Anti-Inflammatory and Physicochemical Characterization of the Croton rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of the Croton Rhamnifolioides Essential Oil
2.3. Preparation of Inclusion Complexes
2.4. Physicochemical Characterization of the Inclusion Complexes
2.5. In Vivo Experimental Protocols
2.6. Animals
2.7. Determination of the Median Lethal Dose (LD50)
2.8. Ear Edema Induced by a Single Administration of Croton Oil
2.9. Paw Edema Induced by an Intraplantar Administration of Carrageenan
2.10. Paw Edema Induced by an Intraplantar Administration of 1% Dextran
2.11. Paw Edema Induced by an Intraplantar Administration of Histamine
2.12. Paw Edema Induced by an Intraplantar Administration of Arachidonic Acid
2.13. Paw Edema Measurement
2.14. Evaluation of Vascular Permeability by Evans Blue Extravasation
2.15. Granuloma Induced by Cotton Pellet Implantation
2.16. Statistical Analysis
2.17. Docking Procedure and Pharmacokinetic Characteristics
3. Results
3.1. Physico-Chemical Characterization of the OEFC Inclusion Complex in β-Cyclodextrin
3.2. Determination of the Median Lethal Dose (LD50)
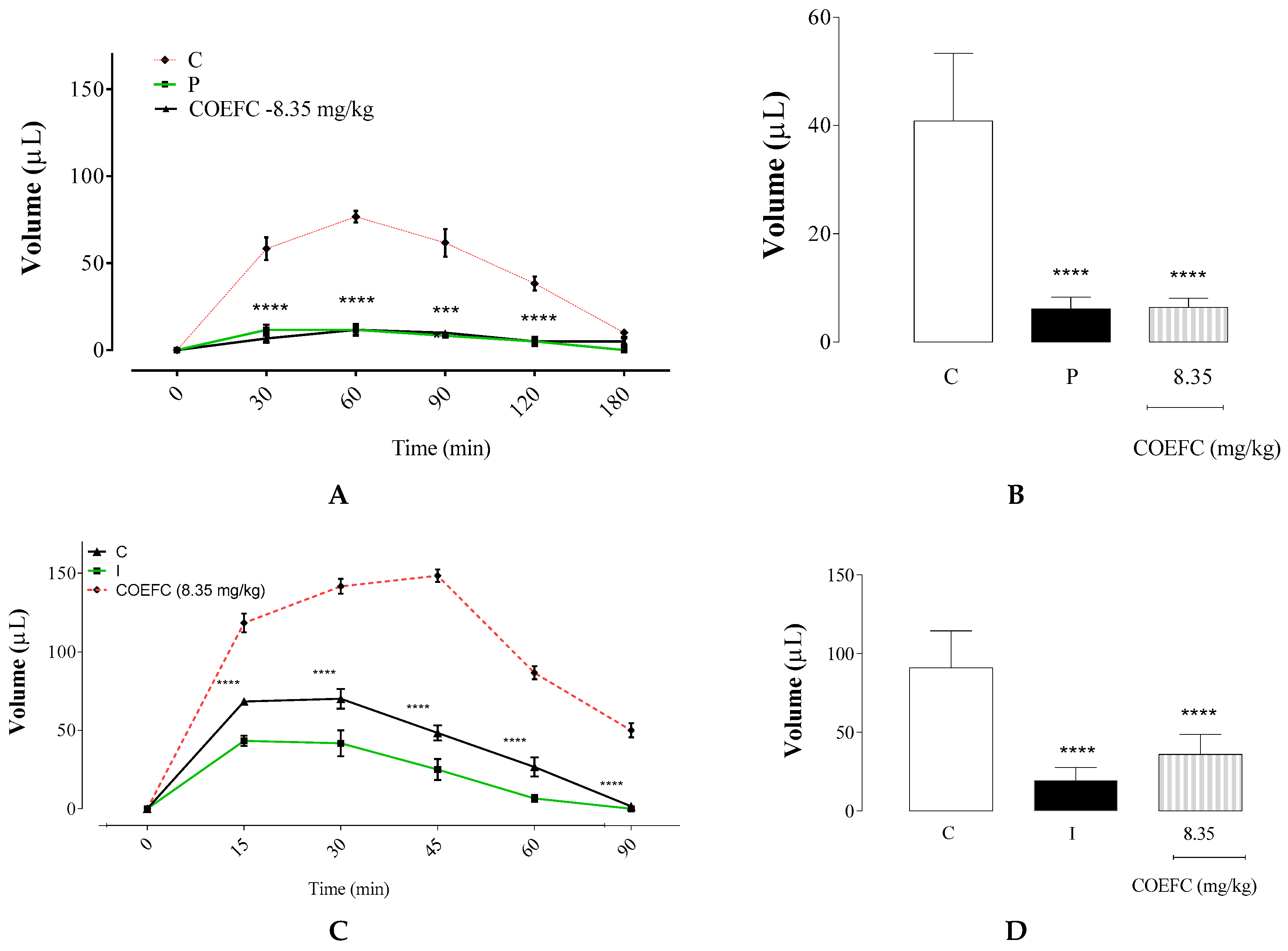
3.3. Ear Edema Induced by a Single Application of Croton Oil
3.4. Paw Edema Induced by an Intraplantar Administration of 1% Carrageenan and Dextran 1%
3.5. Paw Edema Induced by an Intraplantar Administration of Histamine or Arachidonic Acid
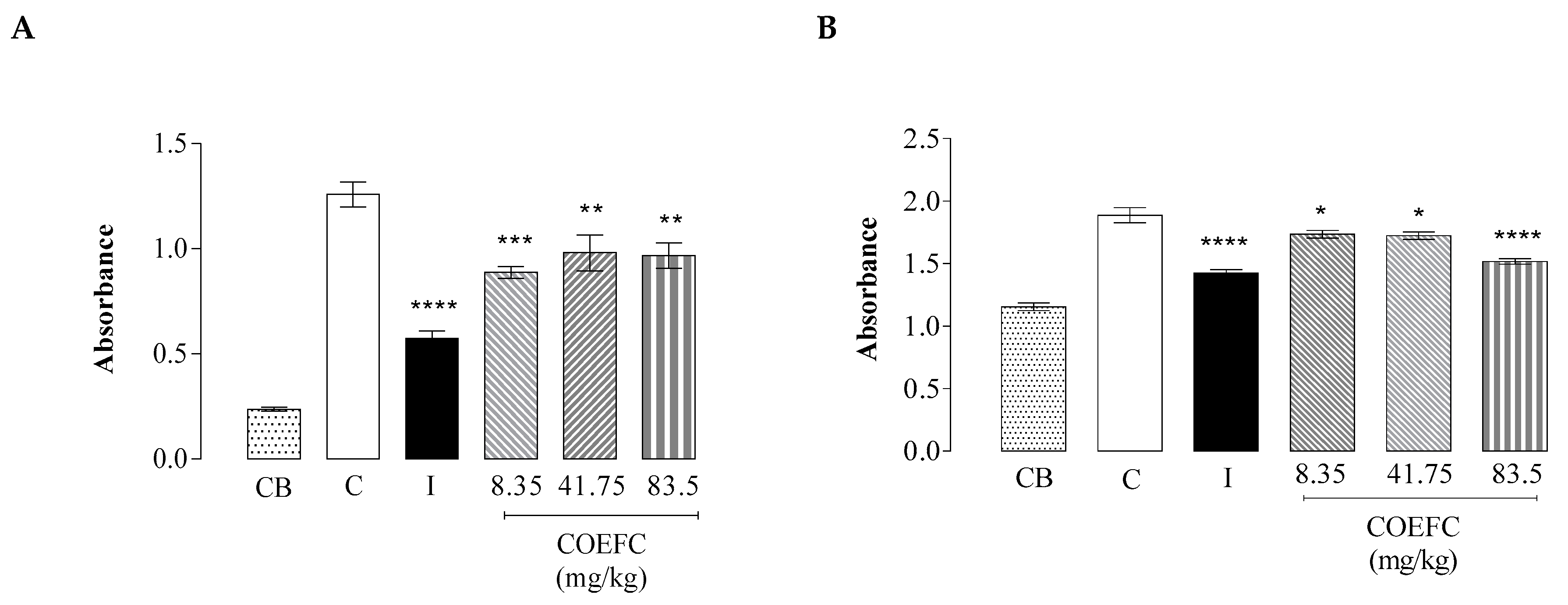
3.6. Vascular Permeability by Evans Blue Extravasation
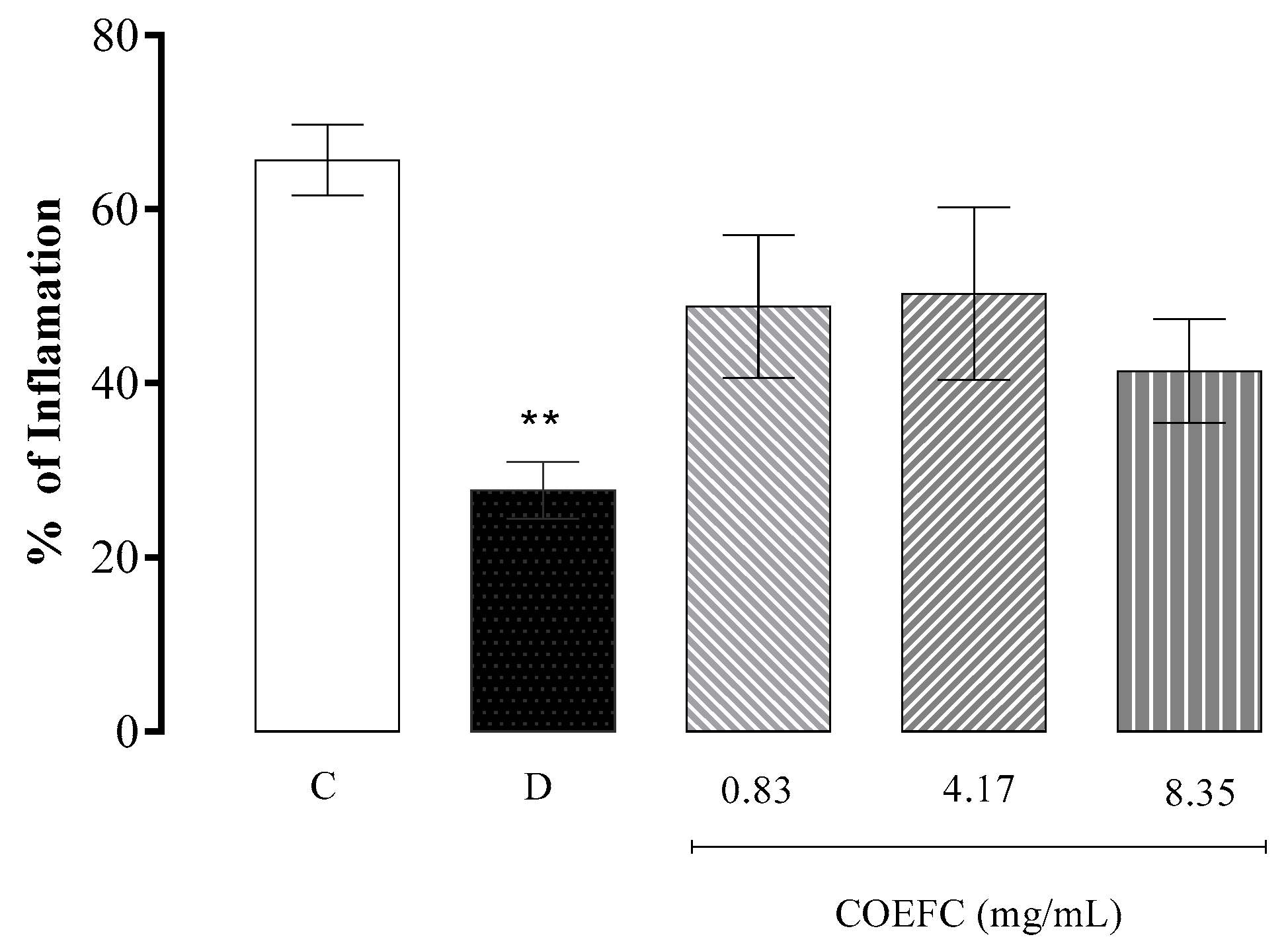
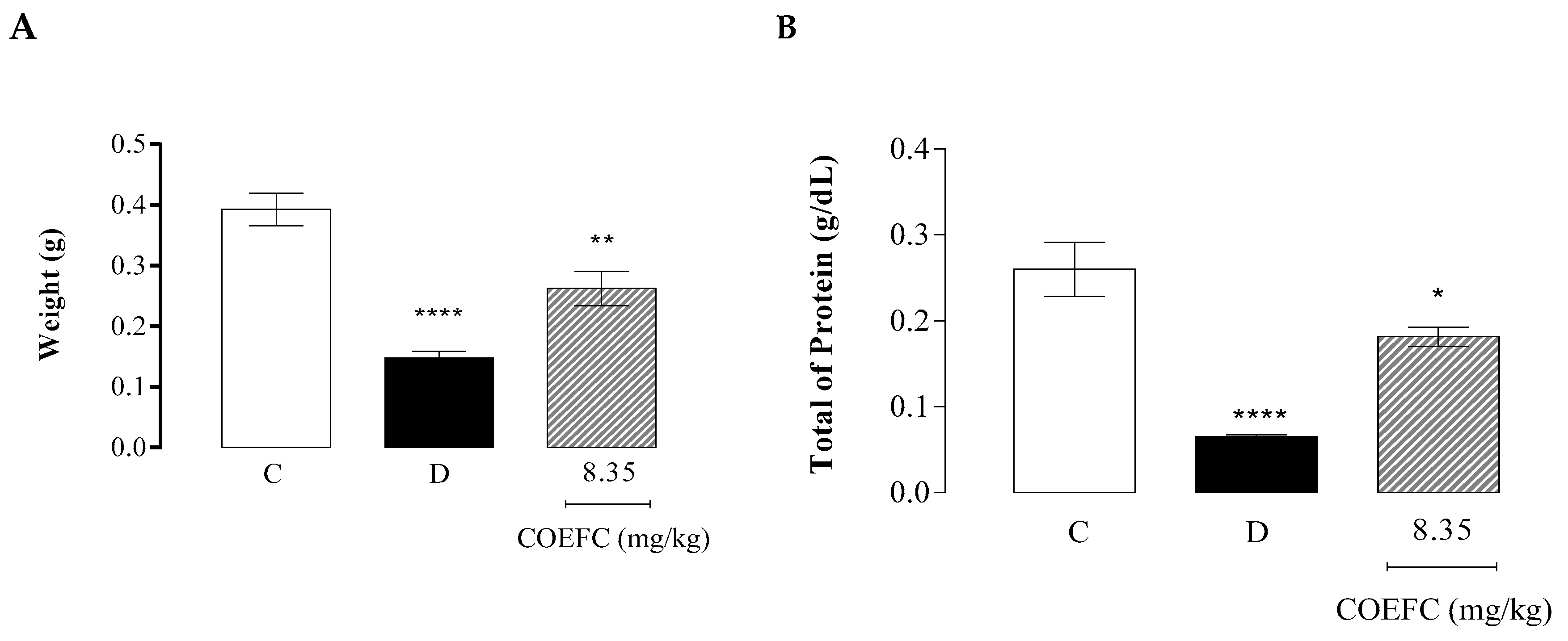
3.7. Granuloma Induced by Cotton Pellet Implantation
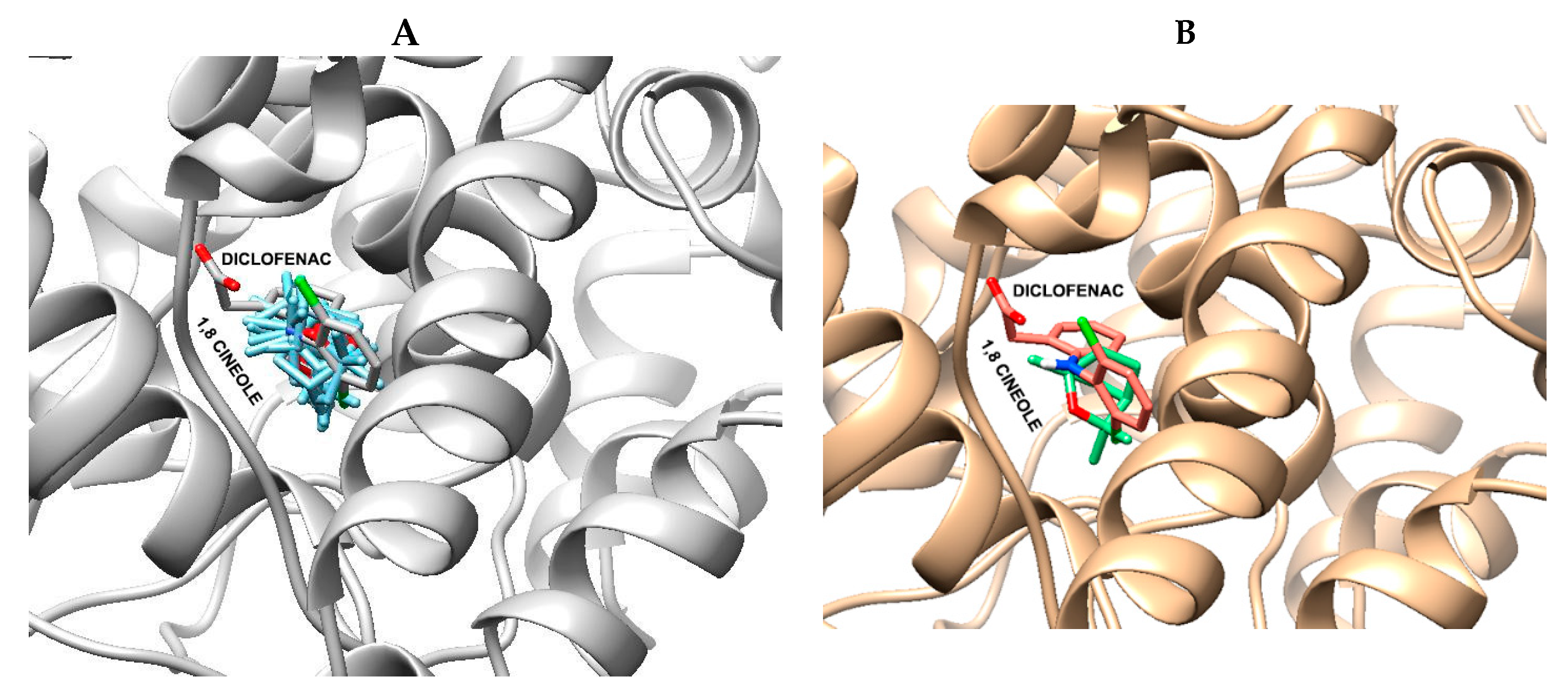
3.8. Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Simões, C.M.O.; Schenkel, E.P.; de Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Do Produto Natural ao Medicamento; Artmed Editora: Porto Alegre, RS, Brasil, 2016; ISBN 8582713657. [Google Scholar]
- Sinha, S.; Jothiramajayam, M.; Ghosh, M.; Mukherjee, A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem. Toxicol. 2014, 68, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Malik, F.; Bhushan, S.; Sethi, V.K.; Shahi, A.K.; Taneja, S.C.; Qazi, G.N.; Singh, J. An essential oil and its major constituent isointermedeol induce apoptosis by increased expression of mitochondrial cytochrome c and apical death receptors in human leukaemia HL-60 cells. Chem. Biol. Interact. 2008, 171, 332–347. [Google Scholar] [CrossRef]
- Santos, P.L.; Brito, R.G.; Quintans, J.S.S.; Araujo, A.A.S.; Menezes, I.R.A.; Brogden, N.K.; Quintans-Junior, L.J. Cyclodextrins as Complexation Agents to Improve the Anti-inflammatory Drugs Profile: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2017, 23, 2096–2107. [Google Scholar] [CrossRef]
- Sithole, M.N.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Development of a Novel Polymeric Nanocomposite Complex for Drugs with Low Bioavailability. AAPS PharmSciTech 2018, 19, 303–314. [Google Scholar] [CrossRef]
- Aguiar, U.N.; de Lima, S.G.; Moura, L.C.B.; de Almeida, L.T.G.; Oliveira, T.M.; de Freitas, R.M.; Silva, R.M.; Rocha, M.S. Preparação e caracterização do complexo de inclusão do óleo essencial de Croton zehntneri com β-Ciclodextrina. Química Nova 2014, 37, 50–55. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- De Bitu, V.C.N.; da Costa Martins, J.G.; Rogrigues, F.F.G.; Colares, A.V.; Coutinho, H.D.M.; Botelho, M.A.; Portela, A.D.C.; de Santana, N.M.; Menezes, I.R.A. Effect of Collection Time on Composition of Essential Oil of Lippia gracilis Schauer (Verbenaceae) Growing in Northeast Brazil. J. Essent. Oil Bear. Plants 2015, 18, 647–653. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Quintans-Júnior, L.J.; Barreto, A.S.; Almeida, J.R.G.S.; Barreto, R.S.S.; Quintans, J.S.S. Neuroprotective effect of natural products on peripheral nerve degeneration: A systematic review. Neurochem. Res. 2016, 41, 647–658. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Gouveia, D.N.; Costa, J.S.; Quintans, J.S.S.; Quintans-Júnior, L.J.; Barreto, R.S.S.; Guimarães, A.G. New perspectives for chronic pain treatment: A patent review (2010–2016). Expert Opin. Ther. Pat. 2017, 27, 787–796. [Google Scholar] [CrossRef]
- Serafini, M.R.; Guimarães, A.G.; Quintans, J.S.S.; Araújo, A.A.S.; Nunes, P.S.; Quintans-Júnior, L.J. Natural compounds for solar photoprotection: A patent review. Expert Opin. Ther. Pat. 2015, 25, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Lima, M.; Quintans-Júnior, L.J.; de Santana, W.A.; Kaneto, C.M.; Soares, M.B.P.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Marilia, S.T.; Adriana, G.G.; Araújo, A.A.S. Monoterpenos com atividade anti-inflamatória: Uma prospecção tecnológica. Rev. GEINTEC Gestão, Inovação E Tecnol. 2014, 4, 867–875. [Google Scholar]
- Vigan, M. Essential oils: Renewal of interest and toxicity. Eur. J. Dermatol. 2010, 20, 685–692. [Google Scholar]
- Höld, K.M.; Sirisoma, N.S.; Casida, J.E. Detoxification of α-and β-Thujones (the active ingredients of absinthe): Site specificity and species differences in cytochrome P450 oxidation in vitro and in vivo. Chem. Res. Toxicol. 2001, 14, 589–595. [Google Scholar] [CrossRef]
- De Araújo Neves, I.; da Camara, C.A.G. Volatile Constituents of Two Croton Species from Caatinga Biome of Pernambuco–Brasil. Rec. Nat. Prod. 2012, 6, 161–165. [Google Scholar]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2009, 81, 413–419. [Google Scholar] [CrossRef]
- Caldas, G.F.R.; da Silva Oliveira, A.R.; Araújo, A.V.; Lafayette, S.S.L.; Albuquerque, G.S.; da Costa Silva-Neto, J.; Costa-Silva, J.H.; Ferreira, F.; da Costa, J.G.M.; Wanderley, A.G. Gastroprotective mechanisms of the monoterpene 1, 8-cineole (eucalyptol). PLoS ONE 2015, 10, e0134558. [Google Scholar]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1, 8-cineole against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef]
- Moon, H.K.; Kang, P.; Lee, H.S.; Min, S.S.; Seol, G.H. Effects of 1, 8-cineole on hypertension induced by chronic exposure to nicotine in rats. J. Pharm. Pharmacol. 2014, 66, 688–693. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2011, 29, 415–422. [Google Scholar]
- De Oliveira, K.Á.R.; de Sousa, J.P.; da Costa Medeiros, J.A.; de Figueiredo, R.C.B.Q.; Magnani, M.; de Siqueira, J.P.; de Souza, E.L. Synergistic inhibition of bacteria associated with minimally processed vegetables in mixed culture by carvacrol and 1, 8-cineole. Food Control 2015, 47, 334–339. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Caldas, G.F.R.; Limeira, M.M.F.; Araújo, A.V.; Albuquerque, G.S.; da Costa Silva-Neto, J.; da Silva, T.G.; Costa-Silva, J.H.; de Menezes, I.R.A.; da Costa, J.G.M.; Wanderley, A.G. Repeated-doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in Wistar rats. Food Chem. Toxicol. 2016, 97, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Randau, K.P.; Florêncio, D.C.; Ferreira, C.P.; Xavier, H.S. Estudo farmacognóstico de Croton rhamnifolius HBK e Croton rhamnifolioides Pax & Hoffm.(EUPHORBIACEAE). Rev. Bras. Farmacogn. 2004, 14, 89–96. [Google Scholar]
- Ribeiro, D.A.; Macêdo, D.G.G.; Oliveira, L.G.S.G.S.; Saraiva, M.E.E.; Oliveira, S.F.F.; Souza, M.M.A.M.A.; Menezes, I.R. Potencial terapêutico e uso de plantas medicinais em uma área de Caatinga no estado do Ceará, nordeste do Brasil. Rev. Bras. Plantas Med. 2014, 16, 912–930. [Google Scholar] [CrossRef]
- Souza, R.K.D.; da Silva, M.A.P.; de Menezes, I.R.A.; Ribeiro, D.A.; Bezerra, L.R.; de Almeida Souza, M.M. Ethnopharmacology of medicinal plants of carrasco, northeastern Brazil. J. Ethnopharmacol. 2014, 157, 99–104. [Google Scholar] [CrossRef]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; de Oliveira, M.R.C.; Tintino, C.D.M.; e Castro, F.F.; Alcântara, I.S.; Fernandes, M.N.M.; de Albuquerque, T.R.; da Silva, M.S.A. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1, 8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; do Carmo Vieira, M.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Chavan, M.J.; Wakte, P.S.; Shinde, D.B. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Kang, Y.; Zou, H.; Cheng, X.; Xie, T.; Shi, L.; Zhang, H. β-elemene attenuates macrophage activation and proinflammatory factor production via crosstalk with Wnt/β-catenin signaling pathway. Fitoterapia 2018, 124, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Held, S.; Schieberle, P.; Somoza, V. Characterization of α-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007, 55, 8040–8046. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Ribeiro-Filho, J.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Quintans Júnior, L.J.; Araújo, A.A.; et al. Anti-inflammatory activity of the essential oil obtained from Ocimum basilicum complexed with β-cyclodextrin (β-CD) in mice. Food Chem. Toxicol. 2017, 109, 836–846. [Google Scholar] [CrossRef]
- Gidwani, B.K.; Bhargava, S.; Rao, S.P.; Majoomdar, A.; Pawar, D.P.; Alaspure, R.N. Analgesic, anti-inflammatory and antihemorrhoidal activity of aqueous extract of Lantana camara Linn. Res. J. Pharm. Technol. 2009, 2, 378–381. [Google Scholar]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- de Oliveira Makson, G.; Guimarães, A.G.; Araújo Adriano, A.; Quintans Jullyana, S.; Santos, M.R.; Quintans-Júnior, L.J. Cyclodextrins: Improving the therapeutic response of analgesic drugs: A patent review. Expert Opin. Ther. Pat. 2015, 25, 897–907. [Google Scholar] [CrossRef]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Menezes, P.P.; Serafini, M.R.; Quintans-Júnior, L.J.; Silva, G.F.; Oliveira, J.F.; Carvalho, F.M.S.; Souza, J.C.C.; Matos, J.R.; Alves, P.B.; Matos, I.L.; et al. Inclusion complex of (−)-linalool and β-cyclodextrin. J. Therm. Anal. Calorim. 2014, 115, 2429–2437. [Google Scholar] [CrossRef]
- Malone, M.H.; Robichaud, R.C. A Hippocratic screen for pure or crude drug materials. In Proceedings of the Lloydia 1962, 25, 320–332. [Google Scholar]
- Malone, M.H. New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity; Wagner, H., Wolff, P., Eds.; Proceedings in Life Sciences; Springer: Berlin/Heidelberg, Germany, 1977; ISBN 978-3-642-66684-1. [Google Scholar]
- Guideline, O.O. 425: Acute oral toxicity—Up-and-down procedure. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2001; Volume 2, pp. 12–16. [Google Scholar]
- Tubaro, A.; Dri, P.; Delbello, G.; Zilli, C.; Loggia, R. Della The Croton oil ear test revisited. Agents Actions 1986, 17, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Maling, H.M.; Webster, M.E.; Williams, M.A.; Saul, W.; Anderson, W. Inflammation induced by histamine, serotonin, bradykinin and compound 48/80 in the rat: Antagonists and mechanisms of action. J. Pharmacol. Exp. Ther. 1974, 191, 300–310. [Google Scholar]
- de Oliveira Ramalho, T.R.; de Oliveira, M.T.P.; de Araujo Lima, A.L.; Bezerra-Santos, C.R.; Piuvezam, M.R. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254. [Google Scholar]
- DiMartino, M.J.; Campbell, G.K.; Wolff, C.E.; Hanna, N. The pharmacology of arachidonic acid-induced rat paw edema. Agents Actions 1987, 21, 303–305. [Google Scholar] [CrossRef]
- Kunnaja, P.; Wongpalee, S.P.; Panthong, A. Evaluation of anti-inflammatory, analgesic, and antipyretic activities of the ethanol extract from Murdannia loriformis (Hassk.) Rolla Rao et Kammathy. BioImpacts BI 2014, 4, 183. [Google Scholar] [CrossRef]
- Lapa, A.J.; Souccar, C.; Lima-Landman, M.T.R.; Castro, M.S.D.A.; Lima, T.C.M. Métodos de avaliação da atividade farmacológica de plantas medicinais. Soc. Bras. Plantas Med. 2003, 64, 66. [Google Scholar]
- Lalitha, K.G.; Sethuraman, M.G. Anti-inflammatory activity of roots of Ecbolium viride (Forsk) Merrill. J. Ethnopharmacol. 2010, 128, 248–250. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. Water content of natural cyclodextrins and their essential oil complexes: A comparative study between Karl Fischer titration and thermal methods. Food Chem. 2012, 132, 1741–1748. [Google Scholar] [CrossRef]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- dos P Menezes, P.; A de S Araujo, A.; Doria, A.A.; Quintans-Junior, L.J.; de Oliveira, M.G.; dos Santos, M.R.; de Oliveira, J.F.; do R Matos, J.; de S Carvalho, F.M.; Alves, P.B.; et al. Physicochemical characterization and analgesic effect of inclusion complexes of essential oil from Hyptis pectinata L. Poit leaves with β-cyclodextrin. Curr. Pharm. Biotechnol. 2015, 16, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N. Host-guest interaction of β-cyclodextrin with isomeric ursolicacid and oleanolic acid: Physicochemical characterization andmolecular modeling study. J. Biomed. Res. 2017, 31, 395. [Google Scholar]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Khuntawee, W.; Wolschann, P.; Rungrotmongkol, T.; Wong-ekkabut, J.; Hannongbua, S. Molecular Dynamics Simulations of the Interaction of Beta Cyclodextrin with a Lipid Bilayer. J. Chem. Inf. Model. 2015, 55, 1894–1902. [Google Scholar] [CrossRef]
- Sherje, A.P.; Kulkarni, V.; Murahari, M.; Nayak, U.Y.; Bhat, P.; Suvarna, V.; Dravyakar, B. Inclusion complexation of etodolac with hydroxypropyl- beta-cyclodextrin and auxiliary agents: Formulation characterization and molecular modeling studies. Mol. Pharm. 2017, 14, 1231–1242. [Google Scholar] [CrossRef]
- Marreto, R.N.; Almeida, E.E.C.V.; Alves, P.B.; Niculau, E.S.; Nunes, R.S.; Matos, C.R.S.; Araújo, A.A.S. Thermal analysis and gas chromatography coupled mass spectrometry analyses of hydroxypropyl-β-cyclodextrin inclusion complex containing Lippia gracilis essential oil. Thermochim. Acta 2008, 475, 53–58. [Google Scholar] [CrossRef]
- De Lima, J.R.; Alves, L.D.S.; de Santana, D.P. Complexos de inclusão como estratégia para veiculação de óleos essenciais. Rev. Bras. Farm 2012, 93, 397–402. [Google Scholar]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Uebelacker, M. Risk assessment of thujone in foods and medicines containing sage and wormwood–evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 2010, 58, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Thuvander, A. Toxicological evaluation of myristicin. Nat. Toxins 1997, 5, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Greyer, H.; Hentschel, H. Nutmeg (myristicin) poisoning—Report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci. Int. 2001, 118, 87–90. [Google Scholar] [CrossRef]
- Darben, T.; Cominos, B.; Lee, C.T. Topical eucalyptus oil poisoning. Australas. J. Dermatol. 1998, 39, 265–267. [Google Scholar] [CrossRef]
- Janes, S.E.J.; Price, C.S.G.; Thomas, D. Essential oil poisoning: N-acetylcysteine for eugenol-induced hepatic failure and analysis of a national database. Eur. J. Pediatr. 2005, 164, 520–522. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Rasheed, A.; Kumar C.K., A.; Sravanthi, V.V.N.S.S. Cyclodextrins as drug carrier molecule: A review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Anjana, M.N.; Nair, S.C.; Joseph, J. An updated review of cyclodextrins-An enabling technology for challenging pharmaceutical formulations. Int. J. Pharm. Pharm. Sci. 2013, 5, 54–58. [Google Scholar]
- Brito, R.G.; Araújo, A.A.S.; Quintans, J.S.S.; Sluka, K.A.; Quintans Júnior, L.J. Enhanced analgesic activity by cyclodextrins—A systematic review and meta-analysis. Expert Opin. Drug Deliv. 2015, 12, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.B.; Vasko, M.R.; Fehrenbacher, J.C. Models of inflammation: Carrageenan air pouch. Curr. Protoc. Pharmacol. 2016, 56, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Wittstock, U.; Lindequist, U.; Teuscher, E. Effects of some Components of the Essential Oil of Chamomile, Chamomilla recutita, on Histamine Release from Rat Mast Cells. Planta Med. 1996, 62, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Rao, V.S.N. Mast cell involvement in the rat paw oedema response to 1,8-cineole, the main constituent of eucalyptus and rosemary oils. Eur. J. Pharmacol. 1997, 331, 253–258. [Google Scholar] [CrossRef]
- Panula, P. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 64, 1–15. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 249–256. [Google Scholar] [CrossRef]
- Chen, S. Natural products triggering biological targets-a review of the anti-inflammatory phytochemicals targeting the arachidonic acid pathway in allergy asthma and rheumatoid arthritis. Curr. Drug Targets 2011, 12, 288–301. [Google Scholar] [CrossRef]
- Beer, A.M.; Zagorchev, P.; Filipova, D.M.; Lukanov, J. Effects of 1, 8-Cineole on the Activity of Cyclooxygenase and Cyclooxygenase 1 and Cyclooxygenase 2 Isoforms. Nat. Prod. Chem. Res. 2017, 5, 2. [Google Scholar]
- Duggan, K.C.; Hermanson, D.J.; Musee, J.; Prusakiewicz, J.J.; Scheib, J.L.; Carter, B.D.; Banerjee, S.; Oates, J.A.; Marnett, L.J. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 2011, 7, 803. [Google Scholar] [CrossRef]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Stallings, W.C.; Kurumbail, R.G.; Marnett, L.J. A Novel Mechanism of Cyclooxygenase-2 Inhibition Involving Interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hermanson, D.J.; Banerjee, S.; Ghebreselasie, K.; Clayton, G.M.; Garavito, R.M.; Marnett, L.J. Oxicams bind in a novel mode to the cyclooxygenase active site via a two-water-mediated H-bonding network. J. Biol. Chem. 2014, 289, 6799–6808. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Carter, J.; Kiefer, J.R.; Kurumbail, R.G.; Pawlitz, J.L.; Brown, D.; Hartmann, S.J.; Graneto, M.J.; Seibert, K.; Talley, J.J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors-part I: The first clinical candidate. Bioorg. Med. Chem. Lett. 2010, 20, 7155–7158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorg. Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef]
- Kiefer, J.R.; Pawiitz, J.L.; Moreland, K.T.; Stegeman, R.A.; Hood, W.F.; Glerse, J.K.; Stevens, A.M.; Goodwin, D.C.; Rowlinson, S.W.; Marnett, L.J.; et al. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 2000, 405, 97–101. [Google Scholar] [CrossRef]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation—The negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef]
- Santos, F.A.A.; Rao, V.S.N.S.N. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phyther. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Júnior, L.J.Q.; da Costa, J.G.M.; Coutinho, H.D.M. Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: In vivo mouse models. Chem. Biol. Interact. 2016, 257, 14–25. [Google Scholar] [CrossRef]
- Linghu, K.; Lin, D.; Yang, H.; Xu, Y.; Zhang, Y.; Tao, L.; Chen, Y.; Shen, X. Ameliorating effects of 1,8-cineole on LPS-induced human umbilical vein endothelial cell injury by suppressing NF-κB signaling in vitro. Eur. J. Pharmacol. 2016, 789, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.; Araújo, A.; Brito, R.; Serafini, M.; Menezes, P.; DeSantana, J.; Júnior, W.; Alves, P.; Blank, A.; Oliveira, R.; et al. Cyclodextrin-Complexed Ocimum basilicum Leaves Essential Oil Increases Fos Protein Expression in the Central Nervous System and Produce an Antihyperalgesic Effect in Animal Models for Fibromyalgia. Int. J. Mol. Sci. 2014, 16, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Lima, P.S.; Brito, R.G.; Araújo-Filho, H.G.; Santos, P.L.; Lucchesi, A.; Araújo, A.A.S.; Menezes, P.P.; Scotti, L.; Scotti, M.T.; Menezes, I.R.A.; et al. Anti-hyperalgesic effect of Lippia grata leaf essential oil complexed with β-cyclodextrin in a chronic musculoskeletal pain animal model: Complemented with a molecular docking and antioxidant screening. Biomed. Pharmacother. 2017, 91, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.; Freitas, T.S.; Araújo, F.O.; Menezes, P.P.; Dória, G.A.A.; Rabelo, A.S.; Quintans-Júnior, L.J.; Santos, M.R.V.; Bezerra, D.P.; Serafini, M.R.; et al. Physico-chemical characterization and antibacterial activity of inclusion complexes of Hyptis martiusii Benth essential oil in β-cyclodextrin. Biomed. Pharmacother. 2017, 89, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Monoterpenes with analgesic activity—A systematic review. Phyther. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef]
- Barreto, R.; Guimarães, A.; Menezes, P.; Araújo, A.; Quintans, J.; Barreto, A.; Guedes, R.; Quintans-Junior, L. β-Cyclodextrin-complexed carvacrol produces antinociceptive effect superior to that of carvacrol in orofacial pain models (657.15). FASEB J. 2014, 28, 615–657. [Google Scholar]
- Araújo-Filho, H.G.; Pereira, E.W.M.; Rezende, M.M.; Menezes, P.P.; Araújo, A.A.S.; Barreto, R.S.S.; Martins, A.O.B.P.B.; Albuquerque, T.R.; Silva, B.A.F.; Alcantara, I.S.; et al. D-limonene exhibits superior antihyperalgesic effects in a β-cyclodextrin-complexed form in chronic musculoskeletal pain reducing Fos protein expression on spinal cord in mice. Neuroscience 2017, 358, 158–169. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Barreto, R.S.S.; Menezes, P.P.; Almeida, J.R.G.S.; Viana, A.F.S.C.; Oliveira, R.; Oliveira, A.P.; Gelain, D.P.; Lucca Júnior, W.; Araújo, A.A.S. β-Cyclodextrin-complexed (−)-linalool produces antinociceptive effect superior to that of (−)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [Google Scholar] [CrossRef]

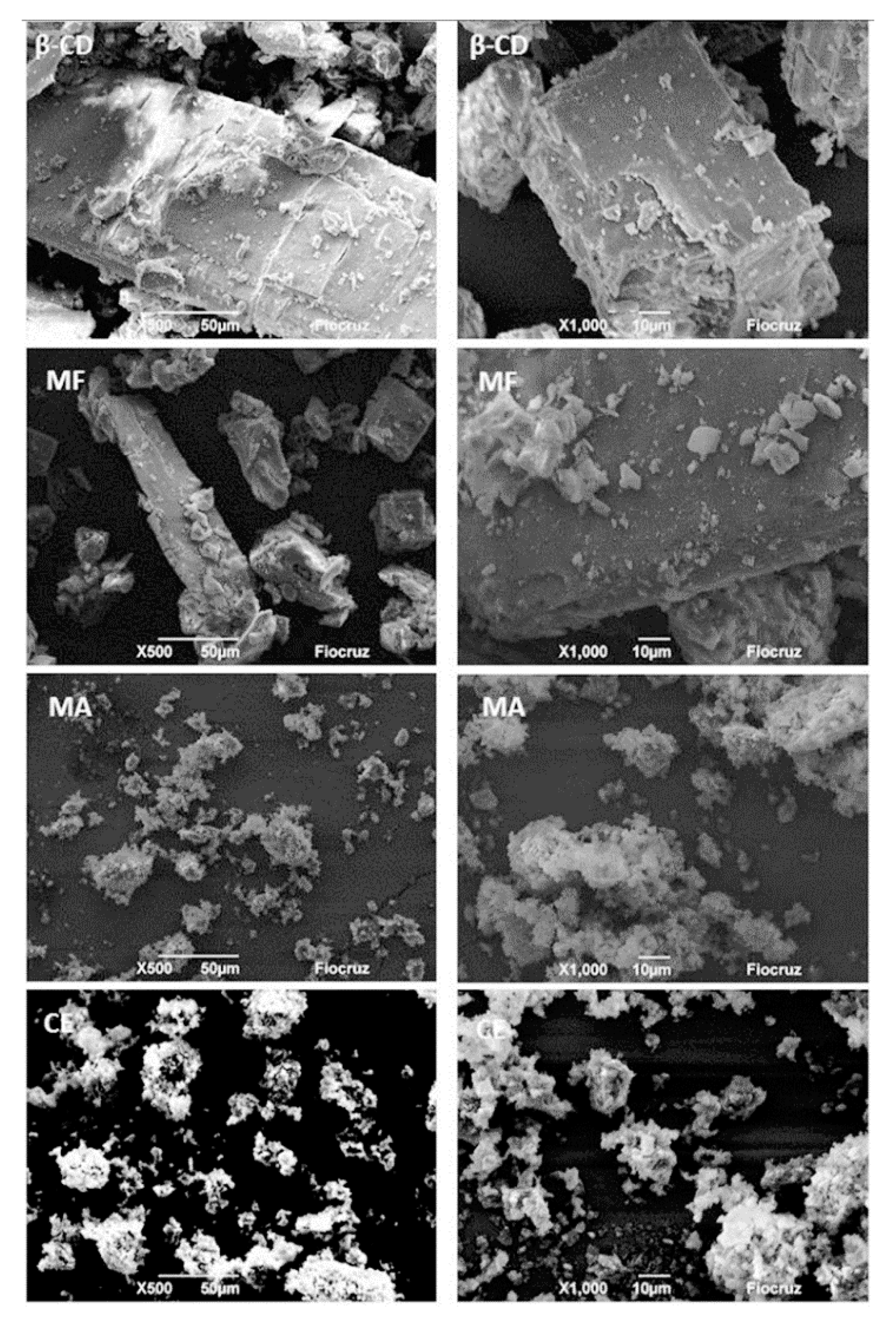

| Sample | 1st Step/% 30–220 °C | 2nd Step/% 220–270 °C | 3rd Step/% 270–380 °C | 4th Step/% 380–500 °C | % H2O |
|---|---|---|---|---|---|
| OEFC | 97.2 ± 1.48 | 1.62 ± 0.35 | 0.88 ± 0.10 | 0.52 ± 0.01 | 0.91 ± 0.06 |
| β-CD | 12.8 ± 1.34 | 0.1 ± 0.07 | 76.6 ± 1.48 | 3.7 ± 0.14 | 13.75 ± 0.39 |
| MF | 19.4 ± 0.07 | 0.5 ± 0.01 | 70.9 ± 1.13 | 2.7 ± 0.98 | 13.45 ± 0.78 |
| MA | 10.1 ± 0.2 | 2.4 ± 0.01 | 92.2 ± 2.92 | 3.2 ± 0.84 | 11.27 ± 0.32 |
| CE | 8.5 ± 1.69 | 2.6 ± 0.28 | 83.4 ± 1.48 | 3.2 ± 0.56 | 12.80 ± 0.27 |
| Ligand | Energy (kcal/mol) | Est. Inhibition Constant, Ki* (µM or ηM) |
|---|---|---|
| Prostaglandin E2 (Natural ligand) | −4.8 | 433.11 µM |
| Celecoxib | −10.4 | 70.80 ηM |
| Diclofenac | −8.4 | 13.2 µM |
| Meloxicam | −9.1 | 3.02 µM |
| Naproxen | −8.4 | 14.26 µM |
| 1,8-Cineole | −6.0 | 31.12 µM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira Brito Pereira Bezerra Martins, A.; Wanderley, A.G.; Alcântara, I.S.; Rodrigues, L.B.; Cesário, F.R.A.S.; Correia de Oliveira, M.R.; Castro, F.F.e.; Albuquerque, T.R.d.; da Silva, M.S.A.; Ribeiro-Filho, J.; et al. Anti-Inflammatory and Physicochemical Characterization of the Croton rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology 2020, 9, 114. https://doi.org/10.3390/biology9060114
Oliveira Brito Pereira Bezerra Martins A, Wanderley AG, Alcântara IS, Rodrigues LB, Cesário FRAS, Correia de Oliveira MR, Castro FFe, Albuquerque TRd, da Silva MSA, Ribeiro-Filho J, et al. Anti-Inflammatory and Physicochemical Characterization of the Croton rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology. 2020; 9(6):114. https://doi.org/10.3390/biology9060114
Chicago/Turabian StyleOliveira Brito Pereira Bezerra Martins, Anita, Almir Gonçalves Wanderley, Isabel Sousa Alcântara, Lindaiane Bezerra Rodrigues, Francisco Rafael Alves Santana Cesário, Maria Rayane Correia de Oliveira, Fyama Ferreira e Castro, Thaís Rodrigues de Albuquerque, Maria Sanadia Alexandre da Silva, Jaime Ribeiro-Filho, and et al. 2020. "Anti-Inflammatory and Physicochemical Characterization of the Croton rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin" Biology 9, no. 6: 114. https://doi.org/10.3390/biology9060114
APA StyleOliveira Brito Pereira Bezerra Martins, A., Wanderley, A. G., Alcântara, I. S., Rodrigues, L. B., Cesário, F. R. A. S., Correia de Oliveira, M. R., Castro, F. F. e., Albuquerque, T. R. d., da Silva, M. S. A., Ribeiro-Filho, J., Coutinho, H. D. M., Menezes, P. P., Quintans-Júnior, L. J., Araújo, A. A. d. S., Iriti, M., Almeida, J. R. G. d. S., & Menezes, I. R. A. d. (2020). Anti-Inflammatory and Physicochemical Characterization of the Croton rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology, 9(6), 114. https://doi.org/10.3390/biology9060114








