Intelligent Control of Seizure-Like Activity in a Memristive Neuromorphic Circuit Based on the Hodgkin–Huxley Model
Abstract
1. Introduction
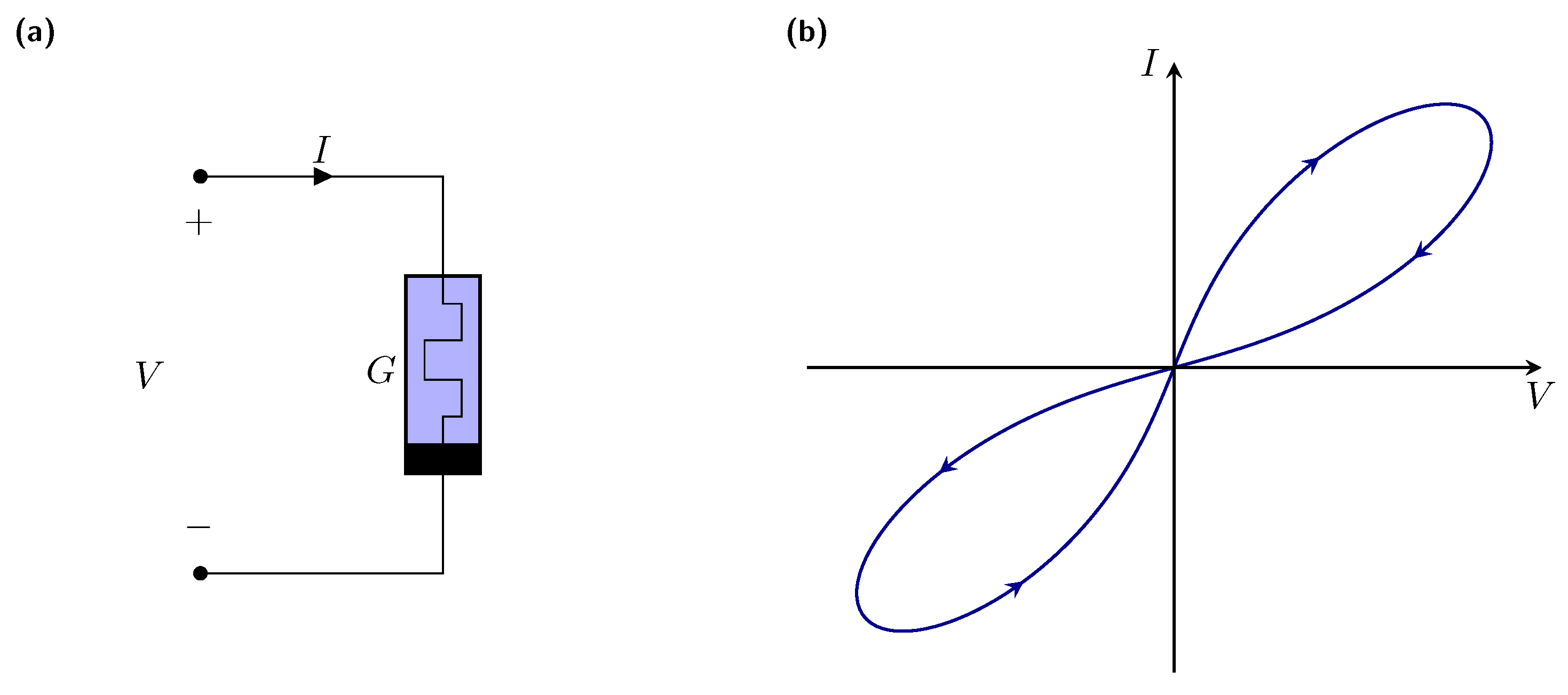
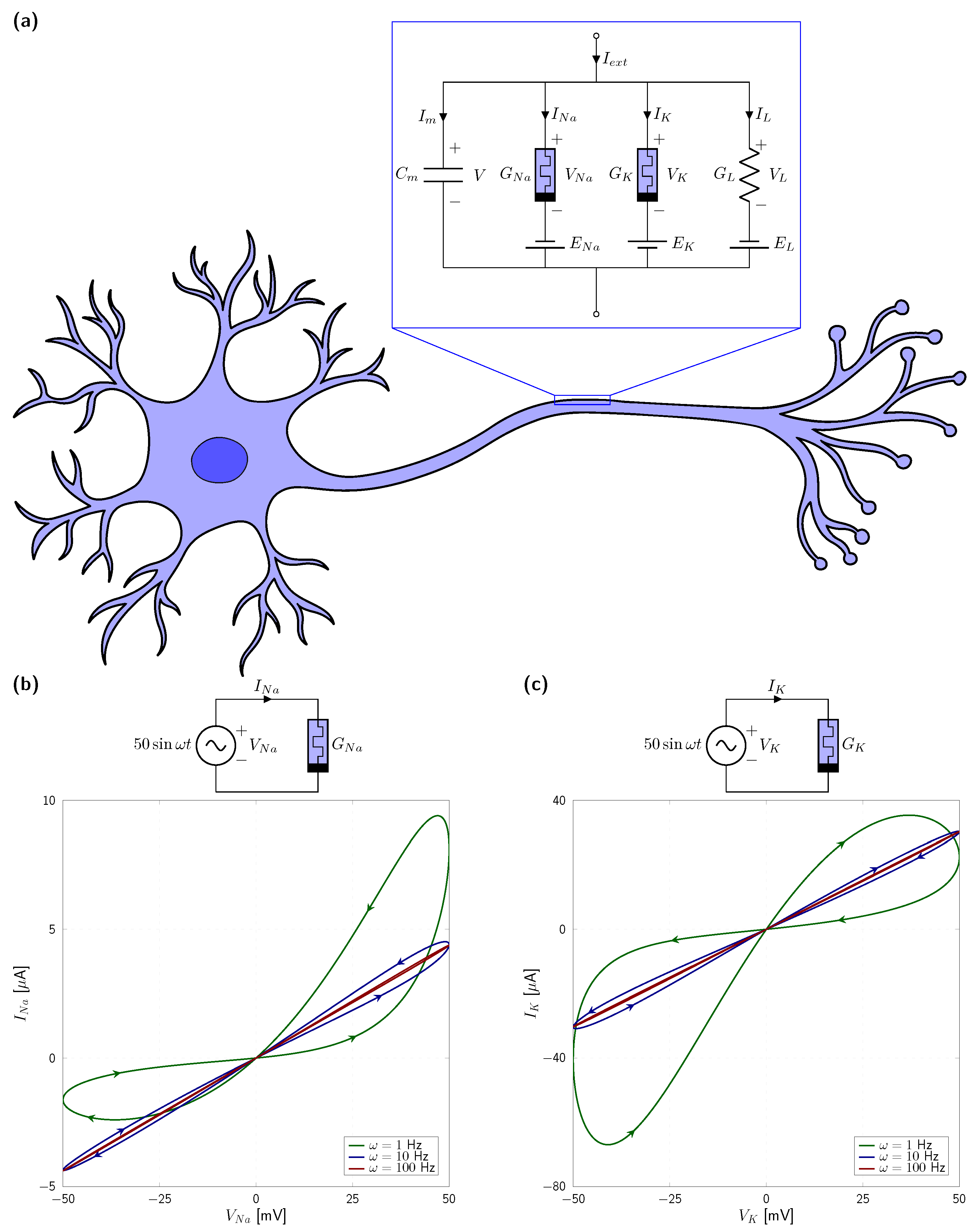
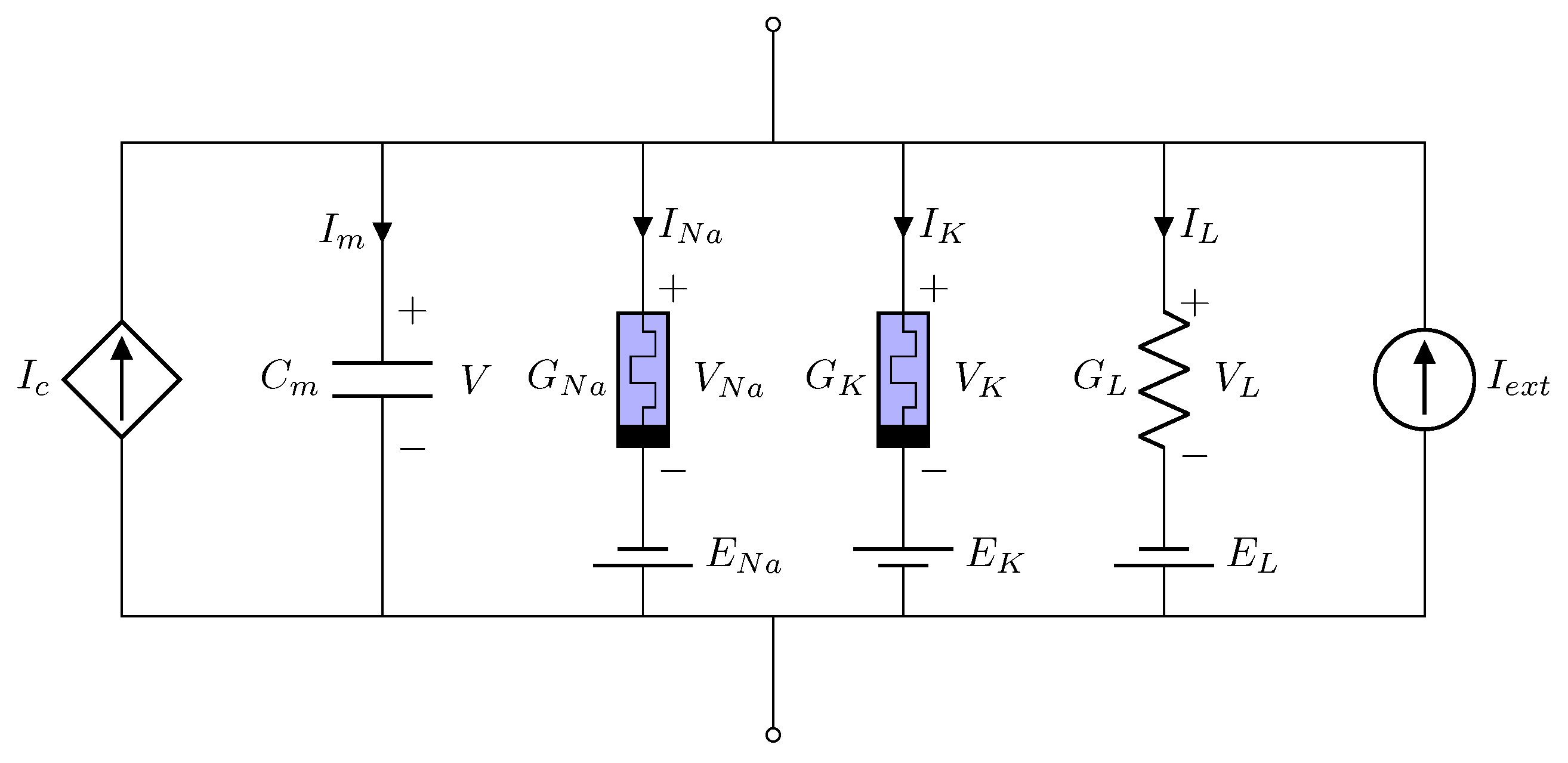
2. Memristive Neuromorphic Circuit
3. Intelligent Control
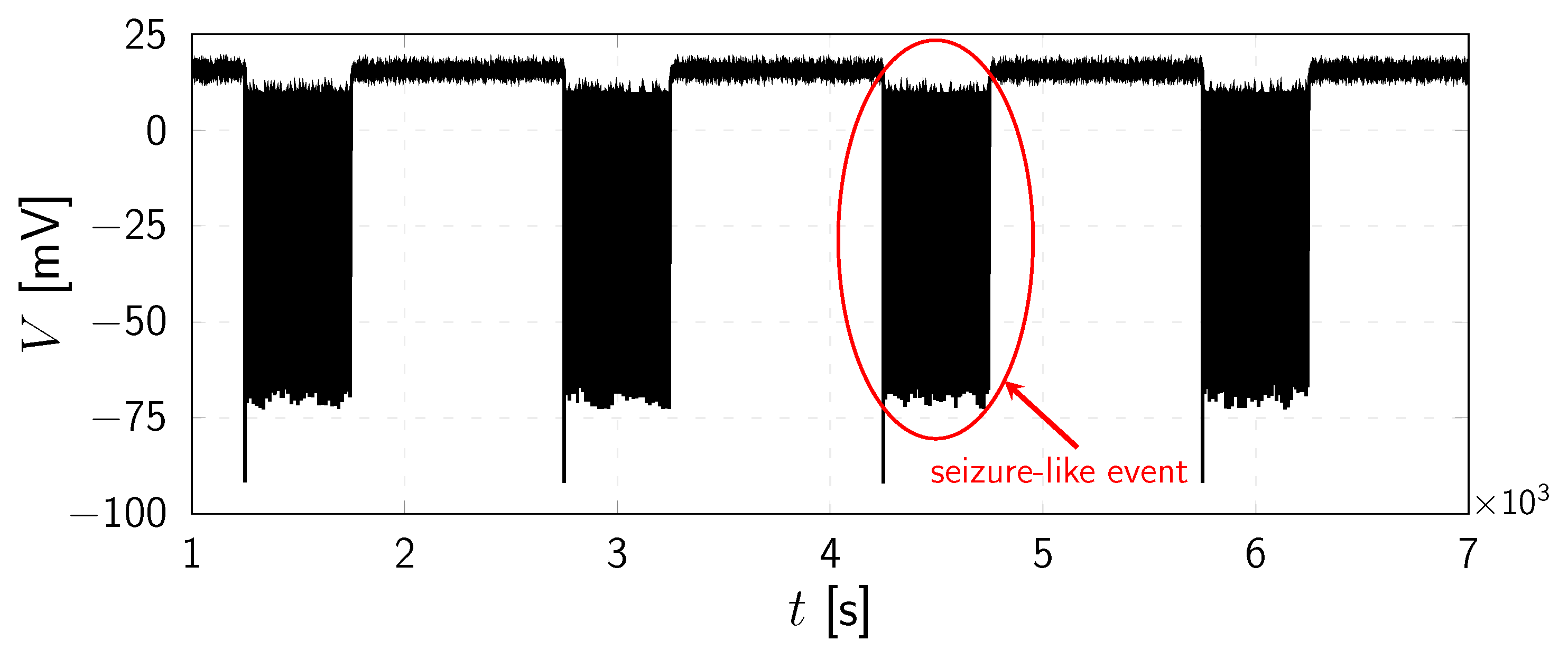
4. Numerical Results
- The intelligent control scheme can adequately handle the unknown external electric current and the uncertainties associated with the unmodeled internal states of the memristors, which in fact may allow other different memristive circuits to be controlled by means of the same control framework;
- Online learning, rather than offline training, is adopted to allow the controller to continuously improve its overall performance, even when subjected to dynamically changing circumstances;
- The neural network can learn to compensate for uncertainties and disturbances by minimizing the control error, making direct measurements of the signal to be estimated (which is not possible in real applications) completely unnecessary;
- By combining the artificial neural network with a nonlinear control method, the computational demands on the ANN are minimized, which allows its implementation on low-power embedded hardware and the adoption of the online learning scheme.
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schuman, C.D.; Kulkarni, S.R.; Parsa, M.; Mitchell, J.P.; Date, P.; Kay, B. Opportunities for neuromorphic computing algorithms and applications. Nat. Comput. Sci. 2022, 2, 10–19. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, S.; Liang, Y.; Wang, G. Neuromorphic dynamics near the edge of chaos in memristive neurons. Chaos Solitons Fractals 2022, 160, 112241. [Google Scholar] [CrossRef]
- Choi, S.; Yang, J.; Wang, G. Emerging Memristive Artificial Synapses and Neurons for Energy-Efficient Neuromorphic Computing. Adv. Mater. 2020, 32, 2004659. [Google Scholar] [CrossRef]
- Zidan, M.A.; Strachan, J.P.; Lu, W.D. The future of electronics based on memristive systems. Nat. Electron. 2018, 1, 22–29. [Google Scholar] [CrossRef]
- George, R.; Chiappalone, M.; Giugliano, M.; Levi, T.; Vassanelli, S.; Partzsch, J.; Mayr, C. Plasticity and Adaptation in Neuromorphic Biohybrid Systems. iScience 2020, 23, 101589. [Google Scholar] [CrossRef]
- An, H.; Li, J.; Li, Y.; Fu, X.; Yi, Y. Three dimensional memristor-based neuromorphic computing system and its application to cloud robotics. Comput. Electr. Eng. 2017, 63, 99–113. [Google Scholar] [CrossRef]
- Liu, T.Y.; Mahjoubfar, A.; Prusinski, D.; Stevens, L. Neuromorphic computing for content-based image retrieval. PLoS ONE 2022, 17, e0264364. [Google Scholar] [CrossRef]
- Buccelli, S.; Bornat, Y.; Colombi, I.; Ambroise, M.; Martines, L.; Pasquale, V.; Bisio, M.; Tessadori, J.; Nowak, P.; Grassia, F.; et al. A Neuromorphic Prosthesis to Restore Communication in Neuronal Networks. iScience 2019, 19, 402–414. [Google Scholar] [CrossRef]
- Isah, A.; Bilbault, J.M. Review on the Basic Circuit Elements and Memristor Interpretation: Analysis, Technology and Applications. J. Low Power Electron. Appl. 2022, 12, 44. [Google Scholar] [CrossRef]
- Chiolerio, A.; Chiappalone, M.; Ariano, P.; Bocchini, S. Coupling Resistive Switching Devices with Neurons: State of the Art and Perspectives. Front. Neurosci. 2017, 11, 70. [Google Scholar] [CrossRef]
- Chua, L. Memristor, Hodgkin-Huxley, and Edge of Chaos. Nanotechnology 2013, 24, 383001. [Google Scholar] [CrossRef]
- Chua, L. Memristor-The missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507–519. [Google Scholar] [CrossRef]
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef]
- Williams, R.S. How We Found The Missing Memristor. IEEE Spectr. 2008, 45, 28–35. [Google Scholar] [CrossRef]
- Dias, C.; Castro, D.; Aroso, M.; Ventura, J.; Aguiar, P. Memristor-Based Neuromodulation Device for Real-Time Monitoring and Adaptive Control of Neuronal Populations. ACS Appl. Electron. Mater. 2022, 4, 2380–2387. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, J.J. Memristive crossbar arrays for brain-inspired computing. Nat. Mater. 2019, 18, 309–323. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, G.; Tian, B.; Dkhil, B.; Duan, C. Research progress on solutions to the sneak path issue in memristor crossbar arrays. Nanoscale Adv. 2020, 2, 1811–1827. [Google Scholar] [CrossRef]
- Chua, L.; Kang, S.M. Memristive devices and systems. Proc. IEEE 1976, 64, 209–223. [Google Scholar] [CrossRef]
- Chua, L.; Sbitnev, V.; Kim, H. Hodgkin-Huxley Axon is Made of Memristors. Int. J. Bifurc. Chaos 2012, 22, 1230011. [Google Scholar] [CrossRef]
- Beeman, D. Hodgkin-Huxley Model. In Encyclopedia of Computational Neuroscience; Springer: New York, NY, USA, 2014; pp. 1–13. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F.; Katz, B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J. Physiol. 1952, 116, 424–448. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 1952, 116, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 1952, 116, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J. Physiol. 1952, 116, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Raman, I.M.; Robinson, H.P.C.; Sejnowski, T.J.; Paulsen, O. The Hodgkin-Huxley Heritage: From Channels to Circuits. J. Neurosci. 2012, 32, 14064–14073. [Google Scholar] [CrossRef]
- Teka, W.; Stockton, D.; Santamaria, F. Power-Law Dynamics of Membrane Conductances Increase Spiking Diversity in a Hodgkin-Huxley Model. PLoS Comput. Biol. 2016, 12, e1004776. [Google Scholar] [CrossRef]
- Sohanian Haghighi, H.; Markazi, A.H.D. A new description of epileptic seizures based on dynamic analysis of a thalamocortical model. Sci. Rep. 2017, 7, 13615. [Google Scholar] [CrossRef]
- Wei, Y.; Ullah, G.; Ingram, J.; Schiff, S.J. Oxygen and seizure dynamics: II. Computational modeling. J. Neurophysiol. 2014, 112, 213–223. [Google Scholar] [CrossRef]
- Holt, A.B.; Netoff, T.I. Computational modeling of epilepsy for an experimental neurologist. Exp. Neurol. 2013, 244, 75–86. [Google Scholar] [CrossRef]
- Wendling, F. Computational models of epileptic activity: A bridge between observation and pathophysiological interpretation. Expert Rev. Neurother. 2008, 8, 889–896. [Google Scholar] [CrossRef]
- Nejad, M.M.; Rotter, S.; Schmidt, R. Basal ganglia and cortical control of thalamic rebound spikes. Eur. J. Neurosci. 2021, 54, 4295–4313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Ling, Z.; Wang, P.; Jian, X. The Influence of Transcranial Magnetoacoustic Stimulation Parameters on the Basal Ganglia-Thalamus Neural Network in Parkinson’s Disease. Front. Neurosci. 2021, 15, 761720. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Deng, B.; Wei, X.; Yu, H.; Li, H.; Fietkiewicz, C.; Loparo, K.A. Closed-Loop Control of Tremor-Predominant Parkinsonian State Based on Parameter Estimation. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Eaton, M.; Kienle, A.; Flockerzi, D.; Yang, Y.; Ramkrishna, D. Examining Sodium and Potassium Channel Conductances Involved in Hyperexcitability of Chemotherapy-Induced Peripheral Neuropathy: A Mathematical and Cell Culture-Based Study. Front. Comput. Neurosci. 2020, 14, 564980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, H.; Ma, K. Dynamical mechanism for conduction failure behavior of action potentials related to pain information transmission. Neurocomputing 2020, 387, 293–308. [Google Scholar] [CrossRef]
- Khodashenas, M.; Baghdadi, G.; Towhidkhah, F. A modified Hodgkin-Huxley model to show the effect of motor cortex stimulation on the trigeminal neuralgia network. J. Math. Neurosci. 2019, 9, 4. [Google Scholar] [CrossRef]
- Aihara, K.; Matsumoto, G. Chaotic oscillations and bifurcations in squid giant axons. In Chaos; Princeton University Press: Princeton, NY, USA, 2014; pp. 257–270. [Google Scholar] [CrossRef]
- Chay, T.; Rinzel, J. Bursting, beating, and chaos in an excitable membrane model. Biophys. J. 1985, 47, 357–366. [Google Scholar] [CrossRef]
- Cressman, J.R.; Ullah, G.; Ziburkus, J.; Schiff, S.J.; Barreto, E. The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. J. Comput. Neurosci. 2009, 26, 159–170. [Google Scholar] [CrossRef]
- Jin, W.Y.; Xu, J.X.; Wu, Y.; Hong, L.; Wei, Y.B. Crisis of interspike intervals in Hodgkin-Huxley model. Chaos Solitons Fractals 2006, 27, 952–958. [Google Scholar] [CrossRef]
- Korn, H.; Faure, P. Is there chaos in the brain? II. Experimental evidence and related models. Comptes Rendus Biol. 2003, 326, 787–840. [Google Scholar] [CrossRef]
- Chua, L. Resistance switching memories are memristors. Appl. Phys. A 2011, 102, 765–783. [Google Scholar] [CrossRef]
- Chua, L.O. The Fourth Element. Proc. IEEE 2012, 100, 1920–1927. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Dingledine, R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J. Neurophysiol. 1988, 59, 259–276. [Google Scholar] [CrossRef]
- Ziburkus, J.; Cressman, J.R.; Barreto, E.; Schiff, S.J. Interneuron and Pyramidal Cell Interplay During In Vitro Seizure-Like Events. J. Neurophysiol. 2006, 95, 3948–3954. [Google Scholar] [CrossRef] [PubMed]
- Bessa, W.M.; Brinkmann, G.; Duecker, D.A.; Kreuzer, E.; Solowjow, E. A Biologically Inspired Framework for the Intelligent Control of Mechatronic Systems and Its Application to a Micro Diving Agent. Math. Probl. Eng. 2018, 2018, 9648126. [Google Scholar] [CrossRef]
- Bessa, W.M.; Kreuzer, E.; Lange, J.; Pick, M.A.; Solowjow, E. Design and Adaptive Depth Control of a Micro Diving Agent. IEEE Robot. Autom. Lett. 2017, 2, 1871–1877. [Google Scholar] [CrossRef]
- Bessa, W.M.; Otto, S.; Kreuzer, E.; Seifried, R. An adaptive fuzzy sliding mode controller for uncertain underactuated mechanical systems. J. Vib. Control 2019, 25, 1521–1535. [Google Scholar] [CrossRef]
- Bessa, W.M.; De Paula, A.S.; Savi, M.A. Adaptive fuzzy sliding mode control of a chaotic pendulum with noisy signals. Z. Angew. Math. Mech. 2014, 94, 256–263. [Google Scholar] [CrossRef]
- Scarselli, F.; Chung Tsoi, A. Universal Approximation Using Feedforward Neural Networks: A Survey of Some Existing Methods, and Some New Results. Neural Netw. 1998, 11, 15–37. [Google Scholar] [CrossRef]
- Tanaka, M.C.; de Macedo Fernandes, J.M.; Bessa, W.M. Feedback Linearization with Fuzzy Compensation for Uncertain Nonlinear Systems. Int. J. Comput. Commun. Control 2013, 8, 736–743. [Google Scholar] [CrossRef][Green Version]
- Ioannou, P.; Fidan, B. Adaptive Control Tutorial; SIAM: Philadelphia, PA, USA, 2006. [Google Scholar] [CrossRef]

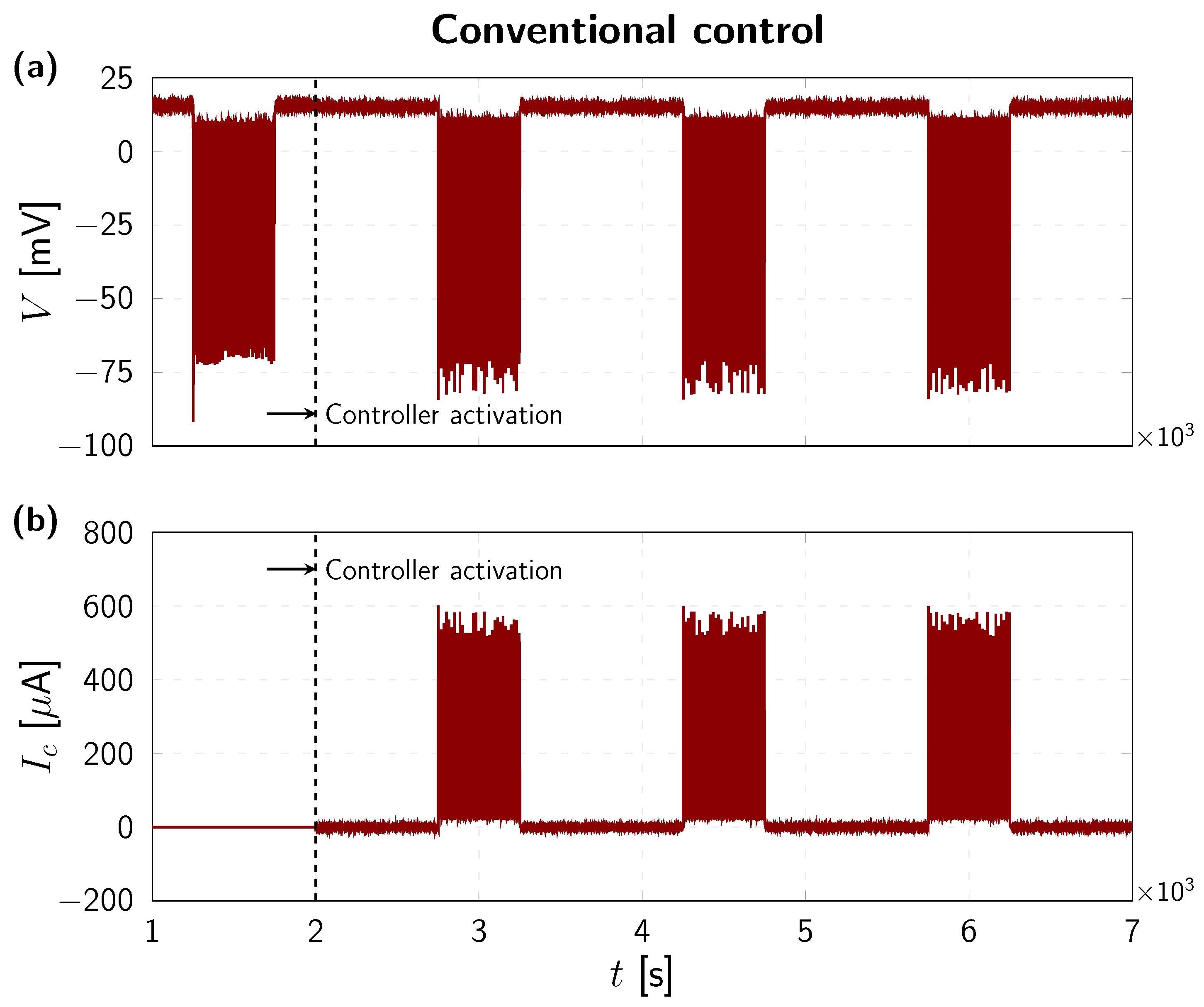
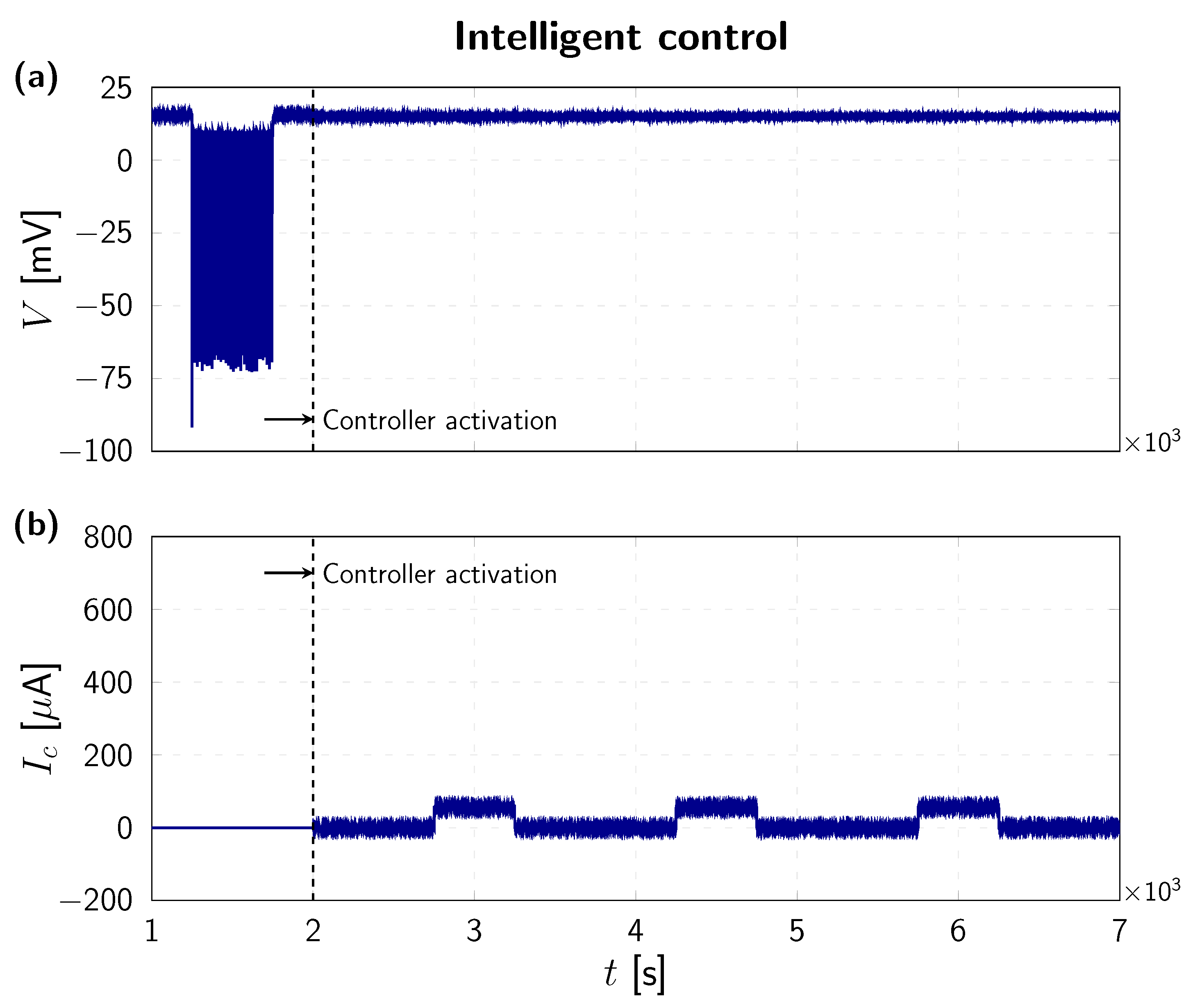
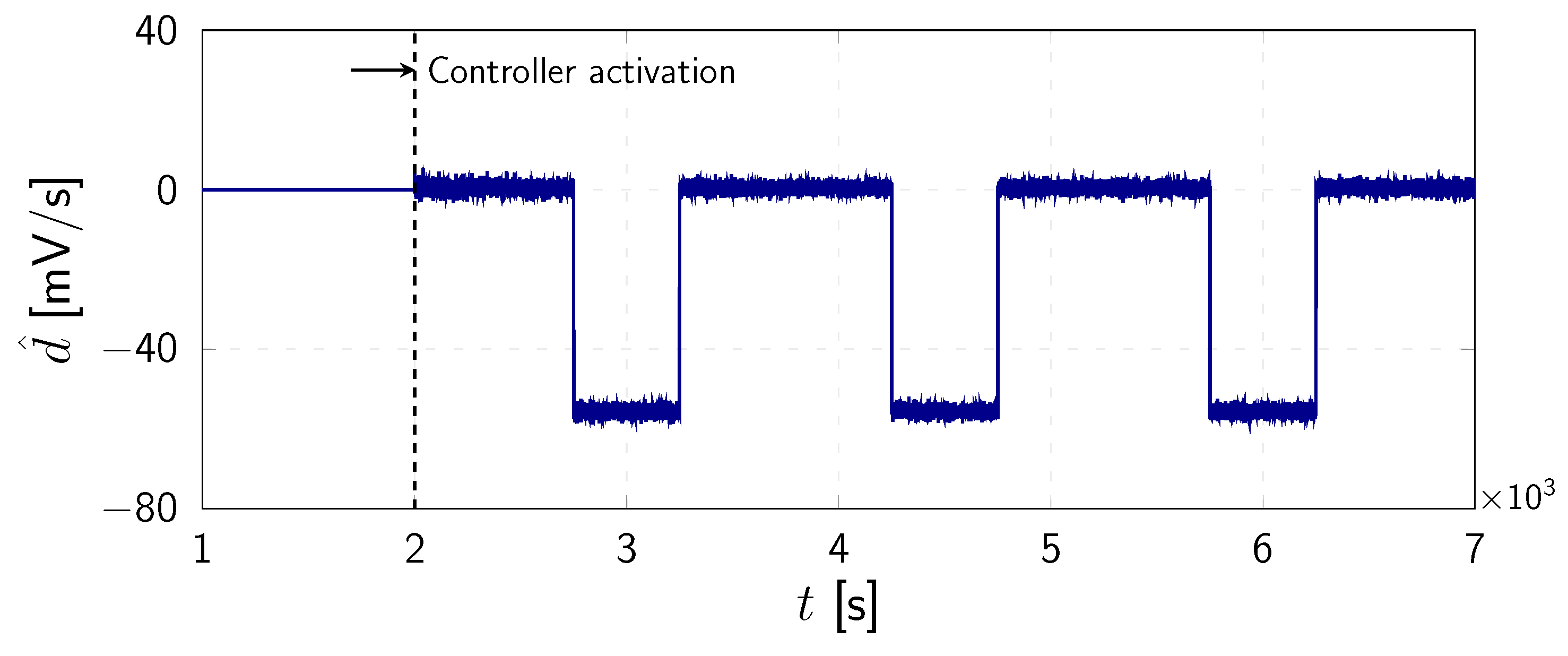
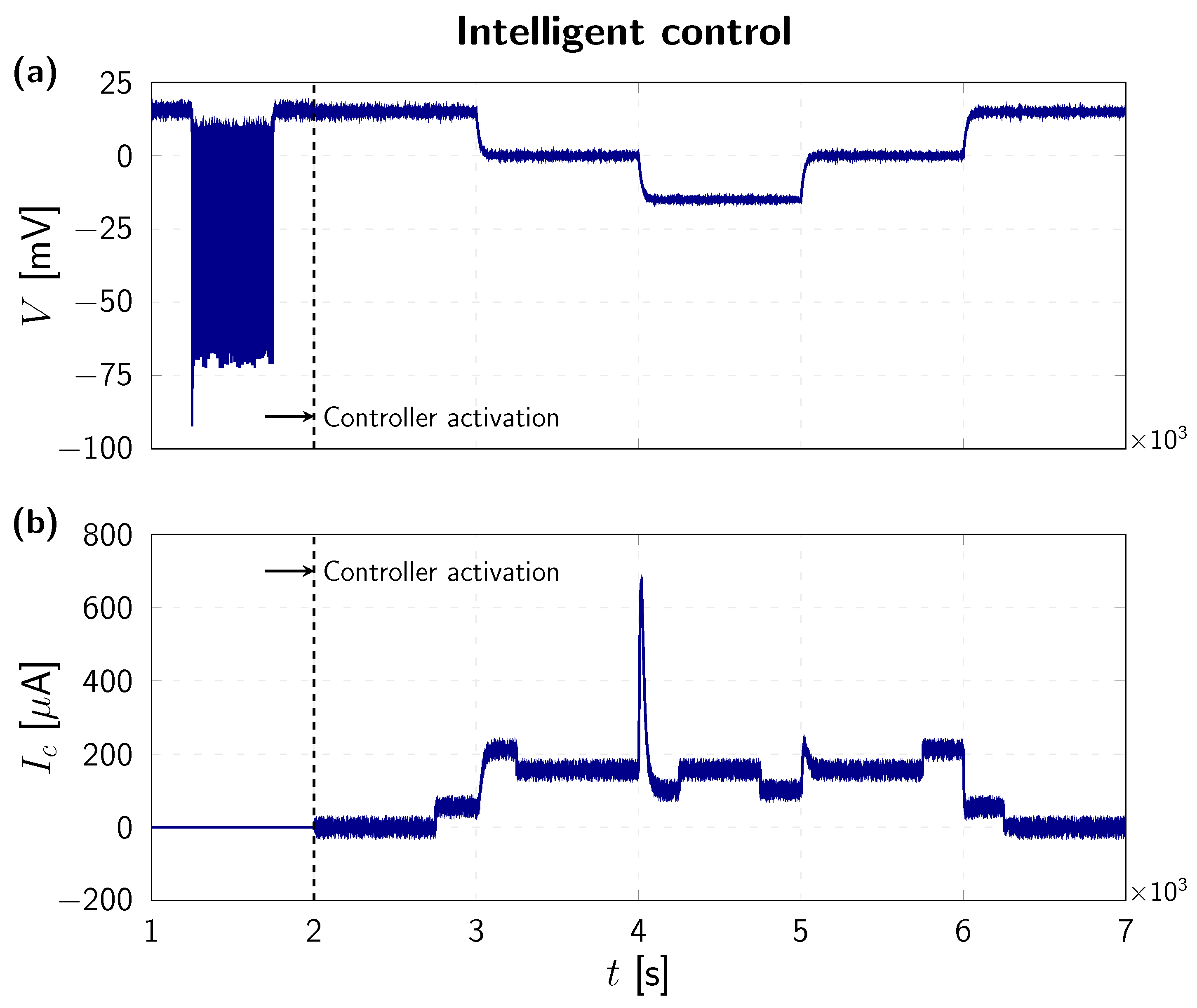
| 1 µF | 115 mV | 12 mV | mV | 120 mS | 36 mS | mS |
| Centers | ||||||
| Widths |
| Conventional | Intelligent | Percentage Reduction | |
|---|---|---|---|
| IAE [mV s] | |||
| IACI [C] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessa, W.M.; Lima, G.d.S. Intelligent Control of Seizure-Like Activity in a Memristive Neuromorphic Circuit Based on the Hodgkin–Huxley Model. J. Low Power Electron. Appl. 2022, 12, 54. https://doi.org/10.3390/jlpea12040054
Bessa WM, Lima GdS. Intelligent Control of Seizure-Like Activity in a Memristive Neuromorphic Circuit Based on the Hodgkin–Huxley Model. Journal of Low Power Electronics and Applications. 2022; 12(4):54. https://doi.org/10.3390/jlpea12040054
Chicago/Turabian StyleBessa, Wallace Moreira, and Gabriel da Silva Lima. 2022. "Intelligent Control of Seizure-Like Activity in a Memristive Neuromorphic Circuit Based on the Hodgkin–Huxley Model" Journal of Low Power Electronics and Applications 12, no. 4: 54. https://doi.org/10.3390/jlpea12040054
APA StyleBessa, W. M., & Lima, G. d. S. (2022). Intelligent Control of Seizure-Like Activity in a Memristive Neuromorphic Circuit Based on the Hodgkin–Huxley Model. Journal of Low Power Electronics and Applications, 12(4), 54. https://doi.org/10.3390/jlpea12040054








