Characterizing the Efficacy of a Film-Forming Antitranspirant on Raspberry Foliar and Fruit Transpiration
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
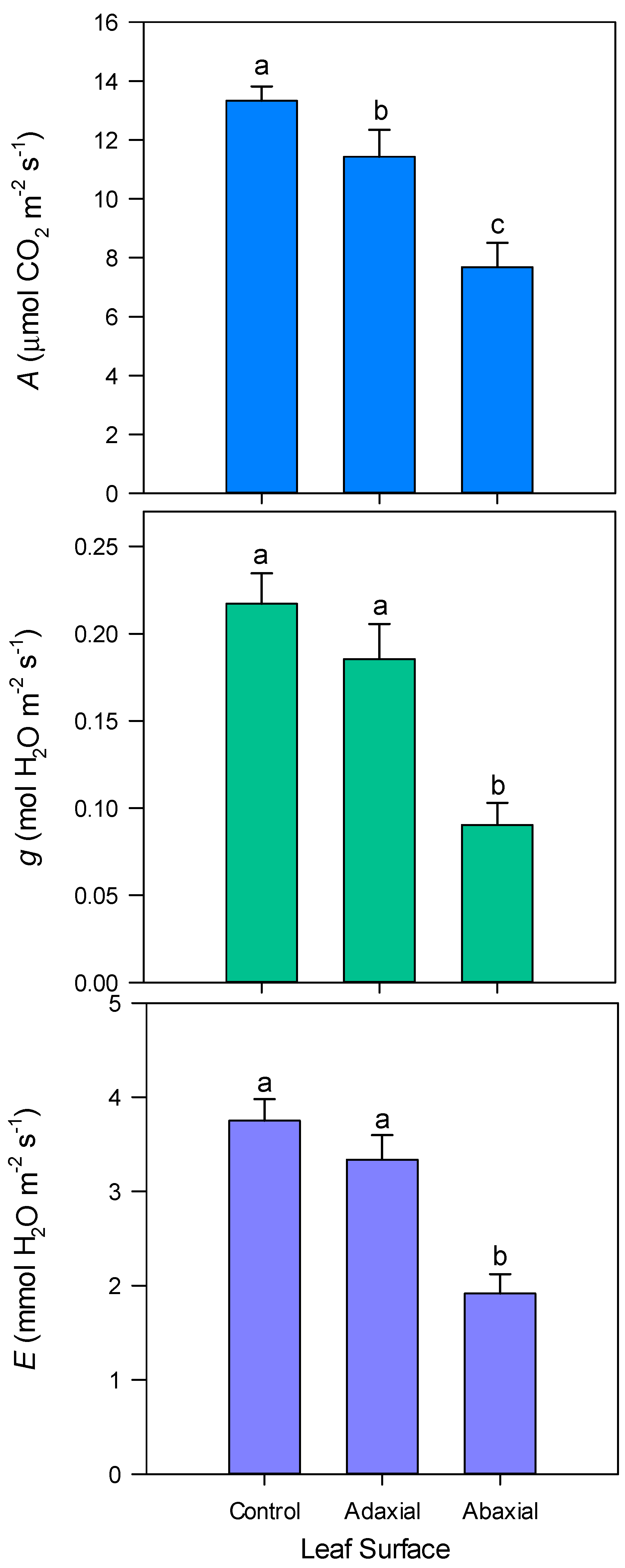
2.2. Leaf Surface Study
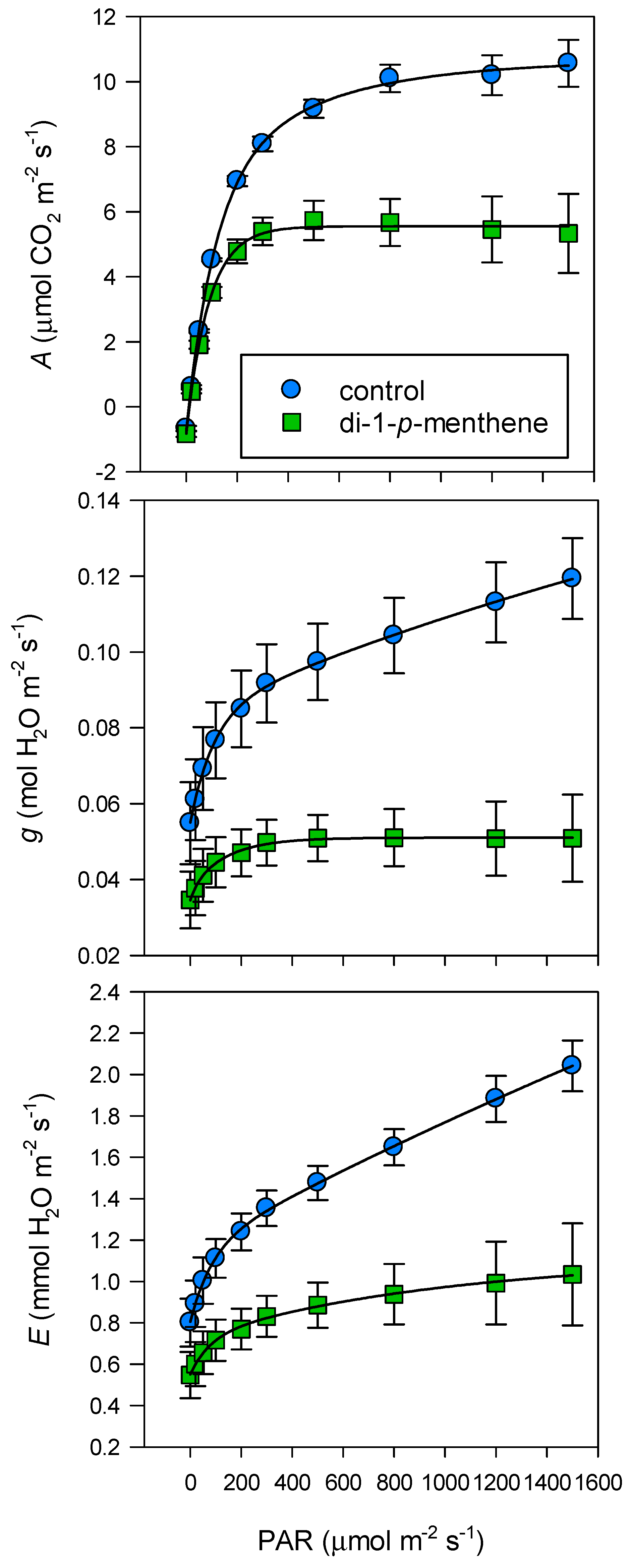
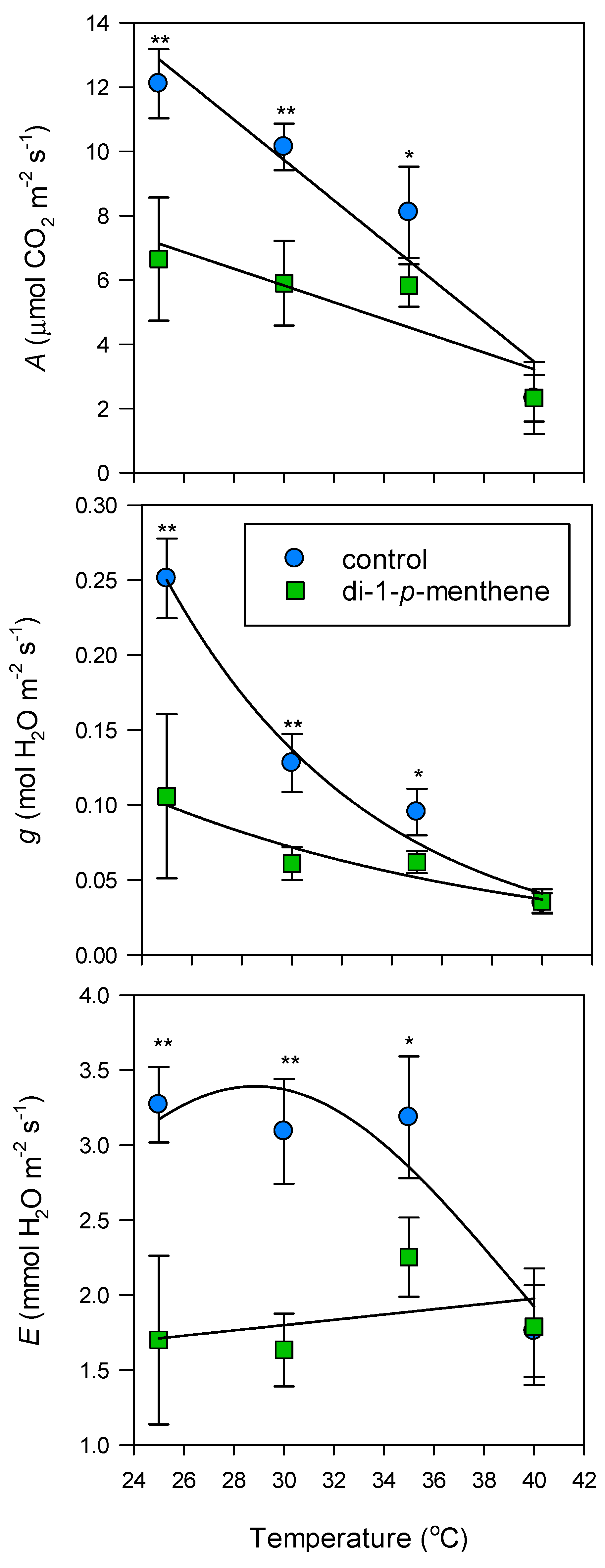
2.3. Light and Temperature Response of Individual Leaves to Infrared Gas Analyser Settings
2.4. Leaf Gas Exchange
2.5. Fruit Transpiration
2.6. Statistical Analysis
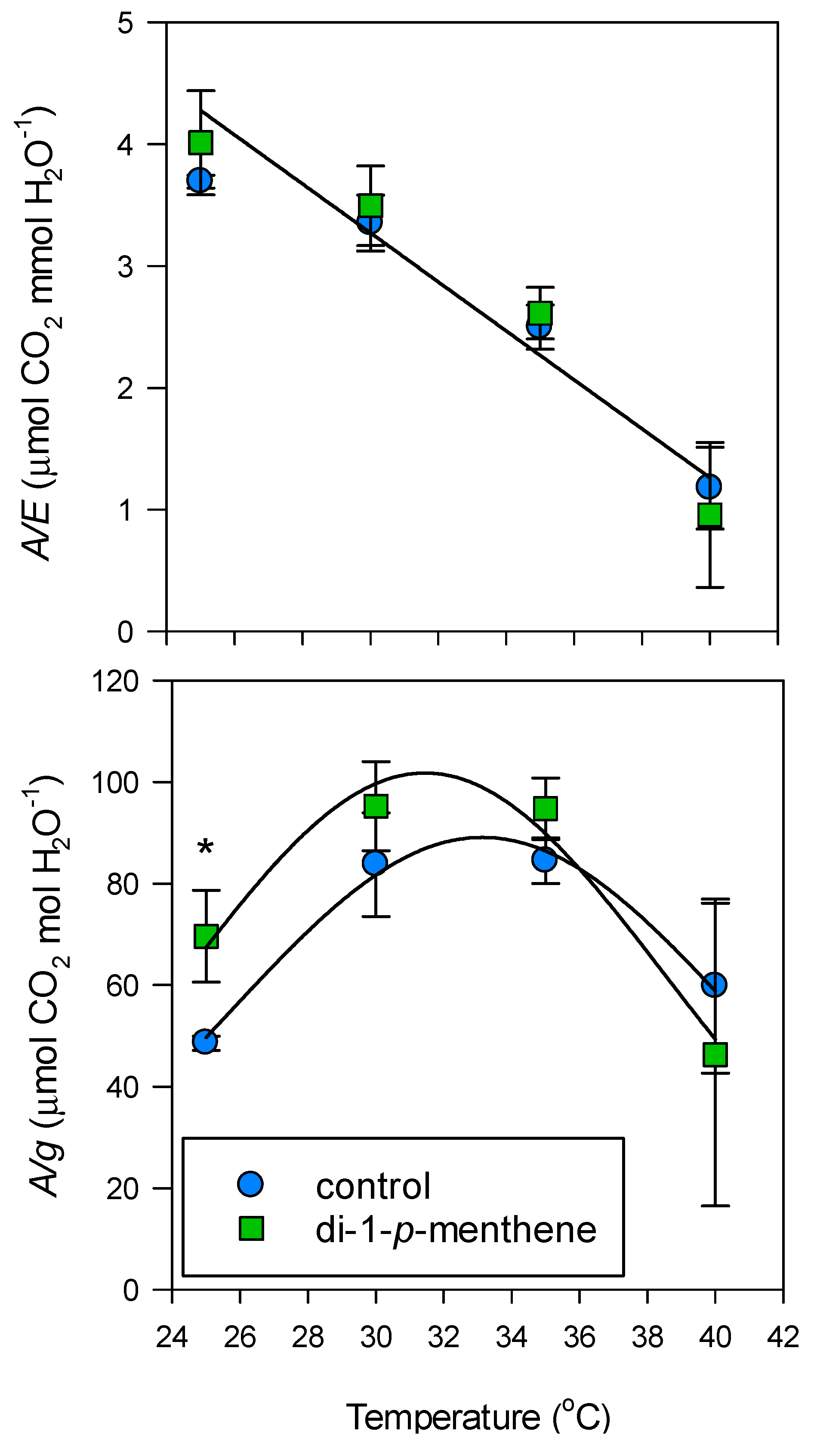
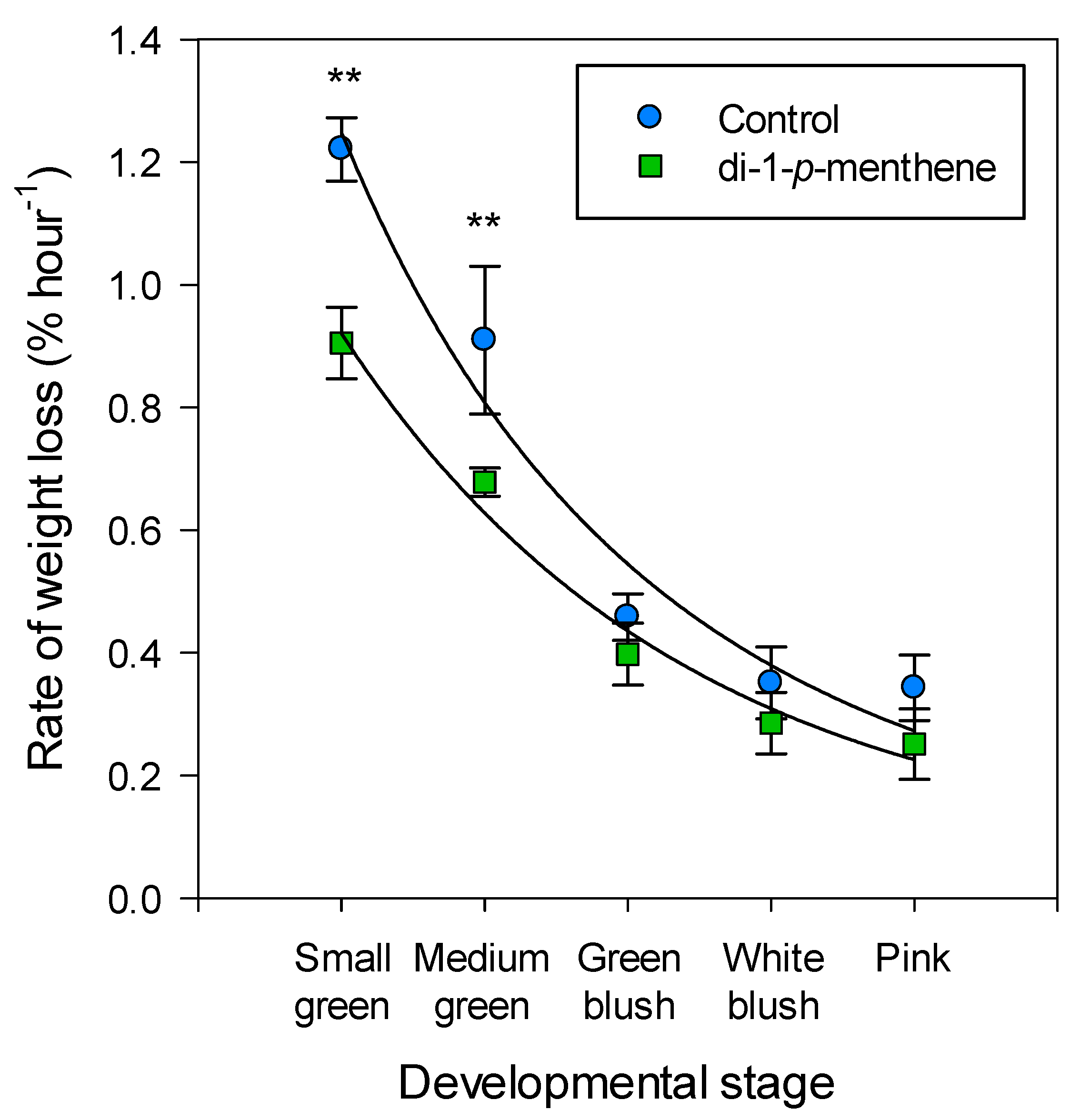
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Totic, I. Raspberry breeding and protection against disease and pests. Bulg. J. Agric. Sci. 2014, 20, 391–404. [Google Scholar]
- Morales, C.G.; Pino, M.T.; del Pozo, A. Phenological and physiological responses to drought stress and subsequent rehydration cycles in two raspberry cultivars. Sci. Hortic. 2013, 162, 234–241. [Google Scholar] [CrossRef]
- Percival, D.C.; Proctor, J.T.; Sullivan, J.A. Cultivar differences in carbon assimilation and partitioning of primocane-fruiting raspberry. J. Am. Pomol. Soc. 2001, 55, 82. [Google Scholar]
- Bryla, D.; Kaufman, D.; Strik, B. Effects of irrigation method and level of water application on fruit size and yield in red raspberry (Rubus idaeus L.) during the first year of full production. HortScience 2008, 43, 1112. [Google Scholar] [CrossRef]
- Carew, J.; Mahmood, K.; Darby, J.; Hadley, P.; Battey, N. The effect of temperature, photosynthetic photon flux density, and photoperiod on the vegetative growth and flowering of ‘Autumn Bliss′ raspberry. J. Am. Soc. Hortic. Sci. 2003, 128, 291–296. [Google Scholar] [CrossRef]
- Gotame, T.; Andersen, L.; Petersen, K.K.; Pedersen, H.L.; Ottosen, C.-O.; Graham, J. Chlorophyll fluorescence and flowering behaviour of annual-fruiting raspberry cultivars under elevated temperature regimes. Eur. J. Hortic. Sci. 2013, 78, 193–202. [Google Scholar]
- Fernandez, G.; Pritts, M. Growth, carbon acquisition and source-sink relationships in ‘Titan’ red raspberry. HortScience 1994, 29, 248b. [Google Scholar] [CrossRef]
- Qiu, C.; Ethier, G.; Pepin, S.; Dubé, P.; Desjardins, Y.; Gosselin, A. Persistent negative temperature response of mesophyll conductance in red raspberry (Rubus idaeus L.) leaves under both high and low vapour pressure deficits: A role for abscisic acid? Plant Cell Environ. 2017, 40, 1940–1959. [Google Scholar] [CrossRef]
- Stafne, E.T.; Clark, J.R.; Rom, C.R. Leaf gas exchange response of ‘Arapaho’ blackberry and six red raspberry cultivars to moderate and high temperatures. HortScience 2001, 36, 880–883. [Google Scholar] [CrossRef]
- Lepaja, K.; Kullaj, E.; Lepaja, L.; Krasniqi, N. Effect of water stress on some physiological indices in raspberry canes. Acta Hortic. 2019, 381–386. [Google Scholar] [CrossRef]
- Percival, D.; Proctor, J.A.; Privé, J.-P. Gas exchange, stem water potential and leaf orientation of Rubus idaeus L. are influenced by drought stress. J. Hortic. Sci. Biotechnol. 1998, 73, 831–840. [Google Scholar] [CrossRef]
- Prive, J.; Janes, D. Evaluation of plant and soil moisture sensors for the detection of drought stress in raspberry. Acta Hortic. 2003. [Google Scholar] [CrossRef]
- Gale, J.; Hagan, R.M. Plant antitranspirants. Ann. Rev. Plant Physiol. 1966, 17, 269–282. [Google Scholar] [CrossRef]
- Mphande, W.; Kettlewell, P.S.; Grove, I.G.; Farrell, A.D. The potential of antitranspirants in drought management of arable crops: A review. Agric. Water Manag. 2020, 236, 106143. [Google Scholar] [CrossRef]
- Plaut, Z.; Magril, Y.; Kedem, U. A new film forming material, which reduces water vapour conductance more than CO2 fixation in several horticultural crops. J. Hortic. Sci. Biotechnol. 2004, 79, 528–532. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef]
- Moftah, A.E.; Al-Humaid, A.I. Effects of Kaolin and Pinolene film-forming polymers on water relations and photosynthetic rate of tuberose (Polianthes tuberosa L.) plants under water deficit conditions. J. Appl. Hortic. 2004, 6, 16–22. [Google Scholar] [CrossRef]
- Palliotti, A.; Poni, S.; Berrios, J.; Bernizzoni, F. Vine performance and grape composition as affected by early-season source limitation induced with anti-transpirants in two red Vitis vinifera L. cultivars. Aust. J. Grape Wine Res. 2010, 16, 426–433. [Google Scholar] [CrossRef]
- Fahey, D.; Rogiers, S. Di-1-p-menthene reduces grape leaf and bunch transpiration. Aust. J. Grape Wine Res. 2019, 25, 134–141. [Google Scholar] [CrossRef]
- Rogiers, S.; Fahey, D.; Holzapfel, B. Mitigating sunburn, dehydration and smoke taint in the vineyard: Is there a role for sunscreens, antitranspirants and film forming barriers? Acta Hortic. 2018, 1274, 71–78. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing kaolin and pinolene to improve sustainable grapevine production during drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Galbignani, M.; Garavani, A.; Bernizzoni, F.; Tombesi, S.; Palliotti, A.; Poni, S. Manipulation of ripening via antitranspirants in cv. Barbera (Vitis vinifera L.). Aust. J. Grape Wine Res. 2016, 22, 245–255. [Google Scholar] [CrossRef]
- Palliotti, A.; Panara, F.; Famiani, F.; Sabbatini, P.; Howell, G.S.; Silvestroni, O.; Poni, S. Postveraison application of antitranspirant di-1-p-menthene to control sugar accumulation in Sangiovese grapevines. Am. J. Enol. Vitic. 2013, 64, 378–385. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Mikiciuk, M.; Ptak, P. The effects of anitranspirant di-1-p-menthene on some physiological traits of strawberry. J. Ecol. Eng. 2015, 16. [Google Scholar] [CrossRef]
- del Amor, F.M.; Cuadra-Crespo, P.; Walker, D.J.; Cámara, J.M.; Madrid, R. Effect of foliar application of antitranspirant on photosynthesis and water relations of pepper plants under different levels of CO2 and water stress. J. Plant Physiol. 2010, 167, 1232–1238. [Google Scholar] [CrossRef]
- Tardieu, F.; Davies, W.J. Stomatal response to abscisic acid is a function of current plant water status. Plant Physiol. 1992, 98, 540–545. [Google Scholar] [CrossRef]
- Dodge, B.O. Effect of the orange rusts of Rubus on the development and distribution of stomata. J. Agric. Res. 1923, 25, 495–500. [Google Scholar]
- Woolley, J.T. Relative permeabilities of plastic films to water and carbon dioxide. Plant Physiol. 1967, 42, 641–643. [Google Scholar] [CrossRef]
- Kettlewell, P.; Heath, W.; Haigh, I. Yield enhancement of droughted wheat by film antitranspirant application: Rationale and evidence. Agric. Sci. 2010, 1, 143–147. [Google Scholar] [CrossRef][Green Version]
- Kattge, J.; Knorr, W. Temperature acclimation in a biochemical model of photosynthesis: A reanalysis of data from 36 species. Plant Cell Environ. 2007, 30, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R. Does growth temperature affect the temperature responses of photosynthesis and internal conductance to CO2? A test with Eucalyptus regnans. Tree Physiol. 2008, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weedon, M.M. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef]
- Latocha, P.; Ciechocinska, M.; Pietkiewicz, S.; Kalaji, M. Preliminary assessment of antitranspirant Vapor Gard influence on Actinidia arguta growing under drought stress conditions. Hortic. Landsc. Archit. 2009, 30, 149–159. [Google Scholar]
- del Amor, F.M.; Rubio, J.S. Effects of antitranspirant spray and potassium: Calcium: Magnesium ratio on photosynthesis, nutrient and water uptake, growth, and yield of sweet pepper. J. Plant Nutr. 2009, 32, 97–111. [Google Scholar] [CrossRef]
- Al-Desouki, M.; El-Rhman, I.; Sahar, A. Effect of some antitranspirants and supplementery irrigation on growth, yield and fruit quality of Sultani fig (Ficus carica) grown in the Egyptian western coastal zone under rainfed conditions. Res. J. Agric. Biol. Sci. 2009, 5, 899–908. [Google Scholar]
- Di Vaio, C.; Marallo, N.; Di Lorenzo, R.; Pisciotta, A. Anti-transpirant effects on vine physiology, berry and wine composition of cv. Aglianico (Vitis vinifera L.) grown in south Italy. Agronomy 2019, 9, 244. [Google Scholar] [CrossRef]
- Rebucci, B.; Poni, S.; Intrieri, C.; Magnanini, E.; Lakso, A. Effects of manipulated grape berry transpiration on post-veraison sugar accumulation. Aust. J. Grape Wine Res. 1997, 3, 57–65. [Google Scholar] [CrossRef]
- Marko, V.; Blommers, L.; Bogya, S.; Helsen, H. Kaolin particle films suppress many apple pests, disrupt natural enemies and promote woolly apple aphid. J. Appl. Entomol. 2008, 132, 26–35. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moroni, F.J.; Gascon-Aldana, P.J.; Rogiers, S.Y. Characterizing the Efficacy of a Film-Forming Antitranspirant on Raspberry Foliar and Fruit Transpiration. Biology 2020, 9, 255. https://doi.org/10.3390/biology9090255
Moroni FJ, Gascon-Aldana PJ, Rogiers SY. Characterizing the Efficacy of a Film-Forming Antitranspirant on Raspberry Foliar and Fruit Transpiration. Biology. 2020; 9(9):255. https://doi.org/10.3390/biology9090255
Chicago/Turabian StyleMoroni, Francesca J., Pedro J. Gascon-Aldana, and Suzy Y. Rogiers. 2020. "Characterizing the Efficacy of a Film-Forming Antitranspirant on Raspberry Foliar and Fruit Transpiration" Biology 9, no. 9: 255. https://doi.org/10.3390/biology9090255
APA StyleMoroni, F. J., Gascon-Aldana, P. J., & Rogiers, S. Y. (2020). Characterizing the Efficacy of a Film-Forming Antitranspirant on Raspberry Foliar and Fruit Transpiration. Biology, 9(9), 255. https://doi.org/10.3390/biology9090255





