3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Evaluation of Cell Viability
2.1.2. Western Blot
2.1.3. Immunofluorescence
2.1.4. The Oil Red O Staining
2.1.5. RT-PCR
2.1.6. Determination of 2-Deoxy-D-Glucose Uptake
2.1.7. Adipocyte Lipolysis
2.1.8. Evaluation of ATP Cell Levels
2.1.9. Statistical Analysis
3. Results
3.1. The Effect of Cell Conditioning with T1AM on BAs’ Viability and Development
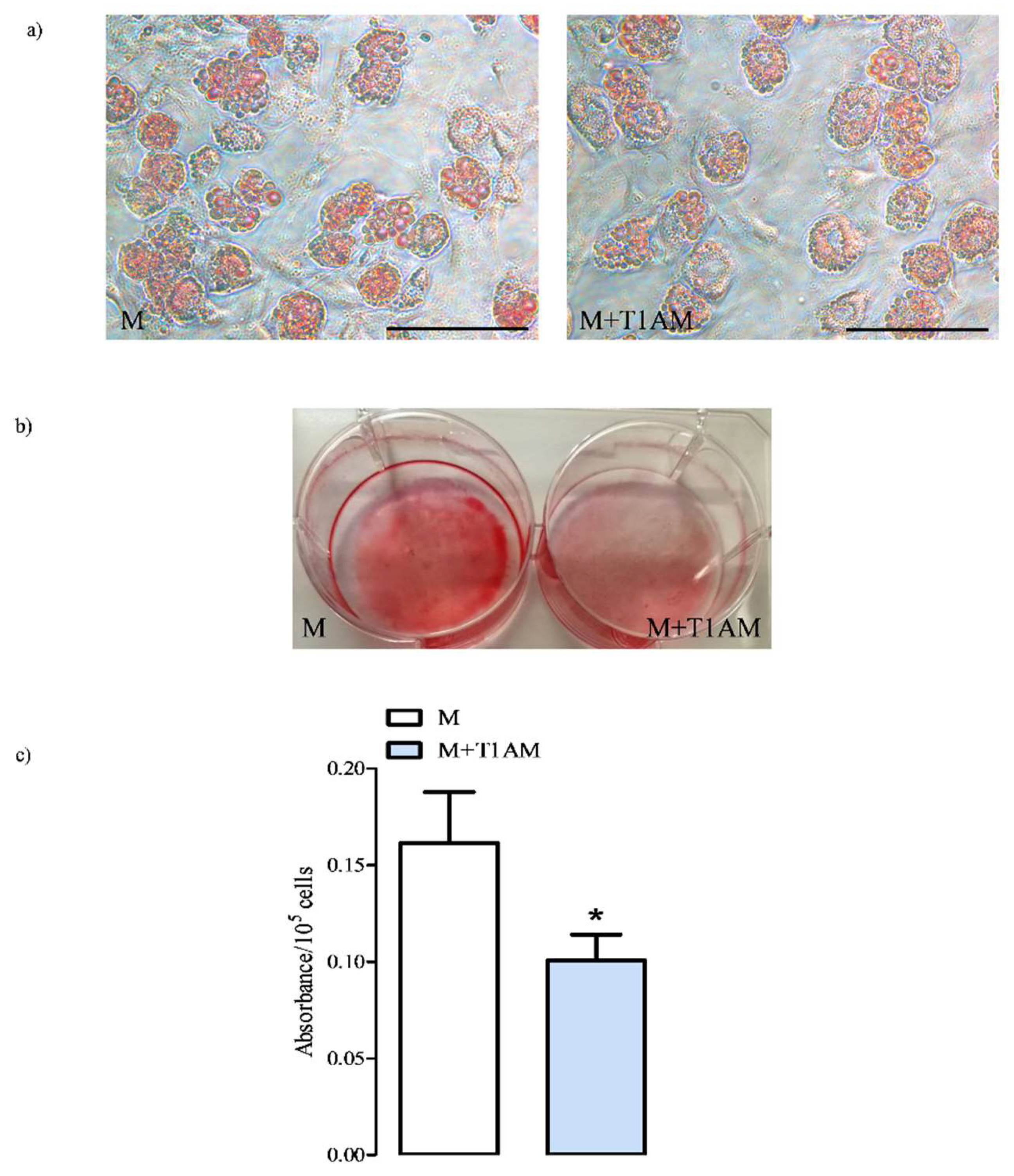
3.1.1. M and M+T1AM Cell Viability
3.1.2. The Estimation of M and M+T1AM Cell Lipid Droplet Content
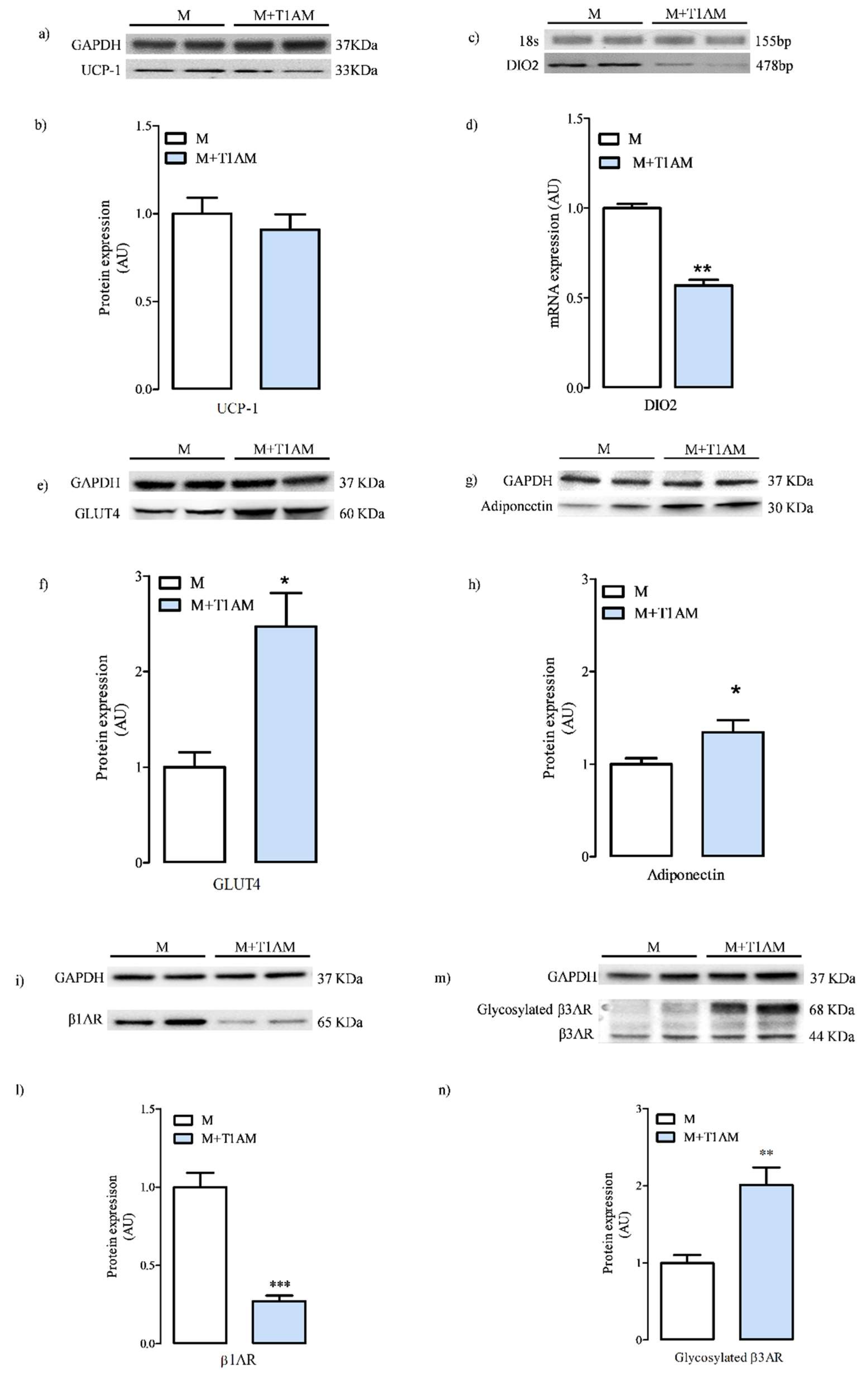
3.1.3. Cell Differentiation Makers
3.2. The Effect of Cell Conditioning with T1AM on Thermogenic Substrates
3.2.1. Glycerol Mobilization in M and M+T1AM Cells
3.2.2. Total and Phosphorylated PKA, CREB, and P38p Levels in M and M+T1AM Cells Reflect the Effect of Beta Adrenergic Agonists
3.3. Basal and Insulin-Stimulated Glucose Uptake in M and M+T1AM Cells
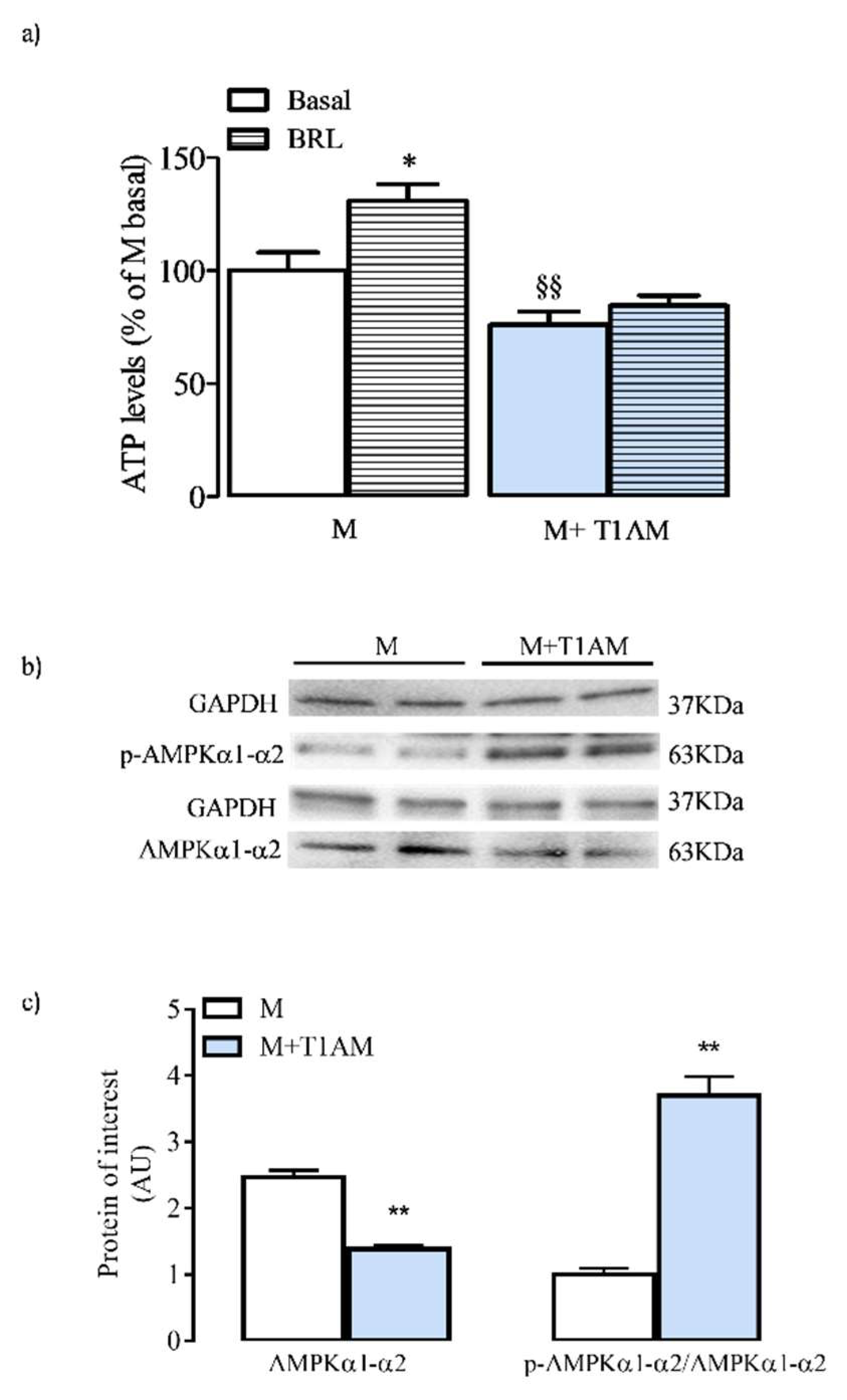
3.4. ATP Cell Levels and AMPK Activation
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiellini, G.; Erba, P.; Carnicelli, V.; Manfredi, C.; Frascarelli, S.; Ghelardoni, S.; Mariani, G.; Zucchi, R. Distribution of exogenous [125I]-3-iodothyronamine in mouse in vivo: Relationship with trace amine-associated receptors. J. Endocrinol. 2012, 213, 223. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Gencarelli, M.; Mazzantini, C.; Laurino, A.; Pellegrini-Giampietro, D.E.; Raimondi, L. N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide (EPPTB) Prevents 3-iodothyronamine (T1AM)-induced Neuroprotection Against Kainic Acid Toxicity. Neurochem. Int. 2019, 129, 104460. [Google Scholar] [CrossRef] [PubMed]
- Laurino, A.; Landucci, E.; Resta, F.; De Siena, G.; Pellegrini-Giampietro, D.E.; Masi, A.; Mannaioni, G.; Raimondi, L. Anticonvulsant and Neuroprotective Effects of the Thyroid Hormone Metabolite 3-Iodothyroacetic Acid. Thyroid 2018, 28, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, L.; Laurino, A.; Sabatini, M.; Sestito, S.; Lenzi, P.; Raimondi, L.; Rapposelli, S.; Biagioni, F.; Fornai, F.; Salvetti, A.; et al. New Insights into the Potential Roles of 3-Iodothyronamine (T1AM) and Newly Developed Thyronamine-Like TAAR1 Agonists in Neuroprotection. Front. Pharmacol. 2017, 8, 905. [Google Scholar] [CrossRef]
- Laurino, A.; Lucenteforte, E.; De Siena, G.; Raimondi, L. The Impact of Scopolamine Pretreatment on 3-iodothyronamine (T1AM) Effects on Memory and Pain in Mice. Horm. Behav. 2017, 94, 93–96. [Google Scholar] [CrossRef]
- Ju, H.; So, H.; Ha, K.; Park, K.; Lee, J.W.; Chung, C.M.; Choi, I. Sustained torpidity following multi-dose administration of 3-iodothyronamine in mice. J. Cell. Physiol. 2011, 226, 853–858. [Google Scholar] [CrossRef]
- Doyle, K.P.; Suchland, K.L.; Ciesielski, T.M.P.; Lessov, N.S.; Grandy, D.K.; Scanlan, T.S.; Stenzel-Poore, M.P. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 2007, 38, 2569–2576. [Google Scholar] [CrossRef]
- Gachkar, S.; Oelkrug, R.; Martinez-Sanchez, N.; Rial-Pensado, E.; Warner, A.; Hoefig, C.S.; López, M.; Mittag, J. 3-iodothyronamine induces tail vasodilation through central action in male mice. Endocrinology 2017, 158, 1977–1984. [Google Scholar] [CrossRef]
- Rogowski, M.; Bellusci, L.; Sabatini, M.; Rapposelli, S.; Rahman, S.M.; Chiellini, G.; Assadi-Porter, F.M. Lipolytic effects of 3-iodothyronamine (T1AM) and a novel thyronamine-like analog SG-2 through the AMPK pathway. Int. J. Mol. Sci. 2019, 20, 4054. [Google Scholar] [CrossRef]
- Biebermann, H.; Kleinau, G. 3-Iodothyronamine Induces Diverse Signaling Effects at Different Aminergic and Non-Aminergic G-Protein Coupled Receptors [published online ahead of print, 2019 Nov 7]. Exp. Clin. Endocrinol. Diabetes 2019. [Google Scholar] [CrossRef]
- Cumero, S.; Fogolari, F.; Domenis, R.; Zucchi, R.; Mavelli, I.; Contessi, S. Mitochondrial F(0) F(1) -ATP synthase is a molecular target of 3-iodothyronamine, an endogenous metabolite of thyroid hormone. Br. J. Pharmacol. 2012, 166, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.P.; Dodson, M.V.; Culver, M.F.; Hausman, G.J. The Increasingly Complex Regulation of Adipocyte Differentiation. Exp. Biol. Med. 2016, 241, 449–456. [Google Scholar]
- Liu, D.; Bordicchia, M.; Zhang, C.; Fang, H.; Wei, W.; Li, J.L.; Czech, M.P.; Collins, S.; Guilherme, A.; Guntur, K. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J. Clin. Investig. 2016, 126, 1704–1716. [Google Scholar] [CrossRef]
- Mittag, J. More Than Fever—Novel Concepts in the Regulation of Body Temperature by Thyroid Hormones [published online ahead of print, 2019 Oct 25]. Exp. Clin. Endocrinol. Diabetes 2019. [Google Scholar] [CrossRef]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, Brown and Pink Adipocytes: The Extraordinary Plasticity of the Adipose Organ. Eur. J. Endocrinol. 2014, 170, R159-71. [Google Scholar] [CrossRef]
- Silvestri, E.; Senese, R.; Cioffi, F.; De Matteis, R.; Lattanzi, D.; Lombardi, A.; Giacco, A.; Salzano, A.M.; Scaloni, A.; Ceccarelli, M.; et al. 3,5-Diiodo-L-Thyronine Exerts Metabolically Favorable Effects on Visceral Adipose Tissue of Rats Receiving a High-Fat Diet. Nutrients 2019, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gomez, G.; Hernàndez, A.; Calvo, R.M.; Martin, E.; Obregón, M.J. Potent thermogenic action of triiodothyroacetic acid in brown adipocytes. Cell. Mol. Life Sci. 2003, 60, 1957–1967. [Google Scholar] [CrossRef]
- Cioffi, F.; Gentile, A.; Silvestri, E.; Goglia, F.; Lombardi, A. Effect of Iodothyronines on Thermogenesis: Focus on Brown Adipose Tissue. Front. Endocrinol. 2018, 9, 254. [Google Scholar] [CrossRef]
- Raimondi, L.; Pirisino, R.; Banchelli, G.; Ignesti, G.; Conforti, L.; Buffoni, F. Cultured preadipocytes produce a semicarbazide-sensitive amine oxidase (SSAO) activity. J. Neural Transm. Suppl. 1990, 32, 331–336. [Google Scholar]
- Landucci, E.; Lattanzi, R.; Gerace, E.; Scartabelli, T.; Balboni, G.; Negri, L.; Pellegrini Giampietro, D.E. Prokineticins are neuroprotective in models of cerebral ischemia and ischemic tolerance in vitro. Neuropharmacology 2016, 108, 39–48. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Alfarano, C.; Sartiani, L.; Nediani, C.; Mannucci, E.; Mugelli, A.; Cerbai, E.; Raimondi, L. Functional coupling of angiotensin II type 1 receptor with insulin resistance of energy substrate uptakes in immortalized cardiomyocytes (HL-1 cells). Br. J. Pharmacol. 2008, 153, 907–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buonvicino, D.; Mazzola, F.; Zamporlini, F.; Resta, F.; Ranieri, G.; Camaioni, E.; Muzzi, M.; Zecchi, R.; Pieraccini, G.; Dölle, C.; et al. Identification of the Nicotinamide Salvage Pathway as a New Toxification Route for Antimetabolites. Cell Chem. Biol. 2018, 25, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Li, M.; Speakman, J.R. Switching on the furnace: Regulation of heat production in brown adipose tissue. Mol. Asp. Med. 2019, 68, 60–73. [Google Scholar] [CrossRef]
- Ju, H.; Shin, H.; Son, C.; Park, K.; Choi, I. 3-Iodothyronamine-mediated metabolic suppression increases the phosphorylation of AMPK and induces fuel choice toward lipid mobilization. Horm. Metab. Res. 2015, 47, 605–610. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Huang, W.; Yang, H. N-glycosylation of the β2adrenergic receptor regulates receptor function by modulating dimerization. FEBS J. 2017, 284, 2004–2018. [Google Scholar] [CrossRef]
- Berg, K.A.; Clarke, W.P. Making Sense of Pharmacology: Inverse Agonism and Functional Selectivity. Int. J. Neuropsychopharmacol. 2018, 21, 962–977. [Google Scholar] [CrossRef]
- Jönsson, C.; Castor Batista, A.P.; Kjølhede, P.; Strålfors, P. Insulin and β-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochem. J. 2019, 476, 2883–2908. [Google Scholar] [CrossRef]
- Arrojo E Drigo, R.; Fonseca, T.L.; Werneck-de-Castro, J.P.; Bianco, A.C. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim. Biophys. Acta 2013, 1830, 3956–3964. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, W.H.; Han, K.L.; Park, J.H.; Lee, J.; Yeo, J.; Jung, M.H. cAMP-response element binding protein (CREB) positively regulates mouse adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2010, 391, 634–639. [Google Scholar] [CrossRef]
- Alves-Wagner, A.B.; Yonamine, C.Y.; de Fatima, L.A.; Festuccia, W.; Machado, U.F. Sympathetic Regulation of Slc2a4 Gene Expression: Participation of a Putative cAMP Responsive Element (CRE) Site in the Slc2a4 Promoter. Cell. Physiol. Biochem. 2019, 52, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Yubero, P.; Barberá, M.J.; Alvarez, R.; Viñas, O.; Mampel, T.; Iglesias, R.; Villarroya, F.; Giralt, M. Dominant negative regulation by c-Jun of transcription of the uncoupling protein-1 gene through a proximal cAMP-regulatory element: A mechanism for repressing basal and norepinephrine-induced expression of the gene before brown adipocyte differentiation. Mol. Endocrinol. 1998, 12, 1023–1037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliver, P.; Lombardi, A.; De Matteis, R. Editorial: Insights into Brown Adipose Tissue Functions and Browning Phenomenon. Front. Physiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]



| Name | Forward Primer 5′ ≥ 3′ | Reverse Primer 3′ ≥ 5′ | Size (Bp) |
|---|---|---|---|
| DIO2 | ACGCCTACAAACAGGTTAAATTGG | ATGCACACACGTTCAAAGGC | 478 |
| 18s | AAACGGCTACCACATCCAAG | CCTCCAATGGATCCTCGTTA | 155 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gencarelli, M.; Laurino, A.; Landucci, E.; Buonvicino, D.; Mazzantini, C.; Chiellini, G.; Raimondi, L. 3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes. Biology 2020, 9, 95. https://doi.org/10.3390/biology9050095
Gencarelli M, Laurino A, Landucci E, Buonvicino D, Mazzantini C, Chiellini G, Raimondi L. 3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes. Biology. 2020; 9(5):95. https://doi.org/10.3390/biology9050095
Chicago/Turabian StyleGencarelli, Manuela, Annunziatina Laurino, Elisa Landucci, Daniela Buonvicino, Costanza Mazzantini, Grazia Chiellini, and Laura Raimondi. 2020. "3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes" Biology 9, no. 5: 95. https://doi.org/10.3390/biology9050095
APA StyleGencarelli, M., Laurino, A., Landucci, E., Buonvicino, D., Mazzantini, C., Chiellini, G., & Raimondi, L. (2020). 3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes. Biology, 9(5), 95. https://doi.org/10.3390/biology9050095






