1. Introduction
A large amount of wastes are generated worldwide annually. In the European Union (EU), approximately 6000 kg of waste is produced by one EU citizen per year, and only a small fraction of such waste is recycled. A notable amount of secondary materials, such as metals and plastics, still end up in landfills [
1]. The EU has implemented regulations on waste management, such as the recovery and valorization of secondary raw materials [
2]. Given the implementation of such regulations and given the public’s preference for more environmentally friendly products to ensure a sustainable future, it is important to identify various means to recycle potentially valuable waste streams.
Electrocoagulation (EC) is a technology involving an electrochemical technique. It has been widely studied and used in a mining wastewater treatment facility in Sydney, Australia, in oil de-emulsification in Maca, Brazil, and in residential construction stormwater treatment in Ridgefield, United States of America. Since EC was first introduced in the late 19th century [
3], it has been widely studied in different water treatment applications. Various models of EC processes [
3,
4,
5,
6,
7] have been published. Most of these studies have focused on the removal of substances, such as nutrients [
8,
9], pharmaceuticals/bacteria [
10,
11], or metals [
12,
13]. Several review studies have already been made on different EC technologies or processes [
4,
5,
6,
14,
15,
16] or on the removal of various substances [
16,
17,
18,
19,
20]. Meanwhile, the demand for recycling has been increasing globally, and environmental regulations for all waste products are becoming stringent worldwide. Therefore, technological developments that will decrease the amounts of wastes that end in landfills are important.
EC removes impurities from waters; however, it generates gases and sludge, which may be considered wastes, rendering studies on sludge valorization important. The possible uses of EC sludge have not yet been widely explored, and only a few studies on sludge utilization or valorization in certain fields have been conducted. While a few studies on the use of sludge as fertilizers, pigments, construction materials, absorbents, and catalysts [
9,
21,
22,
23] and a few studies on EC sludge valorization have been reported, to our best knowledge, no practical reviews on this subject have been published. Thus, this review focuses on the EC sludge valorization studies that were published between 2010 and 2020. Therefore, there is a need for a review of EC sludge valorization studies.
2. Theory of Electrocoagulation
EC was developed in the late 19th century. Different EC systems have been widely studied worldwide. Various types of batch and continuous EC systems exist, all of which are based upon the same principles, which are explained below.
2.1. Description of the EC System
EC systems involve different techniques, such as coagulation, oxidation, flotation, and sedimentation, during water treatment [
6]. As a result, EC has been used in various fields such as metal and pharmaceutical removal [
5,
10,
13,
17,
18,
19,
20,
24,
25]. In an EC system, the arrangement of electrodes could be one of three main types: parallel monopolar electrode connection (MP-P), series monopolar electrode connection (MP-S), and series bipolar electrode connection (BP-S) [
3,
26], all of which use DC current (
Figure 1). When an EC system consists of multiple electrode pairs, the electricity feed system can be any of these types; MP-P is always the electricity feed for a single pair feed system. Additionally, most continuous systems involve the MP-P electricity feed system [
3,
5,
27].
EC can be used either as a batch process or as a continuous process. A batch process usually consists of the following components: a power source, a stirrer, electrodes, and a reactor container. In most batch systems, the electrodes are hanging. The main differences between batch systems are the cap size between electrodes, the electrode materials, and the reactor volume [
5,
7,
17,
18,
19]. The components of continuous systems are the same as above, but their reactor design varies [
5].
2.2. Chemical Reactions in an EC Process
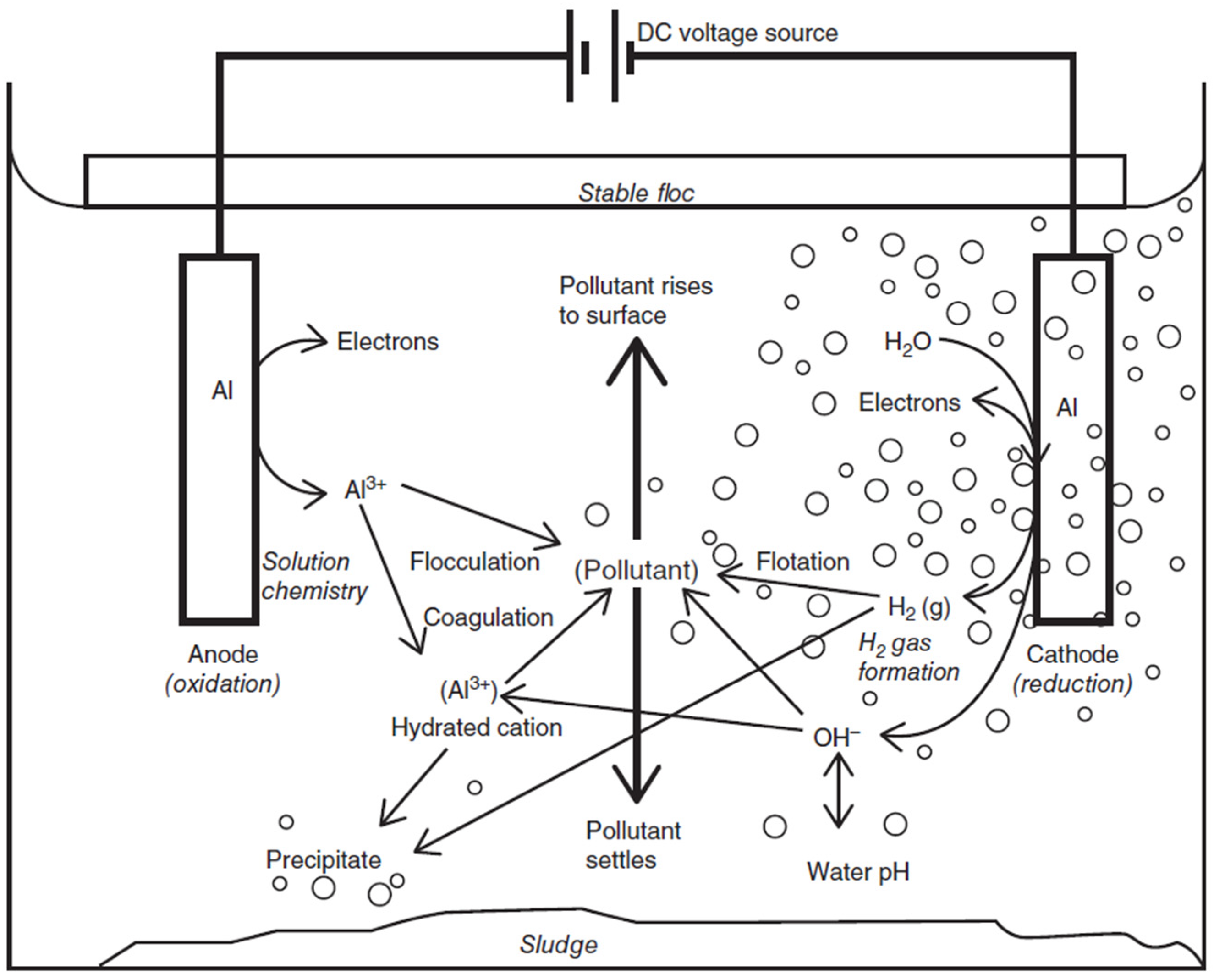
Figure 2 presents the complexity of an EC process [
6], which is influenced by different variables, such as pH, concentration, and current, along with other parameters. The overall mechanism of EC involves the combination of electrochemistry, coagulation, and hydrodynamics. During an EC process, the anode dissolves itself into a solution, whereas the cathode generates gases (mainly hydrogen (H
2)) [
6,
27]. The EC process produces H
2 gas, which could replace up to 13% of the total energy used in EC process [
27]. This information was derived from the process itself.
Figure 2 presents the sludge formation. Metal ions are produced from the dissolving anodes, which react with different pollutants present in the solution. Flocculation and coagulation are the main reactions during sludge formation. Sludge either settles at the bottom or rises to the top, along with the H
2 gas produced during the reaction of the cathode with the wastewater.
Different reactions occur depending on the electrode materials used. The most commonly used electrodes are aluminum (Al) and iron (Fe). Chen [
7] described anode leaching and precipitation under alkaline or acidic conditions in reactions 1–6:
at alkaline conditions
at acidic conditions
For iron anode:
at alkaline conditions
at acidic conditions
In addition to the traditional Fe and Al electrodes, other electrode materials can be used as dissolving electrodes. An anodic electrode, such as magnesium (Mg) anode, can be used as a source of coagulant [
28]. Song et al. [
29] described an anodic dissolution of Mg using the chemical reactions 7–9, as follows:
Reaction 7 is assumed to be the rate-determining step, and Mg+ is oxidized into Mg2+ in reaction 8. Reaction 9 presents the cathodic reduction reaction. These reactions are possibly more complex individually.
A relationship exists between current density (A/cm
2) and the amount of metal dissolved (g/M/cm
3), which is calculated using the Faraday’s Law [
30] and presented in Equation (1):
The efficiencies of different EC processes can be compared based on the following parameters: applied current density, treatment time, pH, cap between electrodes, solution temperature, water flow rate, chemical composition of sludge, and electrode material used. EC offers some advantages over conventional coagulation, such as production of sludge with a better quality and lower volume, easy automation, and use of simple equipment [
15].
2.3. Properties of EC Sludge
Studies [
15,
16,
31] have shown that EC produces less sludge than conventional chemical precipitation (CP). In these studies, the volume of EC sludge is less than 2%. The volume of the sludge differs in different element purifications and methods as shown at
Table 1. Ilhan et al. [
31] studied the impact of time and pH on sludge production. They found that a longer reaction time and an increasing pH correlated with greater sludge volume. Emamjomeh and Sivakumar [
32] studied the effects of current density on sludge volume and found correlation between them. Moreover, a linear relationship was observed between sludge formation and current density at different flow rates.
Mollah et al. [
15] have reported some advantages of EC sludge over the conventional CP sludge. EC is a technique that generates low volume of sludge, which mainly consists of metallic oxide/hydroxide. As a result, EC sludge is easy to de-water, and it settles easily. Additionally, EC flocs are larger than CP flocs, and the sludge contains less water [
33]. Moreover, EC sludge is acid resistant and stable. It is also easily obtained through filtration [
15].
Gomes et al. [
34] characterized EC sludge in their study and found that different metal hydroxides (MH) and metal oxyhydroxides (MOH) form in the sludge. The MH and MOH species that would form depend on the electrode material used; for example, Al electrodes form aluminum hydroxide and/or aluminum oxyhydroxides in the sludge. The sludge produced from Al electrodes display an amorphous or poor crystalline appearance, whereas sludge produced from Fe electrodes display crystalline phases similar to those of magnetite. Other chemical compounds also form in the sludge depending on what was coagulated from the water and on what electrode material was used.
The basic principles of sludge formation can be observed in every EC process, but each sludge must be considered case-specific because its quality depends on different parameters, such as the electrodes used, and the quality of the water being purified. All sludges contain common contents, such as the leached material from electrodes mostly in the form of MH or MOH, as well as the materials coagulated from treated water (e.g., metals). Moreover, all sludges are stable, and EC sludge, in particular, settles easily. Studies cited in this review are research-based. Rajaniemi et al [
9] studied struvite formation and some of the sludge settled to the bottom of the tank. Sahu et al [
16] found that iron electrodes produce heavier sludge than aluminum electrodes. Characteristics of EC sludge is different in different purification method and with different electrodes as shown in
Table 2.
3. Valorization Applications
Chapters 3.1–3.6 present an overview of the sludge valorization studies highlighting different sludge valorization possibilities. In Finland, the waste tax cost is approximately 70 €/ton of landfilled waste. In the future, waste tax will increase. All values presented in the following chapters are obtained from the original studies and have not been treated in any way. In many cases, such as adsorption and catalyst use, EC sludge has to be calcinated before use. As a fertilizer, there is no need for any additional treatment of sludge.
3.1. Valorization of Sludge as Fertilizer
Nitrogen (N) and phosphor (P) are important nutrients for all living organisms. These nutrients promote plant growth and, thereby, help in satisfying the food demand of the ever-increasing human population [
38,
39,
40,
41]. The cycle of N from the atmosphere to land and water bodies proceeds via different routes [
40]; by contrast, the earthly cycle of P is not as easy to describe [
38,
41]. The application of N and P fertilizers has increased quite rapidly. Their prices have also considerably increased, and these fertilizers are currently unavailable to many farmers [
25,
39,
41,
42].
Some studies have investigated struvite precipitation through EC. Struvite is also known as magnesium ammonium phosphate (MAP).
Table 3 presents the recent studies on struvite precipitation using EC systems at pH 5–12.5, but the studies were performed mainly at pH 7–9. The anode material used in one study was Al, whereas Mg was used in all other studies. The cathode materials used in different studies varied. As regards removal efficiency, for P it was over 90% in most studies.
Our research group [
9] compared CP with one batch and one continuous EC method involving Mg anode and cathode in terms of struvite precipitation. In the MINEQL+ program, the optimal conditions for struvite formation were defined in the experiments. The molar N:P:Mg ratio was set to 1:1:1. Authentic water and synthetic water were used. The phosphate removal ranged from 71.6% to 93.6%, and the ammonium removal ranged from 51.5% to 79.4% of their initial concentrations in water. The maximum purity of struvite was 98.1%, and struvite formation was heavily dependent on pH. The struvite yield ranged from 0.61 kg/m
3 to 3.04 kg/m
3, whereas the cost of precipitated struvite ranged from 0.22 €/kg to 0.55 €/kg. Both EC methods were found to be suitable for struvite precipitation from P- and N-rich waters, but costs were slightly higher in EC than in CP.
Kruk et al. [
28] studied struvite precipitation using Mg sacrificial anode and Mg cathode. In this study, two water types were used: pure synthetic water solution and fermented waste activated sludge water obtained from a wastewater treatment plant. They used an N:P ratio of 1.9:1 (mol/mol) in synthetic water, where the concentrations of ammonia (NH
3) and P were 98–490 mg/L and 105–548 mg/L, respectively. In the authentic water, the molar N:P ratio ranged from 1:1.05 to 1:1.16, and the resulting P removal rate ranged from 95% to 98%. This study demonstrated that the batch EC process can be used for high purity struvite precipitation under different pH values. Additionally, it showed that a higher current produces struvite with higher purity. This study was performed using a small (1 L) batch, and comparability with full scale or continuous EC process remains unclear. Moreover, only one molar N:P ratio was used in this study.
Kim et al. [
43] studied Mg–air fuel cell EC in struvite production. They used various N:P ratios, where the N concentration was fixed at 0.277 M and the PO
4-P concentration ranged from 0.006 M to 0.1 M. The phosphate removal efficiency increased with higher Mg:P ratio, and it did not depend on the initial concentration of electrolyte NaCl. The highest P removal of 98% was observed at 0.01 M concentration. They also found that current density and pH influenced struvite formation. The Mg–air fuel cell was found to be similar to conventional Mg EC when used as a struvite recovery method. This study used only synthetic water to optimize the molar N:P ratio. No comparison to any real wastewater in terms of the molar N:P ratio was performed.
The effect of pH on struvite precipitation in EC was investigated by Wang et al. [
44]. Actual swine wastewater was treated with continuous EC using Mg sacrificial anode and carbon cathode; the flow rate was 20 L/h, the phosphate concentrations ranged from 20 mg/L to 100 mg/L, and the pH values ranged from 8.3 to 9.2. The phosphate recovery efficiency was 99.5%, the highest rate achieved after 4 h at pH 9.2. In the actual swine water, the N:P ratio was 2:1, the struvite yield was 21.9 g/m
2 h, and the operational cost was 1.45 ¥/m
3. The above method is suitable for struvite precipitation using continuous EC. The water used in this study was simulated water, and the water concentration was obtained from authentic water, but no tests using authentic water were performed.
Hug and Udert [
45] used urine from men’s collection tank as a water source in their study using a batch EC setup with Mg anode and steel cathode. The reactor contained 1 L of urine stirred at approximately 275 rpm. The optimal Mg:P ratio was calculated to be 1:1 mol/mol theoretically, but in their experiment, the value was closer to 1.5:1. The total phosphate removal rate lies between 59% and 84%. The pH values ranged from 8.9 to 12.8. They found that EC is a feasible process for struvite precipitation, but it is costlier than using MgO. Additionally, they provided a good comparison of the four different Mg sources. EC has a high electricity consumption, increasing its cost. They used a 5.5 cm space between electrodes, and electricity consumption dependent on distances of the electrodes. The use of a smaller cap between electrodes should be studied.
Garcia et al. [
46] studied struvite precipitation from dairy industry wastewater. They used continuous EC with a fluid flow of 75 L/h and with Al anode and Ti/ruthenium oxide (RuO
2) cathode. Struvite precipitation was undertaken from sludge with MgCl
2 and NH
4OH in liquid solution. The reaction pH was controlled within 8.8–9.2. This method is also suitable for struvite precipitation from EC sludge. However, this method does not produce struvite straight from EC, and its economic soundness was not compared with that of other struvite precipitation methods.
Lin et al. [
47] used simulated and real slurry from swine biogas digestion in their EC experiments. They used a novel two-chamber batch electrolysis equipment with Mg anode and stainless-steel cathode. The total volume of the reactor was 1 L. In the simulated slurry, they used the same NH
4:PO
4 ratio as in the real one, but in the absence of other components. They used ion exchange membranes to avoid ion movements and ultimately prevent pH fluctuations. The pH range was 8.0–9.5. They found that the ion exchange membrane increased phosphate removal and that this method is suitable for struvite precipitation. There was a 3.5 cm cap between electrodes, and a membrane was placed between electrodes; a smaller cap uses less energy. This study did not present the economic soundness of the real-life application of this system.
Huang et al. [
48] studied the simultaneous removal of ammonia-nitrogen (NH
3-N) and phosphate recovery using EC. They coupled three reactors and used Mg anode and stainless-steel cathode in their electrolysis reactor within pH 6–9. They managed to produce struvite with 95.7% purity under the optimal conditions of 2 mA/cm
2 current density and 45 min reaction time. A removal rate of 93 was achieved for phosphate and 94 for NH
3-N. They found that the system used was suitable for struvite precipitation from swine wastewater. This study has proven that simultaneous recovery of P and N is possible with batch EC process.
In summary, studies have investigated struvite precipitation during EC treatment, and promising results were obtained. Most of these studies treated only one or a maximum of two water types. Different authentic water types must be investigated using the same EC processes and parameters. Current density, reaction time, and molar P:N ratio in a treated solution are important factors in struvite precipitation. To date, this field remains poorly studied, and more studies from the economic and ecological points of view are warranted. Struvite is a useful slow-release nutrient and an eco-friendly fertilizer [
49], and EC is one easy means to produce it.
3.2. Valorization of Sludge as an Adsorbent and Catalyst
Variables such as pH, initial adsorbate concentration, reaction time, adsorbent surface area, and material to be adsorbed affect the adsorption capacity of an adsorbent material. A catalyst enhances a chemical reaction, but it does not participate in it. Catalysts are characterized based on their activity in a reaction, selectivity, and reusability. The most commonly used catalytic materials are metal catalysts.
3.2.1. Valorization of Sludge as an Adsorbent
The use of EC sludge in anionic dye removal has been studied. Golder et al. [
23] studied red dye removal by using sludge formed during an EC process involving Al electrodes and synthetic aqueous water containing basic chromium sulfate [Cr(OH)SO
4]. The sludge was prepared into an adsorbent by drying it at 105 °C for 12 h and by grinding it into powder form. In the adsorption test, the optimal speed when mixing water solution and the adsorbent was 200 rpm. The dye removal rate was higher under low pH values. Moreover, the removal rate was higher under lower dye concentration and higher adsorbent dosage. The maximum adsorption capacity was 513 mg/g at the initial pH of 3. The EC sludge that is formed in EC processes involving Al electrodes is a suitable adsorbent for red dye. No further characterization of these adsorbent materials was presented.
Golder et al. [
50] studied how EC sludge can be used as an adsorbent for phosphate removal. They used EC sludge generated from an EC process involving Al electrodes and a synthetic solution of chrome sulphate. The sludge was calcinated at 600 °C and then ground into powder. They studied the effects of stirrer speed, initial adsorbent concentration, adsorbent dosage, and pH on phosphate removal. Their results showed that the stirrer speed exerted a small effect or none at all on the removal rate. The increase in initial adsorbent concentration and adsorbent dosage increased the removal rate of initial P. The P removal rate decreased when pH increased. The maximum adsorption capacity was 23.3 mg/g at pH 3. They concluded that electrocoagulated metal hydroxides are suitable adsorbents for phosphate removal.
Yilmaz et al. [
51] studied red textile dye removal by using the EC sludge of geothermal water with Al electrodes. After being filtered and dried, the EC sludge was calcinated at 500 °C for 2 h and then ground. They examined the effect of pH, stirrer speed, dye concentration, adsorbent dosage, and temperature on red dye removal. The lower the pH, the better the red dye removal, and a stirrer speed of 200 was found to be optimal. Dye removal rate was greater under higher dye concentrations. With increasing adsorbent dosage, the percent removal rate for red dye also increased. The temperature data showed that the process was endothermic, and that the removal rate lightly increased with increasing temperature. The maximum adsorption capacity was 192.31 mg/g at 50 °C. Overall, the results showed that the calcinated EC sludge produced after treatment using Al electrodes is suitable as an adsorbent material for red dye removal.
Yilmaz et al. [
52] used EC sludge generated after boron (B) removal using Al electrodes. Their main objective was to use EC sludge as an adsorbent for fluoride (F
−) removal. The treatment of EC sludge was the same as in Yilmaz et al. [
51]. In this study, the optimal pH was 6.0, and the F
− removal rate was 71.2%. The higher the adsorbent dosage, the higher the F
− removal rate. By contrast, a higher initial F
− concentration correlated with lower removal rate. Moreover, a correlation was observed between particle size and removal rate. When the particle size increases, the removal rate decreases. Furthermore, higher temperatures correlated with lower removal rates. The optimal stirrer speed was 300 rpm, and the maximum adsorption capacity was 124.6 mg/g at pH 6.0 with a dosage of 0.4 g/100 mL. They concluded that EC sludge is a promising alternative sorbent for F
− removal.
Table 4 shows the wide range of investigated pH values, and acidic pH values have been found to be optimal for the usage of EC sludge as an adsorbent. Calcination was performed in most studies. All of the studies have used Al electrodes and synthetic solutions in their adsorption experiments. The adsorbent dosing was 0.1–4 g/L, and the adsorption capacity was 23.3–513 mg/g. More studies on the use of EC sludge as an adsorbent for various substances are needed. Most of the studies have found that initial concentration, pH, stirrer speed, and particle sizes affect removal rates.
3.2.2. Valorization of EC Sludge as a Catalyst
Among the commonly used catalysts are iron oxide-based materials, which are used in various applications. EC sludge may contain iron oxide-based materials. Pandey and Thakur [
53] studied the qualities of paper mill EC sludge and found that this sludge can be used as a catalyst because of its high metal content. They only mentioned this idea on the basis of the characteristics that render the sludge suitable for catalytic use, and they did not validate this idea through quantitative assessments.
Ghanbari et al. [
24] studied the removal of titanium dioxide (TiO
2) nanoparticles and the catalytic activity of the generated EC sludge. They used synthetic TiO
2 solution and a 200 mL EC equipment to produce sludge in the laboratory. The sludge was dried at 105 °C for 1 h and then calcinated at 400 °C. This EC sludge was considered a potential catalyst of peroxymonosulfate activation for ciprofloxacin degradation. They studied this EC sludge at different dosages and pH values. The result showed that the removal rate was higher in peroxymonosulphate-containing EC sludge than in either substances alone. They found that the use of EC sludge as a catalyst is a suitable strategy for circular EC sludge management.
Tezcan Un et al. [
54] studied the utilization of sludge from tissue paper wastewater as a catalyst. The sludge was collected from a small laboratory-scale batch EC reactor, which is a small, fixed bed reactor, with Fe electrodes. The EC sludge had a consistency close to that of red mud. Pyrolysis was performed at 500 °C. They found that the quality of the bio-oil improved when EC sludge was used as a catalyst and that it was dependent on the amount of catalyst under atmospheric pressure. They concluded that EC sludge is a suitable catalyst in the catalytic pyrolysis of bio-oil and that it improves the quality of bio-oil.
Shon et al. [
35] studied EC sludge as a photocatalyst. The EC sludge was generated from synthetic wastewater using Ti electrodes. The reactions of the Ti electrodes are presented as reactions 10 (anodic reaction) and 11 (cathodic reaction).
After the EC treatment, the sludge was collected and calcinated at 600 °C to produce a TiO2 photocatalyst. Their laboratory-scale photocatalytic investigation involved a small airtight reactor equipped with two 10 W, 352 nm UVA lamps and a flame ionization detector that could measure changes in acetaldehyde concentration. The results showed that the EC sludge generated using Ti electrodes is comparable to the commercial photocatalyst P-25. They also found that some nanotubes were formed.
Samy et al. [
55] used Fe sludge generated from an EC treatment in a heterogenous photo-Fenton process. Their main goal was to use EC sludge instead of Fe salts in the photo-Fenton process. The sludge was calcinated at 500 °C for 4 h, cooled at room temperature for 24 h, ground, and then sieved using a 100-mesh screen. The sludge particles were spherical and therefore had a high surface area. The photo-Fenton process was performed at pH 2–5. The optimal conditions were as follows: Fe sludge concentration of 150 mg/L, H
2O
2 concentration of 1.5 g/L, presence of light, pH of 3, and an elapsed time of 180 min when the phenol concentration was 50 mg/L. They concluded that EC sludge can be used instead of Fe salts in the photo-Fenton process and that it is more environmentally friendly than Fe salts.
EC sludge offers a potential application as a catalyst. In most studies, EC sludge requires calcination before it could be used as a catalyst, increasing the cost of catalyst preparation. The studies reviewed used Fe and Ti electrodes to produce sludge that was used as a catalyst. There are also other electrode materials, such as Al and copper (Cu), which can be used as electrodes in EC and as a catalytic material. The abovementioned studies did not cover all catalytic processes; thus, more studies in this field are needed.
3.3. Valorization as a Pigment and Construction Material
Iron oxides are among the most commonly used pigments. Sludge produced from EC treatment with Fe electrodes contains iron oxides and thus may be used as a pigment. Meanwhile, Al is an important component of concrete materials. Sludge produced from EC treatment with Al electrodes contains a high Al content and therefore offers a potential application in construction materials.
3.3.1. Valorization of Sludge as an Fe-Based Pigment
Pigments are used in different industries, such as in the concrete, food, textile, and paint industries. As shown in reaction 5 in
Section 2.2, iron oxides form during EC. Iron oxides have been widely used as pigments. The valorization of EC sludge as pigment has been reported in some studies, which are presented below.
Tezcan Un et al. [
22] investigated the valorization of EC sludge as a pigment in the ceramic industry. They treated authentic electroplating wastewater using a batch EC reactor with Fe electrodes at pH 2.4. During the EC process, nearly all Cr(VI) were removed from the water and collected into the sludge. Their XRF and XRD analyses revealed the high amounts of Fe and chromium (Cr) in the sludge. They used the brown and black color mixtures with a sludge amount of 10 wt.% and 20 wt.% to compare these colors. The pigments were calcined at 1250 °C. EC sludge is suitable for use as pigment in the ceramic industry, and it was found to be environmentally friendly.
Tezcan Un and Ozel [
36] studied the use of EC sludge produced during yogurt wastewater treatment as a pigment. They used an EC batch reactor chamber with Fe anodes and cathode. Their XRF analysis showed that the sludge mainly consisted of Fe
2O
3. They used this sludge as a component of two color mixtures, namely, brown (commercially available as Cr
2O
3, Al
2(OH)
2, and ZnO) and black (Co
3O
4). The pigments were calcinated at 1200 °C. The results showed that the EC sludge can be used as a pigment in the ceramic industry.
While many EC studies [
56,
57,
58,
59] have successfully removed dye from wastewater, none have reported the use of sludge as a pigment. Based on the reviewed studies, iron oxides are used as pigments, and they are captured during EC process with Fe as anode. These studies have investigated one pigment color only; however, there are several pigment colors produced by different manufacturers, such as Lanxess (Byeferrox products) and Sclieber and Heyng (Ferroxon products). Studies on the valorization of pigments of different colors must be conducted from the economic and environmental points of view.
3.3.2. Valorization of Sludge as a Construction Material
Starting during the last decade, people tend to prefer more environmentally friendly construction materials, the production of which required considerable efforts from many manufacturers. EU has implemented its own regulations on waste materials and on their valorization [
2]. Studies have investigated the use of sludge generated from different sources as building blocks [
60,
61], which serve as the basic units in constructing various structures (e.g., houses). However, only a few studies on EC sludge valorization as building blocks were found, and no other such studies on construction material exist to our best knowledge.
Sharma and Josh [
21] mainly aimed to utilize EC sludge as a partial replacement for non-structural building blocks. Sludge was generated in a laboratory-scale batch EC reactor with stainless steel anode under the following conditions: pH of 7.8, current density of 154.32 A/m
2, electrode distance of 2.2 cm, and treatment time of 135 min. The EC sludge obtained was oven-dried at 100 °C. They used 0–15% sludge to reduce the amount of cement used in blocks. They found that the EC sludge particles are greater than the cement particles. The wet and dry densities decreased with increasing amount of EC sludge. The compressive strength test results showed that strength decreased when greater than 7.5% EC sludge was used. They concluded that mixing EC sludge with cement is an effective means of utilizing EC sludge in construction materials. In their study, 7.5% of EC sludge was found to be the optimal amount of sludge to be mixed with cement.
Adyel et al. [
37] studied the use of electrocoagulated metal hydroxide sludge (EMHS) produced during the EC treatment of textile industry wastewater as a component of building blocks used in construction. Main characteristics of sludge were Fe (87.1% of total mass of sludge), Mg (7.3%), Si (4.8%) and Ca (0.5%). Other elements were under 0.1% of total mass of sludge. They tested normal and pressurized building blocks containing different amounts of EMHS mixed with clay. They collected wet EMHS from the textile industry and brick-making soil and clay material from a local brick field. They used 10–40% EMHS, along with other materials, and performed several rounds of each different EMHS test. Also, 100% EMHS, clay, and soil samples were tested. The test bricks had a good size and shape, and 30% of the EMHS samples showed suitable weight loss. The tested EMHS samples that adsorbed water were categorized as second-class bricks. In the compressive strength test, only 10% of the tested EMHS samples were classified as first-class bricks. Overall, EMHS can be valorized as a construction material as well as in materials used for non-loading purposes.
EC sludge has the potential to be used as a component of non-structural building materials. This finding represents only one valorization possibility for sludge in the construction industry, and more studies on sludge use in other construction materials should be conducted. Some interesting fields of study include the use of EC sludge in ready-mix concrete and in environmental concrete products, such as speed pumps or driving barrier. Other interesting construction materials where sludge may be used include mineral wool insulation or plastering.
3.4. Other EC Sludge Valorization Studies
Some EC sludge valorization studies have focused on other valorization purposes. Hutnan et al. [
62] studied the anaerobic digestion of sludge that has been formed in municipal wastewater treatment. They used a small EC wastewater treatment plant (SEWWTP) with a treatment capacity of 25 m
3. Six Al and four Fe alternatively poled electrodes were used. Anaerobic digestion was tested in a laboratory. They concluded that the quality of the SEWWTP sludge differs from that of the conventional wastewater treatment plant sludge. The biogas production from the SEWWTP sludge was significantly lower than that in anaerobic digestion. Therefore, SEWWTP sludge is not recommended for anaerobic digestion.
Kathiravan and Muthukumar [
63] used EC sludge in ultrasound-mediated Cr(VI) reduction. The EC sludge was collected during pharmaceutical wastewater treatment using a laboratory-scale 0.5 L equipment with Fe anode and stainless-steel cathode. The Cr(VI) reduction was observed in 250 mL Erlenmeyer flasks containing different Cr(VI) and EC sludge concentrations. The EC sludge contains iron oxide, along with organic pollutants. The increase in EC sludge increased the reduction rate for Cr(VI). Their study showed that EC sludge can be valorized for Cr(VI) reduction with and without ultrasound.
Li et al. [
64] studied nanocrystalline zinc oxide (ZnO), a semiconductor. They used the sludge obtained during the EC treatment of soy sauce wastewater using Zn electrodes. The sludge was washed three times with distilled water, autoclaved at 150 °C for 24 h, centrifuged, and washed prior to XRD characterization. They found that EC sludge can be a source of ZnO nanomaterial, and they demonstrated a possible process to produce ZnO nanowires from EC Sludge.
4. Conclusions
EC sludge valorization studies had been published worldwide in the past decade. Many of these studies were conducted in the Middle East and Asia. It has been found that sludge is valorizable and that it is an ecological and economical alternative for various applications, such as pigments, fertilizer, construction materials, adsorbent, and catalyst. This paper is a review of the EC sludge valorization studies published during the last decade (2010–2020); however, it does not include all such studies published during this period. Publications wherein the full text was inaccessible were excluded from this review.
Many studies have focused on collecting phosphate and/or N into EC sludge, while some studies have investigated phosphate removal only. Many studies conducted during the last decade have confirmed that phosphate and N can be simultaneously collected from wastewater into sludge in the form of struvite, a slow-release fertilizer. Chemical analyses have shown that the struvite formed during an EC process display a considerably good quality, but its effect on the growth of plants or other organisms has not yet been investigated.
As an adsorbent, EC sludge has shown suitability for the removal of some impurities. EC sludge displays some characteristics similar to those of metallic oxides/hydroxides, which are well-known adsorbent materials. In terms of dye removal, EC sludge has been successfully used as an adsorbent, but only a few types of dye have been studied. The pigment and nutrient removal through adsorption using EC sludge as an adsorbent has been successful. In some studies, EC sludge was used as a catalyst, and promising results were achieved. In most cases, EC sludge requires calcination at temperatures above 400 °C for hours. More studies should investigate the other applications of sludge as catalysts.
Only a few publications on the use of EC sludge as pigment were found in the literature. In the future, more studies involving a larger number of colors should be conducted. Studies have shown that EC is suitable for dye removal; however, no studies on the valorization of sludge that already contains some pigments have been found. Valorization of EC sludge is one possible way to recycle Fe-based pigments. A few studies have investigated the use of EC sludge in construction materials. These studies have found that EC sludge could constitute the mass of building blocks to a certain extent. The use of EC sludge in many construction areas remains unexplored, such as in ready-mix concrete, environmental concrete products (e.g., draining channel, kerbstone, and parking element), plastering, and possibly in a new mineral wool product for insulation.
Some studies on other applications, such as nanomaterial or biogas production, have been published. However, on the basis of only one study, it would be difficult to arrive at a conclusion regarding the effectiveness of the valorization of sludge in the above applications. Thus, more studies in these areas should be conducted. A dried EC sludge is easy to modify to form different products. EC sludge possesses chemical properties suitable for use in 3D printing, landfilling, and as road base, to name a few. EC sludge may also be suitable for applications not mentioned herein.
5. Future Research Needs for EC Sludge Valorization
More sludge valorization studies are needed to identify the other possible uses of EC sludge. EC sludge offers a great potential application as a construction material, adsorbent, and catalyst. In these applications characterization of sludge is needed to optimize the valorization of the sludge. This needs more studies also from an economic point of view. Furthermore, sludge has a great potential application as a fertilizer, making it useful in farming, especially in developing countries. The sludge’s use as fertilizer must fulfill the requirements of laws such as EU Fertilizer Directive’s requirements and needs more research. Studies on the impact of EC process parameters (e.g., anode and cathode materials used, pH, current, and settling time) on sludge qualities are needed to acquire a better understanding of sludge characteristics and to obtain EC sludge with a better quality for further use.
The valorization of gases formed in the EC process should be further studied. It has been found that approximately 15% of the total energy used in EC process can be replaced by the energy derived from H2 gas if only it can be collected from EC systems. In the EC process, developments that could bring all resources (i.e., treated water, sludge, and heat and gases) into essential use are desirable. Different EC processes should be compared in order to develop more efficient equipment. The development of an EC process that could optimize sludge quality is one future area of study that deserves attention.
Studies of other sludge valorization possibilities are needed to promote sludge valorization in various fields or countries. Based on the published chemical analyses of various EC sludge samples, EC sludge is potentially suitable for landfill use, as road bases, as a recycled material that is useful in Fe and Al manufacturing, and as a 3D printing material, among other applications. There is a need to study one sludge suitability in relation to several different valorization uses. However, to the best of our knowledge, no studies on EC sludge valorization in any of these areas have ever been made.
Author Contributions
Writing–original draft preparation, K.R.; writing–review and editing, all authors, visualization, K.R.; supervision, S.T. and U.L.; project administration, U.L.; funding acquisition, K.R. and U.L. All authors have read and agreed to the published version of the manuscript.
Funding
Kyösti Rajaniemi acknowledges the funding support extended by the Erkki Paasikivi Foundation, Maa- ja Vesitekniikan tuki ry and Tauno Tönning Foundation. This work was conducted under the auspices of the Waterpro (ERDF project number: A74635, funded by the European Union, European Regional Development Fund, Leverage from the EU Central and Ostrobothnia Regional Council).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article (tables and figures). The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Authors declare no conflict of interest.
Ethics Statements
Manuscript has been prepared in compliance with the Ethics in Publishing Policy.
References
- European Commission. Environment—Waste. 2020. Available online: https://ec.europa.eu/environment/waste/index.htm (accessed on 27 August 2021).
- EU. Directive 2008/98/EC. 2008, Directive on waste and repealing certain Directives. Off. J. Eur. Union 2008, 51, 3–30. [Google Scholar]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modelling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Raulio, M.; Tuomikoski, S.; Lassi, U. Comparison of batch and novel continuous electrocoagulation processes in the treatment of paint industry wash water. Desalin. Water Treat. 2019, 170, 394–404. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Wark, M.; Mitchell, C.A. A quantitative comparison between chemical dosing and electrocoagulation. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 233–248. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Jayaraj, J.; Sozhan, G. Remediation of phosphate-contaminated water by electrocoagulation with aluminium, aluminium alloy and mild steel anodes. J. Hazard. Mater. 2009, 164, 1480–1486. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Hu, T.; Nurmesniemi, E.-T.; Tuomikoski, S.; Lassi, U. Phosphate and Ammonium Removal from Water through Electrochemical and Chemical Precipitation of Struvite. Processes 2021, 9, 150. [Google Scholar] [CrossRef]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of pharmaceutical from water with an electrocoagulation process; effect of various parameters and studies of isotherm and kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Wei, V.; Elektorowicz, M.; Oleszkiewicz, J.A. Influence of electric current on bacterial viability in wastewater treatment. Water Res. 2011, 45, 5058–5062. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of lead and zinc from battery industry wastewater using electrocoagulation process: Influence of direct and alternating current by using iron and stainless steel rod electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Electrochemically assisted coagulation for the removal of boron from water using zinc anode. Desalination 2013, 310, 122–129. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Kuokkanen, V.; Kuokkanen, T.; Rämö, J.; Lassi, U. Recent Applications of Electrocoagulation in Treatment of Water and Wastewater—A Review. Green Sustain. Chem. 2013, 3, 89–121. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M.; Varyani, A.S. Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation (ECF) process. Desalination 2011, 275, 102–106. [Google Scholar] [CrossRef]
- Naje, A.S.; Abbas, S.A. Electrocoagulation technology in wastewater treatment: A review of methods and applications. Civil Environ. Res. 2013, 3, 29–42. [Google Scholar]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, H. Utilization of electrocoagulation-treated spent wash sludge in making building blocks. Int. J. Environ. Sci. Technol. 2016, 13, 349–358. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Onpeker, S.E.; Ozel, E. The treatment of chromium containing wastewater using electrocoagulation and the production of ceramic pigments from the resulting sludge. J. Environ. Manage. 2017, 200, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Golder, A.K.; Samanta, A.N.; Ray, S. Anionic reactive dye removal from aqueous solution using a new adsorbent—sludge generated in removal of heavy metal by electrocoagulation. Chem. Eng. J. 2006, 122, 107–115. [Google Scholar] [CrossRef]
- Ghanbari, F.; Zirrahi, F.; Olfati, D.; Gohari, F.; Hassani, A. TiO2 nanoparticles removal by electrocoagulation using iron electrodes: Catalytic activity of electrochemical sludge for the degradation of emerging pollutant. J. Mol. Liq. 2020, 310, 113217. [Google Scholar] [CrossRef]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.W.; Van Vuuren, D.P.; Willems, J.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Kannan, B.S.; Lakshmi, J.; Mohanraj, S.; Sozhan, G. Effects of alternating and direct current in electrocoagulation process on the removal of fluoride from water. J. Chem. Technol. Biotechnol. 2011, 86, 428–443. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Sukkasem, P.; Mutchimsattha, C. Hydrogen recovery from the electrocoagulation treatment of dye-containing wastewater. Int. J. Hydrogen Energy 2010, 35, 10934–10943. [Google Scholar] [CrossRef]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite precipitation and phosphorus removal using magnesium sacrificial anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A.; St John, D.; Wu, X.; Nairn, J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros. Sci. 1997, 39, 1981–2004. [Google Scholar] [CrossRef]
- Chaturved, S.I. Electrocoagulation: A novel wastewater treatment method. Int. J. Mod. Eng. Res. 2013, 3, 93–100. [Google Scholar]
- Ilhan, F.; Kurt, U.; Apaydin, O.; Gonullu, M.T. Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J. Hazard. Mater. 2008, 154, 381–389. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Fluoride removal by a continuous flow electrocoagulation reactor. J. Environ. Manag. 2009, 90, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Lee, J. Performance evaluation of electrocoagulation and electrode-watering system for reduction of water content in sewage sludge, Korean. J. Chem. Eng. 2006, 23, 188–193. [Google Scholar] [CrossRef]
- Gomes, J.A.G.; Daida, P.; Kesmez, M.; Weir, M.; Moreno, H.; Parga, J.R.; Irwin, G.; McWhinney, H.; Grady, T.; Peterson, E.; et al. Arsenic removal by electrocoagulation using combined Al–Fe electrode system and characterization of products. J. Hazard. Mater. 2007, 139, 220–231. [Google Scholar] [CrossRef]
- Shon, H.K.; Phuntsho, S.; Vigneswaran, S.; Kandasamy, J.; Nghiem, L.D.; Kim, G.J.; Kim, J.B.; Kim, K.J.-H. Preparation of titanium dioxide nanoparticles from electrocoagulated sludge using sacrificial titanium electrodes. Environ. Sci. Technol. 2010, 44, 5553–5557. [Google Scholar] [CrossRef] [PubMed]
- Tezcan Un, U.; Ozel, E. Electrocoagulation of yogurt industry wastewater and the production of ceramic pigments from the sludge. Sep. Purif. Technol. 2013, 120, 386–391. [Google Scholar] [CrossRef]
- Adyel, T.M.; Rahman, S.H.; Zaman, M.M.; Sayem, H.; Khan, M.; Gafur, A.; Islam, S.M.N. Utilization Feasibility of Electrocoagulated Metal Hydroxide Sludge of Textile Industry in the Manufacturing of Building Blocks. J. Waste Manag. 2013, 2013, 686981. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Global anthropogenic phosphorus loads to freshwater and associated grey water footprints and water pollution levels: A high-resolution global study. Water Resour. Res. 2018, 54, 345–358. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total. Environ. 2013, 461–462, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Zengwei, Y.; Songyan, J.; Hu, S.; Xin, L.; Hui, H.; Xuewei, L.; You, Z. Human Perturbation of the Global Phosphorus Cycle: Changes and Consequences. Environ. Sci. Technol. 2018, 52, 2438–2450. [Google Scholar] [CrossRef]
- Talboys, P.; Heppell, J.; Roose, T.; Healey, J.; Jones, D.; Withers, P. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; An, B.M.; Lim, D.H.; Park, J.Y. Electricity production and phosphorous recovery as struvite from synthetic wastewater using magnesium-air fuel cell electrocoagulation. Water Res. 2019, 132, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fu, R.; Lv, H.; Zhu, G.; Lu, B.; Zhou, Z.; Wu, X.; Chen, H. Phosphate Recovery from Swine Wastewater by a Struvite Precipitation Electrolyzer. Sci. Rep. 2019, 9, 8893. [Google Scholar] [CrossRef]
- Hug, A.; Udert, K.M. Struvite precipitation from urine with electrochemical magnesium dosage. Water Res. 2012, 47, 289–299. [Google Scholar] [CrossRef]
- Garcia, F.P.; Hernández, J.C.; Reyes Gruz, V.E.; Santillán, Y.M.; Méndez Marzo, M.A.; Ávila, J.H.; Moreno, F.P. Recovery and Characterization of Struvite from Sediment and Sludge Resulting from the Process of Acid Whey Electrocoagulation. Asian J. Chem. 2013, 25, 8005–8009. [Google Scholar] [CrossRef]
- Lin, X.; Han, Z.; Yu, H.; Ye, Z.; Zhu, S.; Zhu, J. Struvite precipitation from biogas digestion slurry using a two-chamber electrolysis cell with a magnesium anode. J. Clean. Prod. 2017, 174, 1598–1607. [Google Scholar] [CrossRef]
- Huang, H.M.; Zhang, P.; Zhang, Z.; Liu, J.H.; Xiao, J.; Gao, F.M. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Golder, A.; Samanta, A.; Ray, S. Removal of phosphate from aqueous solutions using calcined metal hydroxides sludge waste generated from electrocoagulation. Sep. Purif. Technol. 2006, 52, 102–109. [Google Scholar] [CrossRef]
- Yilmaz, A.E.; Boncukcuoğlu, R.; Kocakerim, M.; Karakaş, İ.H. Waste utilization: The removal of textile dye (Bomaplex Red CR-L) from aqueous solution on sludge waste from electrocoagulation as adsorbent. Desalination 2011, 277, 156–163. [Google Scholar] [CrossRef]
- Yilmaz, A.E.; Fil, B.A.; Bayar, S.; Karcioglu Karakas, Z. A new adsorbent for fluoride removal: The utilization of sludge waste from electrocoagulation as adsorbent. Global NEST J. 2015, 17, 186–197. [Google Scholar]
- Pandey, N.; Thakur, C. Study on treatment of paper mill wastewater by electrocoagulation and its sludge analysis. Chem. Data Collect. 2020, 27, 100390. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Topal, S.; Ates, F. Electrocoagulation of tissue paper wastewater and an evaluation of sludge for pyrolysis. Desalin. Water Treat. 2016, 57, 28724–28733. [Google Scholar] [CrossRef]
- Samy, M.; Alalm, M.G.; Mossad, M. Utilization of Iron Sludge Resulted from Electrocoagulation in Heterogeneous Phot-Fenton process. Water Pract. Technol. 2020, 15, 1228–1237. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Umaiyambika, N.; Gayathri, R. Dye removal from aqueous solution by electrocoagulation process using stainless steel electrodes. Environ. Eng. Manag. J. 2010, 9, 1031–1037. [Google Scholar] [CrossRef]
- Daneshvar, N.; Oladegaragoze, A.; Djafarzadeh, N. Decolorization of basic dye solutions by electrocoagulation: An investigation of the effect of operational parameters. J. Hazard. Mater. 2006, 129, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-C.; Wang, K.-S.; Huang, C.-L.; Chiang, C.-W.; Chang, T.-J.; Lee, S.-S.; Chang, S.-H. Improvement of textile dye removal by electrocoagulation with low-cost steel wool cathode reactor. Chem. Eng. J. 2012, 192, 37–44. [Google Scholar] [CrossRef]
- Golder, A.; Hridaya, N.; Samanta, A.; Ray, S. Electrocoagulation of methylene blue and eosin yellowish using mild steel electrodes. J. Hazard. Mater. 2005, 127, 134–140. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, D.F.; Chiang, P.C. Utilization of sludge as brick materials. Adv. Environ. Res. 2003, 7, 679–685. [Google Scholar] [CrossRef]
- Balasubramanian, J.; Sabumon, P.C.; Lazar, J.U.; Ilangovan, R. Utilization of textile effluent treatment plant sludge in building materials. Waste Manag. 2006, 26, 22–28. [Google Scholar] [CrossRef]
- Hutnan, M.; Drtil, M.; Kalina, A. Anaerobic stabilization of sludge produced during municipal wastewater treatment by electrocoagulation. J. Hazard. Mater. B 2006, 131, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.N.; Muthukumar, K. Ultrasound mediated reduction of Cr(VI) using sludge obtained during electrocoagulation. Environ. Technol. 2011, 13, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, F.; Ma, C.; Elingarami, S.; He, N. Preparation of ZnO Nanowire Using Sludge from Wastewater Treatment. J. Nanosci. Nanotechol. 2013, 13, 5859–5863. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).