Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer?
Abstract
1. Introduction
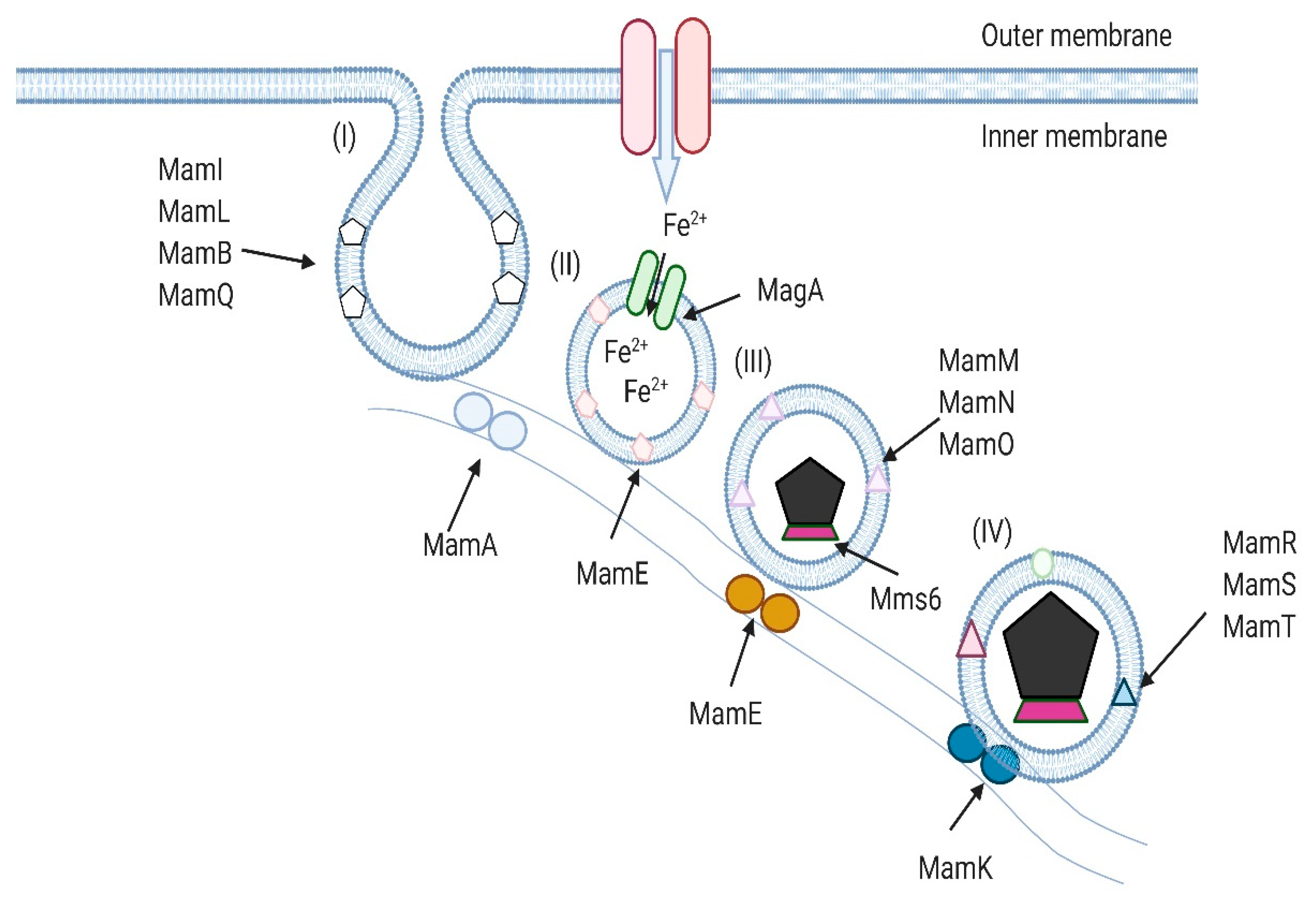
2. Magnetotactic Bacteria and Their Magnetosomes
3. The Applicability of Magnetotactic Bacteria and Magnetosomes in the Treatment of Cancer
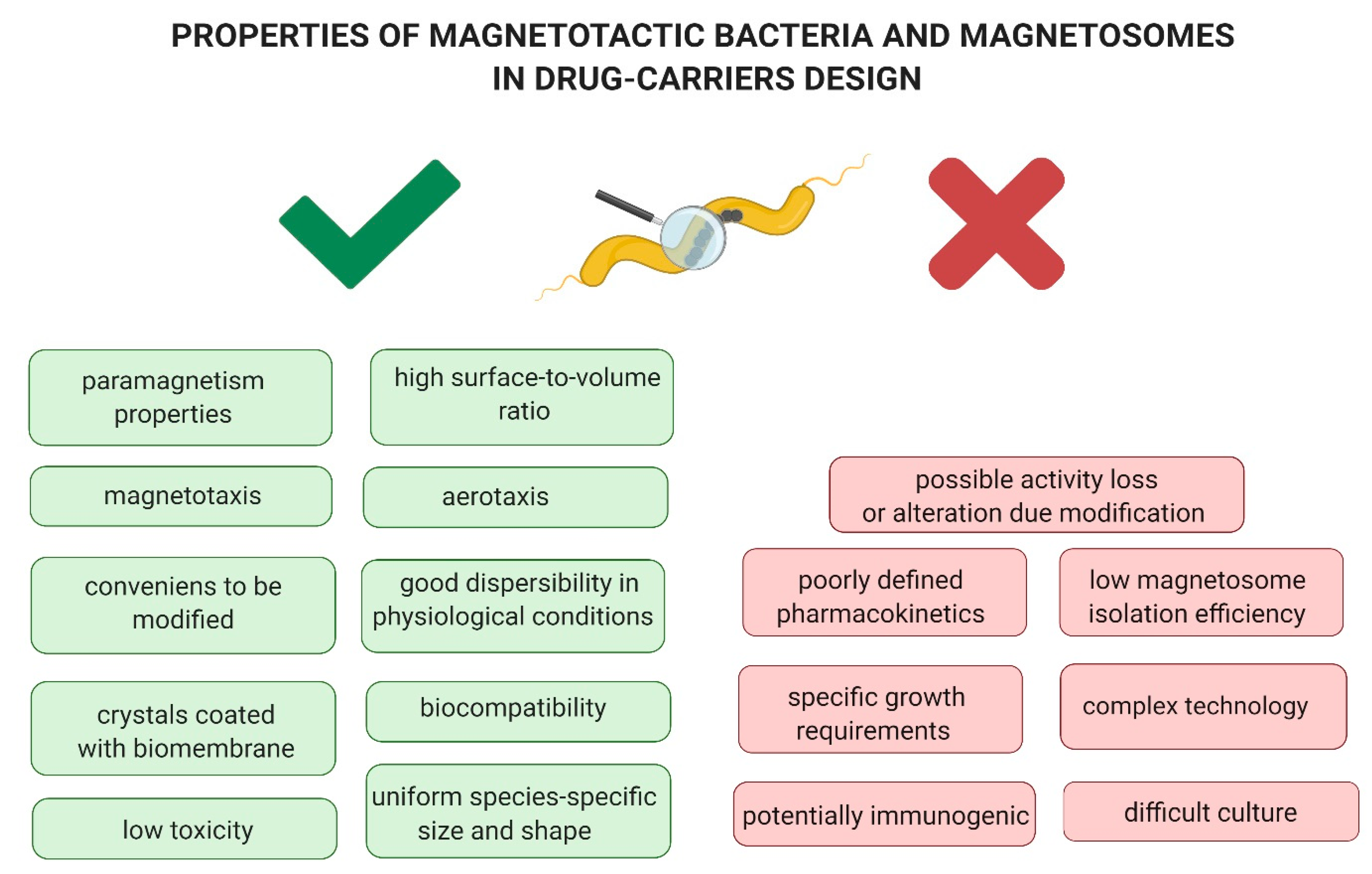
3.1. Biosafety of Magnetotactic Bacteria and Magnetosomes
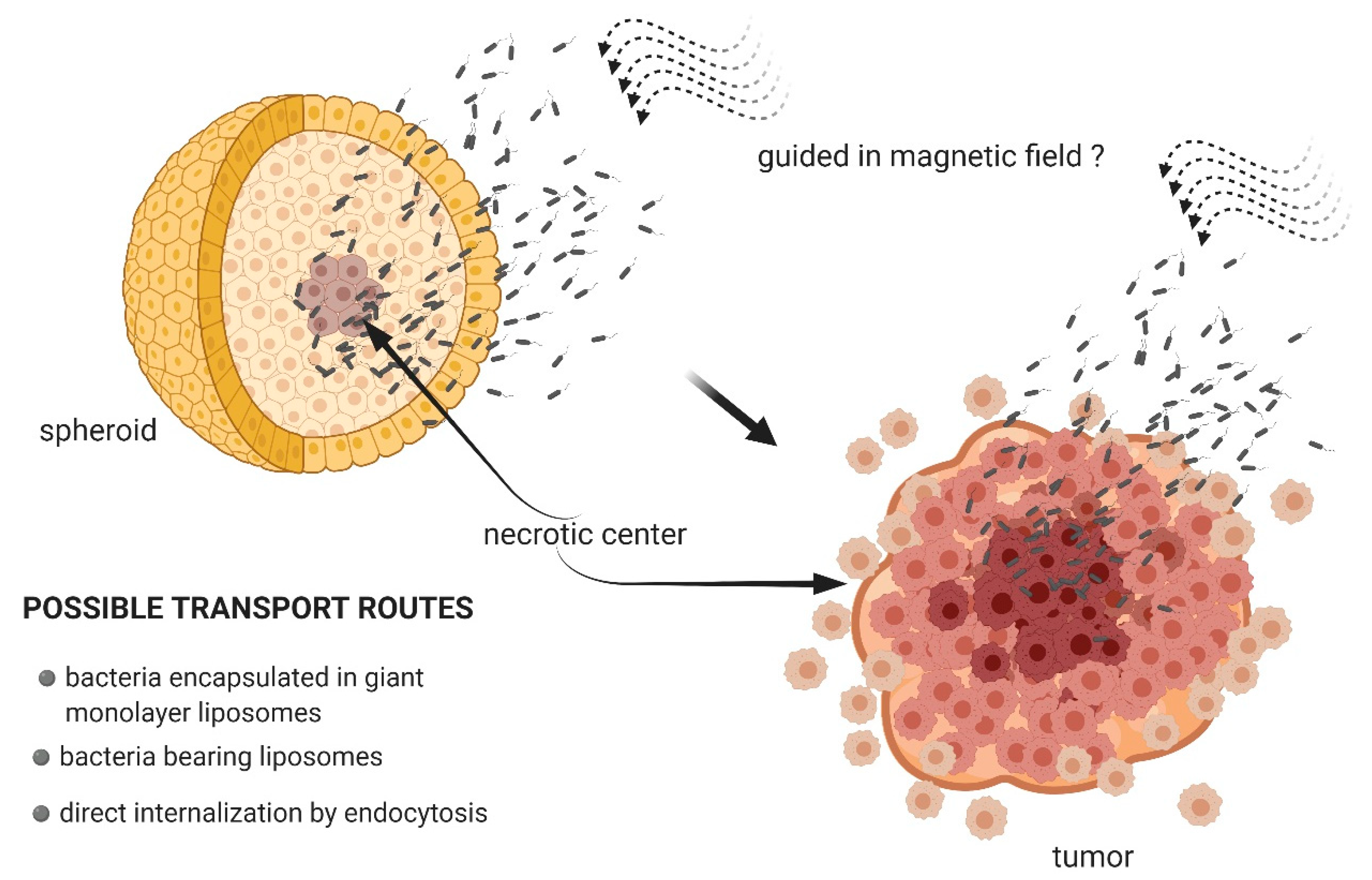
3.2. Magnetotactic Bacteria as Transport Systems
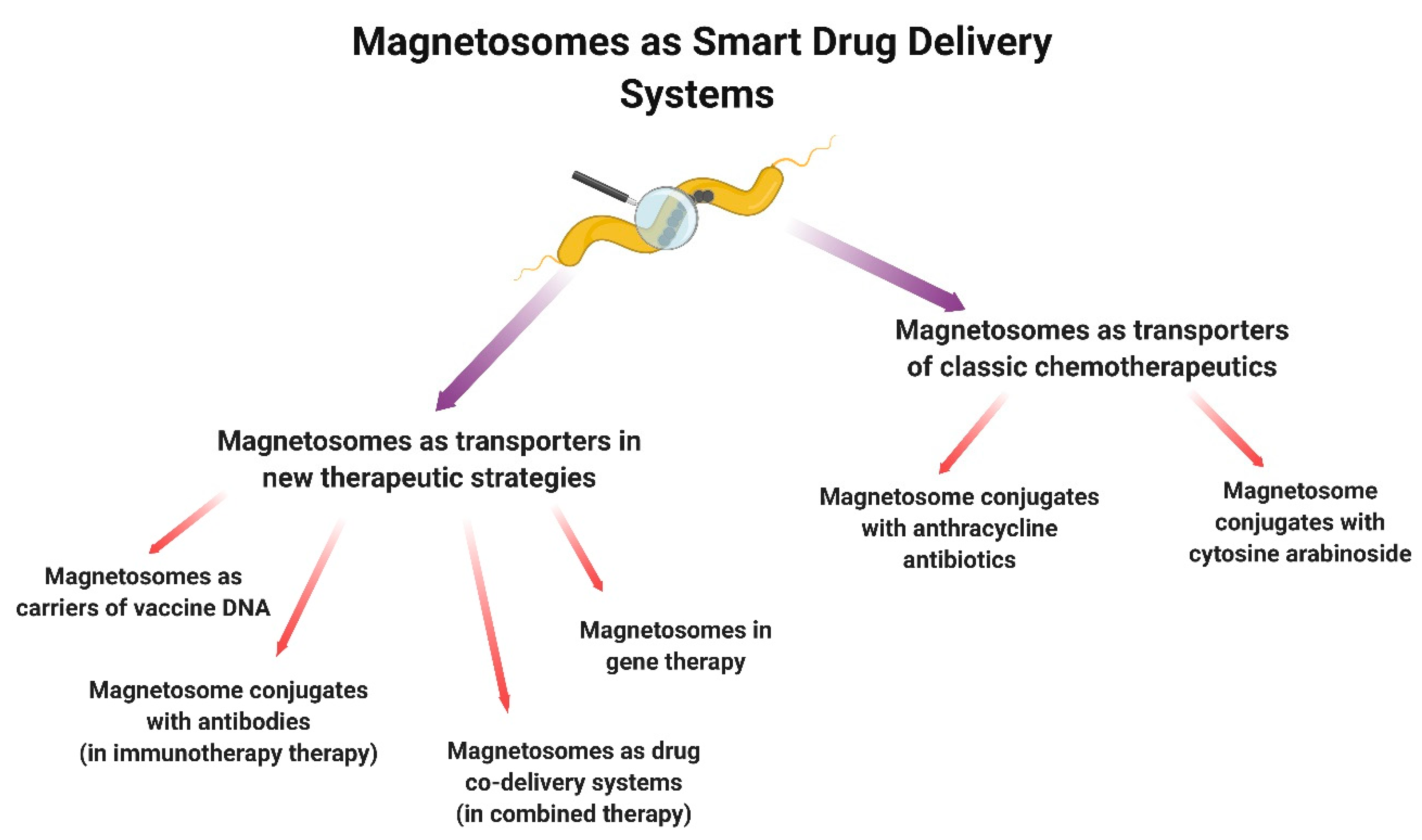
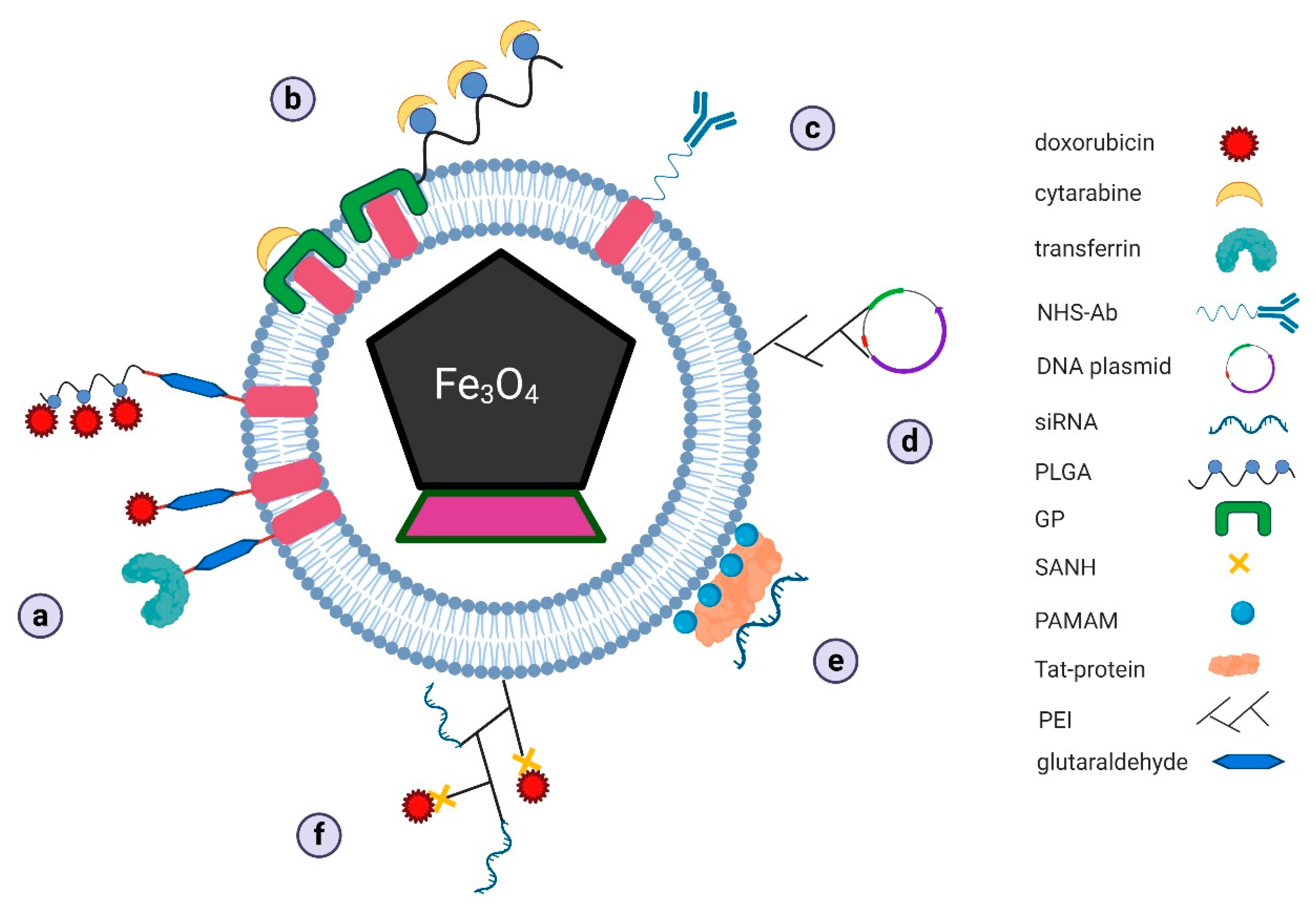
3.3. Magnetosomes as Transport Systems
3.3.1. Magnetosomes as Transporters of Classic Chemotherapeutics
Magnetosome Conjugates with Anthracycline Antibiotics
Magnetosome Conjugates with Cytosine Arabinoside
3.3.2. Magnetosomes as Transporters in New Therapeutic Strategies
Magnetosome Conjugates with Antibodies
Magnetosomes as Carriers of Vaccine DNA
Magnetosomes in Gene Therapy
Magnetosomes as Drug Co-Delivery Systems
4. Limitations in the Use of Magnetotactic Bacteria and Magnetosomes as Drug Delivery Systems
5. Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Damyanov, C.A.; Maslev, I.K.; Pavlov, V.S.; Avramov, L. Conventional treatment of cancer realities and problems. Ann. Complement. Altern. Med. 2018, 1, 1002. [Google Scholar]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Kutova, O.; Guryev, E.; Sokolova, E.A.; Alzeibak, R.; Balalaeva, I. Targeted Delivery to Tumors: Multidirectional Strategies to Improve Treatment Efficiency. Cancers 2019, 11, 68. [Google Scholar] [CrossRef]
- Yandim, M.K.; Adan-Gokbulut, A.; Baran, Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit. Rev. Biotechnol. 2016, 36, 716–726. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef]
- Kim, S.M.; Faix, P.H.; Schnitzer, J.E. Overcoming key biological barriers to cancer drug delivery and efficacy. J. Control. Release 2017, 267, 15–30. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kibria, G.; Hatakeyama, H.; Harashima, H. Cancer multidrug resistance: Mechanisms involved and strategies for circumvention using a drug delivery system. Arch. Pharmacal Res. 2013, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Frankel, R. The discovery of magnetotactic/magnetosensitive bacteria. Chin. J. Oceanol. Limnol. 2009, 27, 1–2. [Google Scholar] [CrossRef]
- Lefèvre, C.T.; Bazylinski, D.A. Ecology, Diversity, and Evolution of Magnetotactic Bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Bazylinski, D.A.; Xiao, T.; Wu, L.-F.; Pan, Y. Life with compass: Diversity and biogeography of magnetotactic bacteria. Environ. Microbiol. 2013, 16, 2646–2658. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.L.; Bazylinski, D.A.; Edwards, K.J. South-Seeking Magnetotactic Bacteria in the Northern Hemisphere. Science 2006, 311, 371–374. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Sadighi, A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid Interface Sci. 2013, 189–190, 1–20. [Google Scholar] [CrossRef]
- Scheffel, A.; Gruska, M.; Faivre, D.; Linaroudis, A.; Plitzko, J.M.; Schüler, D. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 2005, 440, 110–114. [Google Scholar] [CrossRef]
- Murat, D.; Quinlan, A.; Vali, H.; Komeili, A. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc. Natl. Acad. Sci. USA 2010, 107, 5593–5598. [Google Scholar] [CrossRef]
- Nudelman, H.; Zarivach, R. Structure prediction of magnetosome-associated proteins. Front. Microbiol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Dieudonné, A.; Pignol, D.; Prévéral, S. Magnetosomes: Biogenic iron nanoparticles produced by environmental bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 3637–3649. [Google Scholar] [CrossRef]
- Schüler, D. Formation of magnetosomes in magnetotactic bacteria. J. Mol. Microbiol. Biotechnol. 1999, 1, 79–86. [Google Scholar] [PubMed]
- Lefèvre, C.T.; Abreu, F.; Lins, U.; Bazylinski, D.A. A Bacterial Backbone: Magnetosomes in Magnetotactic Bacteria. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 4, pp. 78–102. [Google Scholar]
- Bazylinski, D.A.; Schübbe, S. Controlled Biomineralization by and Applications of Magnetotactic Bacteria. Adv. Appl. Microbiol. 2007, 62, 21–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Liang, X.-J.; Wang, P.C. Bacterial Magnetosome: A Novel Biogenetic Magnetic Targeted Drug Carrier with Potential Multifunctions. J. Nanomater. 2011, 2011, 469031–469043. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Revathy, T.; Jayasri, M.A.; Suthindhiran, K. Toxicity assessment of magnetosomes in different models. 3 Biotech 2017, 7, 126. [Google Scholar] [CrossRef]
- Xiang, L.; Wei, J.; Jianbo, S.; Guili, W.; Feng, G.; Ying, L. Purified and sterilized magnetosomes from Magnetospirillum gryphiswaldense MSR-1 were not toxic to mouse fibroblasts in vitro. Lett. Appl. Microbiol. 2007, 45, 75–81. [Google Scholar] [CrossRef]
- Qi, L.; Lv, X.; Zhang, T.; Jia, P.; Yan, R.; Li, S.; Zou, R.; Xue, Y.; Dai, L. Cytotoxicity and genotoxicity of bacterial magnetosomes against human retinal pigment epithelium cells. Sci. Rep. 2016, 6, 26961. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Endotoxins: Lipopolysaccharides of Gram-Negative Bacteria. Membr. Biog. 2010, 53, 3–25. [Google Scholar] [CrossRef]
- Sun, J.; Tang, T.; Duan, J.; Xu, P.-X.; Wang, Z.; Zhang, Y.; Wu, L.; Li, Y. Biocompatibility of bacterial magnetosomes: Acute toxicity, immunotoxicity and cytotoxicity. Nanotoxicology 2010, 4, 271–283. [Google Scholar] [CrossRef]
- Cypriano, J.; Werckmann, J.; Vargas, G.; Dos Santos, A.L.; Silva, K.T.; Leão, P.; Almeida, F.P.; Bazylinski, D.A.; Farina, M.; Lins, U.; et al. Uptake and persistence of bacterial magnetite magnetosomes in a mammalian cell line: Implications for medical and biotechnological applications. PLoS ONE 2019, 14, e0215657. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.-L.; Duan, J.-H.; Ren, J.; Yang, X.-D.; Dai, S.; Li, Y.; Jian-Bo, S.; Zi-Liang, W.; Jin-Hong, D.; et al. Targeted Distribution of Bacterial Magnetosomes Isolated from Magnetospirillum gryphiswaldense MSR-1 in Healthy Sprague-Dawley Rats. J. Nanosci. Nanotechnol. 2009, 9, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, A.; Wang, H.; Pu, P.; Jiang, X.; Kang, C.; Chang, J. Tat-BMPs-PAMAM Conjugates Enhance Therapeutic Effect of Small Interference RNA on U251 Glioma Cells In Vitro and In Vivo. Hum. Gene Ther. 2010, 21, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Lee, S.; Kim, J.; Choi, H.; Seo, J.; Koo, K.-I. Magnetically guided micro-droplet using biological magnetic material for smart drug delivery system. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 1390–1393. [Google Scholar]
- Lee, S.; Ahn, J.-H.; Choi, H.; Seo, J.M.; Cho, D.-I.D.; Koo, K.-I. Natural magnetic nanoparticle containing droplet for smart drug delivery and heat treatment. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 3541–3544. [Google Scholar]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Development of non-pyrogenic magnetosome minerals coated with poly-l-lysine leading to full disappearance of intracranial U87-Luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials 2017, 141, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Le Fèvre, R.; Durand-Dubief, M.; Chebbi, I.; Mandawala, C.; Lagroix, F.; Valet, J.-P.; Idbaih, A.; Adam, C.; Delattre, J.-Y.; Schmitt, C.; et al. Enhanced antitumor efficacy of biocompatible magnetosomes for the magnetic hyperthermia treatment of glioblastoma. Theranostics 2017, 7, 4618–4631. [Google Scholar] [CrossRef]
- Mandawala, C.; Chebbi, I.; Durand-Dubief, M.; Le Fèvre, R.; Hamdous, Y.; Guyot, F.; Alphandéry, E. Biocompatible and stable magnetosome minerals coated with poly-l-lysine, citric acid, oleic acid, and carboxy-methyl-dextran for application in the magnetic hyperthermia treatment of tumors. J. Mater. Chem. B 2017, 5, 7644–7660. [Google Scholar] [CrossRef]
- Hamdous, Y.; Chebbi, I.; Mandawala, C.; Le Fèvre, R.; Guyot, F.; Seksek, O.; Alphandéry, E. Biocompatible coated magnetosome minerals with various organization and cellular interaction properties induce cytotoxicity towards RG-2 and GL-261 glioma cells in the presence of an alternating magnetic field. J. Nanobiotechnol. 2017, 15, 74. [Google Scholar] [CrossRef]
- Martel, S.; Mohammadi, M.; Felfoul, O.; Lu, Z.; Pouponneau, P. Flagellated Magnetotactic Bacteria as Controlled MRI-trackable Propulsion and Steering Systems for Medical Nanorobots Operating in the Human Microvasculature. Int. J. Robot. Res. 2009, 28, 571–582. [Google Scholar] [CrossRef]
- Lu, Z.; Martel, S. Preliminary Investigation of Bio-carriers Using Magnetotactic Bacteria. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 1, pp. 3415–3418. [Google Scholar]
- Mathuriya, A.S. Magnetotactic bacteria: Nanodrivers of the future. Crit. Rev. Biotechnol. 2015, 36, 788–802. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2018, 116, 206–226. [Google Scholar] [CrossRef]
- Mokrani, N.; Felfoul, O.; Zarreh, F.A.; Mohammadi, M.; Aloyz, R.; Batist, G.; Martel, S. Magnetotactic bacteria penetration into multicellular tumor spheroids for targeted therapy. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 4371–4374. [Google Scholar]
- Afkhami, F.; Taherkhani, S.; Mohammadi, M.; Martel, S. Encapsulation of magnetotactic bacteria for targeted and controlled delivery of anticancer agents for tumor therapy. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; Volume 2011, pp. 6668–6671. [Google Scholar]
- Alsaiari, S.; Ezzedine, A.H.; Abdallah, A.M.; Sougrat, R.; Khashab, N.M. Magnetotactic bacterial cages as safe and smart gene delivery vehicles. OpenNano 2016, 1, 36–45. [Google Scholar] [CrossRef]
- Taherkhani, S.; Mohammadi, M.; Daoud, J.; Martel, S.; Tabrizian, M. Covalent Binding of Nanoliposomes to the Surface of Magnetotactic Bacteria for the Synthesis of Self-Propelled Therapeutic Agents. ACS Nano 2014, 8, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; De Lanauze, D.; Xu, Y.Z.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; LaFleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Mordente, A.; Meucci, E.; Martorana, G.; Tavian, D.; Silvestrini, A. Topoisomerases and Anthracyclines: Recent Advances and Perspectives in Anticancer Therapy and Prevention of Cardiotoxicity. Curr. Med. Chem. 2017, 24, 1607–1626. [Google Scholar] [CrossRef]
- Varela-Lopez, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernández, T.Y.; Romero-Márquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef]
- Guo, L.; Huang, J.; Zhang, X.; Li, Y.; Zheng, L. Bacterial magnetic nanoparticles as drug carriers. J. Mater. Chem. 2008, 18, 5993–5997. [Google Scholar] [CrossRef]
- Lin, W.-L.; Liang, P.-C.; Chen, Y.-C.; Chiang, C.-F.; Mo, L.-R.; Wei, S.-Y.; Hsieh, W.-Y.; Lin, W.L. Doxorubicin-modified magnetic nanoparticles as a drug delivery system for magnetic resonance imaging-monitoring magnet-enhancing tumor chemotherapy. Int. J. Nanomed. 2016, 11, 2021–2037. [Google Scholar] [CrossRef]
- Guo, L.; Huang, J.; Zheng, L.-M. Control generating of bacterial magnetic nanoparticle–doxorubicin conjugates by poly-L-glutamic acid surface modification. Nanotechnologly 2011, 22, 175102. [Google Scholar] [CrossRef]
- Sun, J.; Duan, J.-H.; Dai, S.-L.; Ren, J.; Guo, L.; Jiang, W.; Li, Y. Preparation and anti-tumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: Magnetic nanoparticles as drug carriers isolated fromMagnetospirillum gryphiswaldense. Biotechnol. Bioeng. 2008, 101, 1313–1320. [Google Scholar] [CrossRef]
- Caforio, M.; Sorino, C.; Iacovelli, S.; Fanciulli, M.; Locatelli, F.; Folgiero, V. Recent advances in searching c-Myc transcriptional cofactors during tumorigenesis. J. Exp. Clin. Cancer Res. 2018, 37, 239. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Duan, J.-H.; Dai, S.-L.; Ren, J.; Zhang, Y.-D.; Tian, J.-S.; Li, Y. In vitro and in vivo antitumor effects of doxorubicin loaded with bacterial magnetosomes (DBMs) on H22 cells: The magnetic bio-nanoparticles as drug carriers. Cancer Lett. 2007, 258, 109–117. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Wang, X.; Liu, J.; Zhang, Y.; Niu, W.; Basit, A.; Liu, W.; Jiang, W. Growth-inhibitory effects of anthracycline-loaded bacterial magnetosomes against hepatic cancerin vitroandin vivo. Nanomedicine 2019, 14, 1663–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, Y.; Zhang, Y.; Wang, X.; Liu, J.; Basit, A.; Miao, T.; Liu, W.; Jiang, W. Bacterial magnetosomes loaded with doxorubicin and transferrin improve targeted therapy of hepatocellular carcinoma. Nanotheranostics 2019, 3, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Yan, M. Targeted hepatocellular carcinoma therapy: Transferrin modified, self-assembled polymeric nanomedicine for co-delivery of cisplatin and doxorubicin. Drug Dev. Ind. Pharm. 2016, 42, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.; Lotey, N. Role of circulating tumor cells in future diagnosis and therapy of cancer. J. Cancer Metastasis Treat. 2015, 1, 44–56. [Google Scholar] [CrossRef]
- Rostami, P.; Kashaninejad, N.; Moshksayan, K.; Saidi, M.; Firoozabadi, B.; Nguyen, A.V. Novel approaches in cancer management with circulating tumor cell clusters. J. Sci. Adv. Mater. Devices 2019, 4, 1–18. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; Macdonald, T.; Wu, H.; Provenzale, J.; Peng, X.; Huang, J.; Wang, L.; Wang, A.Y.; Yang, J.; et al. Improving sensitivity and specificity of capturing and detecting targeted cancer cells with anti-biofouling polymer coated magnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2016, 150, 261–270. [Google Scholar] [CrossRef]
- Biglione, C.; Bergueiro, J.; Asadian-Birjand, M.; Weise, C.; Khobragade, V.; Chate, G.; Dongare, M.; Khandare, J.J.; Strumia, M.C.; Calderón, M. Optimizing Circulating Tumor Cells’ Capture Efficiency of Magnetic Nanogels by Transferrin Decoration. Polymers 2018, 10, 174. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Thomas, X.; Calvo, F.; Rousselot, P.; Rabilloud, M.; El Jaffari, A.; Cros, E.; Dumontet, C. In vivo mechanisms of resistance to cytarabine in acute myeloid leukaemia. Br. J. Haematol. 2002, 117, 860–868. [Google Scholar] [CrossRef]
- Sreenivasan, Y.; Sarkar, A.; Manna, S.K. Mechanism of cytosine arabinoside-mediated apoptosis: Role of Rel A (p65) dephosphorylation. Oncogene 2003, 22, 4356–4369. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.-R.; Chen, Q.-Q.; Wang, C.-Y.; Yao, M.-C.; Zhang, W.-J. Exploring the Antitumor Mechanism of High-Dose Cytarabine through the Metabolic Perturbations of Ribonucleotide and Deoxyribonucleotide in Human Promyelocytic Leukemia HL-60 Cells. Molecules 2017, 22, 499. [Google Scholar] [CrossRef] [PubMed]
- Stentoft, J. The Toxicity of Cytarabine. Drug Saf. 1990, 5, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Liu, Y.; Wang, S.; Xie, M.; Wu, S.; Chen, A.; Wu, W. Construction of a Novel Magnetic Targeting Anti-Tumor Drug Delivery System: Cytosine Arabinoside-Loaded Bacterial Magnetosome. Materials 2013, 6, 3755–3763. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, M.; Wang, S.; Zheng, Q.; Chen, A.; Deng, Q. Facile fabrication of high performances MTX nanocomposites with natural biomembrane bacterial nanoparticles using GP. Mater. Lett. 2013, 100, 248–251. [Google Scholar] [CrossRef]
- Dai, Q.; Ma, Y.; Wang, S.; Kankala, R.K.; Liu, Y. Investigation of Various Cross-Linking Methods for the Immobilization of Cytosine Arabinoside on Bacterial Magnetosomes. J. Nanomater. 2017, 2017, 6738484. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Q.-L.; Wang, S.-B.; Deng, Q.-J.; Wu, W.-G.; Chen, A.-Z. Preparation and in vitro antitumor effects of cytosine arabinoside-loaded genipin-poly-l-glutamic acid-modified bacterial magnetosomes. Int. J. Nanomed. 2015, 10, 1387–1397. [Google Scholar] [CrossRef]
- Long, R.; Liu, Y.; Dai, Q.; Wang, S.; Deng, Q.; Zhou, X. A Natural Bacterium-Produced Membrane-Bound Nanocarrier for Drug Combination Therapy. Materials 2016, 9, 889. [Google Scholar] [CrossRef]
- Alissafi, T.; Hatzioannou, A.; Legaki, A.; Varveri, A.; Verginis, P. Balancing cancer immunotherapy and immune-related adverse events: The emerging role of regulatory T cells. J. Autoimmun. 2019, 104, 102310. [Google Scholar] [CrossRef]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef]
- Tang, Y.-S.; Wang, D.; Zhou, C.; Zhang, S. Preparation and anti-tumor efficiency evaluation of bacterial magnetosome–anti-4-1BB antibody complex: Bacterial magnetosome as antibody carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Appl. Biochem. 2019, 66, 290–297. [Google Scholar] [CrossRef]
- Erdal, E.; Demirbilek, M.; Yeh, Y.; Akbal, Ö.; Ruff, L.; Bozkurt, D.; Cabuk, A.; Senel, Y.; Gumuskaya, B.; Algın, O.; et al. A Comparative Study of Receptor-Targeted Magnetosome and HSA-Coated Iron Oxide Nanoparticles as MRI Contrast-Enhancing Agent in Animal Cancer Model. Appl. Biochem. Biotechnol. 2018, 185, 91–113. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef]
- Ghaffarifar, F. Plasmid DNA vaccines: Where are we now? Drugs Today 2018, 54, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Bin, W.; Huali, J.; Wei, J.; Jiesheng, T.; Feng, G.; Li, Y. Bacterial magnetic particles (BMPs)-PEI as a novel and efficient non-viral gene delivery system. J. Gene Med. 2007, 9, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-S.; Wang, D.; Zhou, C.; Ma, W.; Zhang, Y.-Q.; Liu, B.; Zhang, S. Bacterial magnetic particles as a novel and efficient gene vaccine delivery system. Gene Ther. 2012, 19, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, C.; Ma, W.; Wang, N.; Zhang, S. An Enhanced Anti-Tumor Response by Using Bacterial Magnetosomes Gene Administration Platform. Arch. Clin. Biomed. Res. 2018, 2, 145–150. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, C.; Wang, N.; Ma, W.; Lin, C.; Wang, Y.; Liang, X.; Li, J.; Guo, S.; Wang, Y.; et al. Enhancement of DNA vaccine potency by sandwiching antigen-coding gene between secondary lymphoid tissue chemokine (SLC) and IgG Fc fragment genes. Cancer Biol. Ther. 2006, 5, 427–434. [Google Scholar] [CrossRef]
- Sun, W.; Shi, Q.; Zhang, H.; Yang, K.; Ke, Y.; Wang, Y.; Qiao, L. Advances in the techniques and methodologies of cancer gene therapy. Discov. Med. 2019, 27, 45–55. [Google Scholar]
- Yan, C.; Gu, J.; Hou, D.; Jing, H.; Wang, J.; Guo, Y.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improved tumor targetability of Tat-conjugated PAMAM dendrimers as a novel nanosized anti-tumor drug carrier. Drug Dev. Ind. Pharm. 2014, 41, 617–622. [Google Scholar] [CrossRef]
- Dai, Q.; Long, R.; Wang, S.-B.; Kankala, R.K.; Wang, J.; Jiang, W.; Liu, Y. Bacterial magnetosomes as an efficient gene delivery platform for cancer theranostics. Microb. Cell Factories 2017, 16, 216. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Geng, Y.-Y.; Wang, J.-J.; Zhang, X.-M.; Yang, S.-S.; Jiang, W.; Liu, W. An enhanced anti-tumor effect of apoptin-cecropin B on human hepatoma cells by using bacterial magnetic particle gene delivery system. Biochem. Biophys. Res. Commun. 2018, 496, 719–725. [Google Scholar] [CrossRef]
- Birame, B.M.; Jigui, W.; Fuxian, Y.; Jiazeng, S.; Zhili, L.; WeiQuan, L. Potentiation of Apoptin-induced apoptosis by Cecropin B-like antibacterial peptide ABPs1 in human HeLa cervical cancer cell lines is associated with membrane pore formation and caspase-3 activation. J. Microbiol. Biotechnol. 2014, 24, 756–764. [Google Scholar] [CrossRef]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef]
- Long, R.-M.; Dai, Q.-L.; Zhou, X.; Cai, D.-H.; Hong, Y.-Z.; Wang, S.-B.; Liu, Y. Bacterial magnetosomes-based nanocarriers for co-delivery of cancer therapeutics in vitro. Int. J. Nanomed. 2018, 13, 8269–8279. [Google Scholar] [CrossRef]
- Cheng, L.; Ke, Y.; Yu, S.; Jing, J. Co-delivery of doxorubicin and recombinant plasmid pHSP70-Plk1-shRNA by bacterial magnetosomes for osteosarcoma therapy. Int. J. Nanomed. 2016, 11, 5277–5286. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Q.; Wang, X.-S. PLK1, A Potential Target for Cancer Therapy. Transl. Oncol. 2017, 10, 22–32. [Google Scholar] [CrossRef]
- Heyen, U.; Schüler, D. Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl. Microbiol. Biotechnol. 2003, 61, 536–544. [Google Scholar] [CrossRef]
- Faivre, D.; Menguy, N.; Pósfai, M.; Schüler, D. Environmental parameters affect the physical properties of fast-growing magnetosomes. Am. Miner. 2008, 93, 463–469. [Google Scholar] [CrossRef]
- Jajan, L.H.-G.; Hosseini, S.N.; Ghorbani, M.; Mousavi, S.F.; Ghareyazie, B.; Abolhassani, M. Effects of Environmental Conditions on High-Yield Magnetosome Production by Magnetospirillum gryphiswaldense MSR-1. Iran. Biomed. J. 2019, 23, 209–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Jiang, W.; Li, Y.; Li, J. Semicontinuous culture of Magnetospirillum gryphiswaldense MSR-1 cells in an autofermentor by nutrient-balancedand isosmotic feeding strategies. Appl. Environ. Microbiol. 2011, 77, 5851–5856. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, F.; Tang, T.; Jiang, W.; Tian, J.-S.; Li, Y.; Li, J.-L. High-yield growth and magnetosome formation by Magnetospirillum gryphiswaldense MSR-1 in an oxygen-controlled fermentor supplied solely with air. Appl. Microbiol. Biotechnol. 2008, 79, 389–397. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.R.; Guo, F.; Jiang, W.; Li, Y.; Li, J. Large-scale production of magnetosomes by chemostat culture of Magnetospirillum gryphiswaldense at high cell density. Microb. Cell Factories 2010, 9, 99. [Google Scholar] [CrossRef]
- Silva, K.T.; Leão, P.; Abreu, F.; López, J.A.; Gutarra, M.; Farina, M.; Bazylinski, D.A.; Freire, D.M.; Lins, U. Optimization of Magnetosome Production and Growth by the Magnetotactic Vibrio Magnetovibrio blakemorei Strain MV-1 through a Statistics-Based Experimental Design. Appl. Environ. Microbiol. 2013, 79, 2823–2827. [Google Scholar] [CrossRef]
- Kolinko, I.; Lohße, A.; Borg, S.; Raschdorf, O.; Jogler, C.; Tu, Q.; Pósfai, M.; Tompa, E.; Plitzko, J.M.; Brachmann, A.; et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat. Nanotechnol. 2014, 9, 193–197. [Google Scholar] [CrossRef]
- Park, W.; Na, K. Advances in the synthesis and application of nanoparticles for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 494–508. [Google Scholar] [CrossRef]
- Pędziwiatr-Werbicka, E.; Horodecka, K.; Shcharbin, D.; Bryszewska, M. Nanoparticles in combating cancer: Opportunities and limitations. A brief review. Curr. Med. Chem. 2020, 27, 1–13. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzajewska, D.; Wszołek, A.; Żwierełło, W.; Kirczuk, L.; Maruszewska, A. Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer? Biology 2020, 9, 102. https://doi.org/10.3390/biology9050102
Kuzajewska D, Wszołek A, Żwierełło W, Kirczuk L, Maruszewska A. Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer? Biology. 2020; 9(5):102. https://doi.org/10.3390/biology9050102
Chicago/Turabian StyleKuzajewska, Danuta, Agata Wszołek, Wojciech Żwierełło, Lucyna Kirczuk, and Agnieszka Maruszewska. 2020. "Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer?" Biology 9, no. 5: 102. https://doi.org/10.3390/biology9050102
APA StyleKuzajewska, D., Wszołek, A., Żwierełło, W., Kirczuk, L., & Maruszewska, A. (2020). Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer? Biology, 9(5), 102. https://doi.org/10.3390/biology9050102




