Size Selective Harvesting Does Not Result in Reproductive Isolation among Experimental Lines of Zebrafish, Danio rerio: Implications for Managing Harvest-Induced Evolution
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Selection Lines
2.2. Prezygotic Tests
2.3. Spawning Trials and Reproductive Output
2.4. Statistical Analysis
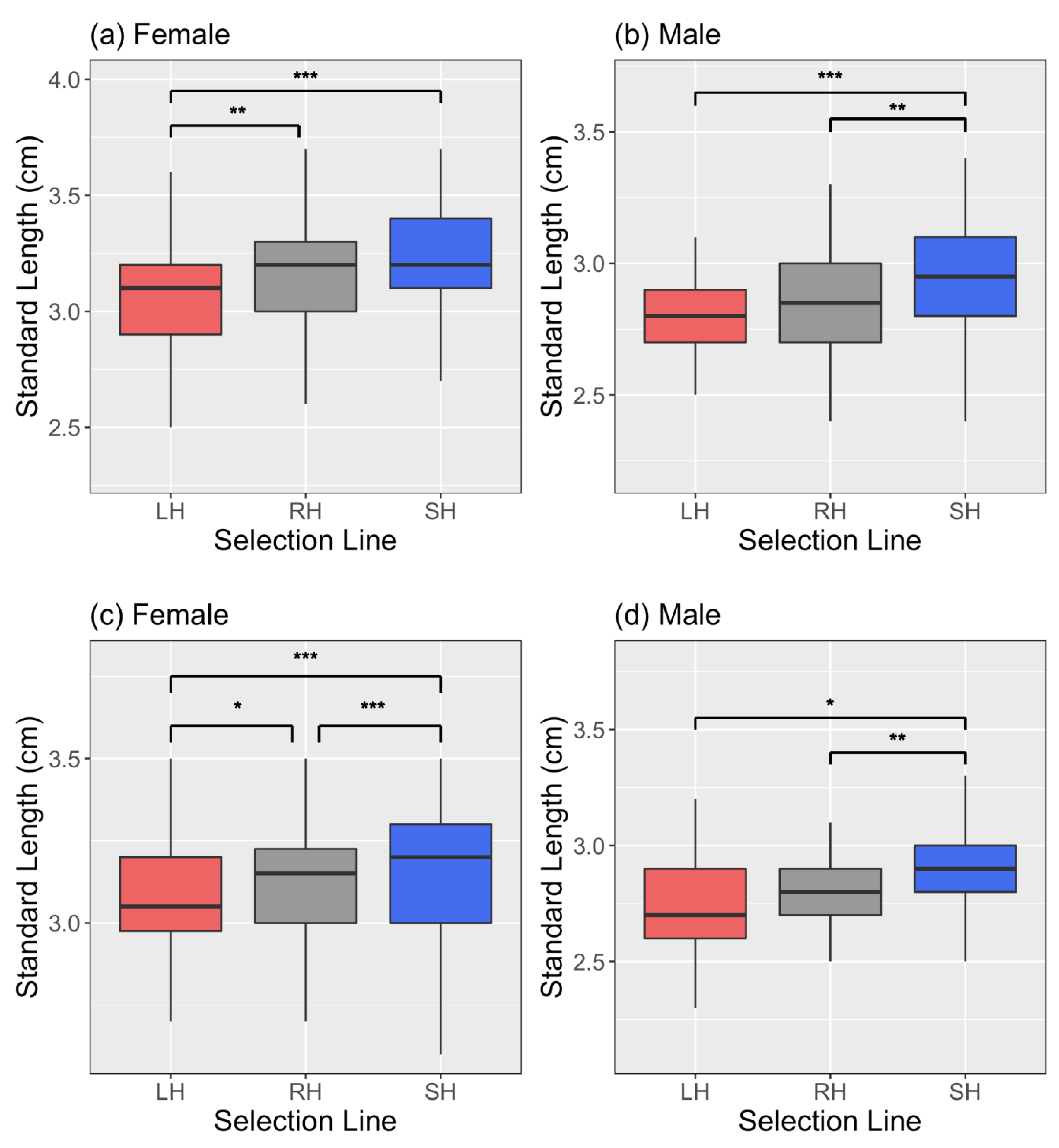
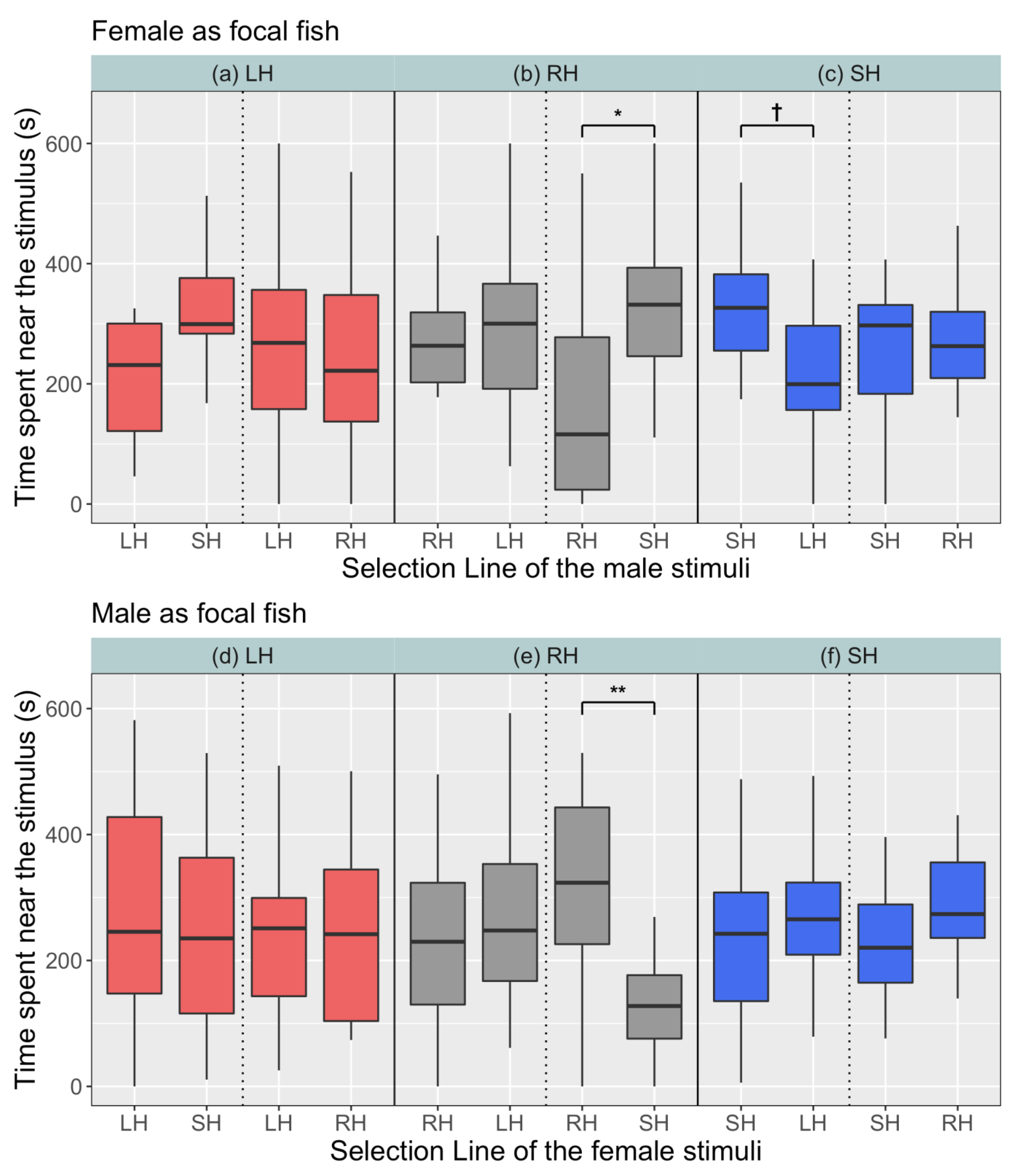
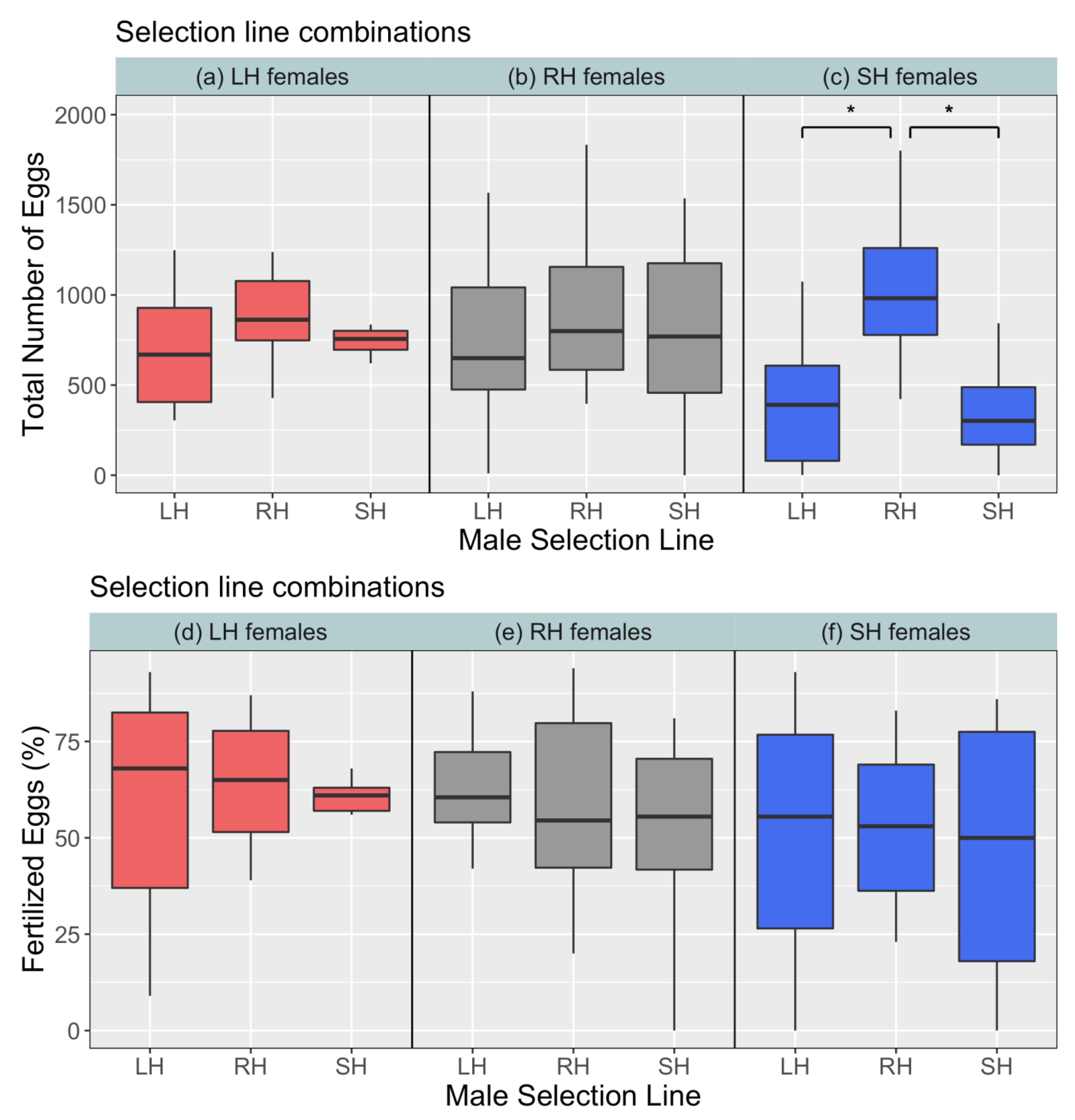
3. Results
4. Discussion
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fugère, V.; Hendry, A.P. Human influences on the strength of phenotypic selection. Proc. Natl. Acad. Sci. USA 2018, 115, 10070–10075. [Google Scholar] [CrossRef]
- Heino, M.; Diaz Pauli, B.; Dieckmann, U. Fisheries-induced evolution. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 461–480. [Google Scholar] [CrossRef]
- Alberti, M.; Correa, C.; Marzluff, J.M.; Hendry, A.P.; Palkovacs, E.P.; Gotanda, K.M.; Hunt, V.M.; Apgar, T.M.; Zhou, Y. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl. Acad. Sci. USA 2017, 114, 8951–8956. [Google Scholar] [CrossRef] [PubMed]
- Hollins, J.; Thambithurai, D.; Koeck, B.; Crespel, A.; Bailey, D.M.; Cooke, S.J.; Lindström, J.; Parsons, K.J.; Killen, S.S. A physiological perspective on fisheries-induced evolution. Evol. Appl. 2018, 11, 561–576. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Hard, J.J. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Natl. Acad. Sci. USA 2009, 106, 9987–9994. [Google Scholar] [CrossRef]
- Ahrens, R.N.; Allen, M.S.; Walters, C.; Arlinghaus, R. Saving large fish through harvest slots outperforms the classical minimum-length limit when the aim is to achieve multiple harvest and catch-related fisheries objectives. Fish Fish. 2020, 21, 483–510. [Google Scholar] [CrossRef]
- Biro, P.A.; Post, J.R. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl. Acad. Sci. USA 2008, 105, 2919–2922. [Google Scholar] [CrossRef]
- Jørgensen, C.; Holt, R.E. Natural mortality: Its ecology, how it shapes fish life histories, and why it may be increased by fishing. J. Sea Res. 2013, 75, 8–18. [Google Scholar]
- Kuparinen, A.; Kuikka, S.; Merilä, J. Estimating fisheries-induced selection: Traditional gear selectivity research meets fisheries-induced evolution. Evol. Appl. 2009, 2, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T. Sexual selection in males and females. Science 2007, 318, 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Iwasa, Y. Sexual selection. Trends Ecol. Evol. 1996, 11, 53–58. [Google Scholar] [CrossRef]
- Rowe, S.; Hutchings, J.A. Mating systems and the conservation of commercially exploited marine fish. Trends Ecol. Evol. 2003, 18, 567–572. [Google Scholar] [CrossRef]
- Dunlop, E.S.; Eikeset, A.M.; Stenseth, N.C. From genes to populations: How fisheries-induced evolution alters stock productivity. Ecol. Appl. 2015, 25, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, J.A.; Rowe, S. Consequences of sexual selection for fisheries-induced evolution: An exploratory analysis. Evol. Appl. 2008, 1, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Heikkilä, S. Implications of size-selective fisheries on sexual selection. Evol. Appl. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sbragaglia, V.; Gliese, C.; Bierbach, D.; Honsey, A.E.; Uusi-Heikkilä, S.; Arlinghaus, R. Size-selective harvesting fosters adaptations in mating behavior and reproductive allocation, affecting sexual selection in fish. J. Anim. Ecol. 2019, 88, 1343–1354. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Lindström, K.; Parre, N.; Arlinghaus, R.; Alós, J.; Kuparinen, A. Altered trait variability in response to size-selective mortality. Biol. Lett. 2016, 12, 20160584. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.L.; Caselle, J.E.; Standish, J.D.; Schroeder, D.M.; Love, M.S.; Rosales-Casian, J.A.; Sosa-Nishizaki, O. Size-selective harvesting alters life histories of a temperate sex-changing fish. Ecol. Appl. 2007, 17, 2268–2280. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Whiteley, A.R.; Kuparinen, A.; Matsumura, S.; Venturelli, P.A.; Wolter, C.; Slate, J.; Primmer, C.R.; Meinelt, T.; Killen, S.S. The evolutionary legacy of size-selective harvesting extends from genes to populations. Evol. Appl. 2015, 8, 597–620. [Google Scholar] [CrossRef]
- Andersen, K.H.; Marty, L.; Arlinghaus, R. Evolution of boldness and life history in response to selective harvesting. Can. J. Fish. Aquat. Sci. 2018, 75, 271–281. [Google Scholar] [CrossRef]
- Roff, D.A. Life history Evolution; Sinauer Associattes: Sunderland, MA, USA, 2002. [Google Scholar]
- Réale, D.; Garant, D.; Humphries, M.M.; Bergeron, P.; Careau, V.; Montiglio, P.-O. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 4051–4063. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef]
- Claireaux, M.; Jørgensen, C.; Enberg, K. Evolutionary effects of fishing gear on foraging behavior and life-history traits. Ecol. Evol. 2018, 8, 10711–10721. [Google Scholar] [CrossRef] [PubMed]
- Mittelbach, G.G.; Ballew, N.G.; Kjelvik, M.K. Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 2014, 71, 927–944. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Gross, M.R. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1992, 250, 57–62. [Google Scholar]
- Riechert, S.E.; Johns, P. Do female spiders select heavier males for the genes for behavioral aggressiveness they offer their offspring? Evolution 2003, 57, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Bierbach, D.; Schulte, M.; Herrmann, N.; Tobler, M.; Stadler, S.; Jung, C.T.; Kunkel, B.; Riesch, R.; Klaus, S.; Ziege, M. Predator-induced changes of female mating preferences: Innate and experiential effects. BMC Evol. Biol. 2011, 11, 1–11. [Google Scholar] [CrossRef]
- Dosen, L.D.; Montgomerie, R. Female size influences mate preferences of male guppies. Ethology 2004, 110, 245–255. [Google Scholar] [CrossRef]
- Baldauf, S.A.; Kullmann, H.; Schroth, S.H.; Thünken, T.; Bakker, T.C. You can’t always get what you want: Size assortative mating by mutual mate choice as a resolution of sexual conflict. BMC Evol. Biol. 2009, 9, 129. [Google Scholar] [CrossRef]
- Rasotto, M.; De Mitcheson, Y.S.; Mitcheson, G. Male body size predicts sperm number in the mandarinfish. J. Zool. 2010, 281, 161–167. [Google Scholar] [CrossRef]
- O’dea, R.; Jennions, M.; Head, M. Male body size and condition affects sperm number and production rates in mosquitofish, Gambusia holbrooki. J. Evol. Biol. 2014, 27, 2739–2744. [Google Scholar] [CrossRef]
- Candolin, U.; Voigt, H.R. Correlation between male size and territory quality: Consequence of male competition or predation susceptibility? Oikos 2001, 95, 225–230. [Google Scholar] [CrossRef]
- Bierbach, D.; Oster, S.; Jourdan, J.; Arias-Rodriguez, L.; Krause, J.; Wilson, A.D.M.; Plath, M. Social network analysis resolves temporal dynamics of male dominance relationships. Behav. Ecol. Sociobiol. 2014, 68, 935–945. [Google Scholar] [CrossRef]
- Kolm, N. Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 2229–2234. [Google Scholar] [CrossRef]
- Berejikian, B.; Tezak, E.; LaRae, A. Female mate choice and spawning behaviour of chinook salmon under experimental conditions. J. Fish Biol. 2000, 57, 647–661. [Google Scholar] [CrossRef]
- Rios-Cardenas, O.; Brewer, J.; Morris, M.R. Maternal investment in the swordtail fish Xiphophorus multilineatus: Support for the differential allocation hypothesis. PLoS ONE 2013, 8, e82723. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Bierbach, D.; Alós, J.; Tscheligi, P.; Wolter, C.; Arlinghaus, R. Relatively large males lower reproductive success in female zebrafish. Environ. Biol. Fishes 2018, 101, 1625–1638. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Kuparinen, A.; Wolter, C.; Meinelt, T.; Arlinghaus, R. Paternal body size affects reproductive success in laboratory-held zebrafish (Danio rerio). Environ. Biol. Fishes 2012, 93, 461–474. [Google Scholar] [CrossRef]
- Herdman, E.J.; Kelly, C.D.; Godin, J.G.J. Male mate choice in the guppy (Poecilia reticulata): Do males prefer larger females as mates? Ethology 2004, 110, 97–111. [Google Scholar] [CrossRef]
- Pélabon, C.; Borg, Å.A.; Bjelvenmark, J.; Forsgren, E.; Barber, I.; Amundsen, T. Do male two-spotted gobies prefer large fecund females? Behav. Ecol. 2003, 14, 787–792. [Google Scholar] [CrossRef]
- Passos, C.; Vidal, N.; D’Anatro, A. Male mate choice in the annual fish Austrolebias reicherti (Cyprinodontiformes: Rivulidae): When size matters. J. Ethol. 2019, 37, 301–306. [Google Scholar] [CrossRef]
- Plath, M.; Seggel, U.; Burmeister, H.; Heubel, K.U.; Schlupp, I. Choosy males from the underground: Male mating preferences in surface-and cave-dwelling Atlantic mollies (Poecilia mexicana). Naturwissenschaften 2006, 93, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.; Quinn, T.; Willson, M. Body size, arrival date, and reproductive success of pink salmon, Oncorhynchus gorbuscha. Ethol. Ecol. Evol. 2002, 14, 29–44. [Google Scholar] [CrossRef]
- Yoneda, M.; Wright, P. Effects of varying temperature and food availability on growth and reproduction in first-time spawning female Atlantic cod. J. Fish Biol. 2005, 67, 1225–1241. [Google Scholar] [CrossRef]
- Dunlop, E.S.; Shuter, B.J.; Dieckmann, U. Demographic and evolutionary consequences of selective mortality: Predictions from an eco-genetic model for smallmouth bass. Trans. Am. Fish. Soc. 2007, 136, 749–765. [Google Scholar] [CrossRef]
- Lane, J.E.; Forrest, M.N.; Willis, C.K. Anthropogenic influences on natural animal mating systems. Anim. Behav. 2011, 81, 909–917. [Google Scholar] [CrossRef]
- Sørdalen, T.K.; Halvorsen, K.T.; Harrison, H.B.; Ellis, C.D.; Vøllestad, L.A.; Knutsen, H.; Moland, E.; Olsen, E.M. Harvesting changes mating behaviour in European lobster. Evol. Appl. 2018, 11, 963–977. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Laskowski, K.L.; Alós, J.; Klefoth, T.; Monk, C.T.; Nakayama, S.; Schröder, A. Passive gear-induced timidity syndrome in wild fish populations and its potential ecological and managerial implications. Fish Fish. 2017, 18, 360–373. [Google Scholar] [CrossRef]
- O’Leary, S.J.; Hice, L.A.; Feldheim, K.A.; Frisk, M.G.; McElroy, A.E.; Fast, M.D.; Chapman, D.D. Severe inbreeding and small effective number of breeders in a formerly abundant marine fish. PLoS ONE 2013, 8, e66126. [Google Scholar] [CrossRef]
- Buchholz-Sørensen, M.; Vella, A. Population structure, genetic diversity, effective population size, demographic history and regional connectivity patterns of the endangered dusky grouper, Epinephelus marginatus (Teleostei: Serranidae), within Malta’s fisheries management zone. PLoS ONE 2016, 11, e0159864. [Google Scholar] [CrossRef]
- Sørdalen, T.K.; Halvorsen, K.T.; Vøllestad, L.A.; Moland, E.; Olsen, E.M. Marine protected areas rescue a sexually selected trait in European lobster. Evol. Appl. 2020. [Google Scholar] [CrossRef]
- Sørdalen, T.K. Marine Reserves and Selective Fishing Shape Mating Behaviour, Secondary Sexual Trait and Growth in European Lobster. Ph.D. Thesis, Faculty of Mathematics and Natural Sciences, University of Oslo, Oslo, Norway, 2019. [Google Scholar]
- Rometsch, S.J.; Torres-Dowdall, J.; Meyer, A. Evolutionary dynamics of pre- and postzygotic reproductive isolation in cichlid fishes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190535. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Kuparinen, A. Implications of fisheries-induced evolution for population recovery: Refocusing the science and refining its communication. Fish Fish. 2020, 21, 453–464. [Google Scholar] [CrossRef]
- Barluenga, M.; Stölting, K.N.; Salzburger, W.; Muschick, M.; Meyer, A. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 2006, 439, 719–723. [Google Scholar] [CrossRef]
- Servedio, M.R.; Van Doorn, G.S.; Kopp, M.; Frame, A.M.; Nosil, P. Magic traits in speciation: ‘Magic’but not rare? Trends Ecol. Evol. 2011, 26, 389–397. [Google Scholar] [CrossRef]
- Kopp, M.; Servedio, M.R.; Mendelson, T.C.; Safran, R.J.; Rodríguez, R.L.; Hauber, M.E.; Scordato, E.C.; Symes, L.B.; Balakrishnan, C.N.; Zonana, D.M. Mechanisms of assortative mating in speciation with gene flow: Connecting theory and empirical research. Am. Nat. 2018, 191, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sbragaglia, V.; Alós, J.; Fromm, K.; Monk, C.T.; Díaz-Gil, C.; Uusi-Heikkilä, S.; Honsey, A.E.; Wilson, A.D.M.; Arlinghaus, R. Experimental Size-Selective Harvesting Affects Behavioral Types of a Social Fish. Trans. Am. Fish. Soc. 2019. [Google Scholar] [CrossRef]
- Sbragaglia, V.; Klamser, P.P.; Romanczuk, P.; Arlinghaus, R. Harvesting-induced evolution of collective behavior in a fish. bioRxiv 2019, 809442. [Google Scholar] [CrossRef]
- Sbragaglia, V.; López-Olmeda, J.F.; Frigato, E.; Bertolucci, C.; Arlinghaus, R. Size-selective mortality induces evolutionary changes in group risk-taking behavior and the circadian system in a fish. J. Anim. Ecol. 2020. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Sävilammi, T.; Leder, E.; Arlinghaus, R.; Primmer, C.R. Rapid, broad-scale gene expression evolution in experimentally harvested fish populations. Mol. Ecol. 2017, 26, 3954–3967. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Heikkilä, S.; Böckenhoff, L.; Wolter, C.; Arlinghaus, R. Differential allocation by female zebrafish (Danio rerio) to different-sized males–an example in a fish species lacking parental care. PLoS ONE 2012, 7, e48317. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Wolter, C.; Meinelt, T.; Arlinghaus, R. Size-dependent reproductive success of wild zebrafish Danio rerio in the laboratory. J. Fish Biol. 2010, 77, 552–569. [Google Scholar] [CrossRef]
- Pyron, M. Female preferences and male male interactions in zebrafish (Danio rerio). Can. J. Zool. 2003, 81, 122–125. [Google Scholar] [CrossRef]
- Roy, T.; Bhat, A. Population, sex and body size: Determinants of behavioural variations and behavioural correlations among wild zebrafish Danio rerio. R. Soc. Open Sci. 2018, 5, 170978. [Google Scholar] [CrossRef]
- Roy, T.; Bhat, A. Repeatability in boldness and aggression among wild zebrafish (Danio rerio) from two differing predation and flow regimes. J. Com. Psychol. 2018, 132, 349. [Google Scholar] [CrossRef]
- Roy, T.; Shukla, R.; Bhat, A. Risk-taking during feeding: Between-and within-population variation and repeatability across contexts among wild zebrafish. Zebrafish 2017, 14, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Smith, C. Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav. Ecol. 2006, 17, 779–783. [Google Scholar] [CrossRef]
- Skinner, A.M.; Watt, P.J. Strategic egg allocation in the zebra fish, Danio rerio. Behav. Ecol. 2007, 18, 905–909. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- Paull, G.C.; Filby, A.L.; Giddins, H.G.; Coe, T.S.; Hamilton, P.B.; Tyler, C.R. Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish 2010, 7, 109–117. [Google Scholar] [CrossRef]
- Moore, M.P.; Whiteman, H.H.; Martin, R.A. A mother’s legacy: The strength of maternal effects in animal populations. Ecol. Lett. 2019, 22, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Sommer-Trembo, C.; Plath, M.; Gismann, J.; Helfrich, C.; Bierbach, D. Context-dependent female mate choice maintains variation in male sexual activity. R. Soc. Open Sci. 2017, 4, 170303. [Google Scholar] [CrossRef]
- Sommer-Trembo, C.; Bierbach, D.; Arias-Rodriguez, L.; Verel, Y.; Jourdan, J.; Zimmer, C.; Riesch, R.; Streit, B.; Plath, M. Does personality affect premating isolation between locally-adapted populations? BMC Evol. Biol. 2016, 16, 138. [Google Scholar] [CrossRef]
- Darrow, K.O.; Harris, W.A. Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish 2004, 1, 40–45. [Google Scholar] [CrossRef]
- Roy, T.; Bhat, A. Can outcomes of dyadic interactions be consistent across contexts among wild zebrafish? R. Soc. Open Sci. 2015, 2, 150282. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing, 3.6.1.; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Mangiafico, S.; Mangiafico, M.S. Package ‘rcompanion’. Cran Repos 2017, 20, 1–71. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Chen, B.-J.; Liu, K.; Zhou, L.-J.; Gomes-Silva, G.; Sommer-Trembo, C.; Plath, M. Personality differentially affects individual mate choice decisions in female and male Western mosquitofish (Gambusia affinis). PLoS ONE 2018, 13, e0197197. [Google Scholar] [CrossRef] [PubMed]
- Kortet, R.; Niemelä, P.T.; Vainikka, A.; Laakso, J. Females prefer bold males; an analysis of boldness, mate choice, and bacterial resistance in the field cricket Gryllus integer. Ecol. Parasitol. Immunol. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Godin, J.-G.; Dugatkin, L.A. Female mating preference for bold males in the guppy, Poecilia reticulata. Proc. Natl. Acad. Sci. USA 1996, 93, 10262–10267. [Google Scholar] [CrossRef] [PubMed]
- Clotfelter, E.D.; Curren, L.J.; Murphy, C.E. Mate choice and spawning success in the fighting fish Betta splendens: The importance of body size, display behavior and nest size. Ethology 2006, 112, 1170–1178. [Google Scholar] [CrossRef]
- Selz, O.M.; Pierotti, M.E.; Maan, M.E.; Schmid, C.; Seehausen, O. Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behav. Ecol. 2014, 25, 612–626. [Google Scholar] [CrossRef]
- Stelkens, R.B.; Seehausen, O. Phenotypic divergence but not genetic distance predicts assortative mating among species of a cichlid fish radiation. J. Evol. Biol. 2009, 22, 1679–1694. [Google Scholar] [CrossRef]
- Bierbach, D.; Penshorn, M.; Hamfler, S.; Herbert, D.B.; Appel, J.; Meyer, P.; Slattery, P.; Charaf, S.; Wolf, R.; Völker, J. Gradient evolution of body colouration in surface-and cave-dwelling Poecilia mexicana and the role of phenotype-assortative female mate choice. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Vines, T.H.; Schluter, D. Strong assortative mating between allopatric sticklebacks as a by-product of adaptation to different environments. Proc. R. Soc. B Biol. Sci. 2006, 273, 911–916. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. Disassortative mating for boldness decreases reproductive success in the guppy. Behav. Ecol. 2013, 24, 1320–1326. [Google Scholar] [CrossRef]
- Jiang, Y.; Bolnick, D.I.; Kirkpatrick, M. Assortative mating in animals. Am. Nat. 2013, 181, E125–E138. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.G.; Ryan, M.J. Assortative preferences for stripes in danios. Anim. Behav. 2005, 70, 1063–1066. [Google Scholar] [CrossRef]
- Vargas, R.; Mackenzie, S.; Rey, S. ‘Love at first sight’: The effect of personality and colouration patterns in the reproductive success of zebrafish (Danio rerio). PLoS ONE 2018, 13, e0203320. [Google Scholar] [CrossRef] [PubMed]
- Bierbach, D.; Sassmannshausen, V.; Streit, B.; Arias-Rodriguez, L.; Plath, M. Females prefer males with superior fighting abilities but avoid sexually harassing winners when eavesdropping on male fights. Behav. Ecol. Sociobiol. 2013, 67, 675–683. [Google Scholar] [CrossRef]
- Spritzer, M.D.; Meikle, D.B.; Solomon, N.G. Female choice based on male spatial ability and aggressiveness among meadow voles. Anim. Behav. 2005, 69, 1121–1130. [Google Scholar] [CrossRef]
- Sommer-Trembo, C.; Schreier, M.; Plath, M. Different preference functions act in unison: Mate choice and risk-taking behaviour in the Atlantic molly (Poecilia mexicana). J. Ethol. 2020, 38, 215–222. [Google Scholar] [CrossRef]
- Scherer, U.; Godin, J.-G.J.; Schuett, W. Do female rainbow kribs choose males on the basis of their apparent aggression and boldness? A non-correlational mate choice study. Behav. Ecol. Sociobiol. 2020, 74, 1–15. [Google Scholar] [CrossRef]
- Magurran, A.E.; Seghers, B.H. A cost of sexual harassment in the guppy, Poecilia reticulata. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 258, 89–92. [Google Scholar]
- Tobler, M.; Schlupp, I.; Plath, M. Costly interactions between the sexes: Combined effects of male sexual harassment and female choice? Behav. Ecol. 2011, 22, 723–729. [Google Scholar] [CrossRef]
- Spence, R.; Smith, C. Male territoriality mediates density and sex ratio effects on oviposition in the zebrafish, Danio rerio. Anim. Behav. 2005, 69, 1317–1323. [Google Scholar] [CrossRef]
- Brand, M.; Granato, M.; Nüsslein-Volhard, C. Keeping and raising zebrafish. In Zebrafish: A Practical Approach; Oxford University Press: Oxford, UK, 2002; pp. 7–37. [Google Scholar]
- Bowles, E.; Marin, K.; Mogensen, S.; MacLeod, P.; Fraser, D.J. Size reductions and genomic changes within two generations in wild walleye populations: Associated with harvest? Evol. Appl. 2020, 13, 1128–1144. [Google Scholar] [CrossRef]
- Arnold, S.J. Constraints on phenotypic evolution. Am. Nat. 1992, 140, S85–S107. [Google Scholar] [CrossRef]
- Renneville, C.; Millot, A.; Agostini, S.; Carmignac, D.; Maugars, G.; Dufour, S.; Le Rouzic, A.; Edeline, E. Anthropogenic selection along directions of most evolutionary resistance. BioRxiv 2018, 498683. [Google Scholar] [CrossRef]
- Enberg, K.; Jørgensen, C.; Dunlop, E.S.; Heino, M.; Dieckmann, U. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evol. Appl. 2009, 2, 394–414. [Google Scholar] [CrossRef]
- Neubauer, P.; Jensen, O.P.; Hutchings, J.A.; Baum, J.K. Resilience and recovery of overexploited marine populations. Science 2013, 340, 347–349. [Google Scholar] [CrossRef] [PubMed]

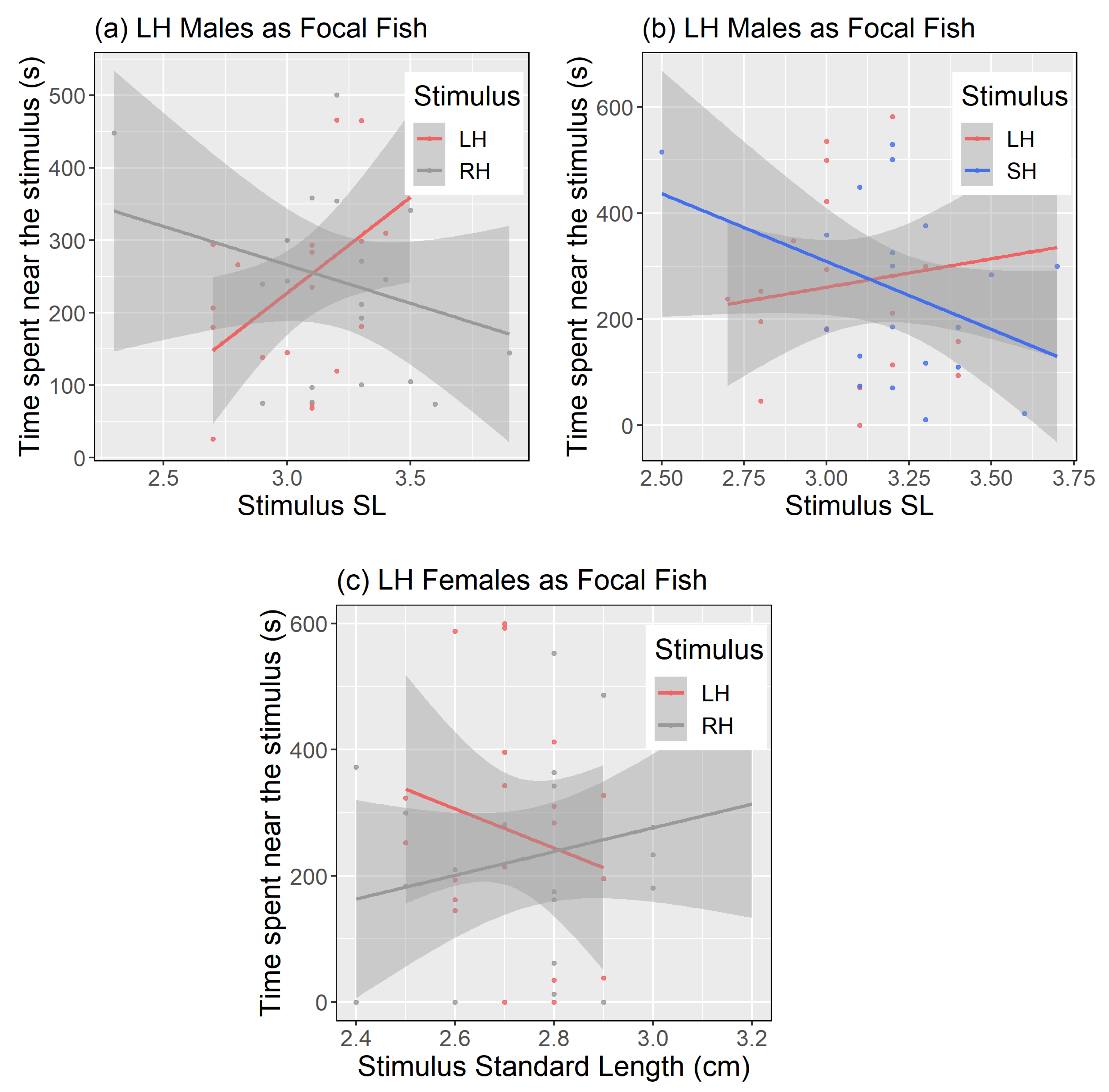
| (a). | |||||||
|---|---|---|---|---|---|---|---|
| Focal Line | Model | Term | Sum Sq. | Mean Sq. | df | F | Pr (>F) |
| LH | Without body size | Stimulus | 44,162 | 22,081 | 2,2 | 1.87 | 0.34 |
| With body size | Stimulus | 49,495 | 24,747.6 | 2,73 | 2.19 | 0.12 | |
| StimulusSL | 1249 | 1249.2 | 1,72 | 0.11 | 0.74 | ||
| Stimulus × Stimulus SL | 51,239 | 25,619.3 | 2,73 | 2.27 | 0.11 | ||
| RH | Without body size | Stimulus | 127,492 | 63,746 | 2,77 | 5.07 | <0.01 |
| With body size | Stimulus | 29,216.4 | 14,608.2 | 2,74 | 1.14 | 0.32 | |
| StimulusSL | 261.6 | 261.6 | 1,74 | 0.02 | 0.89 | ||
| Stimulus × Stimulus SL | 22,587.2 | 11,293.6 | 2,74 | 0.88 | 0.42 | ||
| SH | Without body size | Stimulus | 32,299 | 16,150 | 2,77 | 1.54 | 0.22 |
| With body size | Stimulus | 8022.6 | 4011.3 | 2,74 | 0.37 | 0.69 | |
| StimulusSL | 3521.9 | 3521.9 | 1,74 | 0.33 | 0.57 | ||
| Stimulus × Stimulus SL | 9232.4 | 4616.2 | 2,74 | 0.43 | 0.64 | ||
| (b). | |||||||
| Focal line | Model | Term | Sum Sq. | Mean Sq. | df | F | Pr (>F) |
| LH | Without body size | Stimulus | 143.39 | 71.69 | 2,4 | 0.04 | 0.95 |
| With body size | Stimulus | 12,475.9 | 6238 | 2,74 | 3.92 | 0.02 | |
| StimulusSL | 486.3 | 486.3 | 1,74 | 0.31 | 0.58 | ||
| Stimulus × Stimulus SL | 12,731.7 | 6365.8 | 2,74 | 4.0 | 0.02 | ||
| RH | Without body size | Stimulus | 21,107 | 10,554 | 2,77 | 7.06 | <0.01 |
| With body size | Stimulus | 1097.8 | 548.89 | 2,74 | 0.36 | 0.70 | |
| StimulusSL | 1122.7 | 1122.69 | 1,74 | 0.74 | 0.39 | ||
| Stimulus × Stimulus SL | 1284.3 | 624.17 | 2,74 | 0.41 | 0.66 | ||
| SH | Without body size | Stimulus | 4126 | 2063 | 2,5 | 2.32 | 0.19 |
| With body size | Stimulus | 1978.12 | 989.06 | 2,74 | 1.1 | 0.34 | |
| StimulusSL | 661.42 | 661.42 | 1,74 | 0.73 | 0.4 | ||
| Stimulus × Stimulus SL | 1565.05 | 782.52 | 2,74 | 0.87 | 0.42 | ||
| Metric | Term | Sum Sq. | Mean Sq. | df | F | Pr (>F) |
|---|---|---|---|---|---|---|
| Male | 117,875 | 58,938 | 2,77 | 9.34 | <0.01 | |
| Total no. of eggs | Female | 5493 | 2746 | 2,3 | 0.43 | 0.68 |
| Male × Female | 52,307 | 13,077 | 4,77 | 2.07 | 0.09 | |
| Male | 35,061 | 17,530.4 | 2,77 | 0.57 | 0.57 | |
| % fertilised eggs | Female | 20,845 | 10,422.3 | 2,3 | 0.34 | 0.73 |
| Male × Female | 6777 | 1694.2 | 4,77 | 0.05 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, T.; Fromm, K.; Sbragaglia, V.; Bierbach, D.; Arlinghaus, R. Size Selective Harvesting Does Not Result in Reproductive Isolation among Experimental Lines of Zebrafish, Danio rerio: Implications for Managing Harvest-Induced Evolution. Biology 2021, 10, 113. https://doi.org/10.3390/biology10020113
Roy T, Fromm K, Sbragaglia V, Bierbach D, Arlinghaus R. Size Selective Harvesting Does Not Result in Reproductive Isolation among Experimental Lines of Zebrafish, Danio rerio: Implications for Managing Harvest-Induced Evolution. Biology. 2021; 10(2):113. https://doi.org/10.3390/biology10020113
Chicago/Turabian StyleRoy, Tamal, Kim Fromm, Valerio Sbragaglia, David Bierbach, and Robert Arlinghaus. 2021. "Size Selective Harvesting Does Not Result in Reproductive Isolation among Experimental Lines of Zebrafish, Danio rerio: Implications for Managing Harvest-Induced Evolution" Biology 10, no. 2: 113. https://doi.org/10.3390/biology10020113
APA StyleRoy, T., Fromm, K., Sbragaglia, V., Bierbach, D., & Arlinghaus, R. (2021). Size Selective Harvesting Does Not Result in Reproductive Isolation among Experimental Lines of Zebrafish, Danio rerio: Implications for Managing Harvest-Induced Evolution. Biology, 10(2), 113. https://doi.org/10.3390/biology10020113







