The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk
Abstract
Highlights
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Laser Exposure
2.3. Bacterial Culture Cultivation
2.4. Preparation of Fermented Skimmed Milk by L. casei NRRL-B-1922
2.5. Preparation of Whey Fraction
2.6. Antioxidant Assay
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Determination of Total Antioxidant Capacity
2.7. Fermentation Profile of Fermented Skimmed Milk
2.8. β-Galactosidase Activity of L. casei Strain
2.9. Cholesterol Assimilation by L. casei before and after Treatment
2.10. Determination of Antibacterial Activity
2.11. Determination of Proteolytic Activity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activities of Pre-Treated L. casei with Red Laser
3.2. L. casei Profile of Lactose Fermentation after Red Laser Exposure
3.3. Effect of Red Laser on Antibacterial Activities of L. casei
3.4. Red Laser Enhanced Cholesterol Assimilation and Proteolytic Activities of L. casei
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heller Knut, J. Probiotic Bacteria in Fermented Foods: Product Characteristics and Starter Organisms. Am. J. Clin. Nutr. 2001, 73, 374s–379s. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, I.A.; El-Sayed, E.M.; Hafez, S.A.; El-Zeini, H.M.; Saleh, F.A. Inhibitory Effect of Yoghurt and Soya Yoghurt Containing Bifidobacteria on the Proliferation of Ehrlich Ascites Tumour Cells in Vitro and in Vivo in a Mouse Tumour Model. Br. J. Nutr. 2004, 92, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, T.; Pihlanto, A.; Akkanen, S.; Korhonen, H. Development of Antioxidant Activity in Milk Whey during Fermentation with Lactic Acid Bacteria. J. Appl. Microbiol. 2007, 102, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.M.; Sato, M.; Uzu, K.; Yoshida, T.; Higashi, S.; Kawakami, H.; Kadooka, Y.; Matsuyama, H.; El-Gawad, I.A.A.; Imaizumi, K. Milk Fermented by Lactobacillus Gasseri SBT2055 Influences Adipocyte Size via Inhibition of Dietary Fat Absorption in Zucker Rats. Br. J. Nutr. 2009, 101, 716–724. [Google Scholar] [CrossRef]
- Shah Nagendra, P. Effects of Milk-Derived Bioactives: An Overview. Br. J. Nutr. 2000, 84, 3–10. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Claesson, M.J.; Van Sinderen, D.; O’Toole, P.W. The Genus Lactobacillus—A Genomic Basis for Understanding Its Diversity. FEMS Microbiol. Lett. 2007, 269, 22–28. [Google Scholar] [CrossRef]
- Van Hoorde, K.; Van Leuven, I.; Dirinck, P.; Heyndrickx, M.; Coudijzer, K.; Vandamme, P.; Huys, G. Selection, Application and Monitoring of Lactobacillus Paracasei Strains as Adjunct Cultures in the Production of Gouda-Type Cheeses. Int. J. Food Microbiol. 2010, 144, 226–235. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- El-Dieb, S.M.; Abd Rabo, F.H.R.; Badran, S.M.; Abd El-Fattah, A.M.; Elshaghabee, F.M.F. In Vitro Model for Assessment of the Health Benefits of Some Microbial Strains. Int. J. Probiotics Prebiotics 2010, 5, 157–164. [Google Scholar]
- Wagnerberger, S.; Spruss, A.; Kanuri, G.; Stahl, C.; Schröder, M.; Vetter, W.; Bischoff, S.C.; Bergheim, I. Lactobacillus Casei Shirota Protects from Fructose-Induced Liver Steatosis: A Mouse Model. J. Nutr. Biochem. 2013, 24, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Y.; Wan, Y.P.; Fang, Q.Y.; Lu, W.; Cai, W. Supplementation with Probiotics Modifies Gut Flora and Attenuates Liver Fat Accumulation in Rat Nonalcoholic Fatty Liver Disease Model. J. Clin. Biochem. Nutr. 2012, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- El-Hussein, A.; Marzouk, A.; Harith, M.A. Discriminating Crude Oil Grades Using Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2015, 113, 93–99. [Google Scholar] [CrossRef]
- ElFaham, M.M.; Elthalabawy, W.M.; Elzahed, O.; Zakaria, M.A.; Abdelhamid, M. Mechanical Hardness Estimation of Heat-Treated DIN50Cr3 Spring Steel Utilizing Laser-Induced Breakdown Spectroscopy (LIBS) Inverse Calibration. Appl. Phys. A Mater. Sci. Process. 2020, 126, 167. [Google Scholar] [CrossRef]
- Brassolatti, P.; Bossini, P.S.; Kido, H.W.; Derencio Oliveira, M.C.; Almeida-Lopes, L.; Zanardi, L.M.; Napolitano, M.A.; Retto da Silva de Avó, L.; Araújo-Moreira, F.M.; Parizotto, N.A. Photobiomodulation and Bacterial Cellulose Membrane in the Treatment of Third-Degree Burns in Rats. J. Tissue Viability 2018, 27, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B Biol. 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Chung, W.; Petrofsky, J.S.; Laymon, M.; Logoluso, J.; Park, J.; Lee, J.; Lee, H. The Effects of Low Level Laser Radiation on Bacterial Growth. Phys. Ther. Rehabil. Sci. 2014, 3, 20–26. [Google Scholar] [CrossRef][Green Version]
- Belevich, I.; Borisov, V.B.; Konstantinov, A.A.; Verkhovsky, M.I. Oxygenated complex of cytochrome bd from Escherichia coli: Stability and photolability. FEBS Lett. 2005, 579, 4567–4570. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers: Vs. Light Emitting Diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, W.I.; Lim, S.T. Antioxidant and Anticancer Activities of Organic Extracts from Platycodon Grandiflorum A. de Candolle Roots. J. Ethnopharmacol. 2004, 93, 409–415. [Google Scholar] [CrossRef]
- Kumaran, A.; Joel Karunakaran, R. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Bockelmann, W.; Meske, D.; Vrese, M.d.; Walte, H.G.; Schrezenmeir, J.; Heller, K.J. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H. Enhancement of β-galactosidase activity of lactic acid bacteria in fermented camel milk. Emir. J. Food Agric. 2018, 30, 256–267. [Google Scholar] [CrossRef]
- Mahoney, R.R.; Nickerson, T.A.; Whitaker, J.R. Selection of Strain, Growth Conditions, and Extraction Procedures for Optimum Production of Lactase from Kluyveromyces Fragilis. J. Dairy Sci. 1975, 58, 1620–1629. [Google Scholar] [CrossRef]
- Kimoto, H.; Ohmomo, S.; Nomura, M.; Kobayashi, M.; Okamoto, T. In Vitro Studies on Probiotic Properties of Lactococci. Milchwissenschaft 2000, 55, 245–249. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute—CLSI. M100 Performance Standards for Antimicrobial; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic Activity of Dairy Lactic Acid Bacteria and Probiotics as Determinant of Growth and in Vitro Angiotensin-Converting Enzyme Inhibitory Activity in Fermented Milk. Lait 2007, 87, 21–38. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Saleh, A.M.; Habeeb, T.H.; Wadaan, M.A.M.; AbdElgawad, H. CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem. 2020, 308, 125661. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Sakr, S.; El-Dieb, S.M.; Elkashef, H. Biological Activities of Lactobacilli Relevant to Cardiovascular Health in Skim Milk. Food Sci. Biotechnol. 2017, 26, 1613–1623. [Google Scholar] [CrossRef]
- Warrad, M.; Hassan, Y.M.; Mohamed, M.S.M.; Hagagy, N.; Al-Maghrabi, O.A.; Selim, S.; Saleh, A.M.; AbdElgawad, H. A bioactive fraction from Streptomyces sp. enhances maize tolerance against drought stress. J. Microbiol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Nosrati, F.S.; Sobhani, S.O.; Takantape, S.N.; Amjadi, A. Effect of Low-Level Laser Irradiation on the Function of Glycated Catalase. J. Lasers Med. Sci. 2018, 9, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Obyedul Kalam Azad, M.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 2018, 7, 1–10. [Google Scholar]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech. 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Mende, S.; Jaros, D.; Rohm, H. Fermented Milk Products: Effects of Lactose Hydrolysis and Fermentation Conditions on the Rheological Properties. Dairy Sci. Technol. 2016, 96, 199–211. [Google Scholar] [CrossRef][Green Version]
- Ávila-Pérez, M.; Hellingwerf, K.J.; Kort, R. Blue Light Activates the ΣB-Dependent Stress Response of Bacillus Subtilis via YtvA. J. Bacteriol. 2006, 188, 6411–6414. [Google Scholar] [CrossRef]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Huang, Y.Y.; El-Hussein, A.; Wolf, L.M.; Chiang, L.Y.; Hamblin, M.R. Progressive Cationic Functionalization of Chlorin Derivatives for Antimicrobial Photodynamic Inactivation and Related Vancomycin Conjugates. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Can Light-Based Approaches Overcome Antimicrobial Resistance? Drug Dev. Res. 2019, 80, 48–67. [Google Scholar] [CrossRef]
- Kohli, R.; Gupta, P.K. Irradiance Dependence Fo the He-Ne Laser-Induced Protection against UVC Radiation in E. Coli Strains. J. Photochem. Photobiol. B Biol. 2003, 69, 161–167. [Google Scholar] [CrossRef]
- Huttunen, E.; Noro, K.; Yang, Z. Purification and identification of antimicrobial substances produced by two Lactobacillus casei strains. Int. Dairy J. 1995, 5, 503–513. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Gibson, G.R. Cholesterol Assimilation by Lactic Acid Bacteria and Bifidobacteria Isolated from the Human Gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Tahri, K.; Crociani, J.; Ballongue, J.; Schneider, F. Effects of Three Strains of Bifidobacteria on Cholesterol. Lett. Appl. Microbiol. 1995, 21, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Kolyakov, S.F. Exact Action Spectra for Cellular Responses Relevant to Phototherapy. Photomed. Laser Surg. 2005, 23, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ouf, S.A.; Alsarrani, A.Q.; Al-Adly, A.A.; Ibrahim, M.K. Evaluation of Low-Intensity Laser Radiation on Stimulating the Cholesterol Degrading Activity: Part I. Microorganisms Isolated from Cholesterol-Rich Materials. Saudi J. Biol. Sci. 2012, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Amani, E.; Eskandari, M.H.; Shekarforoush, S. The effect of proteolytic activity of starter cultures on technologically important properties of yogurt. Food Sci. Nutr. 2017, 5, 525–537. [Google Scholar] [CrossRef]
- Edwards, A.M.; Silva, E. Effect of Visible Light on Selected Enzymes, Vitamins and Amino Acids. J. Photochem. Photobiol. B Biol. 2001, 63, 126–131. [Google Scholar] [CrossRef]
- Hutson, M.S.; Tokutake, Y.; Chang, M.S.; Bloor, J.W.; Venakides, S.; Kiehart, D.P.; Edwards, G.S. Forces for Morphogenesis Investigated with Laser Microsurgery and Quantitative Modeling. Science 2003, 300, 145–149. [Google Scholar] [CrossRef]
- El-Hussein, A.; Lam, S.S.K.; Raker, J.; Chen, W.R.; Hamblin, M.R. N-Dihydrogalactochitosan as a Potent Immune Activator for Dendritic Cells. J. Biomed. Mater. Res. Part A 2017, 105, 963–972. [Google Scholar] [CrossRef]
- Ojha, N.K.; Nematian-Ardestani, E.; Neugebauer, S.; Borowski, B.; El-Hussein, A.; Hoshi, T.; Leipold, E.; Heinemann, S.H. Sodium Channels as Gateable Non-Photonic Sensors for Membrane-Delimited Reactive Species. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1412–1419. [Google Scholar] [CrossRef]
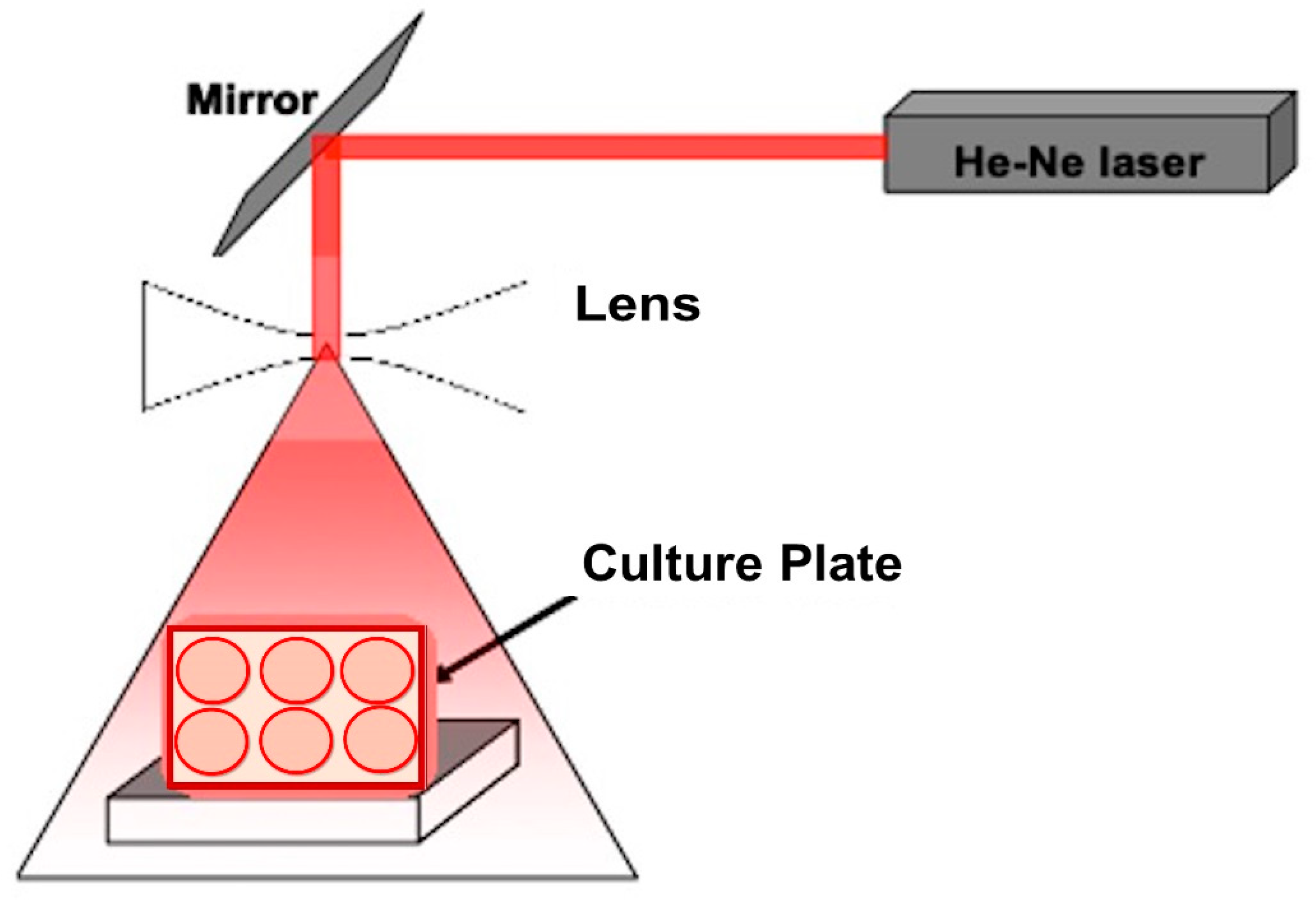
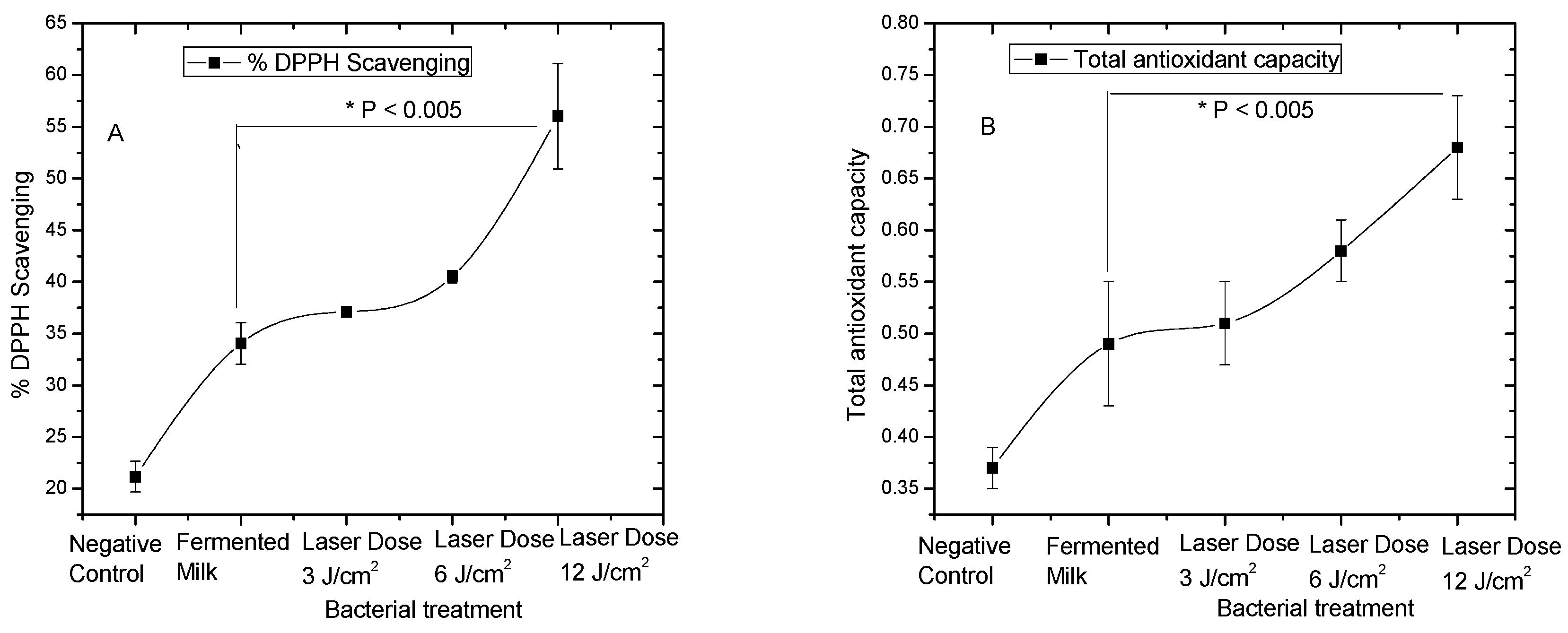
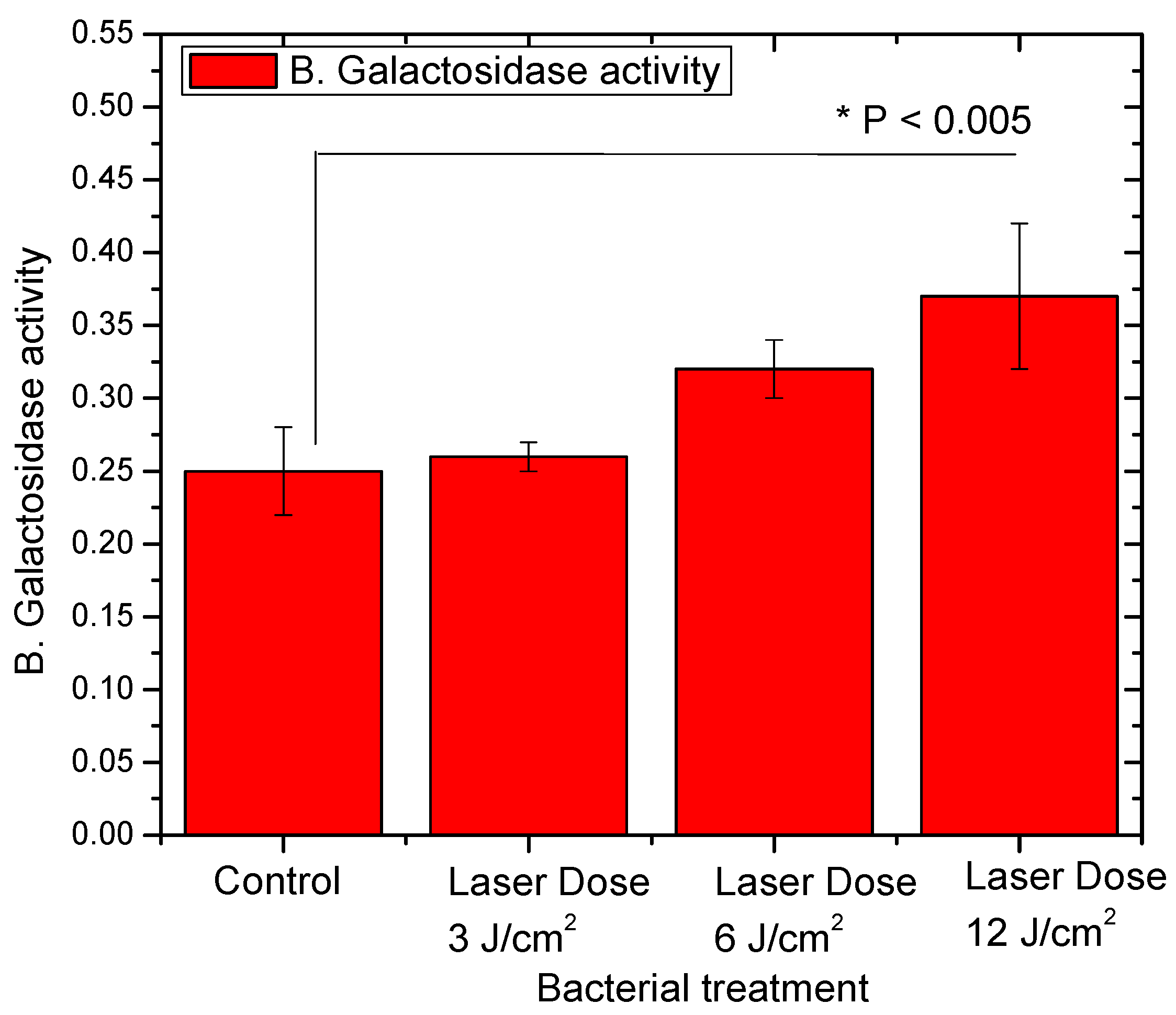
| Treatments | Lactose Residues | Lactic Acid | Acetic Acid |
|---|---|---|---|
| Control | 28.35 ± 0.51 a | 4.82 ± 0.51 a | 0.25 ± 0.06 a |
| 3 J/cm2 | 28.15 ± 0.42 a | 4.80 ± 0.48 a | 0.24 ± 0.05 a |
| 6 J/cm2 | 25.41 ± 0.37 b | 5.70 ± 0.40 b | 0.62 ± 0.03 b |
| 12 J/cm2 | 23.15 ± 0.36 c | 6.12 ± 0.71 c | 0.92 ± 0.10 c |
| Treatments | Staph. aureus | B. subtilis | E. coli |
|---|---|---|---|
| Control | 10.20 ± 0.60 a | 9.30 ± 0.80 a | 7.50 ± 0.20 ab |
| 3 J/cm2 | 10.30 ± 0.40 ab | 9.40 ± 0.35 ab | 7.45 ± 0.60 a |
| 6 J/cm2 | 11.50 ± 0.50 cd | 10.20 ± 0.52 abc | 8.40 ± 0.30 bc |
| 12 J/cm2 | 12.10 ± 0.30 d | 11.00 ± 0.40 cd | 9.15 ± 0.70 cd |
| Ampicillin * | 14.73 ± 0.40 e | 15.93 ± 0.61 e | 12.70 ± 0.52 e |
| Treatments | % Cholesterol Assimilation |
|---|---|
| Control | 38.12 ± 1.20 a |
| 3 J/cm2 | 38.42 ± 0.65 ab |
| 6 J/cm2 | 39.25 ± 1.35 abc |
| 12 J/cm2 | 41.15 ± 0.83 c |
| Treatments | % Proteolytic Activity |
|---|---|
| Control | 5.10 ± 0.40 a |
| 3 J/cm2 | 5.20 ± 0.25 ab |
| 6 J/cm2 | 5.62 ± 0.30 abc |
| 12 J/cm2 | 6.30 ± 0.55 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.S.M.; Elshaghabee, F.M.F.; Alharbi, S.A.; El-Hussein, A. The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk. Biology 2020, 9, 256. https://doi.org/10.3390/biology9090256
Mohamed MSM, Elshaghabee FMF, Alharbi SA, El-Hussein A. The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk. Biology. 2020; 9(9):256. https://doi.org/10.3390/biology9090256
Chicago/Turabian StyleMohamed, Mahmoud S.M., Fouad M.F. Elshaghabee, Sulaiman Ali Alharbi, and Ahmed El-Hussein. 2020. "The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk" Biology 9, no. 9: 256. https://doi.org/10.3390/biology9090256
APA StyleMohamed, M. S. M., Elshaghabee, F. M. F., Alharbi, S. A., & El-Hussein, A. (2020). The Prospective Beneficial Effects of Red Laser Exposure on Lactocaseibacillus casei Fermentation of Skim Milk. Biology, 9(9), 256. https://doi.org/10.3390/biology9090256






