Model and Data Concur and Explain the Coexistence of Two Very Distinct Animal Behavioral Types
Abstract
1. Introduction
2. Material and Methods
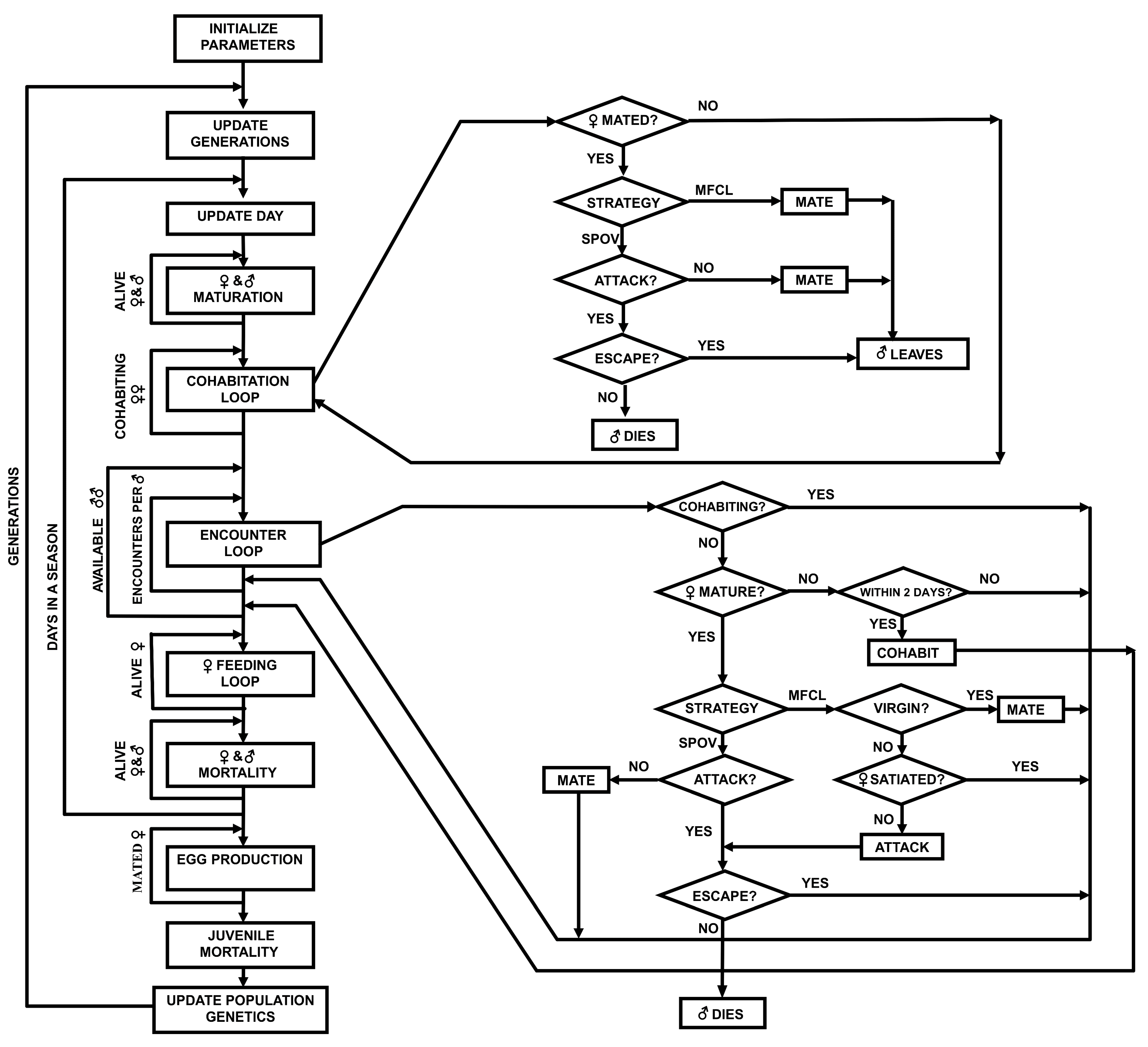
2.1. The Model
2.1.1. Model Assumptions
2.1.2. Modeling Algorithm
2.1.3. Model Parameterization
2.2. Simulations
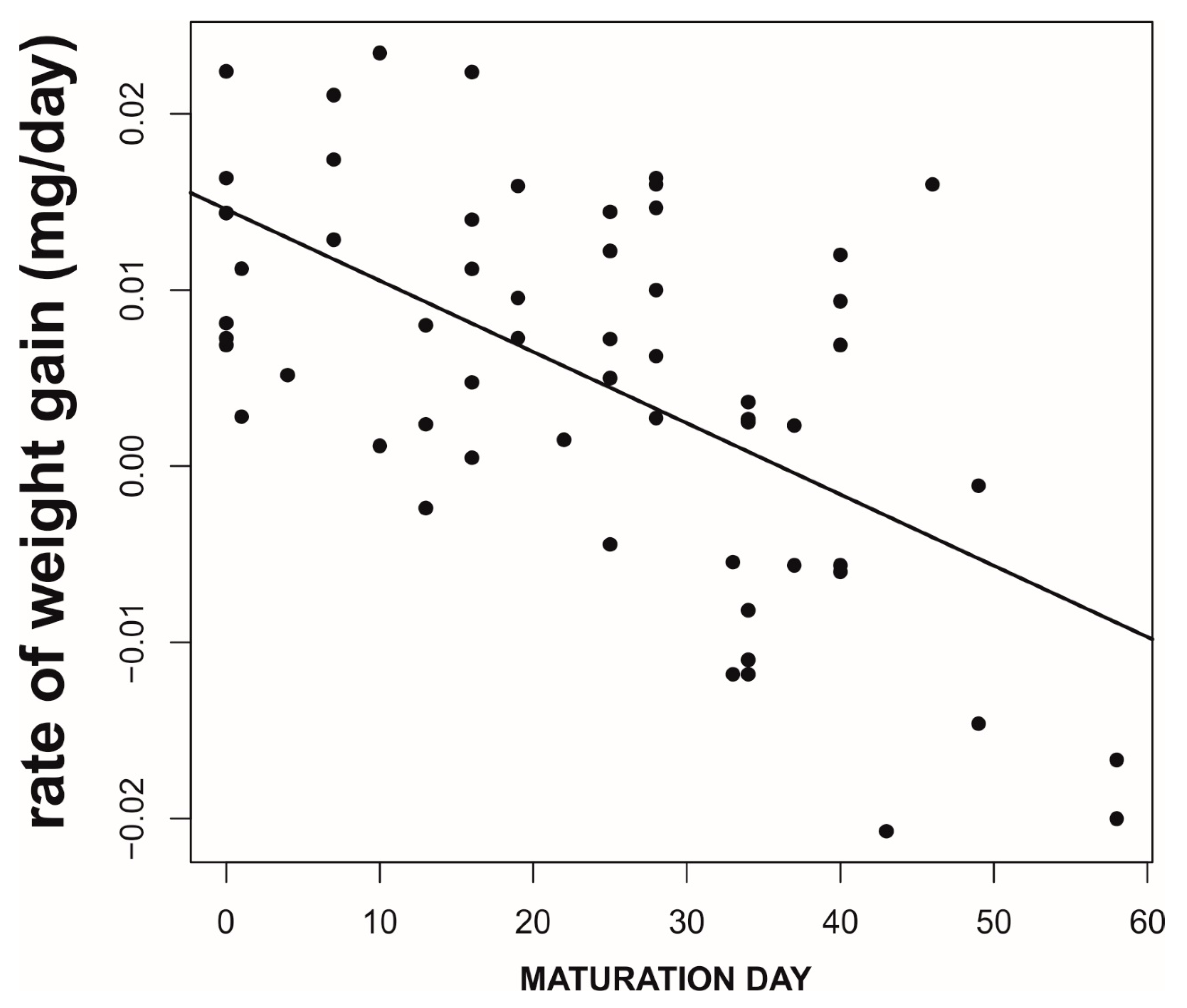
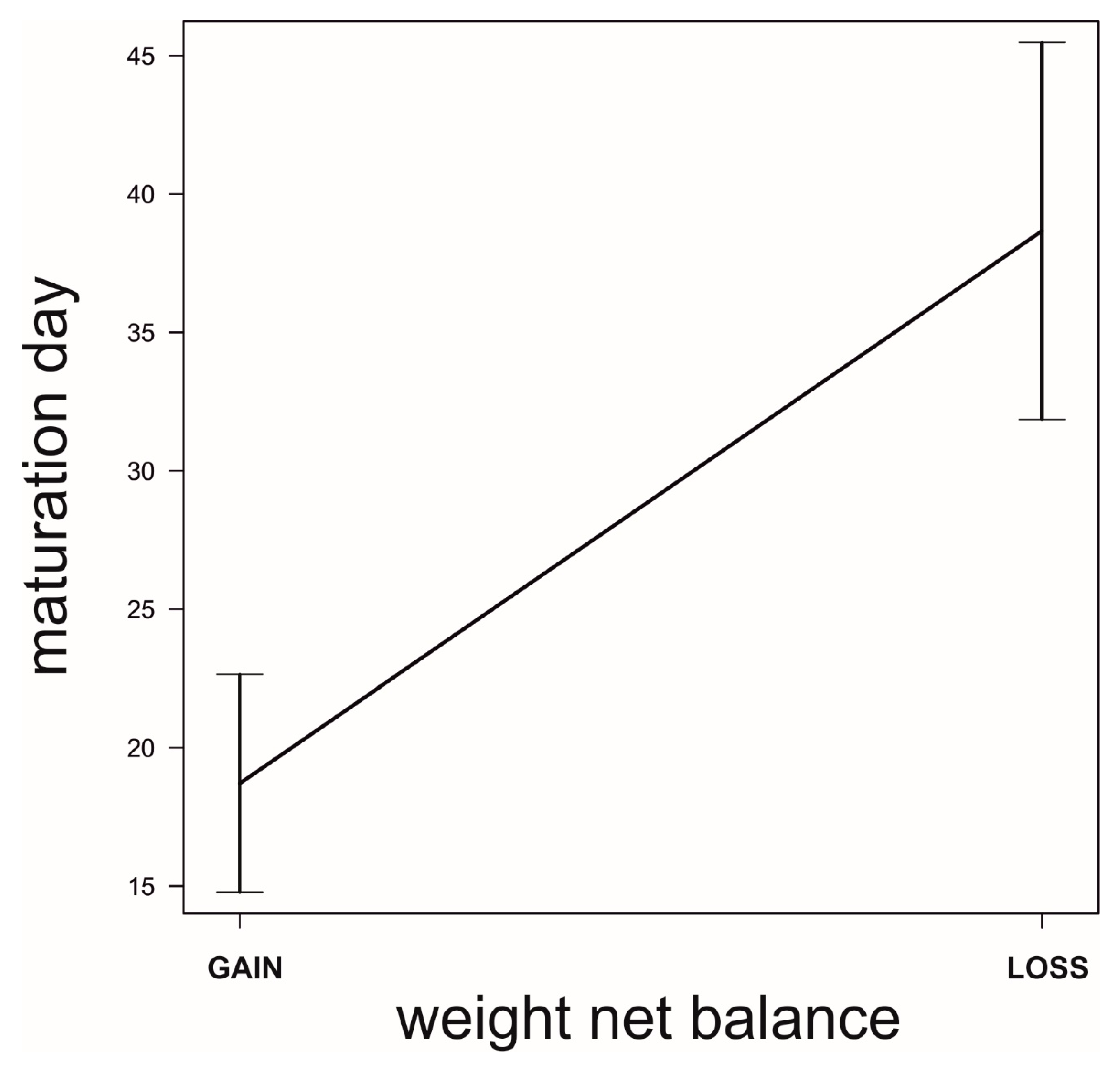
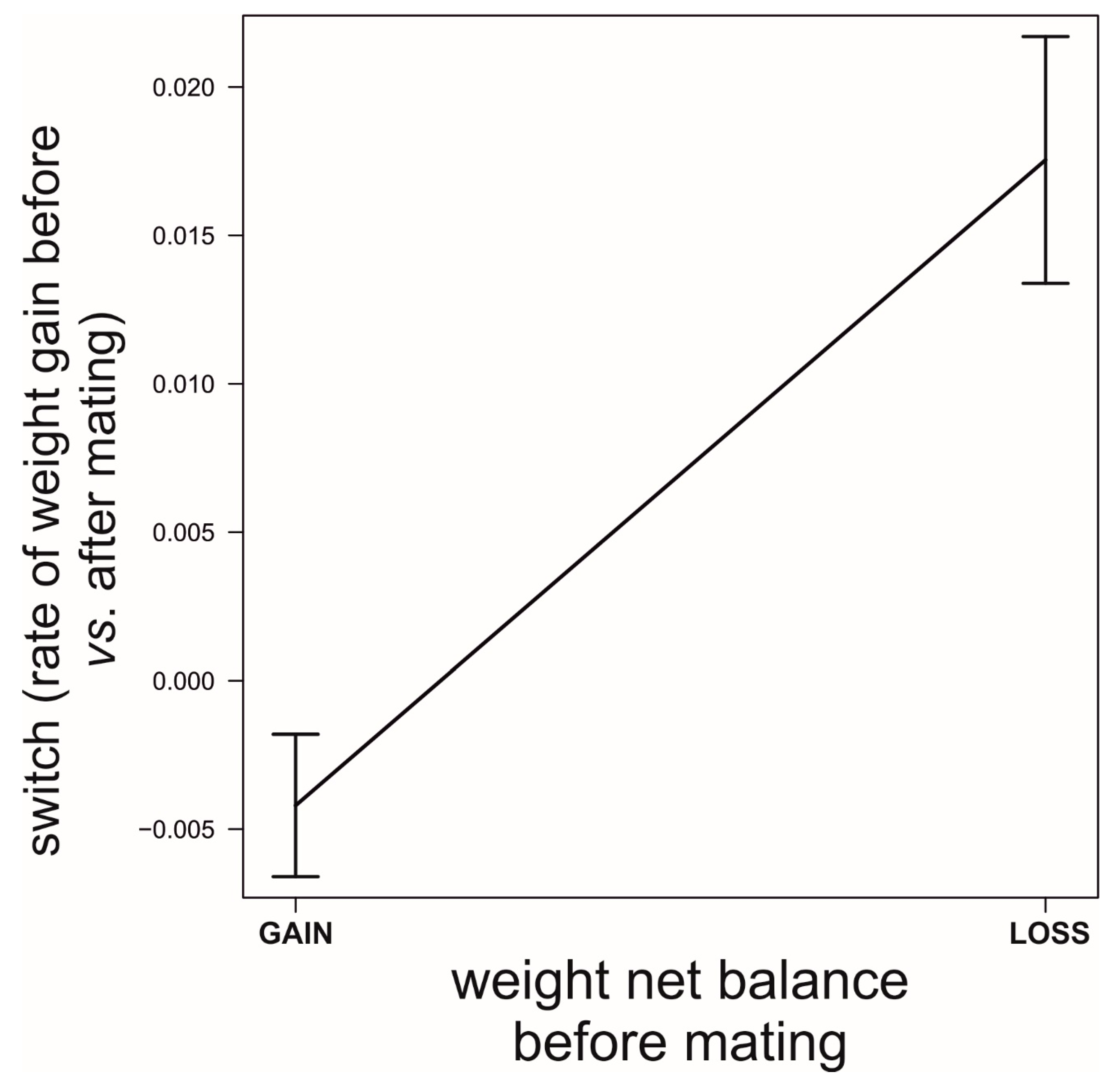
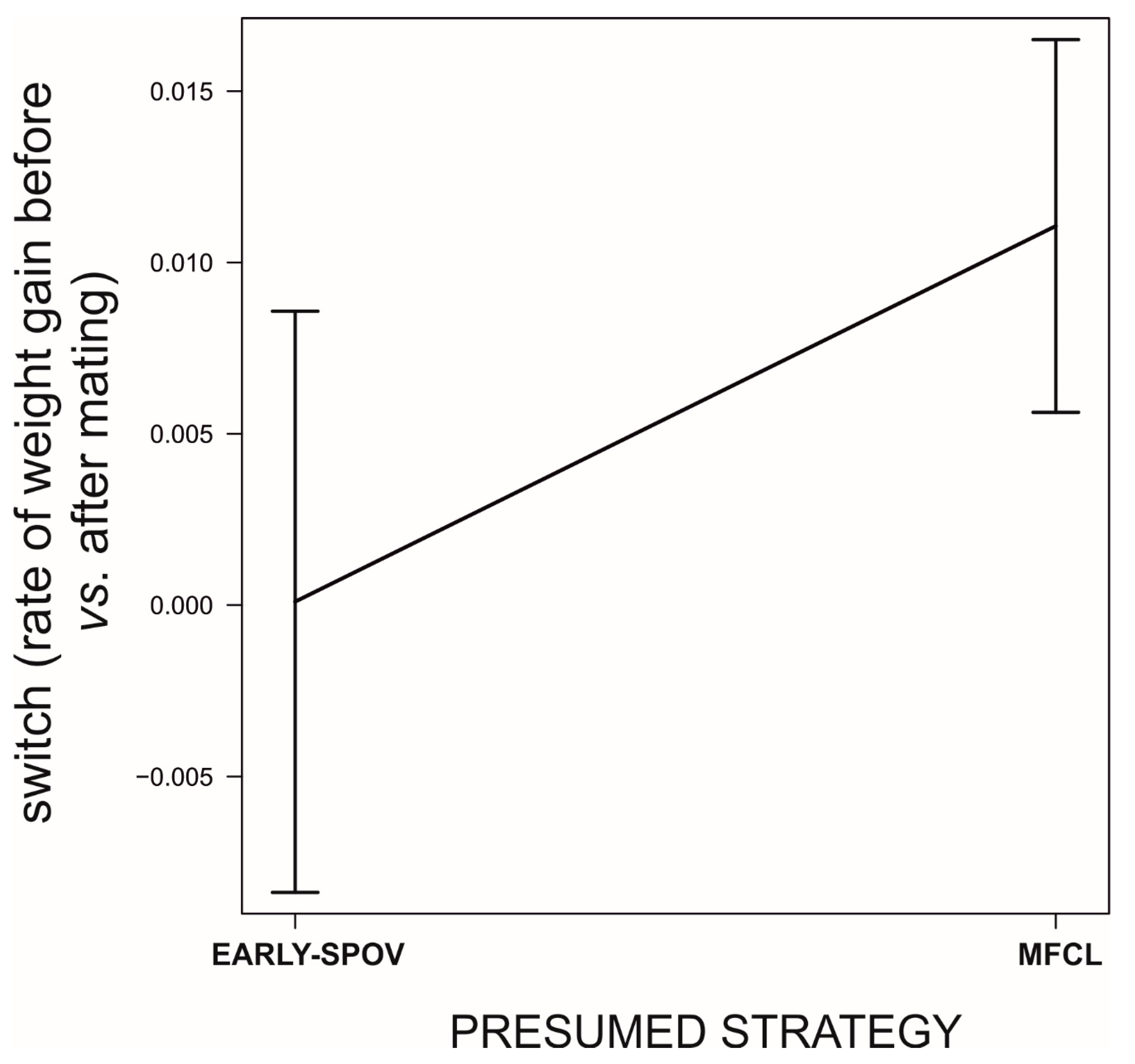
Field Data: Do the Simulated Strategies Coexist in the Wild?
3. Results
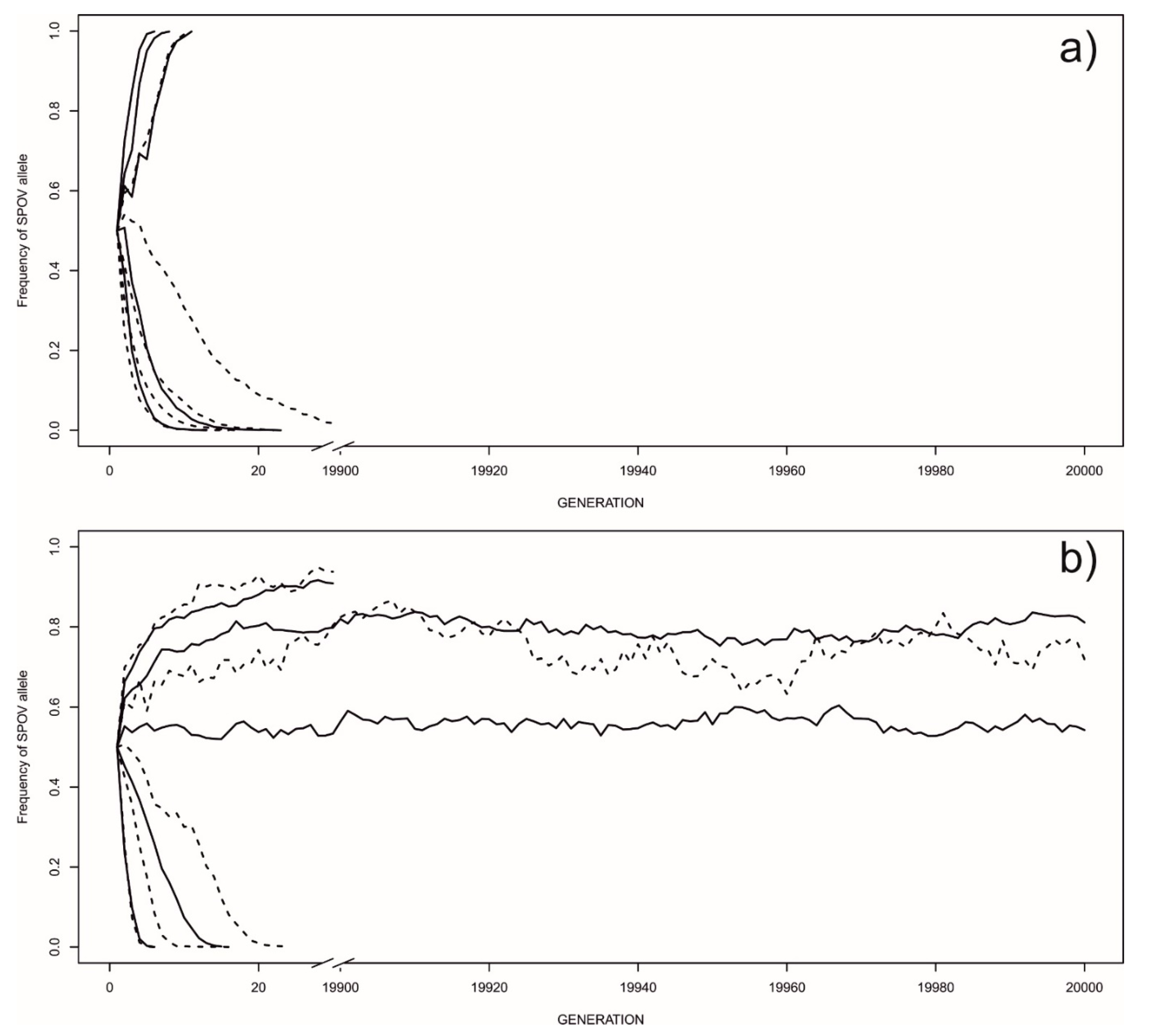
3.1. Ecological Scenarios for the Maintenance of Strategies
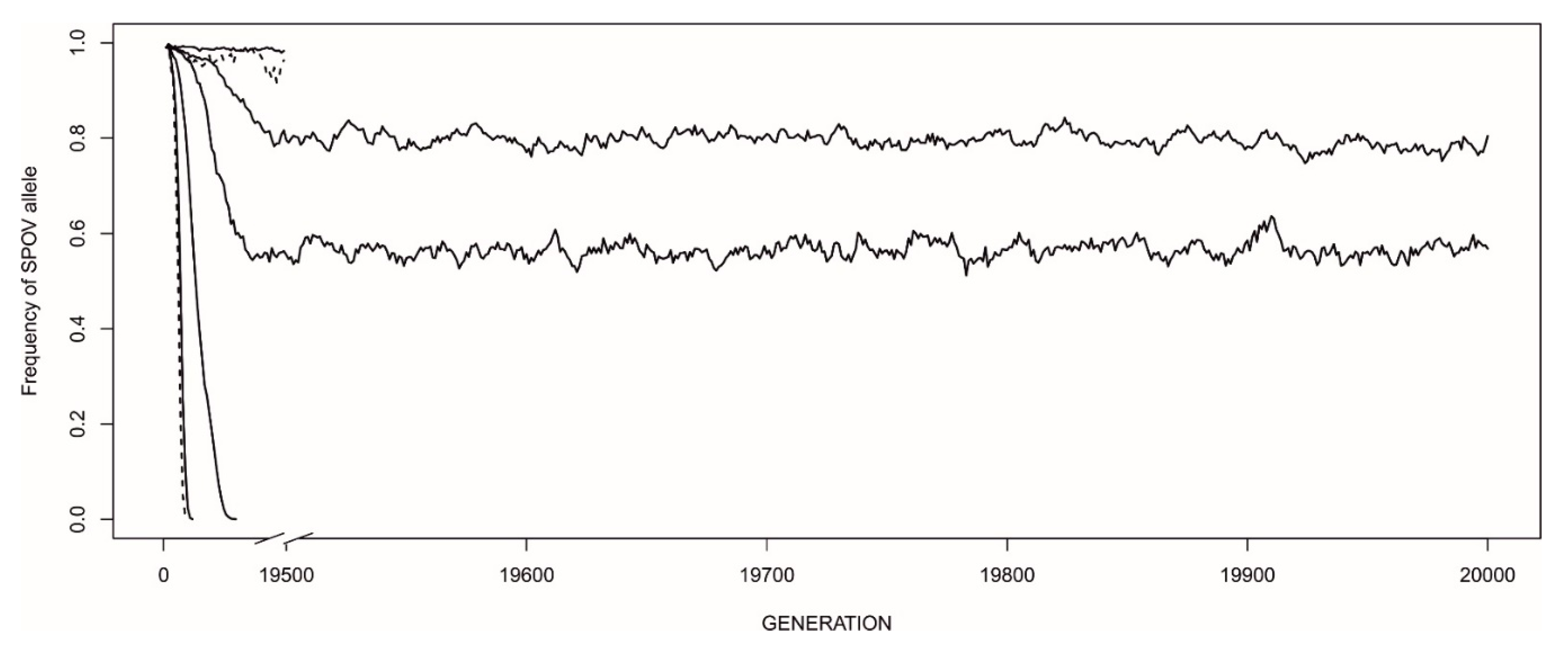
3.2. Frequency-Dependent Equilibria
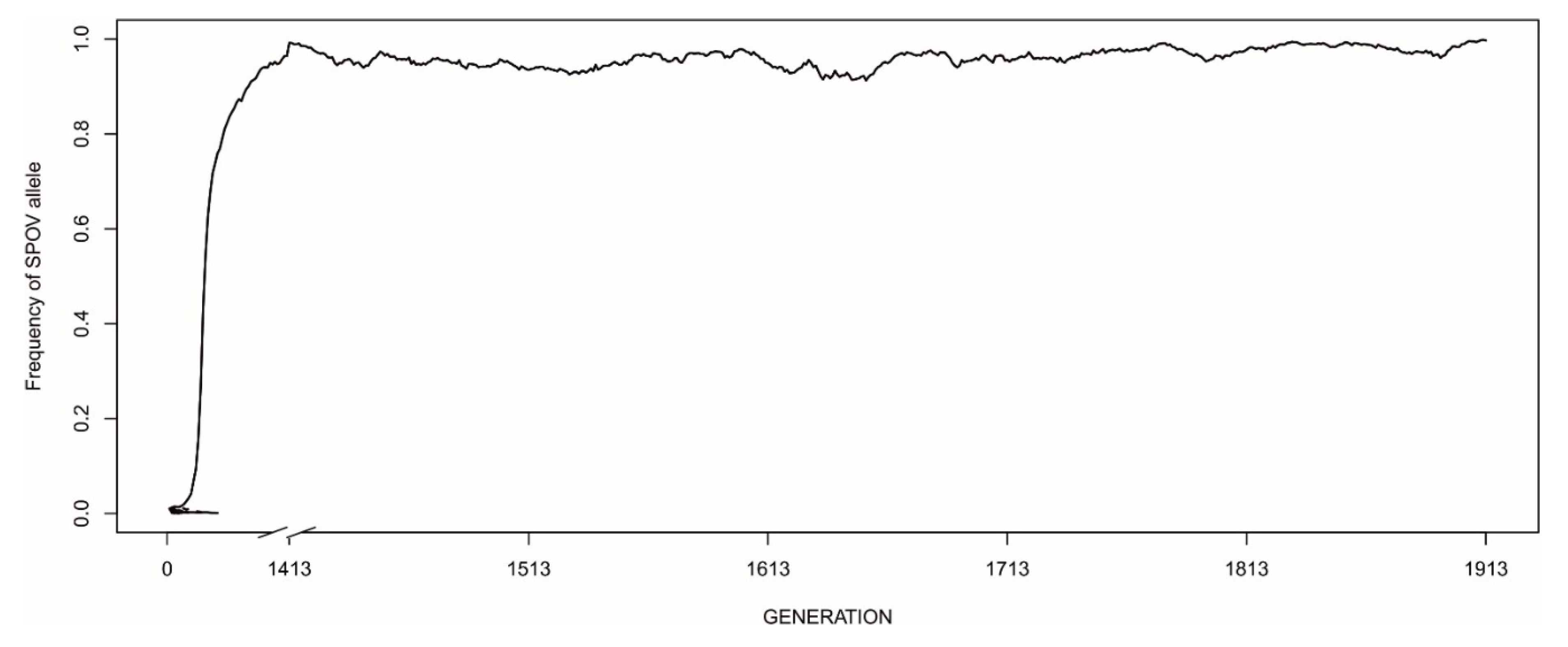
3.3. The Evolution of Strategies—The Invasion of Novel Mutations
Field Data: Do the Simulated Strategies Coexist in the Wild?
4. Discussion
4.1. Ecological Determinants of the Frequency-Dependent Equilibrium
4.2. Behavioral Syndromes and Behavioral Plasticity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lande, R. Natural Selection and Random Genetic Drift in Phenotypic Evolution. Evolution 1976, 30, 314–334. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Bolnick, D.; Svanbäck, R.; Fordyce, J.; Yang, L.; Davis, J.; Hulsey, C.; Forister, M. The Ecology of Individuals: Incidence and Implications of Individual Specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.; Amarasekare, P.; Araújo, M.; Bürger, R.; Levine, J.; Novak, M.; Rudolf, V.; Schreiber, S.; Urban, M.; Vasseur, D. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Des Roches, S.; Post, D.; Turley, N.; Bailey, J.; Hendry, A.; Kinnison, M.; Schweitzer, J.; Palkovacs, E. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Raffard, A.; Santoul, F.; Cucherousset, J.; Blanchet, S. The community and ecosystem consequences of intraspecific diversity: A meta-analysis. Biol. Rev. 2018, 94. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Bilbao-Castro, J.; Barrionuevo Rosales, G.; Ruiz-Lupión, D.; Casado, L.G.; Montserrat, M.; Melian, C.; Magalhaes, S. Eco-Evolutionary Spatial Dynamics: Rapid Evolution and Isolation Explain Food Web Persistence. Adv. Ecol. Res. 2014, 50, 75–143. [Google Scholar] [CrossRef]
- Schoener, T. The Newest Synthesis: Understanding the Interplay of Evolutionary and Ecological Dynamics. Science 2011, 331, 426–429. [Google Scholar] [CrossRef]
- Yoshida, T.; Ellner, S.; Jones, L.; Bohannan, B.; Lenski, R.; Hairston, N. Cryptic Population Dynamics: Rapid Evolution Masks Trophic Interactions. PLoS Biol. 2007, 5, e235. [Google Scholar] [CrossRef]
- Roff, D. Evolutionary Quantitative Genetic. In Evolutionary Quantitative Genetics; Springer US: New York, NY, USA, 1997; Volume 2, p. 493. ISBN 0-412-12971-X. [Google Scholar]
- Dingemanse, N.; Wolf, M. Between-individual differences in behavioural plasticity within populations: Causes and consequences. Anim. Behav. 2013, 85, 1031–1039. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.; Robert, Z. Behavioral syndromes: An integrative overview. Q. Rev. Biol. 2004, 79, 241–277. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J. Behavioral syndrome: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. Future directions in behavioural syndromes research. Proc. Biol. Sci. 2007, 274, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Cote, J.; Evans, M.; Fogarty, S.; Pruitt, J. Ecological implications of behavioural syndromes. Ecol. Lett. 2012, 15, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Weissing, F. Animal personalities: Consequences for ecology and evolution. Trends Ecol. Evol. 2012, 27, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Réale, D.; Reader, S.; Sol, D.; McDougall, P.; Dingemanse, N. Integrating animal temperament within ecology and evolution. Biol. Rev. Camb. Philos. Soc. 2007, 82, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Réale, D.; Garant, D.; Humphries, M.; Bergeron, P.; Careau, V.; Montiglio, P.-O. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 4051–4063. [Google Scholar] [CrossRef]
- Maynard-Smith, J. Evolution and the Theory of Games; Cambridge University Press: Cambridge, UK, 1982; ISBN 9780521288842. [Google Scholar]
- Maynard-Smith, J.; Harper, D. The Evolution of Aggression: Can Selection Generate Variability? [and Discussion]. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988, 319, 557–570. [Google Scholar] [CrossRef]
- Wolf, M.; van Doorn, S.; Weissing, F. Evolutionary emergence of responsive and unresponsive personalities.Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl. Acad. Sci. USA 2008, 105, 15825–15830. [Google Scholar] [CrossRef]
- Wolf, M.; Mcnamara, J.; Wolf, M.; McNamara, J.M. On the evolution of personalities via frequency-dependent selection. Am. Nat. 2012, 179, 679–692. [Google Scholar] [CrossRef]
- Wolf, M.; Weissing, F. An explanatory framework for adaptive personality differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3959–3968. [Google Scholar] [CrossRef]
- Bell, A. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 2005, 18, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, N.; Wright, J.; Kazem, A.; Thomas, D.; Hickling, R.; Dawnay, N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 2007, 76, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, N.; Kazem, A.; Réale, D.; Wright, J. Behavioural reaction norms: Animal personality meets individual plasticity. Trends Ecol. Evol. 2010, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, N.; Bouwman, K.; van de Pol, M.; Overveld, T.; Patrick, S.; Matthysen, E.; Quinn, J. Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J. Anim. Ecol. 2012, 81, 116–126. [Google Scholar] [CrossRef]
- Elgar, M. Sexual cannibalism in spiders and other invertebrates. In Cannibalism: Ecology and Evolution among Diverse Taxa; Oxford University Press: Oxford, UK, 1992; ISBN 0198546505. [Google Scholar]
- Elgar, M.; Schneider, J. Evolutionary Significance of Sexual Cannibalism. In Advances in The Study of Behavior; Elsevier: Amsterdam, The Netherlands, 2004; Volume 34, pp. 135–163. [Google Scholar]
- Arnqvist, G.; Henriksson, S. Sexual cannibalism in fishing spider and a model for the evolution of sexual cannibalism based on genetic constraints. Evol. Ecol. 1997, 11, 255–273. [Google Scholar] [CrossRef]
- Johnson, J.; Sih, A. Fear, food, sex and parental care: A syndrome of boldness in the fishing spider, Dolomedes triton. Anim. Behav. 2007, 74, 1131–1138. [Google Scholar] [CrossRef]
- Johnson, J.; Sih, A. Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): A role for behavioral syndromes. Behav. Ecol. Sociobiol. 2005, 58, 390–396. [Google Scholar] [CrossRef]
- Kralj-Fišer, S.; Schneider, J.; Kuntner, M.; Hauber, M. Challenging the Aggressive Spillover Hypothesis: Is Pre-Copulatory Sexual Cannibalism a Part of a Behavioural Syndrome? Ethology 2013, 119. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Pascual, J.; Wise, D. Mating patterns in late-maturing female Mediterranean tarantulas may reflect the costs and benefits of sexual cannibalism. Anim. Behav. 2003, 66, 469–476. [Google Scholar] [CrossRef][Green Version]
- Rabaneda-Bueno, R.; Aguado, S.; Fernández-Montraveta, C.; Moya-Laraño, J. Does female personality determine mate choice through sexual cannibalism? Ethology 2014, 120. [Google Scholar] [CrossRef]
- Riechert, S.; Hedrick, A. A test for correlations among fitness-linked behavioural traits in the spider Agelenopsis aperta (Araneae, Agelenidae). Anim. Behav. 1993, 46, 669–675. [Google Scholar] [CrossRef]
- Kralj-Fišer, S.; Čandek, K.; Lokovšek, T.; Čelik, T.; Cheng, R.-C.; Elgar, M.; Kuntner, M. Mate choice and sexual size dimorphism, not personality, explain female aggression and sexual cannibalism in raft spiders. Anim. Behav. 2016, 111. [Google Scholar] [CrossRef]
- Newman, J.; Elgar, M. Sexual Cannibalism in Orb-Weaving Spiders: An Economic Model. Am. Nat. 1991, 138, 1372–1395. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Orta-Ocaña, J.; José Antonio, B.; Bach, C.; Wise, D. Intriguing compensation by adult female spiders for food limitation experienced as juveniles. Oikos 2003, 101, 539–548. [Google Scholar] [CrossRef]
- Barry, K.; Holwell, G.; Herberstein, M. Female praying mantids use sexual cannibalism as a foraging strategy to increase fecundity. Behav. Ecol. 2008, 19, 710–715. [Google Scholar] [CrossRef]
- Gavín Centol, P.; Kralj-Fišer, S.; De Mas Castroverde, E.; Ruiz-Lupión, D.; Moya-Laraño, J. Feeding regime, adult age and sexual size dimorphism as determinants of pre-copulatory sexual cannibalism in virgin wolf spiders. Behav. Ecol. Sociobiol. 2017, 71. [Google Scholar] [CrossRef]
- Wilder, S.; Rypstra, A. Sexual size dimorphism mediates the occurrence of state-dependent sexual cannibalism in a wolf spider. Anim. Behav. 2008, 76, 447–454. [Google Scholar] [CrossRef]
- Rabaneda-Bueno, R.; Rodríguez-Gironés, M.; Aguado, S.; Fernández-Montraveta, C.; De Mas Castroverde, E.; Wise, D.; Moya-Laraño, J. Sexual Cannibalism: High Incidence in a Natural Population with Benefits to Females. PLoS ONE 2008, 3, e3484. [Google Scholar] [CrossRef]
- Johnson, J. Sexual cannibalism in fishing spiders (Dolomedes triton): An evaluation of two explanations for female aggression towards potential mates. Anim. Behav. 2001, 61, 905–914. [Google Scholar] [CrossRef]
- Erez, T.; Schneider, J.M.; Lubin, Y. Is Male Cohabitation Costly for Females of the Spider Stegodyphus lineatus (Eresidae)? Ethology 2005, 111, 693–704. [Google Scholar] [CrossRef]
- Dingemanse, N.; Both, C.; Drent, P.; Tinbergen, J. Fitness consequences of avian personalities in a fluctuating environment. Proc. Biol. Sci. 2004, 271, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, N.; Réale, D. Natural selection and animal personality. Behaviour 2005, 142. [Google Scholar] [CrossRef]
- Smith, B.; Blumstein, D. Fitness consequences of personality: A meta-analysis. Behav. Ecol. 2008, 19, 448–455. [Google Scholar] [CrossRef]
- Moya-Laraño, J. Senescence and food limitation in a slowly aging spider. Funct. Ecol. 2002, 16, 734–741. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Orta-Ocana, J.; José Antonio, B.; Bach, C.; Wise, D. Territoriality in a Cannibalistic Burrowing Wolf Spider. Ecology 2002, 83, 356–361. [Google Scholar] [CrossRef]
- Biro, P.; Abrahams, M.; Post, J.; Parkinson, E. Behavioural trade offs between growth and mortality explain evolution of submaximal growth rates. J. Anim. Ecol. 2006, 75, 1165–1171. [Google Scholar] [CrossRef]
- DeAngelis, D.L.; Mooij, W.M. Individual-Based Modeling of Ecological and Evolutionary Processes. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 147–168. [Google Scholar] [CrossRef]
- Deangelis, D.; Grimm, V. Individual-based models in ecology after four decades. F1000Prime Rep. 2014, 6, 39. [Google Scholar] [CrossRef]
- Grimm, V.; Berger, U.; Bastiansen, F.; Eliassen, S.; Ginot, V.; Giske, J.; Goss-Custard, J.; Grand, T.; Heinz, S.; Huse, G.; et al. A Standard Protocol for Describing Individual-Based and Agent Based Models. Ecol. Modell. 2006, 198, 115–126. [Google Scholar] [CrossRef]
- Planas, E.; Fernández-Montraveta, C.; Ribera, C. Molecular systematics of the wolf spider genus Lycosa (Araneae: Lycosidae) in the Western Mediterranean Basin. Mol. Phylogenet. Evol. 2013, 67. [Google Scholar] [CrossRef]
- Riechert, S.; Maynard-Smith, J. Genetic analyses of two behavioural traits linked to individual fitness in the desert spider Agelenopsis aperta. Anim. Behav. 1989, 37, 624–637. [Google Scholar] [CrossRef]
- Král, J.; Musilová, J.; Stahlavsky, F.; Rezác, M.; Akan, Z.; Edwards, R.; Coyle, F.; Ribera, C. Evolution of the karyotype and sex chromosome systems in basal clades of araneomorph spiders (Araneae: Araneomorphae). Chromosome Res. 2006, 14, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montraveta, C.; Ortega, J. Sex differences in the agonistic behaviour of a lycosid spider (Araneae Lycosidae). Ethol. Ecol. Evol. 1993, 5, 293–301. [Google Scholar] [CrossRef]
- Elgar, M. Sperm Competition and Sexual Selection in Spiders and Other Arachnids. In Sperm Competition and Sexual Selection; Academic Press: Cambridge, MA, USA, 1998; pp. 307–339. ISBN 9780121005436. [Google Scholar]
- Orta, J.M.; Moya-Laraño, J.; Barrientos, J.A. Datos fenológicos de una población de Lycosa tarantula fasciiventris L. Dufour, 1835, en el Noroeste de la Península Ibérica (Araneae, Lycosidae). Bolletino Accad. Gioenia Sci. Nat. 1993, 26, 15–26. [Google Scholar]
- Uhl, G.; Gunnarsson, B. Female genitalia in Pityohyphantes phrygianus, a spider with a skewed sex ratio. J. Zool. 2001, 255, 367–376. [Google Scholar] [CrossRef]
- Jakob, E.; Marshall, S.; Uetz, G.; Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating fitness: A comparison of body condition indices. Oikos 1996, 77, 61. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Pascual, J.; Wise, D. Approach Strategy by which Male Mediterranean Tarantulas Adjust to the Cannibalistic Behaviour of Females. Ethology 2004, 110, 717–724. [Google Scholar] [CrossRef]
- Moya-Laraño, J. Limitación por el Alimento, Territorialidad y Canibalismo en la Tarántula Mediterránea, Lycosa tarentula (L.) (Araneae, Lycosidae). Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 1999. [Google Scholar]
- Moya-Laraño, J.; Macías-Ordóñez, R.; Blanckenhorn, W.; Fernández-Montraveta, C. Analysing body condition: Mass, volume or density? J. Anim. Ecol. 2008, 77, 1099–1108. [Google Scholar] [CrossRef]
- Higgins, E.; Rankin, A. Mortality risk of rapid growth in the spider Nephila clavipes. Funct. Ecol. 2001, 15, 24–28. [Google Scholar] [CrossRef]
- Uetz, G. Foraging strategies of spiders. Trends Ecol. Evol. 1992, 7, 155–159. [Google Scholar] [CrossRef]
- Prokop, P.; Václav, R. Seasonal aspects of sexual cannibalism in the praying mantis (Mantis religiosa). J. Ethol. 2008, 26, 213–218. [Google Scholar] [CrossRef]
- Samu, F.; Toft, S.; Kiss, B.; Samu, F.; Toft, S.; Kiss, B. Factors influencing cannibalism in the wolf spider Pardosa agrestis (Araneae, Lycosidae). Behav. Ecol. Sociobiol. 1999, 45, 349–354. [Google Scholar] [CrossRef]
- Wise, D. Spiders in Ecological Webs; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar] [CrossRef]
- Rabaneda-Bueno, R. El Canibalismo Sexual en la Tarántula Ibérica (Lycosa hispanica): Ecología y Evolución de Estrategias Conductuales. Ph.D. Thesis, Autonomous University of Madrid, Madrid, Spain, 2014. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Core: Vienna, Austria, 2014. [Google Scholar]
- Luttbeg, B.; Sih, A. Risk, resources and state-dependent adaptive behavioural syndromes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3977–3990. [Google Scholar] [CrossRef] [PubMed]
- Moya-Laraño, J.; Verdeny-Vilalta, O.; Rowntree, J.; Melguizo-Ruiz, N.; Montserrat, M.; Laiolo, P. Climate Change and Eco-Evolutionary Dynamics in Food Webs. Adv. Ecol. Res. 2012, 47, 1–80. [Google Scholar] [CrossRef]
- Morse, D. A test of sexual cannibalism models, using a sit-and-wait predator. Biol. J. Linn. Soc. 2004, 81, 427–437. [Google Scholar] [CrossRef][Green Version]
- Morse, D. Mating frequencies of male crab spiders, Misumena vatia (Araneae, Thomisidae). J. Arachnol. 2007, 35, 84–88. [Google Scholar] [CrossRef]
- Legrand, R.; Morse, D. Factors driving extreme sexual size dimorphism of a sit-and-wait predator under low density. Biol. J. Linn. Soc. 2008, 71, 643–664. [Google Scholar] [CrossRef]
- Darwin, C. The Descent of Man and Selection in Relation to Sex, 1st ed.; John Murray: London, UK, 1871. [Google Scholar] [CrossRef]
- Kreiter, N.; Wise, D. Age-related changes in movement patterns in the fishing spider, Dolomedes triton (Araneae, Pisauridae). J. Arachnol. 1996, 24, 24–33. [Google Scholar]
- Kreiter, N.; Wise, D. Prey availability limits fecundity and movement patterns of female fishing spiders. Oecologia 2001, 127, 417–424. [Google Scholar] [CrossRef]
- Aisenberg, A.; Viera, C.; Costa, F.G. Daring females, devoted males, and reversed sexual size dimorphism in the sand-dwelling spider Allocosa brasiliensis (Araneae, Lycosidae). Behav. Ecol. Sociobiol. 2007, 62, 29–35. [Google Scholar] [CrossRef]
- Foellmer, M.; Moya-Laraño, J. Sexual size dimorphism in spiders: Patterns and processes. In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism; Oxford University Press: New York, NY, USA, 2007; pp. 71–81. [Google Scholar]
- Hurd, L.; Eisenberg, R.; Fagan, W.; Tilmon, K.; Snyder, W.; Vandersall, K.; Datz, S.; Welch, J. Cannibalism Reverses Male-Biased Sex Ratio in Adult Mantids: Female Strategy against Food Limitation? Oikos 1994, 69, 193. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; Feder, E.; Rowe, L.; Sokolowski, M. Maintaining a behavior polymorphism by frequency-dependent selection on a single gene. Nature 2007, 447, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. On the roles of directed and random changes in gene frequency in the genetics of populations. Evolution 1948, 2, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. The Genetic Theory of Natural Selection; Oxford University Press: Oxford, UK, 1930. [Google Scholar]
- Clarke, B.; O’Donald, P. Frequency-dependent selection. Heredity (Edinb.) 1964, 19, 201–206. [Google Scholar] [CrossRef]
- Wolf, M.; van Doorn, S.; Leimar, O.; Weissing, F. Life history tradeoffs favour the evolution of personality. Nature 2007, 447, 581–584. [Google Scholar] [CrossRef]
- Neff, B.; Sherman, P. Behavioral syndromes versus Darwinian algorithms. Trends Ecol. Evol. 2004, 19. [Google Scholar] [CrossRef]
- Ruiz-Gomez, M.D.L.; Kittilsen, S.; Höglund, E.; Huntingford, F.; Sørensen, C.; Pottinger, T.; Bakken, M.; Winberg, S.; Korzan, W.; Øverli, Ø. Behavioral plasticity in rainbow trout (Oncorhynchus mykiss) with divergent coping styles: When doves become hawks. Horm. Behav. 2008, 54, 534–538. [Google Scholar] [CrossRef]
- Brodin, T. Behavioral syndrome over the boundaries of life-025EFcarryovers from larvae to adult damselfly. Behav. Ecol. 2009, 20, 30–37. [Google Scholar] [CrossRef]
- Minderman, J.; Reid, J.; Evans, P.; Whittingham, M. Personality traits in wild starlings: Exploration behavior and environmental sensitivity. Behav. Ecol. 2009, 20, 830–837. [Google Scholar] [CrossRef]
- Nelson, X.; Wilson, D.; Evans, C. Behavioral Syndromes in Stable Social Groups: An Artifact of External Constraints? Ethology 2008, 114, 1154–1165. [Google Scholar] [CrossRef]
- Wilson, A.; Godin, J.-G. Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav. Ecol. 2009, 20, 231–237. [Google Scholar] [CrossRef]
- Logue, D.; Mishra, S.; McCaffrey, D.; Ball, D.; Cade, W. A behavioral syndrome linking courtship behavior toward males and females predicts reproductive success from a single mating in the hissing cockroach, Gromphadorhina portentosa. Behav. Ecol. 2009, 20, 781–788. [Google Scholar] [CrossRef]
- Gould, S.J. Only his wings remained. Nat. Hist. 1984, 93, 10–18. [Google Scholar]
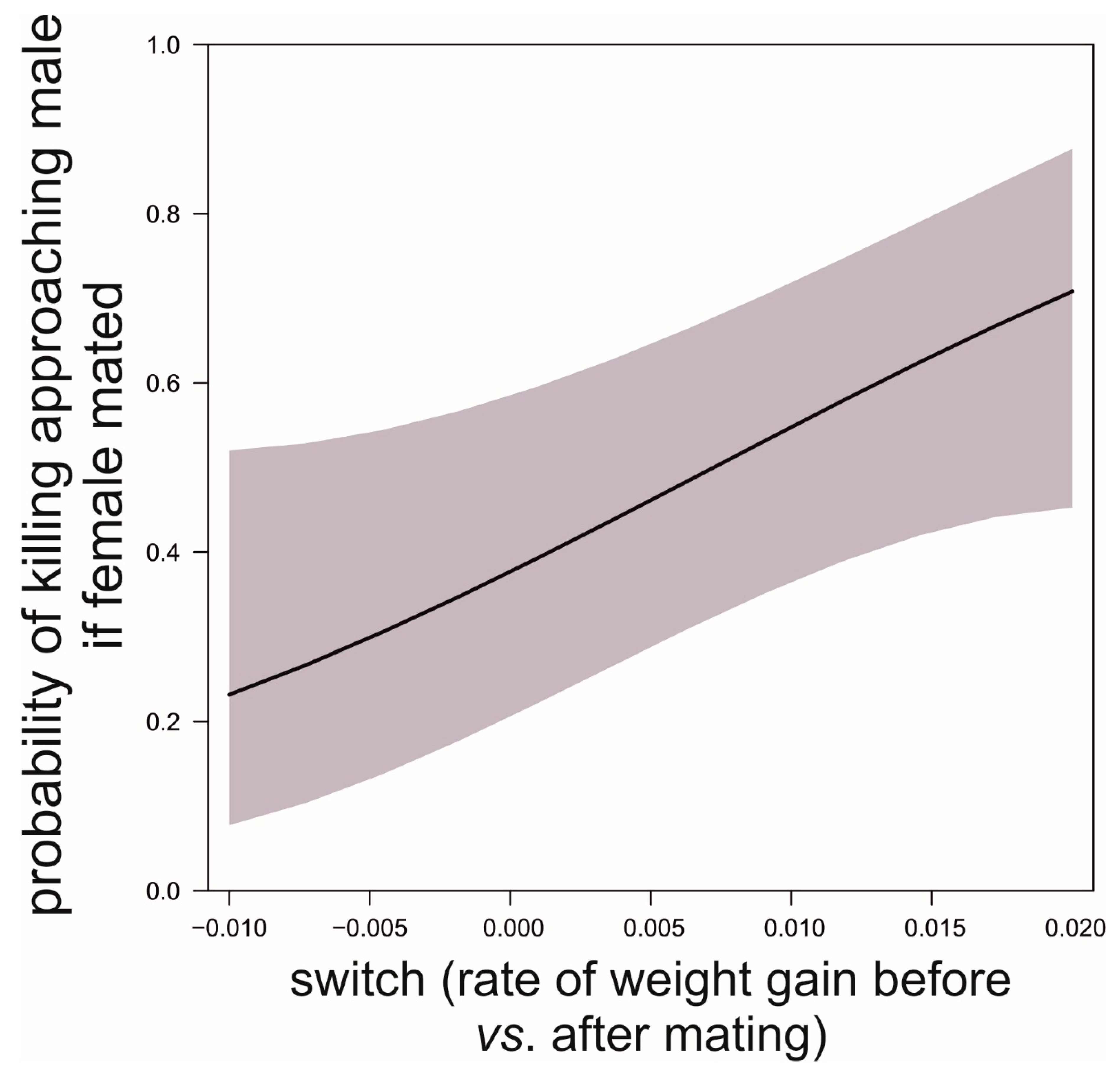
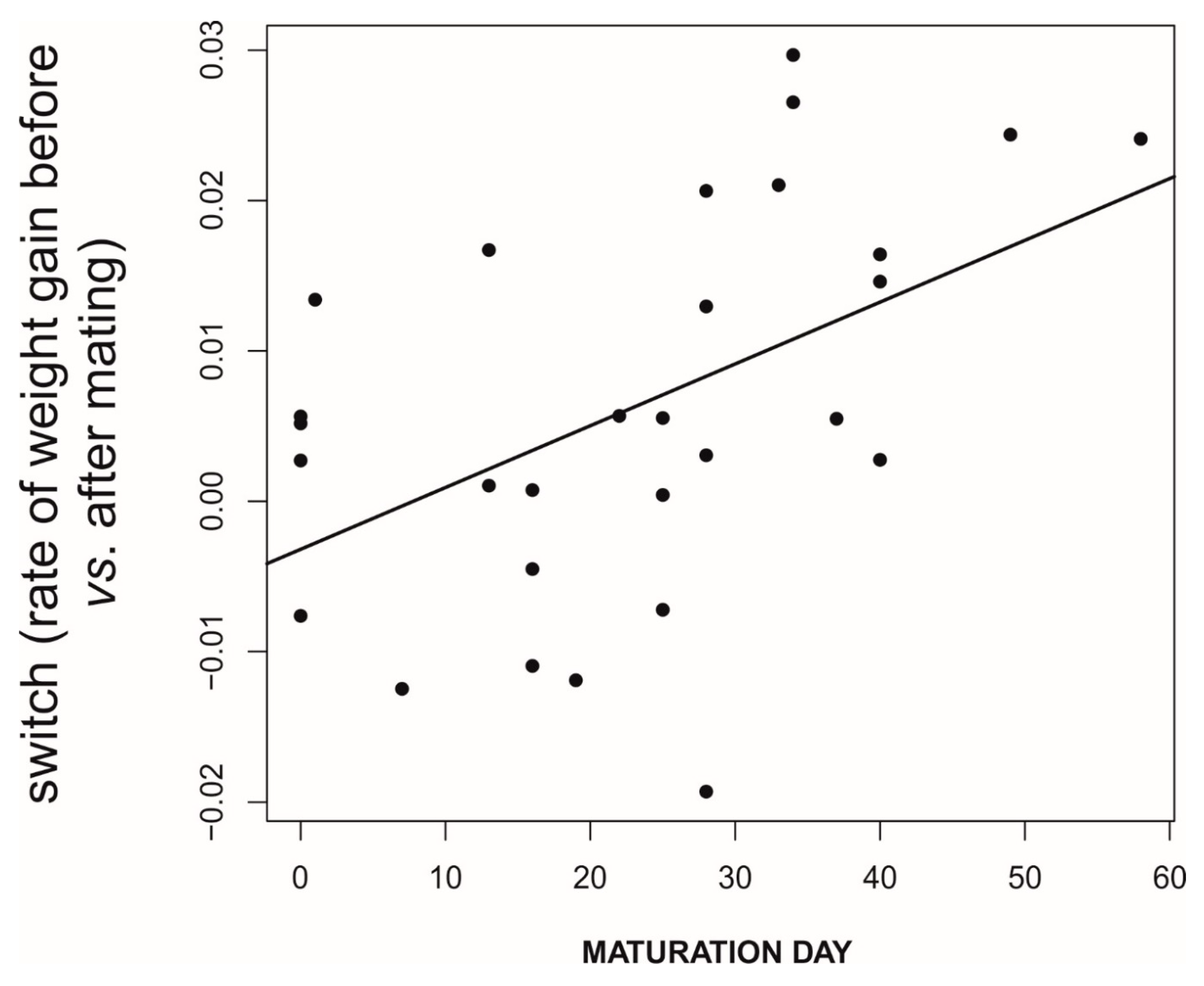
| Parameter | Value/s |
|---|---|
| Date of Season’s Onset | April 23rd |
| Season length | 79 days |
| Background female mortality rate for MFCL | 0.0030 day−1 |
| Background female mortality rate for SPOV, BCD-SPOV, EM-SPOV and BCD EM-SPOV * | 0.0036 day−1 |
| Background male mortality rate | 0.0045 day−1 |
| Female maturation time, “fem_mat” (since April 23rd) for SPOV, BCD-SPOV and MFCL | N|μ = 56, σ = 0.5| |
| Female maturation time, “fem_mat” (since April 23rd) for EM-SPOV and BCD EM-SPOV | N|μ = 39, σ = 0.5| |
| Male maturation time, “mal_mat” (since April 23rd) | N|μ = 36, σ = 0.5| |
| Adult body size, CW (mm) for EM-SPOV and BCD EM-SPOV | N|μ = 3.21 + 0.047 * fem_mat + 0.39 * CL, σ = 0.5066| |
| Adult body size, CW (mm) for MFCL, SPOV and BCD-SPOV | N|μ = 3.21 + 0.024 * fem_mat + 0.39 * CL, σ = 0.4679| |
| Initial condition, CONDo (mm) | N|μ = 3.54 + 0.49 * CW, σ = 0.2671| |
| Threshold level for female satiation (maxCONDf, mm) ** | −38.98 + 11.73 * CW − 0.63 * CW^2 |
| Added value on condition from feeding on a male | 2.39 mm (~0.199 g) |
| Egg sac volume, “vol” (mm3) for SPOV and EM-SPOV | N|μ = −1156.43 + 277.21 * CW, σ = 140.33 |
| Egg sac volume, “vol” (mm3) for BCD SPOV and BCD EM-SPOV | N|μ = −1156.43 + 277.21 * CW + 123.44 * maxCONDf, σ = 132.97| **** |
| Egg sac volume, “vol” (mm3) for MFCL | N|μ = −2297.64 + 217.88 * CW + 123.44 * maxCONDf, σ = 129.59| **** |
| Offspring number (“N”) | N = 57.54 + 0.16 * vol |
| Daily increase in condition (or daily net abdomen growth mm/day) for MFCL | U|0,1| * 0.1482 *** |
| Daily increase in condition (or daily net abdomen growth in mm/day) for SPOV, BCD-SPOV, EM-SPOV and BCD EM-SPOV | 1.5 * U|0,1| * 0.1482 *** |
| Maximum daily rate of encounter with females, “maxenc” | 1, 3 day−1 |
| Probability of an SPOV female attacking a male, “pspov” | 0.5, 0.9 |
| Probability of male escaping from a female attack, “pescape” | exp(−CW * 0.1) |
| Proportion of offspring surviving to maturation in SPOV, BCD-SPOV, EM-SPOV and BCD EM-SPOV relative to MFCL, “different” | 0.1, 0.3, 0.5, 0.7, 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya-Laraño, J.; Rabaneda-Bueno, R.; Morrison, E.; Crowley, P.H. Model and Data Concur and Explain the Coexistence of Two Very Distinct Animal Behavioral Types. Biology 2020, 9, 241. https://doi.org/10.3390/biology9090241
Moya-Laraño J, Rabaneda-Bueno R, Morrison E, Crowley PH. Model and Data Concur and Explain the Coexistence of Two Very Distinct Animal Behavioral Types. Biology. 2020; 9(9):241. https://doi.org/10.3390/biology9090241
Chicago/Turabian StyleMoya-Laraño, Jordi, Rubén Rabaneda-Bueno, Emily Morrison, and Philip H. Crowley. 2020. "Model and Data Concur and Explain the Coexistence of Two Very Distinct Animal Behavioral Types" Biology 9, no. 9: 241. https://doi.org/10.3390/biology9090241
APA StyleMoya-Laraño, J., Rabaneda-Bueno, R., Morrison, E., & Crowley, P. H. (2020). Model and Data Concur and Explain the Coexistence of Two Very Distinct Animal Behavioral Types. Biology, 9(9), 241. https://doi.org/10.3390/biology9090241





