Systems-Oriented Modelling Methods in Preventing and Controlling Emerging Infectious Diseases in the Context of Healthcare Policy: A Scoping Review
Abstract
1. Introduction
Research Question
- What was the context in which the systems-oriented study was conducted?
- Who were the target population?
- What was the systems-oriented aim?
- What were the main complex-systems features considered?
- What were the system’s main elements?
- What were the systems-oriented methods used?
- What challenges related to systems modelling did the authors face?
- Who were the main stakeholder and how were they involved?
- What were the key lessons learned from using the complex systems approach?
2. Materials and Methods
2.1. Preparation
2.2. Identifying Keywords and MeSH Terms
2.3. Identifying Relevant Studies
2.4. Study Selection
2.5. Data Charting
- Numerosity: complex systems involve many interactions among many components.
- Disorder and diversity: the interactions in a complex system are not coordinated or controlled centrally, and the components may differ.
- Feedback: the interactions in complex systems are iterated so that there is feedback from previous interactions on a timescale relevant to the system’s emergent dynamics.
- Non-equilibrium: complex systems are open to the environment and are often driven by something external.
- Spontaneous order and self-organisation: complex systems exhibit structure and order that arises out of the interactions among their parts.
- Nonlinearity: complex systems exhibit nonlinear dependence on parameters or external drivers.
- Robustness: the structure and function of complex system is stable under relevant perturbations.
- Nested structure and modularity: there may be multiple scales of structure, clustering and specialisation of function in complex systems.
- History and memory: complex systems often require a very long history to exist and also store information about history.
- Adaptive behaviour: complex systems are often able to modify their behaviour depending on the state of the environment and the predisposition they make about it.
2.6. Results Summary and Dissemination
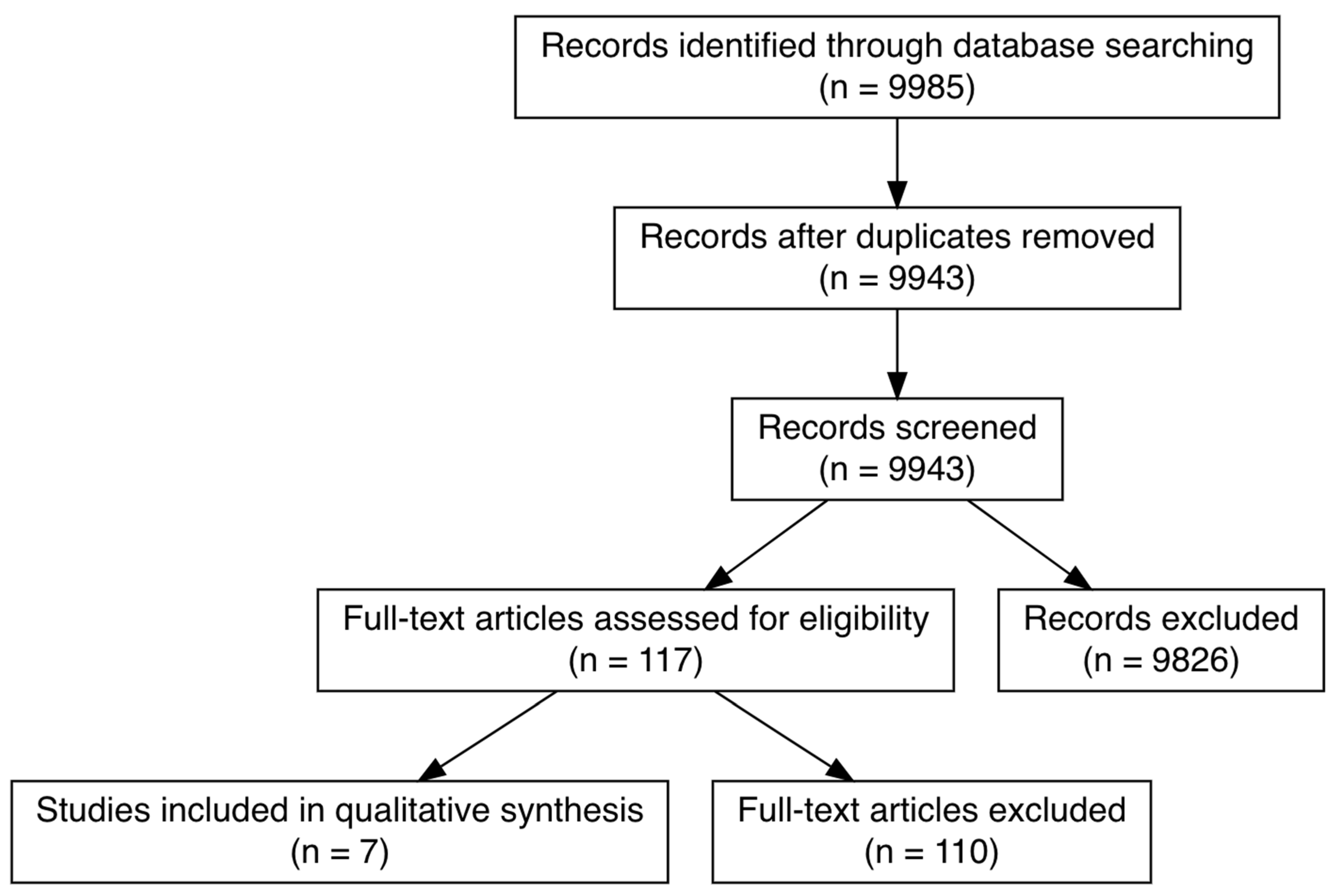
3. Results
3.1. Search and Selection of Citations
3.2. General Characteristics of Included Citations
3.3. Methodological Characteristics of Included Studies
3.3.1. How Are Systems-Oriented Modelling Methods Used to Investigate How to Prevent and Control Emerging Infectious Diseases (EIDs)?
3.3.2. In What Contexts Were the Systems-Oriented Studies Conducted?
3.3.3. Who Was the Target Population?
3.3.4. What Were the Main Complex-Systems Features?
3.3.5. What Was the Systems-Oriented Aim?
3.3.6. What Were the Main Systems Elements?
3.3.7. What Were Systems-Oriented Methods Used?
3.3.8. What Challenges Related to Systems-Modelling Did the Authors Face?
3.3.9. Who Were the Main Stakeholders? Moreover, How Were They Involved?
3.3.10. What Were the Key Lessons Learned from Using the Complex Systems Approach?
4. Discussion
Limitations and Strengths
5. Conclusions
Recommendations for Future Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Keywords Search across Databases
Appendix A.1. PubMed 23/3/21, 1905 Results
Appendix A.2. Web of Science 23/3/21, 1880 Results
Appendix A.3. Scopus 23/3/21, 9230 Results
References
- Nandi, A.; Allen, L.J.S. Probability of a zoonotic spillover with seasonal variation. Infect. Dis. Model. 2021, 6, 514–531. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization ROfS-EA. A brief guide to emerging infectious diseases and zoonoses. WHO Reg. Off. South-East Asia 2014, 1, 1–2. [Google Scholar]
- McArthur, D.B. Emerging infectious diseases. Nurs. Clin. N. Am. 2019, 54, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.J.; Aguilera, X.; Heymann, D.; Wilder-Smith, A.; Bausch, D.G.; Briand, S.; Bruschke, C.; Carmo, E.H.; Cleghorn, S.; Dandona, L.; et al. Preparedness for emerging epidemic threats: A Lancet Infectious Diseases Commission. Lancet Infect. Dis. 2020, 20, 17–19. [Google Scholar] [CrossRef]
- (CDC) CfDCaP. Division of Preparedness and Emerging Infections (DPEI). 2021. Available online: https://www.cdc.gov/ncezid/dpei/eip/index.html (accessed on 21 April 2022).
- Meadows, D.H. Thiniking in Systems; Earthscan: London, UK, 2009. [Google Scholar]
- Meadows, D. Leverage Points: Places to Intervene in a System. 1999. Available online: https://donellameadows.org/archives/leverage-points-places-to-intervene-in-a-system/ (accessed on 26 May 2021).
- Joanna Briggs Institute. Joanna Briggs Institute Reviewer’s Manual. 2019. Available online: https://wiki.joannabriggs.org/display/MANUAL/11.1+Introduction+to+Scoping+reviews (accessed on 21 April 2022).
- Mansouri, M.A.; Kee, F.; Garcia, L.; Bradley, D.T. Role of systems science in preventing and controlling emerging infectious diseases: Protocol for a scoping review. BMJ Open 2021, 11, e046057. [Google Scholar] [CrossRef] [PubMed]
- WHO. Prioritizing Diseases for Research and Development in Emergency Contexts; WHO: Geneva, Switzerland, 2021. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 26 May 2021).
- Ladyman, J.; Wiesner, K. What Is a Complex System? Yale University Press: New Haven, CT, USA; London, UK, 2020. [Google Scholar]
- Mutanga, S.S.; Ngungu, M.; Tshililo, F.P.; Kaggwa, M. Systems dynamics approach for modelling South Africa’s response to COVID-19: A “What if” Scenario. J. Public Health Res. 2021, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Roobaswathiny, A.; Lakshmi Priyadarsini, S. A study on the factors that influence the agility of COVID-19 hospitals. Int. J. Healthc. Manag. 2021, 14, 290–299. [Google Scholar] [CrossRef]
- Shin, N.; Kwag, T.; Park, S.; Kim, Y.H. Effects of operational decisions on the diffusion of epidemic disease: A system dynamics modeling of the MERS-CoV outbreak in South Korea. J. Theor. Biol. 2017, 421, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Parr, T.; Zeidman, P.; Razi, A.; Flandin, G.; Daunizeau, J.; Hulme, O.J.; Billig, A.J.; Litvak, V.; Price, C.J.; et al. Effective immunity and second waves: A dynamic causal modelling study. Wellcome Open Res. 2020, 5, 204. [Google Scholar] [CrossRef] [PubMed]
- Scabini, L.F.S.; Ribas, L.C.; Neiva, M.B.; Junior, A.G.B.; Farfán, A.J.F.; Bruno, O.M. Social interaction layers in complex networks for the dynamical epidemic modeling of COVID-19 in Brazil. Phys. A Stat. Mech. Appl. 2021, 564, 125498. [Google Scholar] [CrossRef] [PubMed]
- Weixing, W.; Yanli, L.; Jinjin, Z. System dynamics modeling of SARS transmission—A case study of Hebei Province. In Proceedings of the 2009 International Conference on Management and Service Science, Beijing, China, 20–22 September 2009. [Google Scholar]
- Silva, P.C.L.; Batista, P.V.C.; Lima, H.S.; Alves, M.A.; Guimarães, F.G.; Silva, R.C.P. COVID-ABS: An agent-based model of COVID-19 epidemic to simulate health and economic effects of social distancing interventions. Chaos Solitons Fractals 2020, 139, 110088. [Google Scholar] [CrossRef] [PubMed]
- Lanza, F.; Seidita, V.; Chella, A. Agents and robots for collaborating and supporting physicians in healthcare scenarios. J. Biomed. Inform. 2020, 108, 103483. [Google Scholar] [CrossRef] [PubMed]
- REALKM. The Mathematics of Modeling: Differential Equations and System Dynamics [Systems Thinking & Modelling Series]. 2017. Available online: https://realkm.com/2017/11/28/the-mathematics-of-modeling-differential-equations-and-system-dynamics-systems-thinking-modelling-series/ (accessed on 29 July 2022).
- Bradley, D.T.; Mansouri, M.A.; Kee, F.; Garcia, L.M.T. A systems approach to preventing and responding to COVID-19. eClinicalMedicine 2020, 21, 100325. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannis, T. A qualitative model of patterns of resilience and vulnerability in responding to a pandemic outbreak with system dynamics. Saf. Sci. 2021, 134, 105077. [Google Scholar] [CrossRef]
- Sahin, O.; Salim, H.; Suprun, E.; Richards, R.; MacAskill, S.; Heilgeist, S.; Rutherford, S.; Stewart, R.A.; Beal, C.D. Developing a preliminary causal loop diagram for understanding the wicked complexity of the COVID-19 pandemic. Systems 2020, 8, 20. [Google Scholar] [CrossRef]
- Klement, R.J. Systems thinking about SARS-CoV-2. Front. Public Health 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Rahmandad, H.; Sterman, J. Reporting guidelines for simulation-based research in social sciences. Syst. Dyn. Rev. 2012, 28, 396–411. [Google Scholar] [CrossRef]
- Cassidy, R.; Singh, N.S.; Schiratti, P.-R.; Semwanga, A.; Binyaruka, P.; Sachingongu, N.; Chama-Chiliba, C.M.; Chalabi, Z.; Borghi, J.; Blanchet, K. Mathematical modelling for health systems research: A systematic review of system dynamics and agent-based models. BMC Health Serv. Res. 2019, 19, 845. [Google Scholar] [CrossRef] [PubMed]
| Concept | Search Terms |
|---|---|
| Systems modelling methods | Complex* systems OR system dynamic* OR agent?based OR stochastic OR network* OR compartmental model* OR multi?agent OR multi-compartment model* |
| Emerging infectious diseases | Emerging infectious diseases OR coronavirus OR MERS-CoV, COVID-19 OR severe acute respiratory syndrome OR SARS-CoV-2 OR SARS OR Ebola OR zika OR dengue OR Nipah OR pandemic * OR influenza OR outbreak* OR Crimean-Congo haemorrhagic fever OR rift valley fever or “diseases X”* OR Lassa fever |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Peer-reviewed reports published since 1 January 2000 | Abstract-only reports |
| Studies related to health systems preparedness and response | Studies that do not include healthcare system element |
| Emerging infectious diseases included in the prioritising diseases for research and development in emergency context list by The World Health Organisation [10] | Studies on non-emerging infectious diseases |
| Studies conducted to investigate preparedness, prevention and response to EIDs that affect human populations | Studies that do not include the human population |
| Considered the dynamic relationships between elements of the system (e.g., feedback loops, network effects) | Studies of mathematical models that do not account for dynamic relationships between elements of the system outside the epidemic model |
| Seasonal influenza |
| Characteristic | Number (n = 7) |
|---|---|
| Publication year | |
| 2009 | 1 |
| 2017 | 1 |
| 2020 | 4 |
| 2021 | 1 |
| Publication type | |
| Journal article | 7 |
| Main stakeholders | |
| Academics Healthcare policymakers | 1 |
| Government | 3 |
| Healthcare administrators | 2 |
| Healthcare policymakers | 5 |
| Politicians | 2 |
| Systems methods used | |
| Agent-based modelling | 1 |
| Dynamic causal modelling | 1 |
| Multilayer complex network | 1 |
| System dynamics modelling | 3 |
| Total interpretive structural modelling | 1 |
| Complex systems feature used | |
| Adaptive behaviour | 6 |
| Disorder | 7 |
| Feedback | 7 |
| History and memory | 0 |
| Nested structure and modularity | 6 |
| Non-equilibrium | 5 |
| Non-linearity | 6 |
| Numerosity | 7 |
| Robustness | 4 |
| Spontaneous | 7 |
| Study | Publication Year | Country | Disease | Target Population | Aims |
|---|---|---|---|---|---|
| Friston [15] | 2020 | US, Brazil, UK, France, Spain, Italy, Mexico, Belgium, Germany, Canada | COVID-19 | Local population of each country investigated | To estimate the duration of population immunity and the latent states and mechanisms that affect the rate of new cases and deaths under the most likely loss of immunity. |
| Mutanga [12] | 2021 | South Africa | COVID-19 | National population | To assess the range of systems dynamics modelling ability in forecasting COVID-19 dynamic and investigate the adequacy of government enforced restriction measures to control the pandemic using different “what if” scenarios. To predict the next wave of COVID-19 infection. |
| Scabini [16] | 2020 | Brazil | COVID-19 | National population | To analyse COVID-19 dynamics in Brazil and to investigate the implications of future actions by the government on the healthcare system |
| Shin [14] | 2017 | South Korea | MERS-CoV | Healthcare staff, patients and visitors in hospitals during MERS-CoV outbreak | To investigate the effect of healthcare policy to control MERS-CoV in South Korea on terms of patient care and diseases spread in hospitals. |
| Silva [18] | 2020 | Brazil | COVID-19 | National population data | To simulate COVID-19 dynamics and the economic impact during different restriction scenarios. |
| Suresh [13] | 2020 | India | COVID-19 | Healthcare workers (physicians, nurses, health inspectors, paramedics, hospital operation and administrative staff) | To analyse the key factors contributing to the agility of the healthcare system in controlling COVID-19 in the context of available resources during the disease dynamics. |
| Weixing [17] | 2009 | China | SARS | Population of Hubei Province | To simulate SARS-CoV-1 spread and evaluate control measures to mitigate further spread of the pathogen. |
| Study | Main Stakeholders | Methods | System’s Elements | Challenges and Potential Solutions | Key Lessons |
|---|---|---|---|---|---|
| Friston [15] | Policymakers and academics | Dynamic causal modelling | The local population is assigned a state in four distinct attributes (location, infection state, symptoms, and testing). 24 parameters specify aspects associated with state transition probabilities (e.g., the effective number of contacts, transmission strength, the efficacy of tracking and tracing). | Modelling process did not account for geospatial aspects, waves of infection or any interactions with seasonal influenza (no potential solution discussed). Inaccuracy of population demography data. Solution: building a model that accounts to population heterogeneity at a coarse-grained level by using a series of bipartitions of the latent states. | “The rate at which immunity is lost is important because it constrains the onset of any putative second wave.” “…the UK might expect a second wave in around January 2021. This is important because there is a window of opportunity in the next few months during which nonpharmacological interventions—especially tracking and tracing—will, in principle, be in a position to defer or delay the second wave indefinitely.” |
| Mutanga [12] | National authorities | System dynamics model | They divided the national population into stocks (susceptible, exposed, infected, recovered and deceased), with flows between them representing the time in which individuals will move from one stock to the other. The model also contained multiple connected variables (e.g., R0, restriction measures, rate of contacts within the community, diseases duration and rates of individuals moving from one stock to another). | The modellers estimated homogenous population mixing, which might not represent the actual magnitude of COVID-19 spread in South Africa. The authors also mentioned that the national data might be sub-optimal due to the novelty of the pathogen. Solution: to replicate the model using current knowledge of COVID-19 and using a different timeline where data aggregation, including reporting and testing, are more accurate. | The systems dynamics model conducted in the study was proven beneficial to inform policymakers about prediction, prevention and control of COVID-19 with a small yet acceptable error. The study supports lockdown as a measure to prevent healthcare systems from collapsing. |
| Scabini [16] | Healthcare policymakers and government | Multilayer complex network | The model’s layers represent the social interactions/activities between the population, including home, work, transport, school, religious activities and random. The nodes represent people, and the edges are social contacts between the nodes. The epidemic dynamic was also considered. Individuals were categorised as susceptible, infected-asymptomatic, infected-mild, infected-sever, infected-critical, recovered and dead. | The main challenge the authors faced was related to Brazil’s geographic and demographic nature. Other challenges included insufficient data and lack of testing. Solution: This study can be repeated in other countries to check if the results are replicated. | The isolation measures in the study are insufficient and could significantly burden the healthcare system and mortality in Brazil. Social distancing is significant to reduce the peak of the pandemic curve. Returning to “normality” would cause a new peak in the pandemic’s wave and the need for ICU beds would surpass the country’s capacity. |
| Shin [14] | Health care policymakers and administrators in government and private sectors | System dynamics model | Model A: Stocks represent the susceptible and infected population at emergency rooms and the flow represent the infectious rate. The variables in model A represent types and frequencies of contact between people in the emergency room (ER occupancy rate, number of contacts made in the ER, susceptible contacts at ER, contact between infected and uninfected people at ER, probability of contact with infected patient at ER, total population at ER, patient arrival at ER, number of visitors at ER, number of visitors per patient) and infectivity of MERS. Model B: Stock represents the general ward’s susceptible and infected population. The variables represent infectivity of MERS, room occupancy, fractions of rooms with different frequencies, type and probability of contact and visitors. | The author reported a cultural challenge where family members in South Korea are expected to attend to patients even when healthcare staff are available which might lead to an increase in new cases. Solution: To understand the mental model for the studied population and find leverage points for a desirable outcome. | In hospitals, the number of MERS-CoV infections showed no significant difference between single and multiple room occupancy during the low infectivity period. However, it was increased between patients during the high infectivity period. High emergency room occupancy was associated with a higher risk of infection when compared to low occupancy emergency rooms. The number of visitors was directly related to increased infections among inpatients. |
| Silva [18] | Politicians, healthcare policymakers | Agent-based model | Agents that make up the society in the model are people and their environment. The agents were grouped into families, business and government. The model contained input parameters (e.g., epidemiology, socioeconomic and demographic) and output parameters. | The scenarios of this study were done on a simulated society; the situation might differ slightly if the author considered confounding factors from real society. Solution: to validate the results by simulating the scenarios for real-world populations. | Lockdown and partial lockdown are best-case scenarios to mitigate the risk of COVID-19 in the context of human lives and health but have a significant impact on the economy. Vertical isolation (isolating infected individuals and high-risk groups) and “Do nothing” approaches had the worst income. The best scenarios were partial isolation (restricting the movement of the agents), using facemasks and social distancing. |
| Suresh [13] | Healthcare managers and government | Total interpretive structural modelling (TISM) | Factors that make up the agility system in hospitals including building a Rapid Response Team (RRT), leadership support for the RRT, readiness for change, team members’ adaptability, strategy fit to match the demand and capacity, accessibility and availability of the required resources, training and development, collaboration and resilience, embracing technology and innovations, multi-tasking and decision making, biomedical waste management, cost-effectiveness, and their interrelationships. Those factors are categorised into five groups according to their influence on the overall hospital agility. | Presenting the interaction of the factors within the model is not very clear at first sight. Solution: feedback back and forth between two factors can be presented with two arrows rather than one. | Using a framework like TISM can help increase agility in the hospitals and improve managers’ decision-making when the most influencing factors and their interrelations are mapped and leverage points are explored rather than making decisions based on instinct and experience that might be suitable to the problem at hand. In this paper, the authors indicated that availability of resources, proper training and collaboration, and resilience are key factors in improving agility in hospitals. |
| Weixing [17] | Healthcare policymakers and government | System dynamics model | They divided the local population into a community, quarantine areas and hospital compartments. Each compartment contains individuals divided into susceptible, latent, infected, recovered and deceased, with flows between them. | The results indicated that most SARS-CoV-1 cases were imported to Hubei from nearby regions. However, events from transportation were not considered in the model. Solution: incorporating modes of transportation in and out of Hubei into future models. | Healthcare in Hubei province is adequate and could control and mintages the risk of SARS-CoV-1. The optimal priority is to quarantine infected patients and reduce the time delay between diagnosis and hospitalisation. Most of the new cases in Hubei were imported from nearby regions. |
| Systems Feature | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Numerosity | Disorder and Diversity | Feedback | Non-Equilibrium | Spontaneous Order and Self-Organisation | Non-Linearity | Robustness | Nested Structure and Modularity | History and Memory | Adaptive Behaviour |
| Friston [15] | X | X | X | X | X | X | ||||
| Mutanga [12] | X | X | X | X | X | X | X | X | X | |
| Scabini [16] | X | X | X | X | X | X | X | X | X | |
| Shin [14] | X | X | X | X | X | X | X | |||
| Silva [18] | X | X | X | X | X | X | X | X | ||
| Suresh [13] | X | X | X | X | X | X | X | X | ||
| Weixing [17] | X | X | X | X | X | X | X | X | X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansouri, M.A.; Garcia, L.; Kee, F.; Bradley, D.T. Systems-Oriented Modelling Methods in Preventing and Controlling Emerging Infectious Diseases in the Context of Healthcare Policy: A Scoping Review. Systems 2022, 10, 182. https://doi.org/10.3390/systems10050182
Mansouri MA, Garcia L, Kee F, Bradley DT. Systems-Oriented Modelling Methods in Preventing and Controlling Emerging Infectious Diseases in the Context of Healthcare Policy: A Scoping Review. Systems. 2022; 10(5):182. https://doi.org/10.3390/systems10050182
Chicago/Turabian StyleMansouri, Mariam Abdulmonem, Leandro Garcia, Frank Kee, and Declan Terence Bradley. 2022. "Systems-Oriented Modelling Methods in Preventing and Controlling Emerging Infectious Diseases in the Context of Healthcare Policy: A Scoping Review" Systems 10, no. 5: 182. https://doi.org/10.3390/systems10050182
APA StyleMansouri, M. A., Garcia, L., Kee, F., & Bradley, D. T. (2022). Systems-Oriented Modelling Methods in Preventing and Controlling Emerging Infectious Diseases in the Context of Healthcare Policy: A Scoping Review. Systems, 10(5), 182. https://doi.org/10.3390/systems10050182







