAE kg−1 DM, followed by the peel and flesh with values of 88.7 and 88.2 g GAE kg−1 DM, respectively (Table 1), and TotF showed the same trend. Conversely, the TotP content of P. pyraster was high in the flesh, 88.7 g GAE kg−1 DM, followed by the peel and core, 81.0 and 63.7 g GAE kg−1 DM, respectively. The antioxidant capacity followed the same trend.
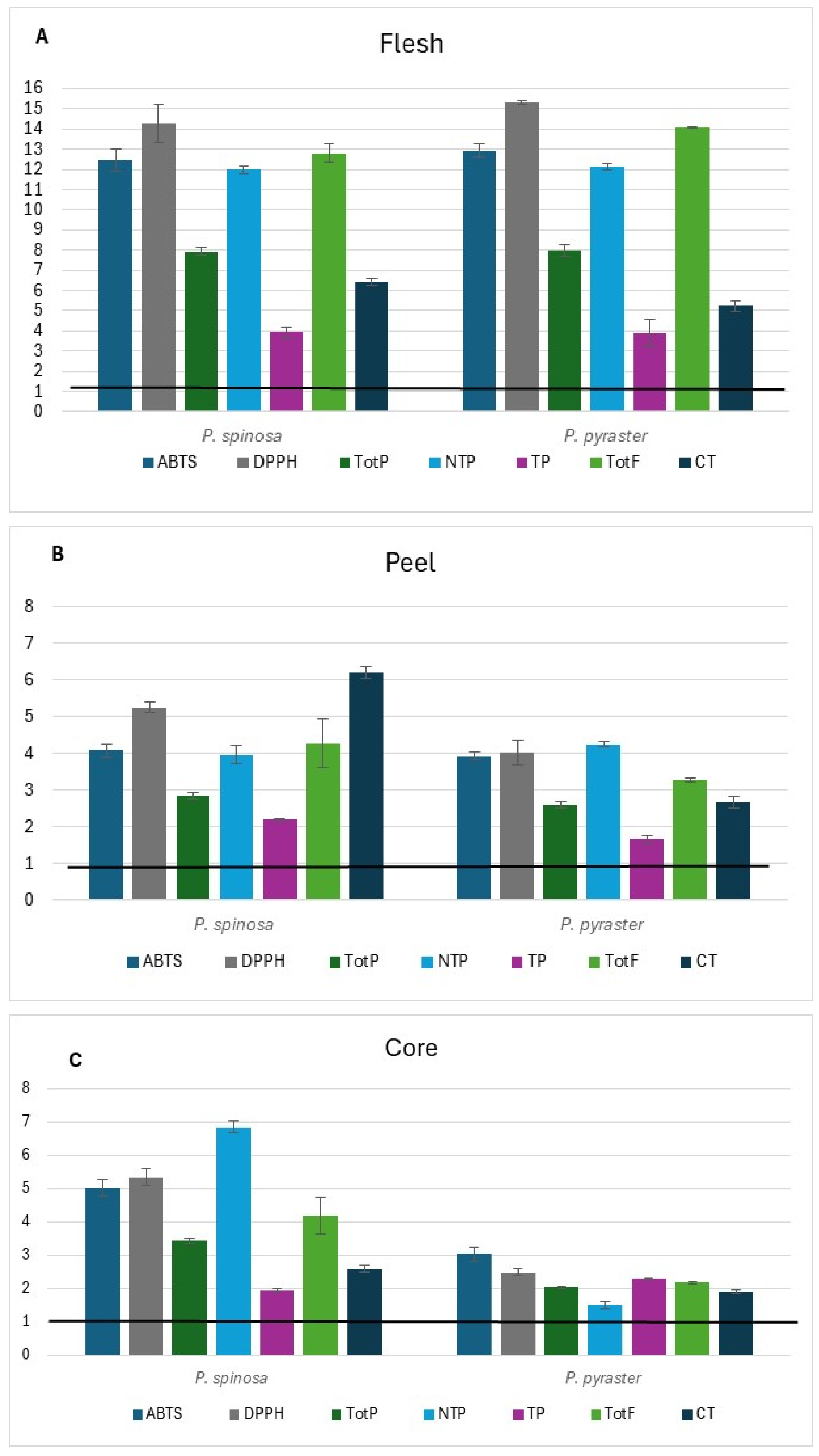
Comparing the antioxidant capacity and the phenolic content of the two wild Pyrus spp. with the corresponding traits of P. communis cultivars of germplasm (PCG) grown in the same study area and previously published [16] (see Tables S1–S3 at Supplementary Materials), it is worth noting that in wild pears the antioxidant capacity (DPPH) and TotF in flesh (Figure 2A) resulted up to 15-fold greater than in PCG. Unlike flesh, the relative variation of peel and core (Figure 2B,C) was 2 to 7-fold greater.
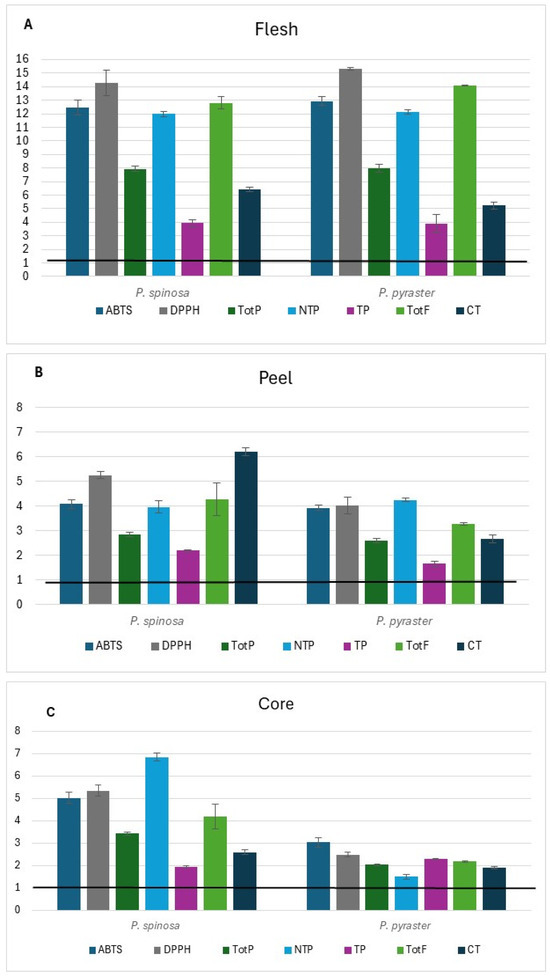
Figure 2.
Relative variation in antioxidant capacity and phenolic class contents of wild pear fruit for flesh (A), peel (B), and core (C). Value 1 is the baseline for the same traits of germplasm pear cultivars* grown in the same study area. * (Mean values from Buttiru, Camusina, and Spadona according to Piluzza et al. [16]; data are available in Supplemental Materials).
3.2. Analysis of Individual Phenolic Compounds by Reverse Phase-High-Performance Liquid Chromatography (RP-HPLC)
The concentration of the main individual phenolic compounds in the wild pear extracts was determined by RP-HPLC. Eleven individual phenolic compounds were identified and quantified in the fruit flesh, 14 in both peel and core. Arbutin, gallic and chlorogenic acids, catechin and epicatechin, quercetin 3-galattoside, and quercetin 3-glucoside were identified with authentic standards and quantified using the external method curve for each standard. Based on the UV spectra, two molecules were identified as isorhamnetin derivatives (tR 23.97 and 24.40), quantified as isorhamnetin 3-rutinoside, and one as a di-O-caffeolylquinic acid derivative (tR 23.50), quantified as 3-5-di-O-caffeolylquinic acid. Two peaks were identified by the UV spectra as flavanols (tR 31.9 and 39.0) and quantified as epicatechin equivalents. For both species, arbutin and chlorogenic acid were the main phenolic compounds quantified in the flesh (Table 2), followed by epicatechin and two quercetin glycosides. In addition, the molecule identified as a flavanol (tR = 31.9) was present in large quantities in the flesh of both species. The sum of the phenolic compound content showed that the flesh of P. spinosa had higher levels than P. pyraster, and the major differences found were in the content of arbutin (about 3-fold) and flavanol, tR = 31.9 (almost 2-fold). It is worth noting that the quercetin 3-galattoside and the flavanol tR = 39.0 were detected only in P. spinosa, whereas the isorhamnetin derivative only in P. pyraster.

Table 2.
HPLC analysis of individual phenolic compounds (mg g−1 DM) in the fruit flesh of the investigated wild pear species.
Even in the peel, arbutin and chlorogenic acid were the most representative phenolic compounds in the analyzed wild pear fruits, followed by the two quercetin glycosides (Table 3). P. spinosa showed higher contents of arbutin and chlorogenic acid, while the content of quercetin 3-glucoside was similar between species. As in the flesh and also in the peel, the flavanol tR = 31.9 was present in large quantity, with the highest content in P. spinosa. Other than the individual compounds found in the flesh, three additional compounds were detected in the peel, namely rutin, a flavonol (tR = 18.6) identified by the UV spectra and quantified as quercetin 3-glucoside, and isorhamnetin 3-rutinoside. On the contrary, the di-O-caffeolylquinic acid derivative was not detected in the peel. P. spinosa peel showed a high content of quercetin 3-galattoside, which was not detected in P. pyraster, as well as rutin and quercetin 3-glucoside. Regarding the sum of individual phenolics in the peel, again, the highest content was detected in P. spinosa, with the same ratio (1.8-fold higher) found in the pulp.

Table 3.
HPLC analysis of individual phenolic compounds (mg g−1 DM) in the fruit peel of the investigated wild pear species.
Compared to the flesh and peel, the core contained a di-O-caffeolylquinic acid (tR = 23.5) derivative, which was quantified as 3,5-di-O-caffeolylquinic acid equivalent, having the same UV spectrum (Table 4). As observed in the peel, arbutin, chlorogenic acid, and flavanol (tR = 31.9) were the most represented compounds in the core of both species. Regarding the core, P. spinosa showed a 2-fold higher sum of phenolic compounds than P. pyraster, which is attributable to the high content of arbutin and flavanol (tR = 31.9), 3.1 and 3.5-fold higher than P. pyraster, respectively.

Table 4.
HPLC analysis of individual phenolic compounds (mg g−1 DM) in the fruit core of the investigated wild pear species.
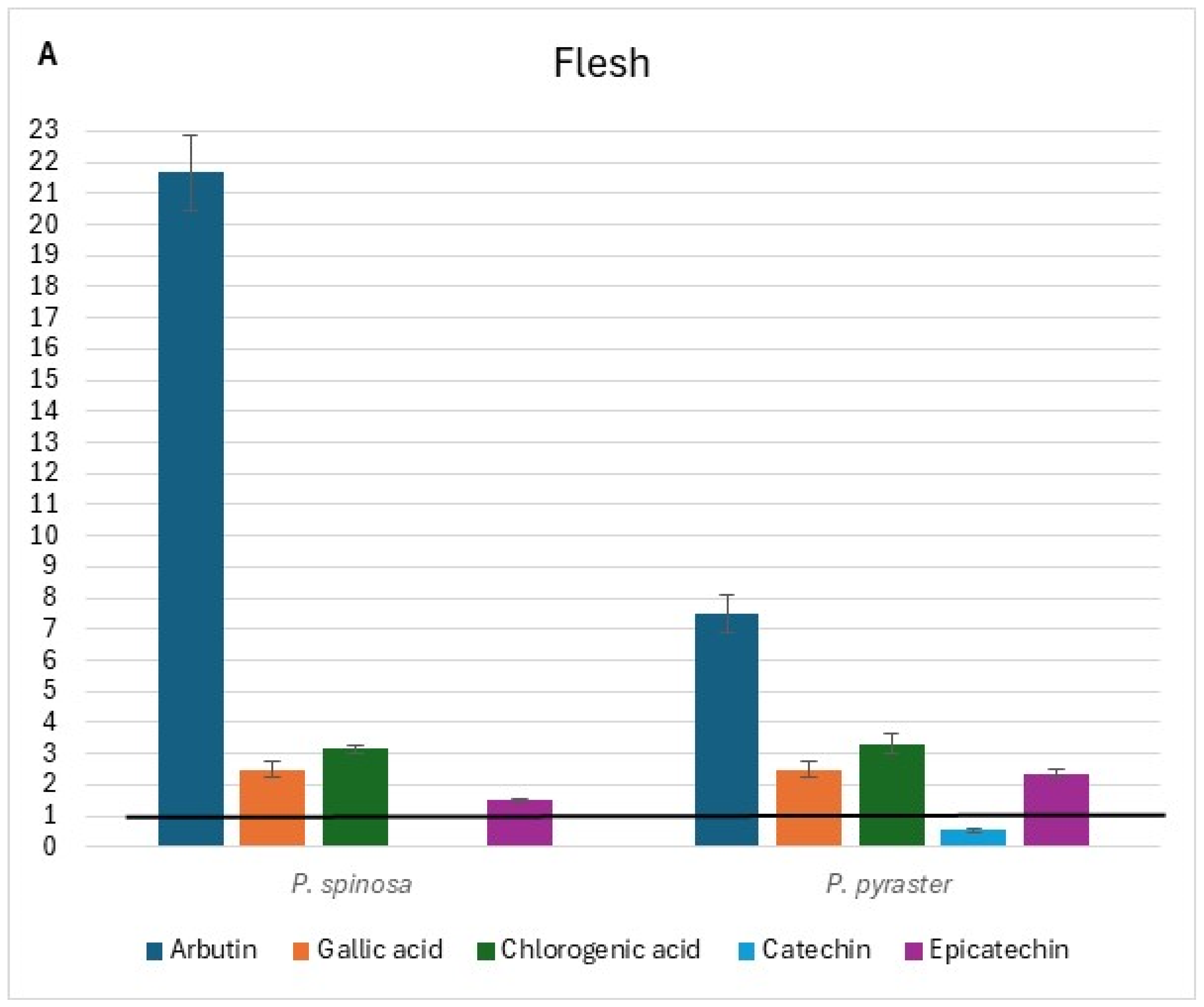
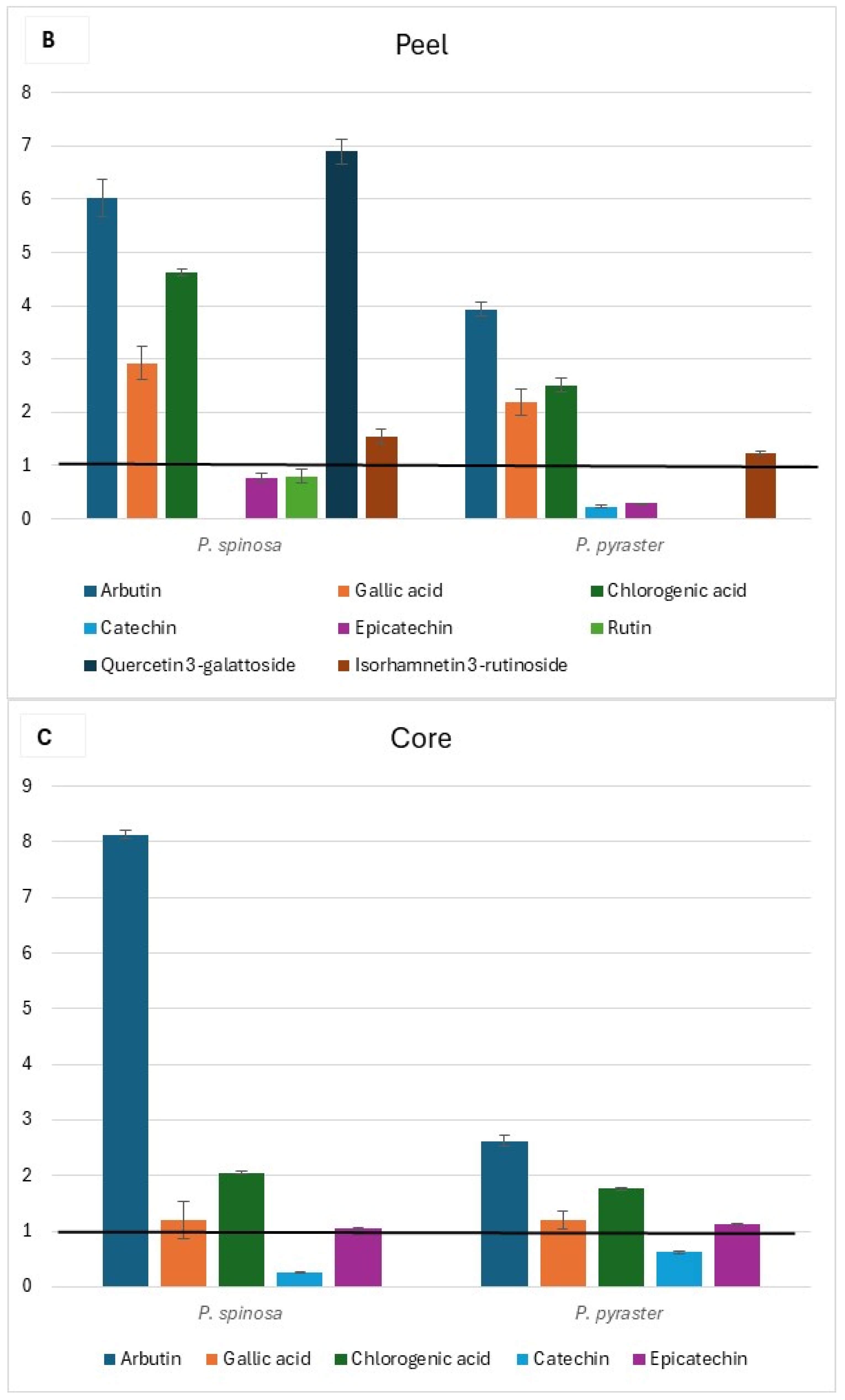
The flesh, peel, and core chromatograms of P. spinosa are reported in Figure S1. Comparing the phenolic profiles of the two wild Pyrus spp. with those of PCG [16] (see Tables S5–S7 at Supplementary Materials), it was found that flavonol glycosides (isorhamnetin derivative tR = 23.97), quercetin glycosides (tR = 20.90 and 21.4), and flavanols (tR = 31.9 and 39.0) were not detected in the flesh of fruit belonging to PCG cultivars, but only in wild pears. Alike, flavanols were undetected in the peel of the fruit of PCG. Conversely, rutin was present only in the fruit flesh of PCG and not in the fruit of wild pears; no flavonol glycosides or quercetin glycosides were detected in the fruit core of PCG. The di-O-caffeolylquinic acid derivative (tR = 23.5) was instead detected in the core of the fruit of both wild pears and PCG. When comparing the content of individual phenolic compounds detected in the fruit of wild pears with the corresponding values in the fruit of PCG, a wide range (from values close to the baseline up to 21-fold higher) of relative variation was observed (Figure 3). Additionally, relevant differences were found between the two wild pear species for the content of arbutin in each fruit component and quercetin 3-galattoside, the latter only present in the peel of P. spinosa (Figure 3B).
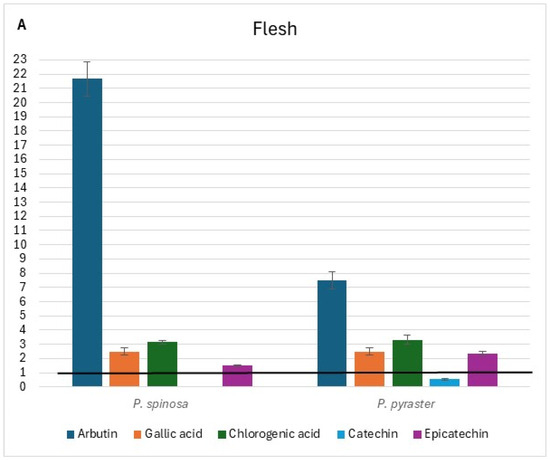
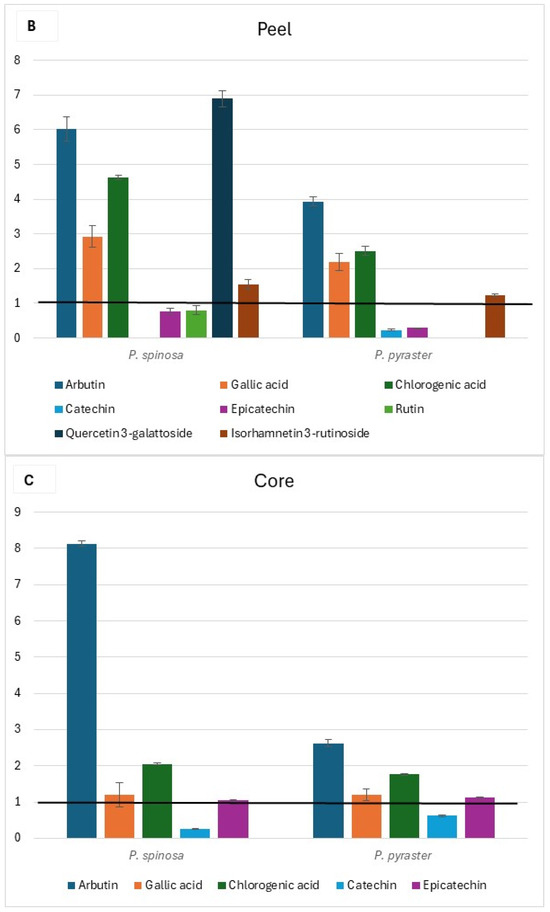
Figure 3.
Relative variation in the content of individual phenolic compounds of wild pear fruit for flesh (A), peel (B), and core (C). Value 1 is the baseline for the same traits of ancient pear cultivars* grown in the same study area. * (Mean values from Butirra, Camusina, and Spadona; source: Piluzza et al. [16].
In the same geographical territory, the comparison between the phenolic fingerprinting and antioxidant capacity results of wild pear fruits and those reported by Piluzza et al. [16] for P. communis cultivar germplasm evidenced a notably higher content of phenolic compounds in wild pears.
3.3. Correlations and Multivariate Analysis
A Pearson linear correlation analysis was performed to explore the potential relationship between the phenolic compounds and antioxidant capacity of wild pears. (Table 5 and Table 6). Interestingly, the correlations between the measured variables differed between species. In P. pyraster, the Pearson matrix highlighted the closest links between antioxidant capacity and phenolic classes (Table 5). Several individual phenolic compounds have also shown a high and significant correlation with antioxidant capacity and phenolic content, in particular catechin, epicatechin, and quercetin 3-galattoside. On the contrary, in P. spinosa, the antioxidant capacity (ABTS assay) was mainly linked to TotP and CT, regarding the phenolic compounds, and was linked to catechin and arbutin when considering individual compounds (Table 6). The antioxidant capacity (DPPH assay) was significantly linked to TP and CT, and epicatechin, arbutin, and chlorogenic acid, for phenolic classes and individual phenolic compounds, respectively.

Table 5.
Pearson correlation coefficients between antioxidant capacity, phenolic classes, and individual phenolic compounds of P. pyraster.

Table 6.
Pearson correlation coefficients between antioxidant capacity, phenolic classes, and individual phenolic compounds of P. spinosa.
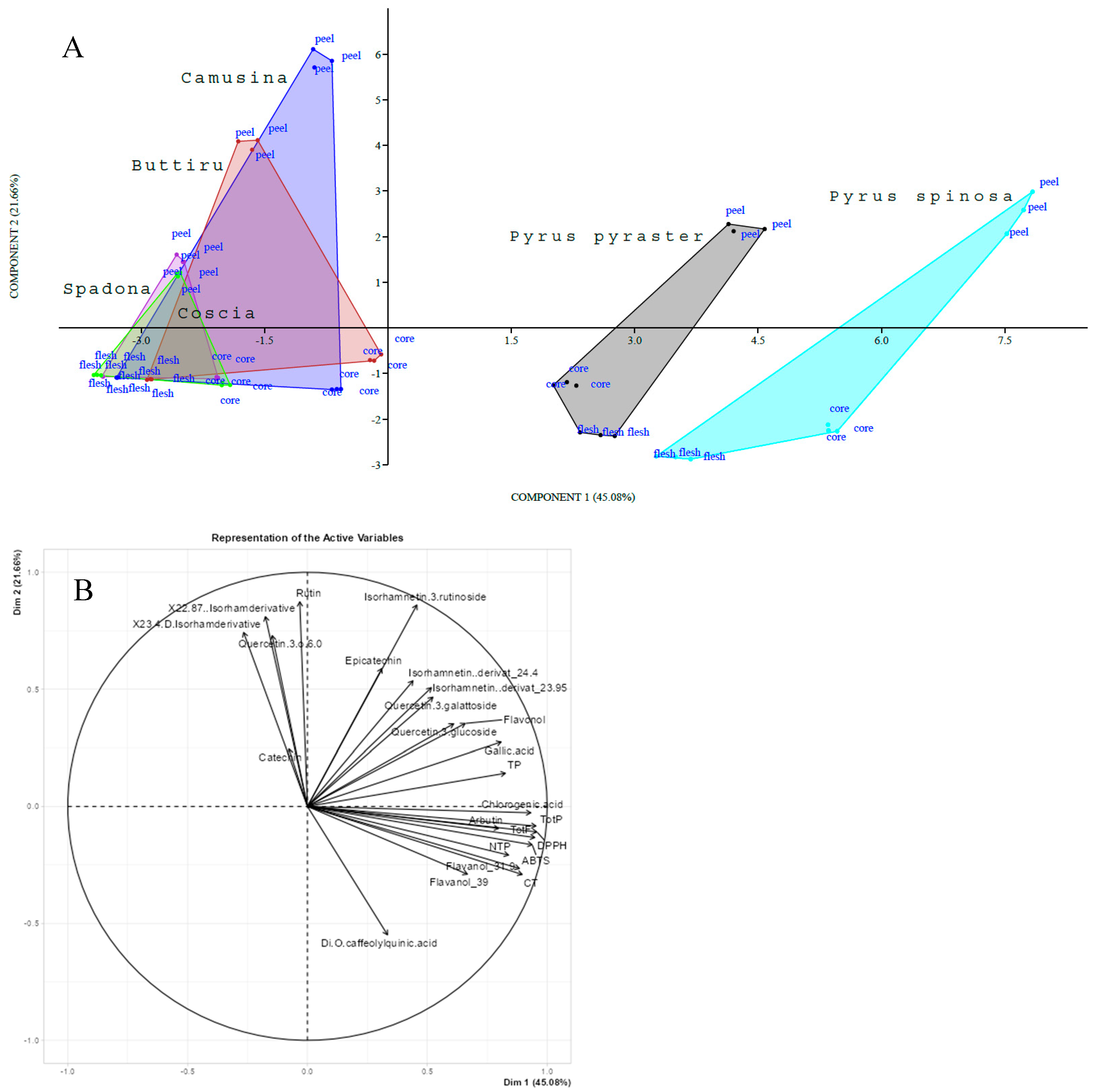
Principal Component Analysis (PCA) was then performed to reveal the interaction between phenolics and antioxidant capacity within the flesh, peel, and core of wild pears, and PCG investigated by Piluzza et al. [16]. From the monitored variables, the PCA provided a first evaluation of the selected traits, visualizing trends in the data and evidencing three distinctive groupings among the compared Pyrus species. Based on the PCA score plot shown in Figure 4A, two main groups can be clearly distinguished: the wild types (P. spinosa and P. pyraster) differed from each other, and both were different from PCG. Within the PCG, two different groupings were identified: Camusina with Buttiru and Spadona with Coscia (an Italian cultivar used as a control). Spadona was the closest to Coscia. Notably, the wild pear group is positioned on the positive side of PC1, while the cultivated pear group is on the negative side of PC1.
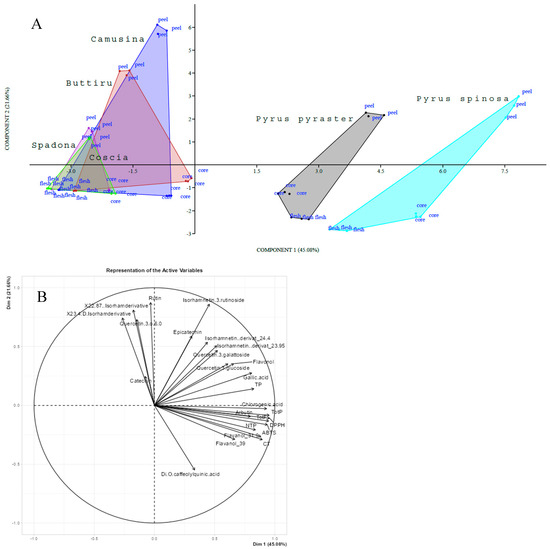
Figure 4.
Principal component analysis score of samples of Pyrus spp. collected in the same field (A) and factor loading plot of variables (B).
The results of PCA analysis (Table 7) indicated that a large portion of the variance (94.35%) could be explained by the first six principal components (PCs). The first PC, with an eigenvalue of 11.27, captured the most variability (45.08%). The second PC explained 21.66% of the variance, with an eigenvalue of 5.41. The first three PCs together explained 80.11% of the variance.

Table 7.
Eigenvalues and standardized coefficients of the correlation matrix from the principal component analysis.
Among all principal components (PCs), the first one (PC1) was the most important and closely related to the antioxidant capacity, TotP, TotF, and chlorogenic acid. The second component (PC2) was mainly influenced by two variables: rutin and isorhamnetin 3-rutinoside. Finally, the third component (PC3) was primarily defined by the levels of quercetin 3-glucoside and epicatechin. Concerning the fruit components, variations between species and within species were revealed. The two wild pears showed specific phytochemical profiles, such that they must be considered distinct entities. Notably, within the same PCA score plot, it was evident how the fruit fractions were also affected by the principal components. This was particularly striking for the peel component, which fell on the positive side of PC2, whereas the core and flesh resided on the negative side. The characteristics of the chemical traits (Figure 4B) showed that almost all chemical traits dominated the positive direction of PC1, which was vastly connected to the wild pear samples. The results of the PCA analysis showed significant variations in the antioxidant capacity, phenolic classes, and individual phenolic compounds of the wild pears and the PCG.
4. Discussion
Wild pears are valuable native resources with high exploitation potential due to their useful traits for both humans and the environment [10,20,29]. Wild pears are important for forestry, ecological conservation, and ornamental purposes, and they also provide genetic resources for cultivated pear varieties and pear breeding [7,30] as well as rootstocks. Additionally, wild pears exhibit traits such as cold and drought resistance, disease resistance, and high adaptability [1]. Leaf and fruit morphometric studies on the two species have been carried out by Vidaković et al. [9,10]. Pear fruits are rich in phenolic compounds and are excellent sources of phytochemicals and natural antioxidants. Phenolic compounds are commonly found in both edible and non-edible parts of pear plants, and they have been reported to have multiple biological effects [30].
Papers reporting on P. spinosa or P. pyraster fruit phenolic composition are modest, but none of them investigated the phenolics allocated in the different parts of pear fruit. To our knowledge, this is the first study regarding total and individual phenolic compounds in different fruit parts (e.g., flesh, peel, and core) of P. pyraster and P. spinosa from the Mediterranean area. The available literature mainly deals with the genetic characterization of wild pear species or with the chemical characterization of different organs of the pear tree [7,12,31]. In fruit peel extracts of Pyrus spp. from China, Li et al. [32] found a TotP content ranging from 263.6 to 1121.5 mg of gallic acid 100 g−1 dry weight (DW), while TotF ranged from 281.2 to 1682.7 mg of rutin 100 g−1 DW, which are lower than our results. It is worth noting that both Pyrus spp. and the methodology of sample preparation and extraction were different. In the peel and flesh of P. ussuriensis, TEAC values with DPPH assay ranged from 13.5 to 36.9 and from 3.1 to 8.7 μmol TE g−1, respectively [30], highlighting variations in antioxidant capacity between peel and flesh as our results but with lower values. Fatty acid components, the antimicrobial activity of fruit seed and pulp of P. spinosa, as well as the antioxidant activity, were evaluated by Özderin [4]; however, a different analytical approach does not allow direct comparison with our data on antioxidant capacity. Tzanakis et al. [33] investigated the TotP in P. amygdaliformis (=P. spinosa) fresh entire fruits from Greece and found TotP values from 165 to 846 mg kg−1 of fresh weight, lower than our results. The difference in the values may be explained because they analyzed the entire fruit without distinction between peel and flesh and also fresh fruit instead of freeze-dried samples. Other studies on Pyrus spp. take into account different organs of the pear tree, like leaves and bark. The total phenolics in leaves of P. spinosa populations from different Greek locations were evaluated by Alexandri et al. [5]; their values ranged from 6.25 to 46.32 mg GAE g−1 of fresh weight and were much lower than in fruits. Ušjak et al. [34] investigated barks of P. pyraster and P. spinosa and found a content of total phenolics of 533.4 and 436.2 mg GAE g−1, respectively, values being much higher than in fruits. According to Kundaković et al. [29], the methanolic extracts of leaf and bark from P. pyraster exhibited significant cytotoxic effects towards human melanoma Fem-x cells, whereas the corresponding methanolic extracts of P. spinosa did not show cytotoxic activity against the Fem-x and human embryonic lung fibroblast MRC-5 cell lines. The same authors evidenced that the entire wild pear’s fruit extracts and the arbutin standard showed antibacterial activity against all bacteria species, demonstrating that the arbutin contained in wild pear extracts possessed biological activity. As the relationship between the fruit phenolic contents of wild pears and pear cultivar germplasm was already investigated [16] in the same area, our research revealed key differences. The greatest differences between wild and cultivated species referred to the phytochemical partitioning among the three examined fruit components, in terms of both qualitative and quantitative variations. The PCG flesh was relatively poor in TotP and individual phenolic compounds compared to the peel and core, the latter being the richest in phenolics (Tables S1–S7). On the contrary, the two wild pear species exhibited very similar antioxidant capacity, TotP and TotF content, in both flesh and peel, whereas the TP content was lower in the flesh than in the peel.
Phenolic acids are biologically active molecules; they are present in a wide range of plants and have commercial value in the cosmetic, health, and medicine industries due to their antiaging, antitumor, antimicrobial, and anti-inflammatory properties. Contardi et al. [35] supported the importance and role of hydroxycinnamic acids in treating dermatological conditions (burns, wound healing, psoriasis, dermatitis, inflammation) and mentioned chlorogenic acid as being beneficial for prolonging the shelf life of food due to its antibacterial and antioxidant properties.
According to Kolniak-Ostek [36], P. communis fruits contain phenolic compounds such as hydroxycinnamic acids (primarily chlorogenic acid), hydroquinones (arbutin being the most abundant), flavanols (catechin, epicatechin, and procyanidins), and flavonols (mainly quercetin and isorhamnetin glycosides). In the entire fruits of P. pyraster, Stoenescu et al. [21] detected gallic acid 0.2 mg g−1 and chlorogenic acid 0.51 mg g−1; our results evidenced a higher content of chlorogenic acid in the flesh, peel, and core 1.9, 1.9, and 2.4-folds, respectively. The same authors did not detect neochlorogenic, vanillic, caffeic, or siringic acids, in agreement with our results. In P. amygdaliformis (=P. spinosa) fruit (without distinction of peel and pulp) from Iraq, Saadatian et al. [20] found gallic and chlorogenic acids and rutin, but with values lower than our results. Peel and flesh extracts from Pyrus spp. originating from China were analyzed by Li et al. [32]. The study identified chlorogenic acid and arbutin as the most abundant compounds, as our results show, along with phenolic acids such as p-coumaric, vanillic, gallic, and ferulic acids. It is worth noting that p-coumaric, vanillic, and ferulic acids were not detected in our study, but they analyzed different Pyrus species.
Concerning the two flavanols detected at tR 31.9 and 39.0 in the chromatogram registered at 280 nm in all examined fruit parts, we hypothesize that these peaks could be oligomeric compounds formed from catechin and epicatechin molecules. The literature supports our hypothesis; in fact, Jeong et al. [37] isolated from the fruit peels of P. pyrifolia five proanthocyanidins, two dimers, and three trimers of catechin and epicatechin, and their structures were determined by nuclear magnetic resonance and mass spectrometry. Wang et al. [30] identified seven flavanols, and among these, one procyanidin dimer and one procyanidin trimer were found in five different Australian grown pear varieties. In addition, He et al. [38] detected five procyanidin dimers in pear juices. The same authors affirmed that procyanidins were the predominant phenolic group in pear fruit (as supported by our quantitative analysis results, where the flavanols weigh from 40 to 80% of the total estimated phenolic content for pulp, peel, and core), and their concentration can exceed 90% of total quantified phenolic compounds. The same results were previously reported by Braham et al. [39] in European and Tunisian pear cultivars and in pear fruits, juices, and pomaces of Plant De Blanc and De Cloche ripe and overripe pear cultivars [40]. Furthermore, five individual phenolic compounds were detected in PCG flesh (Table S5) compared to eleven molecules identified in the flesh of wild species (Table 2). Within individual phenolics, arbutin had a notably higher content in the three examined fruit fractions of wild pears compared to PCG fruits (Figure 3). Otherwise, the content of quercetin 3-galattoside in P spinosa peel substantially differed from that of PCG fruits. Finally, all the examined fruit components of wild pears contained a great quantity of flavanol at tR 31.9, which was detected in much lower concentrations only in the core of Buttiru among PCG (Table S7). The differences in qualitative and quantitative values, compared with literature data, could be explained by genetic factors as well as environmental and pedoclimatic conditions. All these factors can influence the levels and detection of metabolites [9,41]. In our research, sampling of both wild and domesticated pear fruit was carried out at the same site, so the variability due to agronomic and environmental conditions was negligible. The comparison of the antioxidant capacity, phenolic classes, and individual phenolic compounds of wild pear fruit with the corresponding traits in PCG evidenced relevant differences both in quantitative and qualitative terms at species level inside the Pyrus genus, highlighting the remarkable phytochemical content of wild pears compared to P. communis cultivars. The PC analysis indicated good separation of the pear samples in the score plot. Notably, the PCG samples formed a separate cluster from the two wild pear groups, maintaining their distinct characteristics; the replicates clustered tightly in the score plot, indicating consistent results within each treatment group. However, only a few variables showed significant differences in their contribution (loading values) across the three main components (PC1, PC2, and potentially PC3). This identified differences within our dataset warranting further investigation in the antioxidant capacity, phenolic fingerprinting, and content of pear fruit for the classification and differentiation between domestic and wild species. Starting from the hypothesis that the phenolic profile might differentiate between species, as highlighted by several authors [32,42,43], our study suggests the usefulness of characterizing species and highlighting a greater phenotypic proximity among the accessions belonging to the same geographical area [44].
5. Conclusions
The results obtained from this research provide novel information on phenolic compounds in the flesh, peel, and core of P. pyraster and P. spinosa fruits and evidence the high variability between species. Our study confirms the effectiveness of chemometric approaches in distinguishing between Pyrus species. Notably, the investigated wild pear species emerged as promising sources of health-promoting phenolic compounds. The two wild pear species demonstrated a different antioxidant capacity and content of phenolic compounds at the level of species and fruit components (flesh, peel, and core). Moreover, the multivariate analysis also revealed the great differences between P. communis. The comparison between Pyrus spp. in the same geographical territory highlighted that the wild pear species were richer in phenolic compounds than P. communis cultivars.
Due to the potential health benefits and applications in food science and cosmetics, wild pear fruits hold promise as functional foods, dietary supplements, and even as a source of antioxidants for the cosmetic industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/resources13060072/s1, Table S1: Total antioxidant capacity (TEAC) by ABTS and DPPH methods, total phenolics (TotP), non-tannic phenolics (NTP), tannic phenolics (TP), total flavonoids (TotF), and condensed tannins (CT) in the fruit flesh of the investigated pear cultivars; Table S2: Total antioxidant capacity (TEAC) by ABTS and DPPH methods, total phenolics (TotP), non-tannic phenolics (NTP), tannic phenolics (TP), total flavonoids (TotF), and condensed tannins (CT) in the fruit peel of the investigated pear cultivars; Table S3: Total antioxidant capacity (TEAC) by ABTS and DPPH methods, total phenolics (TotP), non-tannic phenolics (NTP), tannic phenolics (TP), total flavonoids (TotF), and condensed tannins (CT) in the fruit core of the investigated pear cultivars; Table S4: Chromatographic parameters of the quantitative evaluation of phenolic compounds; Table S5: HPLC analysis of individual phenolic compounds (mg g−1 DM) in fruit flesh of the investigated pear cultivars; Table S6: HPLC analysis of individual phenolic compounds (mg g−1 DM) in fruit peel of the investigated pear cultivars; Table S7: HPLC analysis of individual phenolic compounds (mg g−1 DM) in fruit core of the investigated pear cultivars; Figure S1: HPLC chromatogram at 280 nm of Pyrus spinosa peel (A), flesh (B), and core (C) 1: arbutin, 2: gallic acid, 3: chlorogenic acid, 4: catechin, 5: epicatechin, 6: flavonol (tR: 18.60), 7: rutin, 8: quercetin 3-galattoside, 9: quercetin 3-glucoside, 10: isorhamnetin 3-rutinoside; 11: di-o-caffeolylquinic acid, 12: isorhamnetin derivative (tR: 23.97), 13: isorhamnetin derivative (tR: 24.40), 14: flavanol (tR: 31.90), and 15: flavanol (tR: 39.00).
Author Contributions
Conceptualization, L.S., G.D., M.G.M. and G.P.; methodology L.S., M.G.M. and G.P.; formal analysis, M.G.M. and G.P.; investigation, L.S., M.G.M. and G.P.; resources, L.S. and G.D.; data curation, L.S., M.G.M., G.P. and F.S.; writing—original draft preparation, L.S., M.G.M., F.S. and G.P.; writing—review and editing, L.S., M.G.M., G.P., G.D., G.A.R. and F.S.; supervision, L.S., M.G.M., G.P. and G.D.; project administration, L.S. and G.D.; funding acquisition, L.S. and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Agricultural, Food, and Forestry Policies, project “FOR[m]AGE, BEES & FRUITS”: bee-fruit synergies with forage farming systems in rainfed Mediterranean environment “(4APIFRUT, decree number 89233, 2019)”.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to all farmers involved in on-farm safeguard and reestablishment of pear biodiversity, especially those who provided in kind the fruit samples that made possible this work. The authors also thank Maria Maddalena Sassu at CNR ISPAAM for her precious assistance in the laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Simionca Mărcășan, L.I.; Pop, R.; Somsai, P.A.; Olteanu, I.; Popa, S.; Sestraş, A.F.; Militaru, M.; Mihai, B.; Sestraş, R.E. Comparative Evaluation of Pyrus Species to Identify Possible Resources of Interest in Pear Breeding. Agronomy 2023, 13, 1264. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Wang, T.; Gao, W. Nutritional composition of pear cultivars (Pyrus spp.). In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2016; pp. 573–608. [Google Scholar]
- Cedro, A.; Antkowiak, W. Dendroclimatological analysis of wild pear Pyrus pyraster (L.) Burgsd. from Biedrusko Military area (West Poland)—Preliminary study. Geochronometria 2016, 43, 18–23. [Google Scholar] [CrossRef]
- Özderin, S. Determination of some chemical properties of wild pear (Pyrus spinosa Forsk.). BioResources 2022, 17, 1659. [Google Scholar] [CrossRef]
- Alexandri, S.; Tsaktsira, M.; Hatzilazarou, S.; Kostas, S.; Nianiou-Obeidat, I.; Economou, A.; Scaltsoyiannes, A.; Tsoulpha, P. Selection for Sustainable Preservation through In Vitro Propagation of Mature Pyrus spinosa Genotypes Rich in Total Phenolics and Antioxidants. Sustainability 2023, 15, 4511. [Google Scholar] [CrossRef]
- Arrigoni, P.V. Flora dell’Isola di Sardegna; Carlo Delfino Editore: Sassari, Italy, 2006; Volume 1, pp. 55–58. [Google Scholar]
- Wolko, Ł.; Bocianowski, J.; Antkowiak, W.; Słomski, R. Genetic diversity and population structure of wild pear (Pyrus pyraster (L.) Burgsd.) in Poland. Open Life Sci. 2014, 10, 19–29. [Google Scholar] [CrossRef]
- Reim, S.; Lochschmidt, F.; Proft, A.; Wolf, H.; Wolf, H. Species delimitation, genetic diversity and structure of the European indigenous wild pear (Pyrus pyraster) in Saxony, Germany. Genet. Resour. Crop Evol. 2017, 64, 1075–1085. [Google Scholar] [CrossRef]
- Vidaković, A.; Šatović, Z.; Tumpa, K.; Idžojtić, M.; Liber, Z.; Pintar, V.; Radunić, M.; Runjić, T.N.; Runjić, M.; Rošin, J.; et al. Phenotypic variation in European wild pear (Pyrus pyraster (L.) Burgsd.) populations in the North-Western Part of the Balkan Peninsula. Plants 2022, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Vidaković, A.; Poljak, I. Fruit morphological variability and chemical composition in European wild pear (Pyrus pyraster (L.) Burgsd.) natural populations. Genet. Resour. Crop Evol. 2024, 2024, 1–16. [Google Scholar]
- Puddu, G.; Falcucci, A.; Maiorano, L. Forest changes over a century in Sardinia: Implications for conservation in a Mediterranean hotspot. Agrofor. Syst. 2012, 85, 319–330. [Google Scholar] [CrossRef]
- Sau, S.; Pastore, C.; D’hallewin, G.; Dondini, L.; Bacchetta, G. Characterisation of microsatellite loci in Sardinian pears (Pyrus communis L. and P. spinosa Forssk.). Sci. Hortic. 2020, 270, 109443. [Google Scholar] [CrossRef]
- Chessa, I.; Nieddu, G. Analysis of diversity in the fruit tree genetic resources from a Mediterranean island. Genet. Resour. Crop Evol. 2005, 52, 267–276. [Google Scholar] [CrossRef]
- Atzei, A.D. Le Piante nella Tradizione Popolare della Sardegna: Documentazione Sugli Usi Alimentari, Aromatizzanti, Profumieri, Artigianali, Cosmetici, Medicinali, Veterinari, Magici, Ornamentali, Rituali, Religiosi, Tintori, Antiparassitari e Vari, delle Piante; Carlo Delfino Editore: Sassari, Italy, 2003; pp. 386–387. [Google Scholar]
- Versini, G.; Franco, M.A.; Moser, S.; Manca, G. Characterisation of pear distillates from wild and cultivated varieties in Sardinia. Int. J. Food Sci. Technol. 2012, 47, 2519–2531. [Google Scholar] [CrossRef]
- Piluzza, G.; Campesi, G.; D’hallewin, G.; Molinu, M.G.; Re, G.A.; Sanna, F.; Sulas, L. Antioxidants in Fruit Fractions of Mediterranean Ancient Pear Cultivars. Molecules 2023, 28, 3559. [Google Scholar] [CrossRef] [PubMed]
- Sulas, L.; Re, G.A.; D’hallewin, G. Agroforestry & Transumance in Sardinia. In World Agroforestry in Practice Platform; Association Française d‘Agroforesterie: Auch, France, 2019; Available online: https://www.agroforesterie.fr/World-Agroforestry-In-Practice.php (accessed on 1 February 2024).
- Loru, L.; D’hallewin, G.; Satta, A.; Sulas, L.; Molinu, M.G.; Piluzza, G.; Pusceddu, M.; Pantaleoni, R.A. FOR[m]AGE, BEES & FRUITS: Bee-fruit synergies with forage farming systems in rainfed Mediterranean environment. In Proceedings of the Agroforestry for the Transition towards Sustainability and Bioeconomy, P3.3-5_216, 5th European Agroforestry Conference, Nuoro, Italy, 17–19 May 2021. [Google Scholar]
- Bueno, R.S.; Badalamenti, E.; Gristina, L.; Novara, A.; La Mantia, T. The Role of Almond-Leaved Pear Pyrus spinosa Forssk. in Mediterranean Pasturelands Carbon Storage and Woodlands Restoration. Land 2023, 12, 2135. [Google Scholar] [CrossRef]
- Saadatian, M.; Mohammad, R.H.; Jumaah, A.A.; Oagaz, J.A. Evaluation of Nutritional Value, Fatty Acids and Polyphenols Profiles of Pyrus amygdaliformis L. Grown in North-East Kurdistan Regional Government, Iraq. J. Oleo Sci. 2022, 71, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Stoenescu, A.M.; Trandafir, I.; Cosmulescu, S. Determination of phenolic compounds using HPLC-UV method in wild fruit species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Sulas, L.; Petretto, G.L.; Pintore, G.; Piluzza, G. Bioactive compounds and antioxidants from a Mediterranean garland harvested at two stages of maturity. Nat. Prod. Res. 2017, 31, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- Re, G.A.; Piluzza, G.; Sulas, L.; Franca, A.; Porqueddu, C.; Sanna, F.; Bullitta, S. Condensed tannin accumulation and nitrogen fixation potential of Onobrychis viciifolia Scop. grown in a Mediterranean environment. J. Sci. Food Agric. 2014, 94, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Sulas, L.; Bullitta, S. Dry matter yield, feeding value, and antioxidant activity in Mediterranean chicory (Cichorium intybus L.) germplasm. Turk. J. Agric. For. 2014, 38, 506–514. [Google Scholar]
- Re, G.A.; Piluzza, G.; Sanna, F.; Molinu, M.G.; Sulas, L. Polyphenolic composition and antioxidant capacity of legume-based swards are affected by light intensity in a Mediterranean agroforestry system. J. Sci. Food Agric. 2019, 99, 191–198. [Google Scholar] [CrossRef]
- StatPoint Technologies Inc. Statgraphics Centurion XVI, User Manual; StatPoint Technologies Inc.: Warrenton, VA, USA, 2009. [Google Scholar]
- Øyvind, H.; Harper, D.A. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- The Jamovi Project. Jamovi, Version 2.3. 2022. Computer Software. Available online: https://www.jamovi.org (accessed on 1 February 2024).
- Kundaković, T.; Ciric, A.; Stanojkovic, T.; Sokovic, M.; Kovacevic, N. Cytotoxicity and antimicrobial activity of Pyrus pyraster Burgsd. and Pyrus spinosa Forssk. (Rosaceae). Afr. J. Microbiol. Res. 2014, 8, 511–518. [Google Scholar]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A Comparative Investigation on Phenolic Composition, Characterization and Antioxidant Potentials of Five Different Australian Grown Pear Varieties. Antioxidant 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Postman, J.; Bassil, N.V.; Neale, D.B. Reconstruction of the largest pedigree network for pear cultivars and evaluation of the genetic diversity of the USDA-ARS national Pyrus collection. G3 Genes Genomes Genet. 2020, 10, 3285–3297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Tzanakis, E.; Kalogeropoulos, T.; Tzimas, S.; Chatzilazarou, A.; Katsoyannos, E. Phenols and antioxidant activity of apple, quince, pomegranate, bitter orange and almond-leaved pear methanolic extracts. J. Sci. Technol. 2006, 1, 16–28. [Google Scholar]
- Ušjak, L.J.; Milutinović, V.M.; Đorđić Crnogorac, M.J.; Stanojković, T.P.; Niketić, M.S.; Kukić-Marković, J.M.; Petrović, S.D. Barks of Three Wild Pyrus Taxa: Phenolic Constituents, Antioxidant Activity, and in Vitro and in Silico Investigations of α-Amylase and α-Glucosidase Inhibition. Chem. Biodivers. 2021, 18, e2100446. [Google Scholar] [CrossRef]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic acids and derivatives formulations for skin damages and disorders: A review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.E.; Cho, J.Y.; Lee, Y.G.; Jeong, H.Y.; Lee, H.J.; Moon, J.H. Isolation of five proanthocyanidins from pear (Pyrus pyrifolia Nakai) fruit peels. Food Sci. Biotechnol. 2017, 26, 1209–1215. [Google Scholar] [CrossRef]
- He, W.; Laaksonen, O.; Tian, Y.; Haikonen, T.; Yang, B. Chemical composition of juices made from cultivars and breeding selections of European pear (Pyrus communis L.). J. Agric. Food Chem. 2022, 70, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Braham, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Brahem, M.; Eder, S.; Renard, C.M.; Loonis, M.; Le Bourvellec, C. Effect of maturity on the phenolic compositions of pear juice and cell wall effects on procyanidins transfer. LWT-Food Sci. Technol. 2017, 85, 380–384. [Google Scholar] [CrossRef]
- Vidaković, A.; Liber, Z.; Šatović, Z.; Idžojtić, M.; Volenec, I.; Zegnal, I.; Pintar, V.; Radunić, M.; Poljak, I. Phenotypic diversity of almond-leaved pear (Pyrus spinosa Forssk.) along Eastern Adriatic Coast. Forests 2021, 12, 1630. [Google Scholar] [CrossRef]
- Mitic, V.; Ilic, M.; Dimitrijevic, M.; Cvetkovic, J.; Ciric, S.; Jovanovic, V.S. Chemometric characterization of peach, nectarine and plum cultivars according to fruit phenolic content and antioxidant activity. Fruits 2016, 71, 57–66. [Google Scholar] [CrossRef]
- Guimarães, A.C.G.; de Souza Gomes, M.; Zacaroni Lima, L.M.; Sales, P.F.; da Cunha, M.C.; Rodrigues, L.J.; de Barros, H.; Pires, C.R.F.; dos Santos, V.F.; Lima, N.; et al. Application of Chemometric Techniques in The Evaluation of Bioactive Compounds and Antioxidant Activity of Fruit From Brazilian Cerrado. J. Food Meas. Charact. 2023, 17, 2095–2106. [Google Scholar] [CrossRef]
- Zarei, A.; Erfani-Moghadam, J.; Jalilian, H. Assessment of variability within and among four Pyrus species using multivariate analysis. Flora 2019, 250, 27–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).