Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Microalgal Strain
2.2. Cultivation Conditions
2.3. Cultivation in a Photobioreactor
2.4. Measurement of Algal Growth and Biomass Dry Weight
2.5. Measurement of Pigment Concentrations
2.6. Ion Chromatography
2.7. Data Analysis
3. Results and Discussion
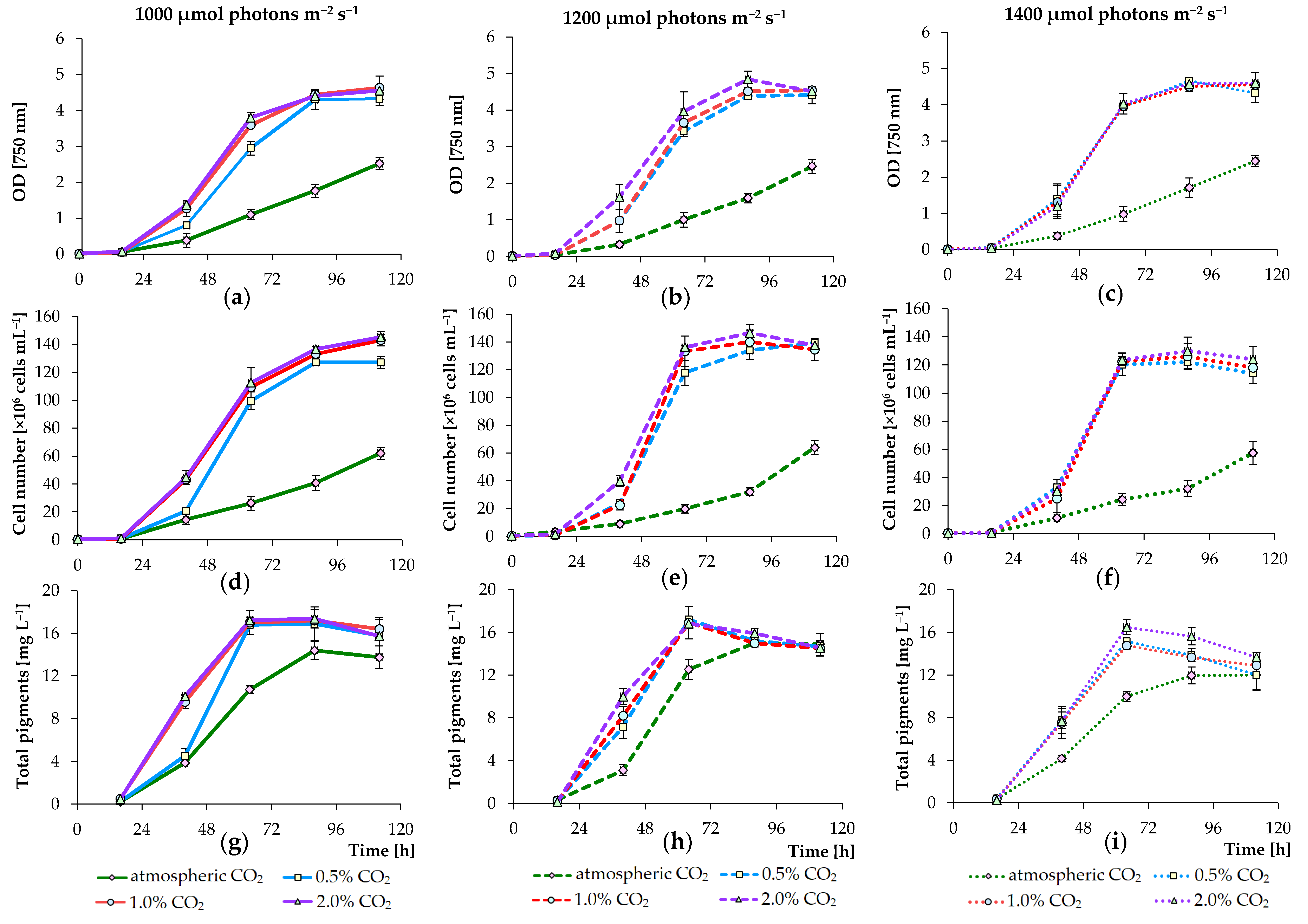
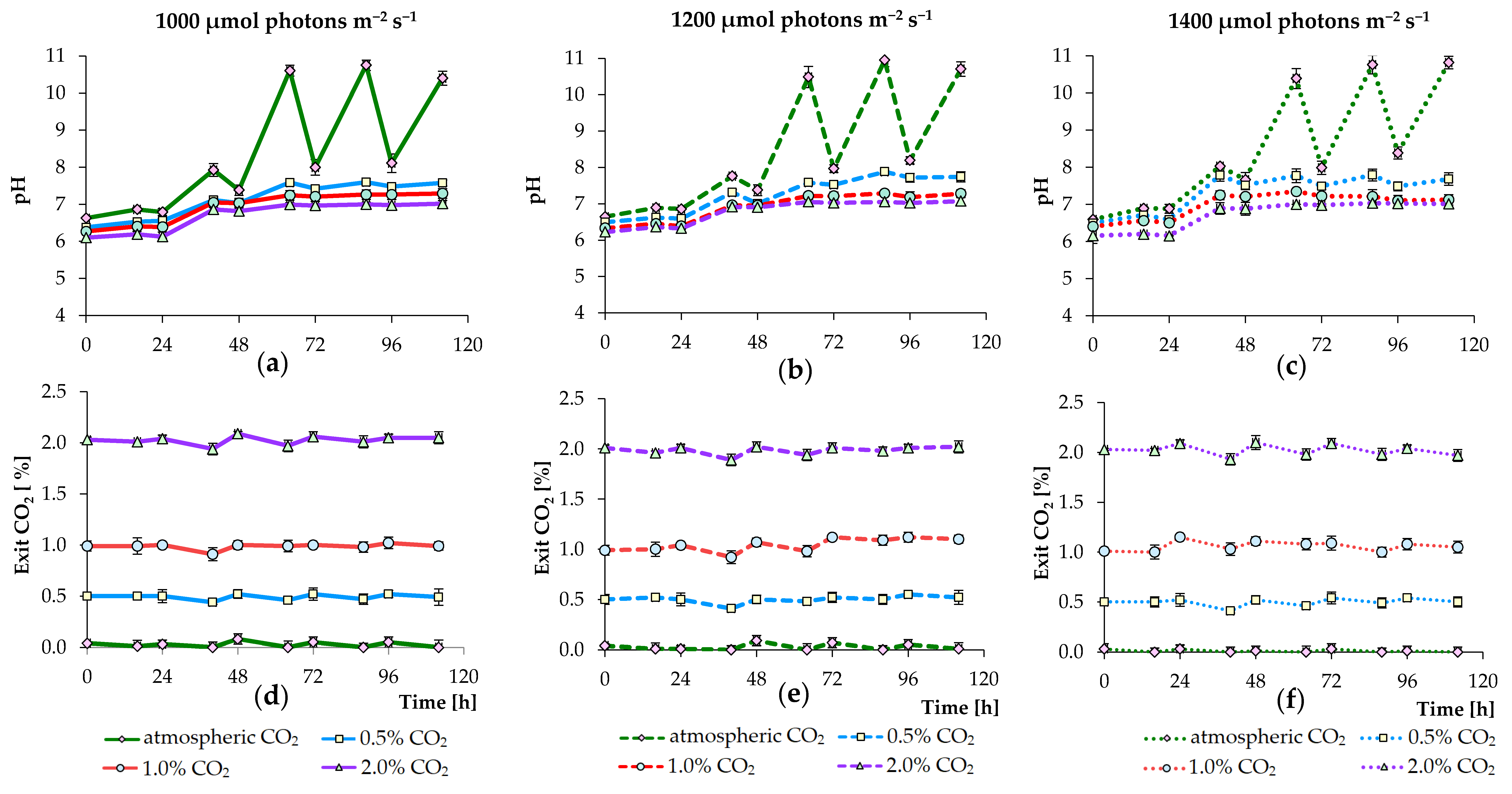
3.1. Effects of PPFD and CO2 Concentration Values on Growth of Algae
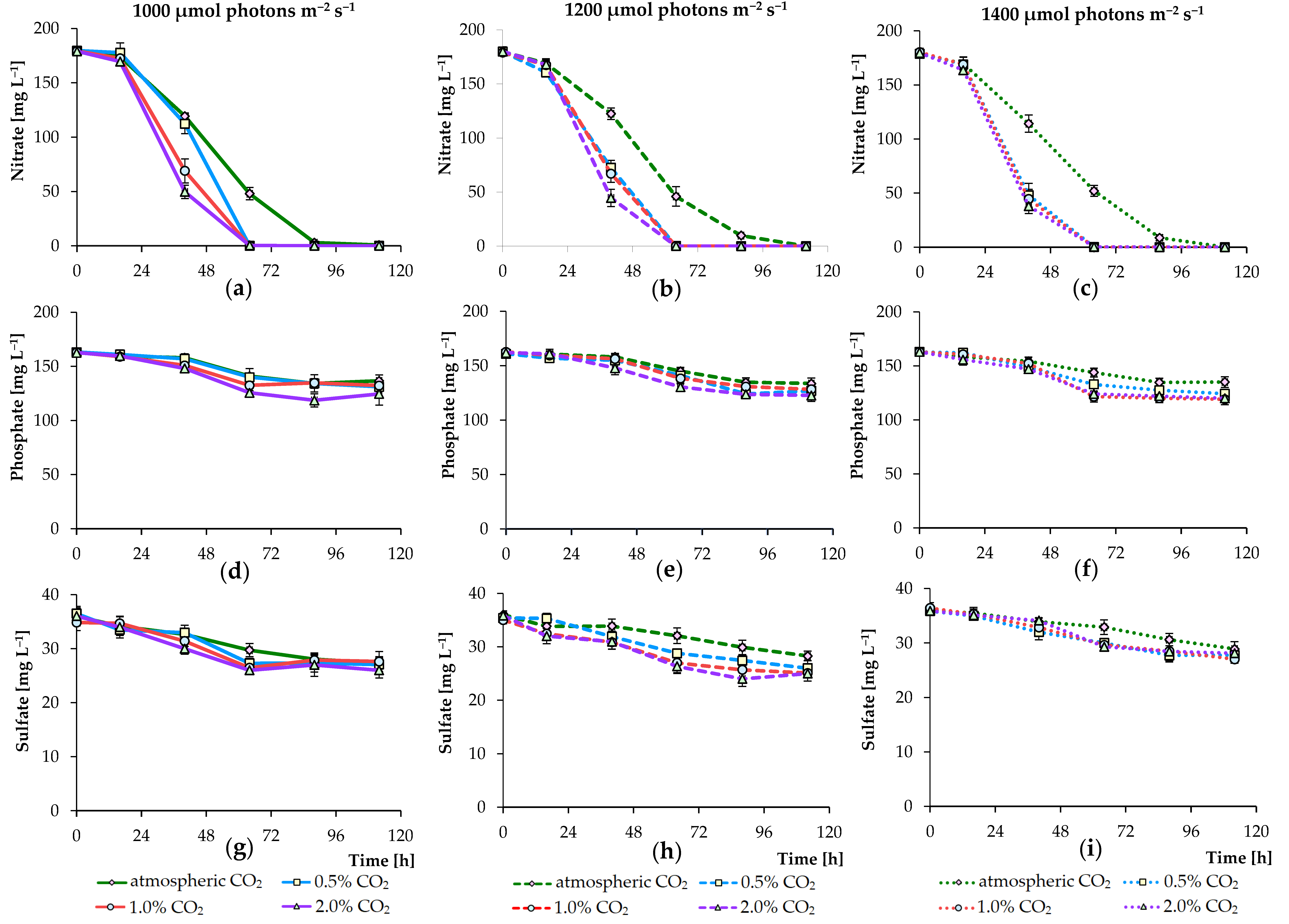
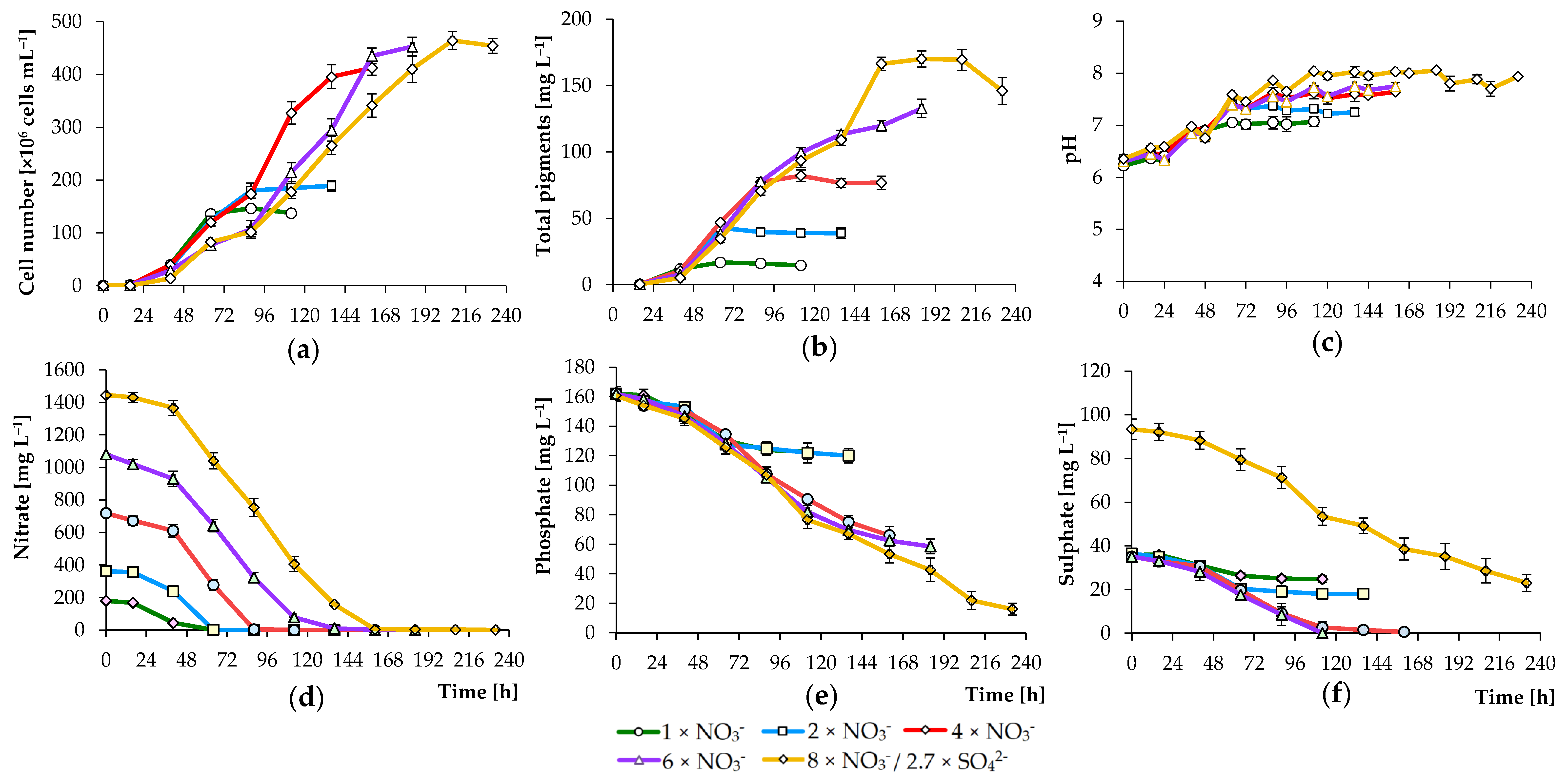
3.2. Effects of Nutrient Concentration on Algal Growth
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammed, A.M.; Prajapati, S.K.; Simsek, S.; Simsek, H. Growth regime and environmental remediation of microalgae. Algae 2016, 31, 189–204. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products—A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and biodegradation of the environmental hormone nonylphenol by four marine microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Dahms, H.; Won, E.; Lee, J.; Shin, K. Microalgae—A promising tool for heavy metal remediation. Ecotox. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.E.; Cho, Y.B.; Hwang, S.J. Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour. Technol. 2013, 144, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Shi, J.; Huang, T.; Guo, Y.; Wei, J.; Guo, M.; Li, L.; Dou, S.; Liu, L.; Liu, G. Characterization of Chlorella sorokiniana growth properties in monosaccharide-supplemented batch culture. PLoS ONE 2018, 17, e0199873. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular basis of autotrophic vs mixotrophic growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, C.C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018, 262, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Lohman, E.J.; Gardner, R.D.; Pedersen, T.; Peyton, B.M.; Cooksey, K.E.; Gerlach, R. Optimized inorganic carbon regime for enhanced growth and lipid accumulation in Chlorella vulgaris. Biotechnol. Biofuels 2015, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Negi, S.; Barry, A.N.; Friedland, N.; Sudasinghe, N.; Subramanian, S.; Pieris, S.; Holguin, F.O.; Dungan, B.; Schaub, T.; Sayre, R. Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. J. Appl. Phycol. 2016, 28, 803–812. [Google Scholar] [CrossRef]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Niu, Y.; Yang, J.; Zhou, J.; Yuan, Y. Metabolic profiling reveals growth related FAME productivity and quality of Chlorella sorokiniana with different inoculum sizes. Biotechnol. Bioeng. 2012, 109, 1651–1662. [Google Scholar] [CrossRef]
- Sorokin, C.; Myers, J. A high-temperature strain of Chlorella. Science 1953, 117, 330–331. [Google Scholar] [CrossRef]
- Sorokin, C.; Kraus, R.W. Maximum growth rates of Chlorella in steady-state and in synchronized cultures. Proc. Nat. Acad. Sci. USA 1959, 45, 1740–1744. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. Effects of temperature and illuminance of Chlorella growth uncoupled from cell division. Plant Physiol. 1962, 37, 37–42. [Google Scholar] [CrossRef]
- Sorokin, C.; Krauss, R.W. The dependence of cell division in Chlorella on temperature and light intensity. Am. J. Bot. 1965, 52, 331–339. [Google Scholar] [CrossRef]
- Min, M.; Hu, B.; Zhou, W.; Li, Y.; Chen, P.; Ruan, R. Mutual influence of light and CO2 on carbon sequestration via cultivating mixotrophic alga Auxenochlorella protothecoides UMN280 in an organic carbon-rich wastewater. J. Appl. Phycol. 2012, 24, 1099–1105. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Salih, F.M. Microalgae tolerance to high concentrations of carbon dioxide: A review. J. Environ. Prot. 2011, 2, 648–654. [Google Scholar] [CrossRef]
- Pavlik, D.; Zhong, Y.; Daiek, C.; Liao, W.; Morgan, R.; Clary, W.; Liu, Y. Microalgae cultivation for carbon dioxide sequestration and protein production using a high-efficiency photobioreactor system. Algal Res. 2017, 25, 413–420. [Google Scholar] [CrossRef]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Vieira, H.H.; Bagatini, I.L.; Guinart, C.M.; Vieira, A.A.H. tufA gene as molecular marker for freshwater Chlorophyceae. Algae 2016, 31, 155–165. [Google Scholar] [CrossRef]
- Hadi, S.I.I.A.; Santana, H.; Brunale, P.P.M.; Gomes, T.G.; Oliveira, M.D.; Matthiensen, A.; Oliveira, M.E.C.; Silva, F.C.P.; Brasil, B.S.A.F. DNA barcoding green microalgae isolated from Neotropical Inland waters. PLoS ONE 2016, 11, e0149284. [Google Scholar] [CrossRef] [PubMed]
- Fama, P.; Wysor, B.; Kooistra, W.H.C.F.; Zuccarello, G.C. Molecular phylogeny of the genus Caulerpa (Caulerpales, Chlorophyta) inferred from chloroplast tufA gene. J. Phycol. 2002, 38, 1040–1050. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. New Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef]
- Cuaresma, M.; Janssen, M.; Vílchez, C.; Wijffels, R.H. Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol. Bioeng. 2009, 104, 352–359. [Google Scholar] [CrossRef]
- Lizzul, A.; Hellier, P.; Purton, S.; Baganz, F.; Ladommatos, N.; Campos, L. Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Front. Microbiol. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Ramanna, L.; Guldhe, A.; Rawat, I.; Bux, F. The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour. Technol. 2014, 168, 127–135. [Google Scholar] [CrossRef]
- Gilmour, D.J.; Hipkins, M.F.; Boney, A.D. The effect of osmotic and ionic stress on the primary processes of photosynthesis in Dunaliella tertiolecta. J. Exp. Bot. 1984, 35, 18–27. [Google Scholar] [CrossRef]
- Ordog, V.; Stirk, W.A.; Balint, P.; Staden, J.; Lovasz, C. Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J. Appl. Phycol. 2012, 24, 907–914. [Google Scholar] [CrossRef]
- Janssen, J.H.; Wijffels, R.H.; Barbosa, M.J. Lipid production in Nannochloropsis gaditana during nitrogen starvation. Biology 2019, 8, 5. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Liu, K.; Nasr, L.K.; Byers, N.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Bouwer, E.J. Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl. Microbiol. Biotechnol. 2015, 99, 6139–6154. [Google Scholar] [CrossRef]
- Mayhead, E.; Silkina, A.; Llewellyn, C.A.; Fuentes-Grünewald, C. Comparing nutrient removal from membrane filtered and unfiltered domestic wastewater using Chlorella vulgaris. Biology 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
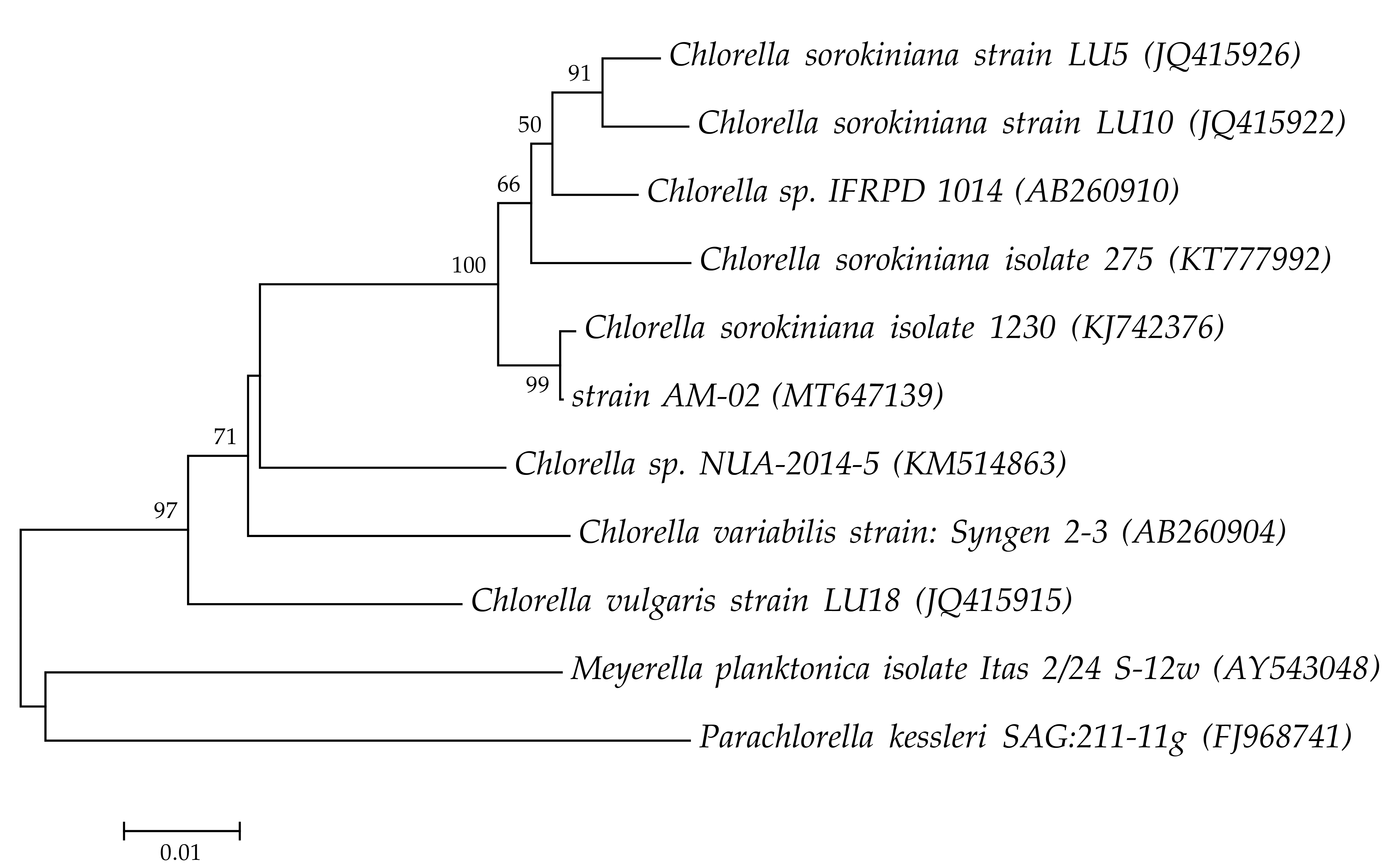
| Molecular Marker | Primers | Sequence 5′ → 3′ | Reference |
|---|---|---|---|
| rbcL (ribulose bisphosphate carboxylase large subunit) | rbcL-M379 F | GGTTTCAAAGCTYTWCGTGC | [26] |
| rbcLFP R | GTAAATACCACGGCTACGRTCTT | ||
| Fw_rbcl_192 | GGTACTTGGACAACWGTWTGGAC | [27] | |
| Rv_rbcL_657 | GAAACGGTCTCKCCARCGCAT | ||
| tufA (translational elongation factor Tu) | tufA F | GGNGCNGCNCAAATGGAYGG | [28] |
| tufA R | CCTTCNCGAATMGCRAAWCGC |
| Regimen | Dry Weight (g L−1) | ||
|---|---|---|---|
| PPFD (μmol m−2 s−1) | Initial NO3– (mg L−1) | Initial CO2 (%) | |
| 1000 | 180 | ~0.04 | 0.43 ± 0.02 |
| 1000 | 180 | 0.5 | 1.07 ± 0.03 |
| 1000 | 180 | 1.0 | 1.08 ± 0.02 |
| 1000 | 180 | 2.0 | 1.02 ± 0.01 |
| 1200 | 180 | ~0.04 | 0.45 ± 0.02 |
| 1200 | 180 | 0.5 | 1.02 ± 0.02 |
| 1200 | 180 | 1.0 | 1.08 ± 0.04 |
| 1200 | 180 | 2.0 | 1.10 ± 0.03 |
| 1400 | 180 | ~0.04 | 0.44 ± 0.01 |
| 1400 | 180 | 0.5 | 1.05 ±0.02 |
| 1400 | 180 | 1.0 | 1.08 ±0.01 |
| 1400 | 180 | 2.0 | 1.07 ±0.02 |
| 1200 | 180 | 2.0 | 1.10 ± 0.03 |
| 1200 | 360 | 2.0 | 1.76 ± 0.08 |
| 1200 | 720 | 2.0 | 2.53 ± 0.12 |
| 1200 | 1080 | 2.0 | 3.11 ± 0.21 |
| 1200 | 1440 | 2.0 | 3.45 ± 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity. Biology 2020, 9, 169. https://doi.org/10.3390/biology9070169
Ziganshina EE, Bulynina SS, Ziganshin AM. Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity. Biology. 2020; 9(7):169. https://doi.org/10.3390/biology9070169
Chicago/Turabian StyleZiganshina, Elvira E., Svetlana S. Bulynina, and Ayrat M. Ziganshin. 2020. "Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity" Biology 9, no. 7: 169. https://doi.org/10.3390/biology9070169
APA StyleZiganshina, E. E., Bulynina, S. S., & Ziganshin, A. M. (2020). Comparison of the Photoautotrophic Growth Regimens of Chlorella sorokiniana in a Photobioreactor for Enhanced Biomass Productivity. Biology, 9(7), 169. https://doi.org/10.3390/biology9070169




