Managing Rhizoctonia Damping-Off of Rocket (Eruca sativa) Seedlings by Drench Application of Bioactive Potato Leaf Phytochemical Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extracts Preparation
2.2. Fungal Pathogen
2.3. In Vitro Determination of the Antifungal Activity
2.4. In Vivo Estimation of Damping-Off Control
2.5. Total Phenol Content and Free Radical Scavenging Capacity
2.6. Phytochemical Analysis of Extracts
2.7. Data Analysis
3. Results
3.1. In Vitro Antifungal Activity of Extracts
3.2. Control Assays of Rocket Seedling Damping-Off
3.3. Metabolite Profiling of Extracts
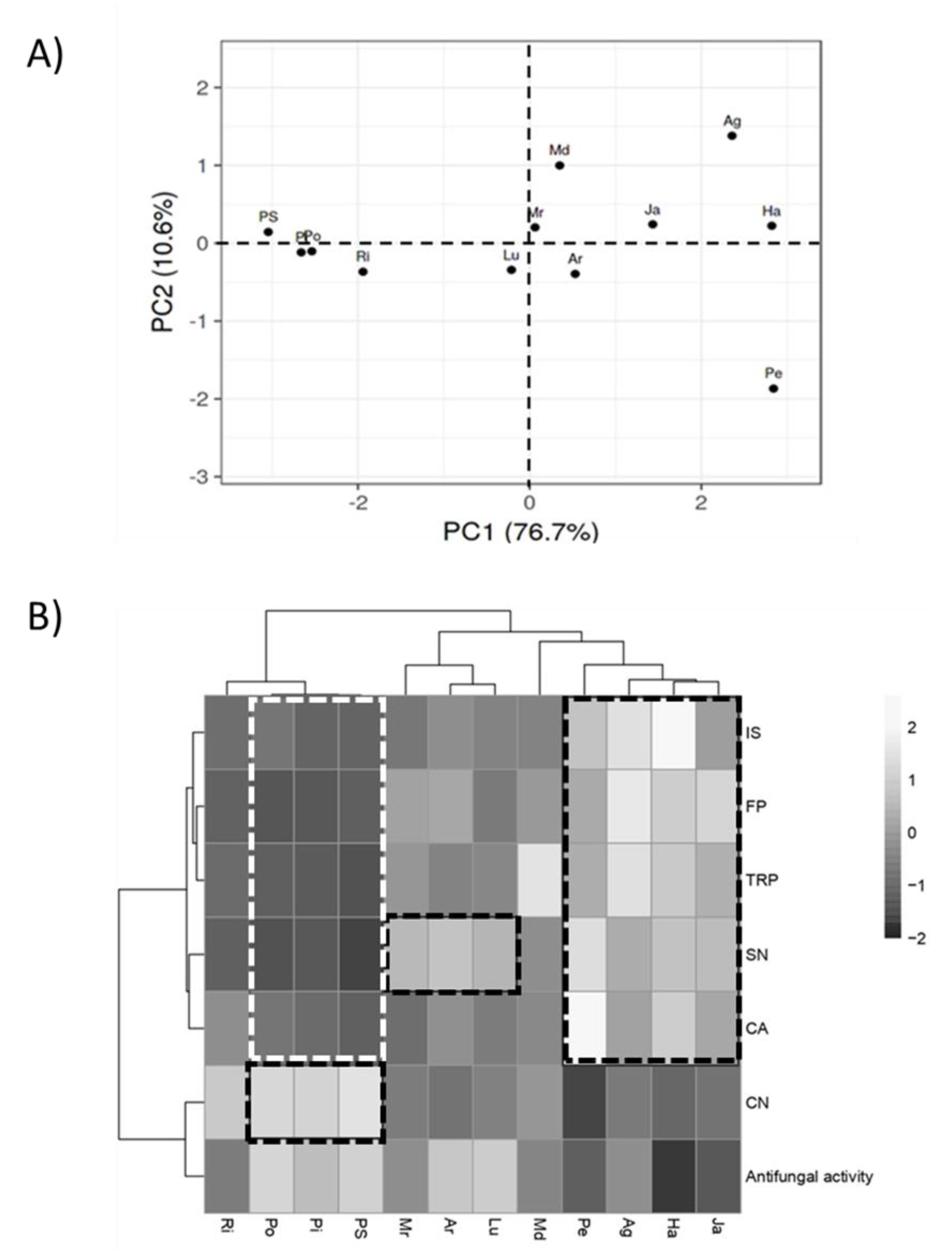
3.4. Biochemical Correlations
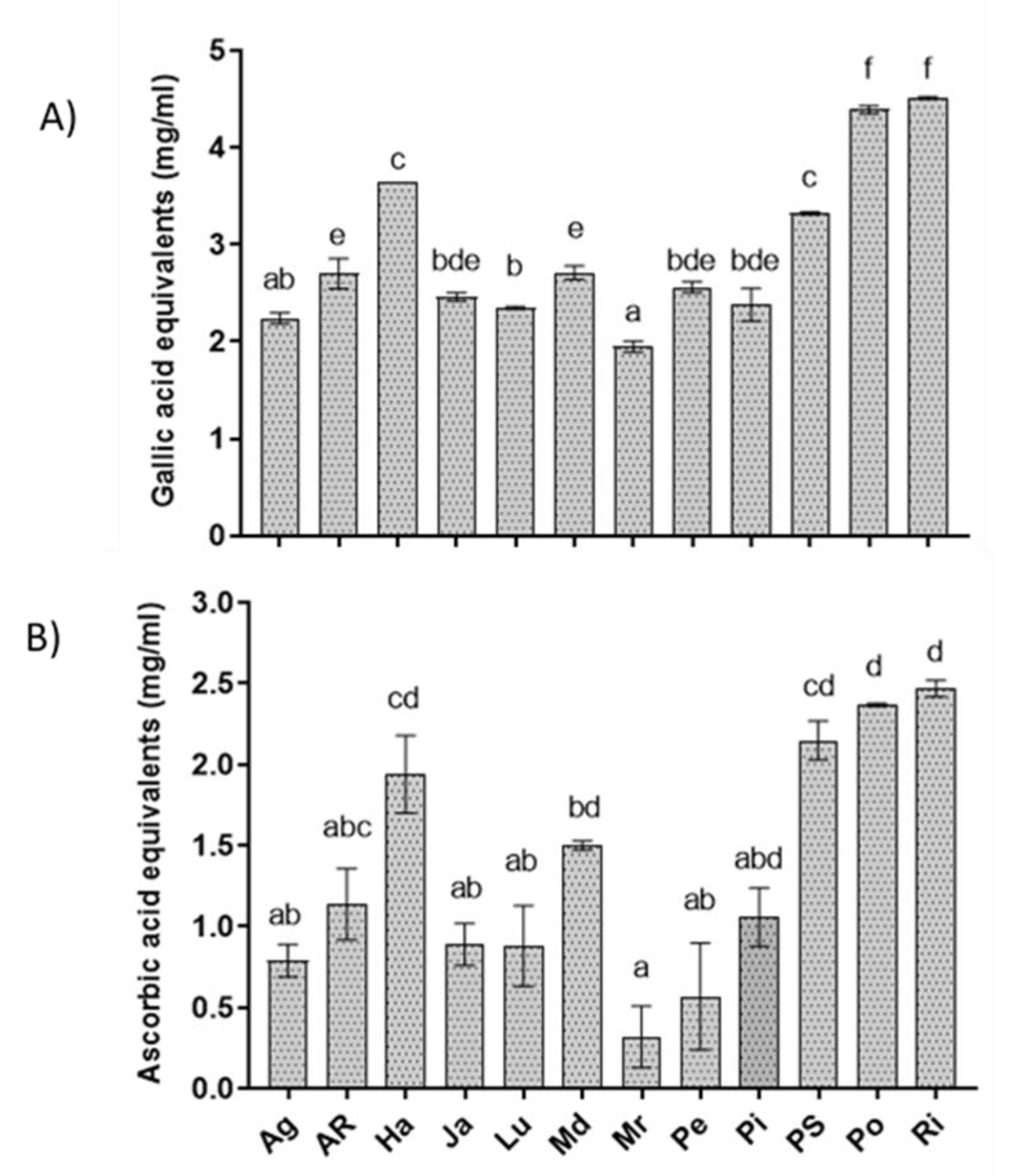
3.5. Total Phenol Content and Antioxidant Activity of Extracts
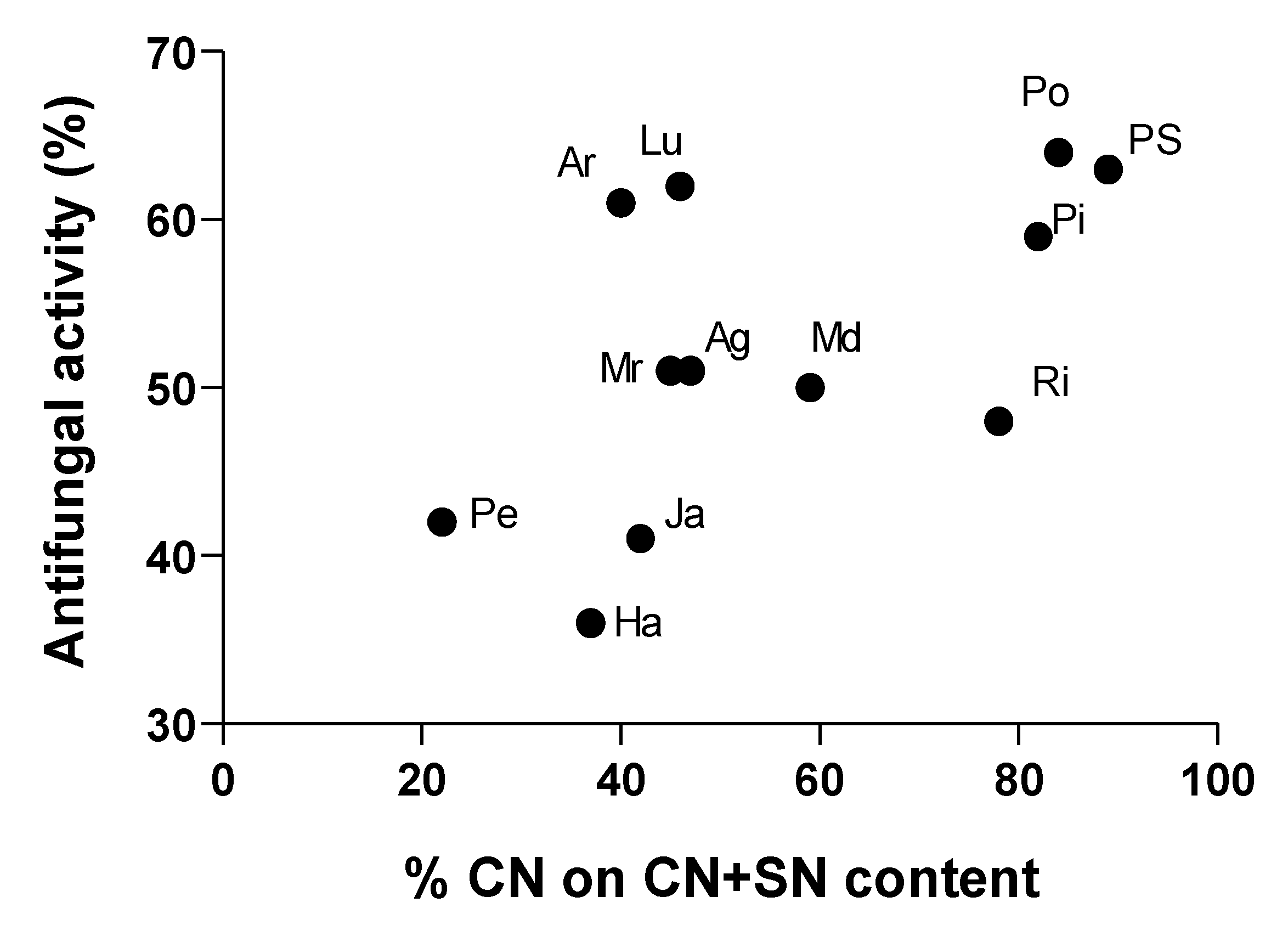
3.6. Regression and Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pane, C.; Sigillo, L.; Caputo, M.; Serratore, G.; Zaccardelli, M.; Tripodi, P. Response of rocket salad germplasm (Eruca and Diplotaxis spp.) to major pathogens causing damping-off, wilting and leaf spot diseases. Arch. Phytopathol. Plant Prot. 2017, 50, 167–177. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, V.; Messéan, A.; Aubertot, J.N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Dis. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Pandey, A.K.; Burlakoti, R.R.; Kenyon, L.; Nair, R.M. Perspectives and challenges for sustainable management of fungal diseases of mungbean [Vigna radiata (L.) R. Wilczek var. radiata]: A Review. Front. Environ. Sci. 2018, 6, 53. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; Lãpez-Ibáñez, S.; Miguélez, E.M.; Villar, C.J.; Lombã, F. Plant phytochemicals in food preservation: Antifungal bioactivity: A review. J. Food. Prot. 2020, 83, 163–171. [Google Scholar] [CrossRef]
- Derbalah, A.S.; Dewir, Y.H.; El-Sayed, A.E.B. Antifungal activity of some plant extracts against sugar beet damping-off caused by Sclerotium rolfsii. Ann. Microbiol. 2012, 62, 1021–1029. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Antifungal effect of powdered spices and their extracts on growth and activity of some fungi in relation to damping-off disease control. J. Plant Prot. Res. 2007, 47, 267–277. [Google Scholar]
- Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M.; Morsy, K.M. Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop Prot. 2011, 30, 185–191. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I. Broad-spectrum antibacterial and antifungal properties of certain traditionally used Indian medicinal plants. World J. Microbiol. Biotechnol. 2003, 19, 653–657. [Google Scholar] [CrossRef]
- Pane, C.; Fratianni, F.; Parisi, M.; Nazzaro, F.; Zaccardelli, M. Control of Alternaria post-harvest infections on cherry tomato fruits by wild pepper phenolic-rich extracts. Crop Prot. 2016, 84, 81–87. [Google Scholar] [CrossRef]
- Pane, C.; Francese, G.; Raimo, F.; Mennella, G.; Zaccardelli, M. Activity of foliar extracts of cultivated eggplants against Sclerotinia lettuce drop disease and their phytochemical profiles. Eur. J. Plant Pathol. 2017, 148, 687–697. [Google Scholar] [CrossRef]
- Pane, C.; Fratianni, F.; Raimo, F.; Nazzaro, F.; Zaccardelli, M. Efficacy of phenolic-rich extracts from leaves of pepper landraces against Alternaria leaf blight of tomato. J. Plant Pathol. 2017, 99, 239–244. [Google Scholar]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) burs extracts and functional compounds: UHPLC-UV-HRMS profiling, antioxidant activity, and inhibitory effects on phytopathogenic fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape cane waste as a source of trans-resveratrol and trans-viniferin: High-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind. Crop. Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Pare, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Mithen, R.; Raybould, A.F.; Giamoustaris, A. Divergent selection for secondary metabolites between wild populations of Brassica oleracea and its implications for plant-herbivore interactions. Heredity 1995, 75, 472–484. [Google Scholar] [CrossRef]
- Hack, H.; Gall, H.; Klemke, T.; Klose, R.; Meier, U.; Stauss, R.; Witzenberger, A. The BBCH-scale for phenological growth stages of potato (Solanum tuberosum L.). In Proceedings of the 12th Annual Congress of the European Association for Potato Research, Paris, France, 18–23 July 1993; pp. 153–154. [Google Scholar]
- Pane, C.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Disease suppressiveness of agricultural greenwaste composts as related to chemical and bio-based properties shaped by different on-farm composting methods. Biol. Control 2019, 137, 104026. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Foti, M.C. Use and abuse of DPPH radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49. [Google Scholar]
- Docimo, T.; Francese, G.; Ruggiero, A.; Batelli, G.; De Palma, M.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Mennella, G.; Tucci, M. Phenylpropanoids accumulation in eggplant fruit: Characterization of biosynthetic genes and regulation by a myb transcription factor. Front. Plant Sci. 2016, 6, 1233. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, D.; Yogendra, K.N.; Gunnaiah, R.; Kushalappa, A.C.; Murphy, A. Identification of late blight resistance-related metabolites and genes in potato through nontargeted metabolomics. Plant Mol. Biol. Rep. 2014, 32, 584–595. [Google Scholar] [CrossRef]
- Tai, H.H.; Worrall, K.; Pelletier, Y.; De Koeyer, D.; Calhoun, L.A. Comparative metabolite profiling of Solanum tuberosum against six wild Solanum species with Colorado potato beetle resistance. J. Agric. Food Chem. 2014, 62, 9043–9055. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; Guerra-Hernández, E.; Cerretani, L.; García-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef]
- Wu, S.B.; Meyer, R.S.; Whitaker, B.D.; Litt, A.; Kennelly, E.J. A new liquid chromatography-mass spectrometry-based strategy to integrate chemistry, morphology, and evolution of eggplant (Solanum) species. J. Chromatogr. A 2013, 1314, 154–172. [Google Scholar] [CrossRef]
- Delledonne, M.; Dal Molin, A.; Minio, A.; Ferrarini, A.; Tononi, P.; Zamperin, G.; Toppino, L.; Sala, T.; Barchi, L.; Comino, C.; et al. A high quality eggplant (Solanum melongena L.) genome draft and its use for mapping metabolic QTLs. In Proceedings of the 11th Solanaceae Conference, SOL 2014, Porto Seguro–Bahia, Brazil, 2–6 November 2014; p. 99. [Google Scholar]
- SAS Institute. JMP Statistics and Graphics Guide; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Palmer-Young, E.C.; Sadd, B.M.; Irwin, R.E.; Adler, L.S. Synergistic effects of floral phytochemicals against a bumble bee parasite. Ecol. Evol. 2017, 7, 1836–1849. [Google Scholar] [CrossRef]
- Zeng, S.L.; Duan, L.; Chen, B.Z.; Li, P.; Liu, E.H. Chemicalome and metabolome profiling of polymethoxylated flavonoids in Citri Reticulatae Pericarpium based on an integrated strategy combining background subtraction and modified mass defect filter in a Microsoft Excel Platform. J. Chromatogr. A 2017, 1508, 106–120. [Google Scholar] [CrossRef]
- Adandonon, A.; Aveling, T.A.; Labuschagne, N.; Tamo, M. Biocontrol agents in combination with Moringa oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur. J. Plant Pathol. 2006, 115, 409–418. [Google Scholar] [CrossRef]
- Al-Askar, A.; Rashad, Y. Efficacy of some plant extracts against Rhizoctonia solani on pea. J. Plant Prot. Res. 2010, 50, 239–243. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; El-Gamal, N.G. Effects of aqueous extracts of some plant species against Fusarium solani and Rhizoctonia solani in Phaseolus vulgaris plants. Arch. Phytopathol. Plant Prot. 2011, 44, 1–16. [Google Scholar] [CrossRef]
- Mangang, H.C.; Chhetry, G.K.N. Antifungal properties of certain plant extracts against Rhizoctonia solani causing root rot of French bean in organic soil of Manipur. Int. J. Sci. Res. Publ. 2012, 2, 1–4. [Google Scholar]
- Islam, M.T.; Faruq, A.N. Effect of some medicinal plant extracts on damping-off disease of winter vegetable. World Appl. Sci. J. 2012, 17, 1498–1503. [Google Scholar]
- Choudhury, D.; Anand, Y.R.; Kundu, S.; Nath, R.; Kole, R.K.; Saha, J. Effect of plant extracts against sheath blight of rice caused by Rhizoctonia solani. J. Pharmacogn. Phytochem. 2017, 6, 399–404. [Google Scholar]
- Malmberg, A. N-Feruloylputrescine in infected potato tubers. Acta Chem. Scand. B 1984, 38, 153–155. [Google Scholar] [CrossRef]
- Macoy, D.M.; Kim, W.; Lee, S.Y.; Kim, M.G. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Fadl Almoulah, N.; Voynikov, Y.; Gevrenova, R.; Schohn, H.; Tzanova, T.; Yagi, S.; Thomas, J.; Mignard, B.; Ahmed, A.A.A.; El Siddig, M.A.; et al. Antibacterial, antiproliferative and antioxidant activity of leaf extracts of selected Solanaceae species. S. Afr. J. Bot. 2017, 112, 368–374. [Google Scholar] [CrossRef]
- Thompson, M.D.; Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Rush, D.K.; Holm, D.G.; Stushnoff, C. Functional food characteristics of potato cultivars (Solanum tuberosum L.): Phytochemical composition and inhibition of 1-methyl-1-nitrosourea induced breast cancer in rats. J. Food Compos. Anal. 2009, 22, 571–576. [Google Scholar] [CrossRef]
- Salawu, S.O.; Boligon, A.A.; Athayde, M.L. Evaluation of antioxidant potential and nutritional values of white skinned sweet potato-unripe plantain composite flour blends. Int. J. Appl. Res. Nat. Prod. 2014, 7, 11–20. [Google Scholar]
- Koïta, K.; Neya, F.B.; Opoku, N.; Baissac, Y.; Campa, C.; Sankara, P. Phytochemical analysis of Ziziphus mucronata Willd. extract and screening for antifungal activity against peanut pathogens. Afr. J. Plant Sci. 2017, 11, 394–402. [Google Scholar]
- Castillo, F.; Hernández, D.; Gallegos, G.; Mendez, M.; Rodríguez, R.; Reyes, A.; Aguilar, C.N. In vitro antifungal activity of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kühn. Ind. Crops Prod. 2010, 32, 324–328. [Google Scholar] [CrossRef]
- Friedman, M.; McDonald, G.M. Potato glycoalkaloids: Chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E. Ecofriendly synthesis of silver nanoparticles using potato steroidal alkaloids and their activity against phytopathogenic fungi. Braz. Arch. Biol. Technol. 2018, 61, e18180013. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G. Interactive antifungal activity of the glycoalkaloids α-solanine and α-chaconine. Phytochemistry 1993, 33, 323–328. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G. Potato glycoalkaloid impairment of fungal development. Mycol. Res. 1997, 101, 597–603. [Google Scholar] [CrossRef]
- Smith, D.B.; Roddick, J.G.; Jones, J.L. Synergism between the potato glycoalkaloids α-chaconine and α-solanine in inhibition of snail feeding. Phytochemistry 2001, 57, 229–234. [Google Scholar] [CrossRef]
- Yamashoji, S.; Matsuda, T. Synergistic cytotoxicity induced by alpha-solanine and alpha-chaconine. Food Chem. 2013, 141, 669–674. [Google Scholar] [CrossRef]
- Roddick, J.G.; Rijnenberg, A.L.; Osman, S.F. Synergistic interaction between potato glycoalkaloids α-solanine andα-chaconine in relation to destabilization of cell membranes: Ecological implications. J. Chem. Ecol. 1988, 14, 889–902. [Google Scholar] [CrossRef]
- López-García, B.; Harries, E.; Carmona, L.; Campos-Soriano, L.; López, J.J.; Manzanares, P.; Gandía, M.; Coca, M.; Marcos, J.F. Concatemerization increases the inhibitory activity of short, cell-penetrating, cationic and tryptophan-rich antifungal peptides. Appl. Microbiol. Biotechnol. 2015, 99, 8011–8021. [Google Scholar] [CrossRef]
- Consonni, C.; Bednarek, P.; Humphry, M.; Francocci, F.; Ferrari, S.; Harzen, A.; van Themaat, E.V.L.; Panstruga, R. Tryptophan-derived metabolites are required for antifungal defense in the Arabidopsis mlo2 mutant. Plant Physiol. 2010, 152, 1544–1561. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P.; Tarighi, S. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 2014, 118, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y. Antifungal activity of Salvia miltiorrhiza against Candida albicans is associated with the alteration of membrane permeability and (1,3)-β-D-glucan synthase activity. J. Microbiol. Biotechnol. 2016, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, Z.G.; Riley-Saldaña, C.A.; González-Esquinca, A.R.; Castro-Moreno, M.; de-la-Cruz-Chacón, I. Antifungal activity of Solanum extracts against phytopathogenic Curvularia lunata. J. Plant Prot. Res. 2018, 58, 311–315. [Google Scholar]
- Yu, J.O.; Yap, A.L.; Tuason, A.A.; Tan, C.C.; Tan, H.T.; Tan, L.V.; Tan, N.G.; Tan, R.T.; Tangkusan, D.I.; Tiosin, J.S.; et al. Antifungal activity of crude glycolated extracts of Solanum tuberosum L. (white potato) peelings against Candida and Aspergillus species. Acta Med. Philipp. 2019, 53, 67–72. [Google Scholar]
- Silveyra, M.X.; Lanteri, M.L.; Damiano, R.B.; Andreu, A.B. Bactericidal and cytotoxic activities of polyphenol extracts from Solanum tuberosum spp. tuberosum and spp. andigena cultivars on Escherichia coli and human neuroblastoma SH-SY5Y cells in vitro. J. Nutr. Metab. 2018, 2018, 8073679. [Google Scholar] [PubMed]
- De Masi, L.; Bontempo, P.; Rigano, D.; Stiuso, P.; Carafa, V.; Nebbioso, A.; Piacente, S.; Montoro, P.; Aversano, R.; D’Amelia, V.; et al. Comparative phytochemical characterization, genetic profile, and antiproliferative activity of polyphenol-rich extracts from pigmented tubers of different Solanum tuberosum varieties. Molecules 2020, 25, 233. [Google Scholar] [CrossRef]
- Bowers, J.H.; Locke, J.C. Effect of botanical extracts on the population density of Fusarium oxysporum in soil and control of Fusarium wilt in the greenhouse. Plant Dis. 2000, 84, 300–305. [Google Scholar] [CrossRef]
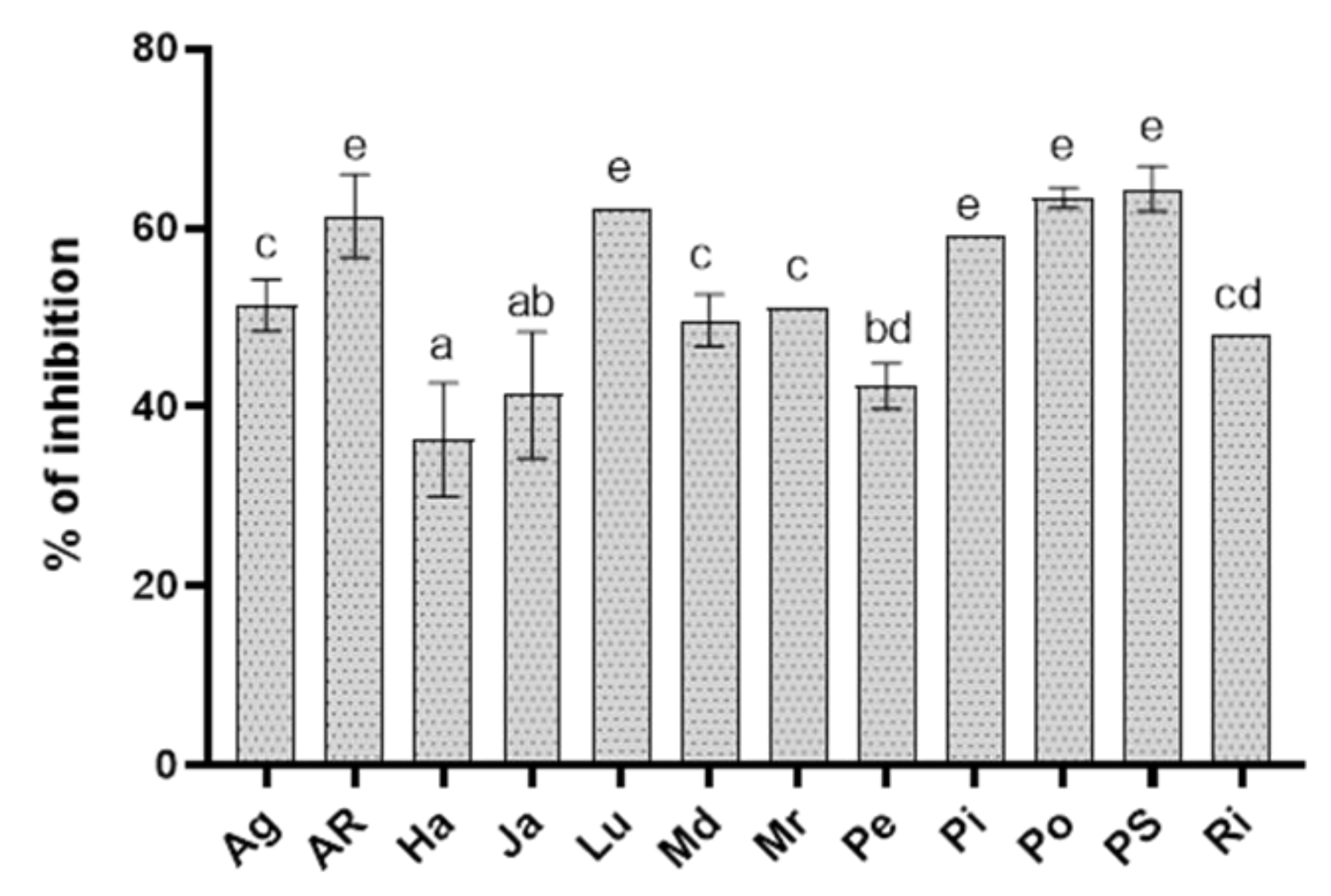
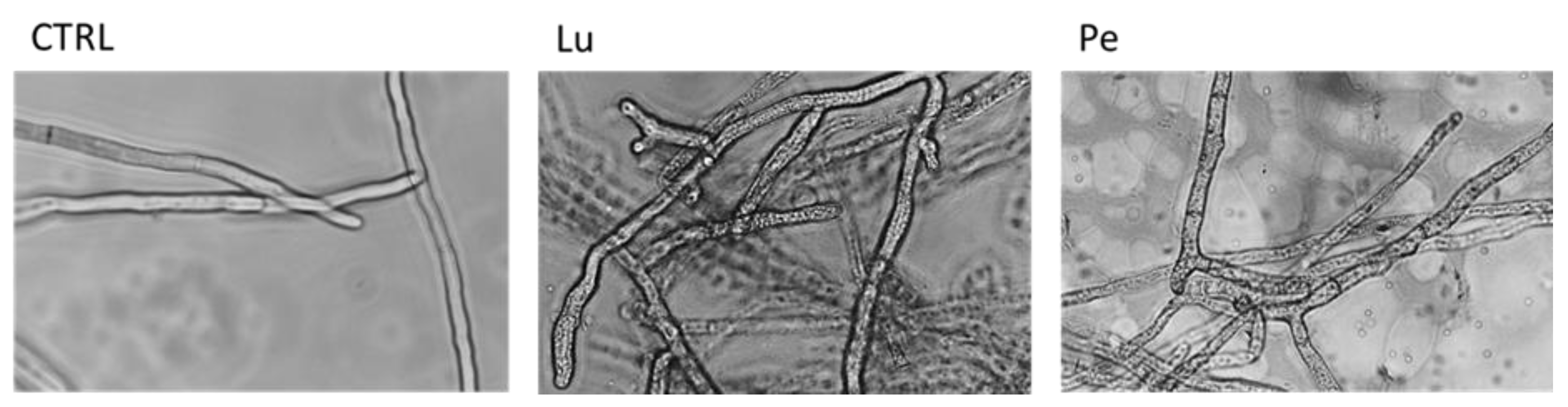
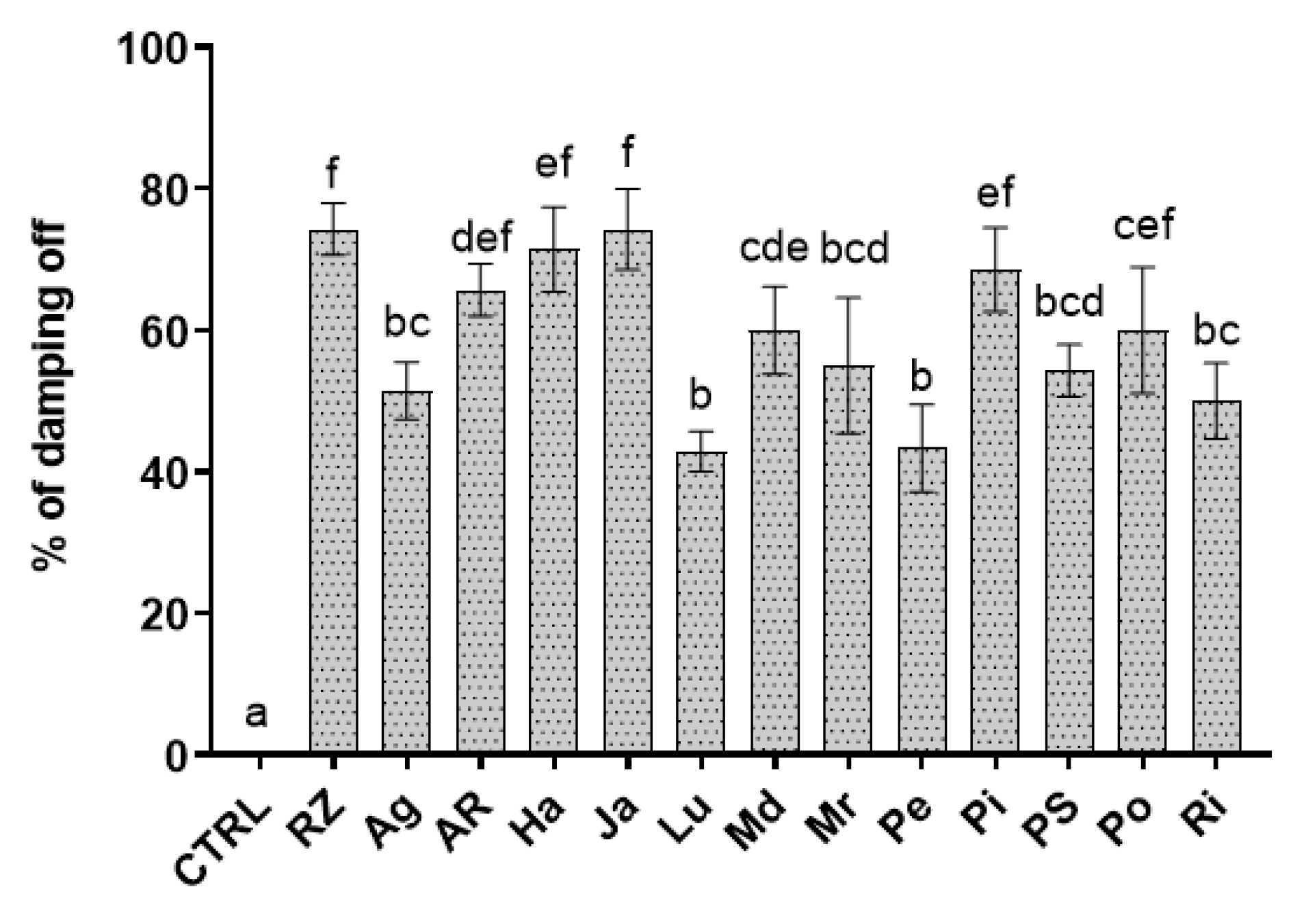
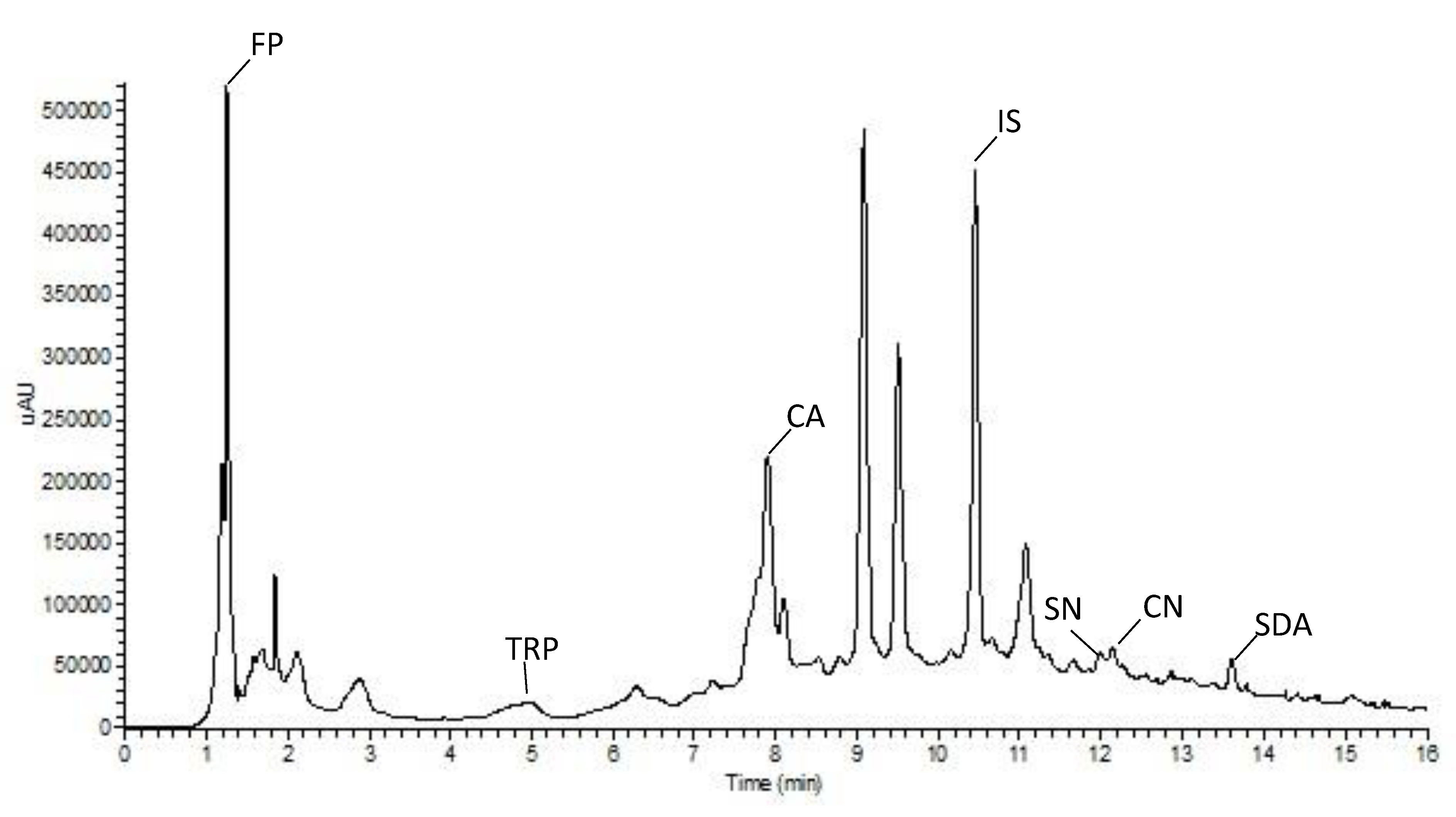
| Extract | EC50 (mg mL−1) |
|---|---|
| Ag | 8.7 |
| AR | 10.1 |
| Ha | >20 |
| Ja | >20 |
| Lu | 3.4 |
| Md | >20 |
| Mr | >20 |
| Pe | >20 |
| Pi | 12.7 |
| Po | <5 |
| PS | 10.5 |
| Ri | 14.9 |
| Extract | Chlorogenic Acid (µg mL−1) | α-Solanine (µg mL−1) |
|---|---|---|
| Ag | 16.0 gh | 205.2 h |
| AR | 28.7 e | 561.6 c |
| Ha | 41.4 cd | 385.6 e |
| Ja | 15.0 h | 200.3 h |
| Lu | 19.2 f | 492.4 d |
| Md | 17.1 g | 250.3 gh |
| Mr | 7.4 i | 324.0 ef |
| Pe | 42.8 c | 289.5 fg |
| Pi | 39.6 d | 685.6 ab |
| PS | 15.2 h | 347.8 ef |
| Po | 72.8 b | 749.5 a |
| Ri | 83.7 a | 631.0 b |
| Differential Metabolites | Retention Time (min) | m/z [M + H]+ | Cultivar/Mean Peak Area a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | AR | Ha | Ja | Lu | Md | Mr | Pe | Pi | PS | Po | Ri | |||
| n-feruloylputrescine | 0.96 | 265 | 259,690 | 350,220 | 341,280 | 196,770 | 199,220 | 209,140 | 204,680 | 154,370 | 329,850 | 335,740 | 365,070 | 357,190 |
| Tryptophan | 5.06 | 205 | 1,388,890 | 1,342,250 | 1,865,080 | 809,840 | 1,392,820 | 1,053,690 | 2,181,940 | 897,950 | 2,019,490 | 1,159,930 | 3,013,390 | 2,499,820 |
| Chlorogenic Acid | 7.97 | 355 | 625,420 | 1,121,230 | 1,615,650 | 585,590 | 749,440 | 288,190 | 668,030 | 1,669,850 | 1,547,820 | 591,670 | 2,843,520 | 3,266,750 |
| Isoquercitrin | 10.90 | 465 | 151,410 | 126,060 | 305,690 | 62,150 | 98,460 | 43,620 | 67,280 | 109,670 | 81,640 | 67,140 | 301,140 | 150,930 |
| α-solanine | 12.04 | 869 | 10,794,330 | 29,544,990 | 20,285,630 | 10,539,390 | 25,904,160 | 17,044,190 | 13,169,040 | 15,227,780 | 36,067,120 | 18,297,530 | 39,425,850 | 33,195,010 |
| α-chaconine | 12.20 | 852 | 9,445,350 | 19,896,460 | 12,034,560 | 7,576,490 | 22,501,550 | 13,829,460 | 18,599,510 | 4,338,610 | 165,521,180 | 149,545,270 | 211,153,460 | 118,210,830 |
| Solasodoside | 14.04 | 1031 | 278,860 | 5,380,510 | 908,010 | 279,170 | 628,970 | 11,197,310 | 572,650 | 611,320 | 598,380 | 121,228,390 | 625,520 | 377,770 |
| CA | SDA | CN | SN | IS | FP | TRP | |
|---|---|---|---|---|---|---|---|
| CA | |||||||
| SDA | −0.27 ns | ||||||
| CN | 0.57 * | 0.34 ns | |||||
| SN | 0.72 ** | −0.14 ns | 0.74 ** | ||||
| IS | 0.62 ** | −0.25 ns | 0.27 ns | 0.41 * | |||
| FP | 0.56 * | 0.23 ns | 0.67 ** | 0.69 ** | 0.55 * | ||
| TRP | 0.74 ** | −0.25 ns | 0.65 ** | 0.69 ** | 0.60 ** | 0.60 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pane, C.; Caputo, M.; Francese, G.; Manganiello, G.; Lo Scalzo, R.; Mennella, G.; Zaccardelli, M. Managing Rhizoctonia Damping-Off of Rocket (Eruca sativa) Seedlings by Drench Application of Bioactive Potato Leaf Phytochemical Extracts. Biology 2020, 9, 270. https://doi.org/10.3390/biology9090270
Pane C, Caputo M, Francese G, Manganiello G, Lo Scalzo R, Mennella G, Zaccardelli M. Managing Rhizoctonia Damping-Off of Rocket (Eruca sativa) Seedlings by Drench Application of Bioactive Potato Leaf Phytochemical Extracts. Biology. 2020; 9(9):270. https://doi.org/10.3390/biology9090270
Chicago/Turabian StylePane, Catello, Michele Caputo, Gianluca Francese, Gelsomina Manganiello, Roberto Lo Scalzo, Giuseppe Mennella, and Massimo Zaccardelli. 2020. "Managing Rhizoctonia Damping-Off of Rocket (Eruca sativa) Seedlings by Drench Application of Bioactive Potato Leaf Phytochemical Extracts" Biology 9, no. 9: 270. https://doi.org/10.3390/biology9090270
APA StylePane, C., Caputo, M., Francese, G., Manganiello, G., Lo Scalzo, R., Mennella, G., & Zaccardelli, M. (2020). Managing Rhizoctonia Damping-Off of Rocket (Eruca sativa) Seedlings by Drench Application of Bioactive Potato Leaf Phytochemical Extracts. Biology, 9(9), 270. https://doi.org/10.3390/biology9090270







